Abstract
To assess multi-gene panel testing in an ethnically diverse clinical cancer genetics practice.
We conducted a retrospective study of individuals with a personal or family history of cancer undergoing clinically indicated multi-gene panel tests of 6–110 genes, from six commercial laboratories. The 475 patients in the study included 228 Hispanics (47.6%), 166 non-Hispanic Whites (35.4%), 55 Asians (11.6%), 19 Blacks (4.0%), and seven others (1.5%).
Panel testing found that 15.6% (74/475) of patients carried deleterious mutations for a total of 79 mutations identified. This included 7.4% (35/475) of patients who had a mutation identified that would not have been tested with a gene-by-gene approach. The identification of a panel-added mutation impacted clinical management for most of cases (69%, 24/35), and genetic testing was recommended for the first degree relatives of nearly all of them (91%, 32/35). Variants of uncertain significance (VUSs) were identified in a higher proportion of tests performed in ethnic minorities. Multi-gene panel testing increases the yield of mutations detected and adds to the capability of providing individualized cancer risk assessment. VUSs represent an interpretive challenge due to less data available outside of White, non-Hispanic populations. Further studies are necessary to expand understanding of the implementation and utilization of panels across broad clinical settings and patient populations.
Keywords: hereditary cancer, cancer risk assessment, multi-gene panels, variants of uncertain significance, ethnic minorities
INTRODUCTION
Over the last twenty years, hereditary cancer risk assessment has evaluated individuals using a syndrome-by-syndrome and gene-by-gene testing approach. After the formation of a differential diagnosis, the most probable condition has been routinely evaluated first with subsequent analyses performed sequentially, guided by clinical judgment and often by insurance coverage and patient motivation. However, the rapid integration of next-generation sequencing (NGS) technologies into the practice of clinical cancer genetics has allowed for simultaneous assessment of multiple syndromes and genes in one analysis.
An emerging body of literature has begun to report on findings from these multi-gene assays in the research, clinical, and laboratory settings. The cohorts studied have included mostly White, non-Hispanic populations and have varied from large laboratory based reports(1–4) to small highly-selected clinical cohorts. (5–8) Additionally, registry based cohorts have looked at non-BRCA gene mutations in previously tested negative patients.(9–11) While these studies have varied in their methodologies and assays, most have found that a panel testing approach identifies mutations that would not have been identified with a syndrome-by-syndrome approach as well as a many variants of uncertain significance (VUSs). (1,2,4,9–12)
The additional yield of mutations identified via multi-gene panel testing in a clinical setting has not yet been well defined, especially among under-represented minority populations. Despite the ethnic and racial diversity of the US population, to date, all of the published cohorts are predominately Caucasian, of non-Hispanic ancestry. We report on the largest multi-ethnic cohort to-date of individuals evaluated for mixed clinical indications by twenty different laboratory assays offered by six CLIA-approved commercial laboratories. In this retrospective study we assessed the additional yield of panel testing by cancer site as well as the clinical characteristics of mutation carriers in a diverse clinic population.
MATERIALS and METHODS
Participants
Study participants were seen for clinical cancer genetic counseling at two University of Southern California (USC) Cancer Genetics Program sites, USC Norris Cancer Hospital and the Los Angeles County + USC Medical Center. A retrospective IRB-approved chart review was conducted of 475 cancer genetics clinic patients who underwent genetic testing with a multi-gene hereditary cancer panels. Individuals were eligible for inclusion if a multi-gene panel was ordered from any laboratory on or before 7/14/14. Genetic test results and clinicopathologic characteristics of each patient were reviewed. All patients received pre- and post-test genetic counseling from genetics professionals.
Genetic testing multi-gene panels
Tests were selected on the basis of clinical indication and insurance coverage. Panel tests ordered in the participants were performed by Myriad Genetics (n=354), Ambry Genetics (n=100), Fulgent Diagnostics (n=17), University of Washington Genetics Lab (n=2), City of Hope Molecular Diagnostics Laboratory (n=1), and Baylor Genetics Laboratory (n=1). Ambry panels included CancerNext™ (n=62), OvaNext™ (n=6), BreastNext™ (n=10), ColoNext™ (n=11), PGLNext™ (n=1), RenalNext™ (n=3), and BRCAPlus™ (n=7); from all other labs, only one hereditary cancer panel was ordered. Some panels had more than one version as laboratories added more genes to existing panels. Genes included on panels ordered are detailed in the Supplemental Material. All laboratories utilized in this cohort report variants of uncertain significance.
Review of mutations and variants
Each case was discussed at the time of assessment in a multidisciplinary cancer genetics case conference, and a differential diagnosis was formed. For each deleterious mutation or suspected deleterious mutation identified on a panel, the mutation was then classified as being either as a “target-gene” or “panel-added” mutations. If the gene was included in the differential diagnosis and would have been part of a syndrome-by-syndrome testing approach, then the mutation was categorized as being in a “target-gene.” If the gene would not have been tested using a syndrome-by-syndrome approach, then the mutation was categorized as “panel-added.” MUTYH mutations were considered “target-gene” mutations in colon cancer cases or families with colon cancer. PALB2 was considered a target gene if there was a combination of breast and pancreatic cancer in the family. TP53 was considered a target gene in breast cancer diagnosed under age 35. CDH1 was considered a target gene if the hereditary diffuse gastric cancer testing criteria were met or in a patient with lobular breast cancer with any gastric cancer in the family. Mutations in genes that were not routinely targeted prior to the clinical availability of panels, such as CHEK2, ATM, BARD1, RAD51C, and others were all considered “panel-added” mutations.
The number of variants of uncertain significance (VUSs) detected for each patient was counted. Any variants that were reported as “likely polymorphisms” were omitted from this analysis. If the reporting laboratory reclassified a variant before 12/31/14, the updated classification was used for this analysis.
The mutation and variants reported in our analysis are all described in accordance with the interpretation provided by lab report. Public databases were not routinely searched to further interpret the laboratory classification. However, the clinical interpretation was altered in two cases. One patient with a MSH2 VUS was counseled mutation-positive due to personal and family phenotype of Lynch syndrome and an informative segregation analysis. One patient, known to have a 56 base pair deletion in BRCA1, underwent a panel test with a different laboratory due to suspicion of Lynch syndrome, but the NGS panel was unable to detect the previously identified BRCA1 mutation.
Statistical Analysis
Using STATA (Stata Statistical Software: Release 13 College Station, TX: StataCorp LP) the odds ratio of detecting a mutation by gender, race/ethnicity, and whether the proband had a diagnosis of cancer was determined. The odds ratio of detecting a VUS was also determined for each of these three predictors.
The correlation between the number of genes on a panel and the likelihood of identifying a variant of uncertain significance was measured using simple linear regression to estimate the slope and to measure R2. Seven panel groups were created by collapsing the 14 panels with multiple versions from the same laboratory into the same group. The panel groups included, Ambry BRCAplus™ v1, Ambry ColoNext™ v1, Ambry BreastNext™ (3 versions), Ambry OvaNext™ (3 versions), Ambry CancerNext™ (3 versions), Myriad myRisk™ v1, and Fulgent (2 versions); an average number of genes tested was determined for each panel group. Ambry PGLNext,™ Ambry RenalNext™, the Baylor High Risk Colorectal Cancer Panel, the City of Hope Molecular Diagnostic Lab Breast Cancer Susceptibility Panel panel, and the two University of Washington BROCA™ panels were excluded because they were used infrequently and there was very little overlap of the composition of the panels. The proportion of panels with a mutation identified or with a variant of uncertain significance was determined for each panel group. Data was analyzed separately for mutations and for VUSs. This analysis was performed both with and without the Fulgent panels, since the Fulgent panels contained far more genes than any other panel.
RESULTS
Demographics
The study cohort consisted of 475 patients assessed through the USC Norris Cancer Genetics Program at two different sites, Los Angeles County + USC Medical Center (n=253) and USC Norris Cancer Hospital (n=222). The mean age at assessment was 48.9 years old (SD ± 12.3). The majority of the cohort was female (86.3%) and had a previous or current cancer diagnosis (77.5%). (Table 1) The most common cancer (41.4%) was breast cancer, followed by colorectal cancer (18.1%), ovarian cancer (8.6%), endometrial cancer (5.1%), and gastric cancer (3.4%). Almost 12% of the cohort had another type of cancer. There were 54 (11.3%) individuals with two or more primary cancer diagnoses, excluding non-melanoma skin cancer, and 18 (3.8%) individuals had three or more primaries. Individuals with multiple primaries were counted in more than one primary tumor site category.
Table 1.
Deleterious mutations & variant of uncertain significance (VUS) detection overall, by gender, race/ethnicity, and cancer status (N=475)
| N,% | Patients with one or more deleterious mutation N, % |
OR, 95% CI |
p- value |
Patients with one or more VUS N, % |
OR, 95% CI |
p- value |
Patients with two or more VUSs N, % |
OR, 95% CI |
p- value |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 475 | 74 (15.6) | - | 205 (43.2) | - | 63 (13.3) | - | - | |||
| Gender | Female | 410 (86.3) | 60 (14.6) | Ref | 177 (43.2) | Ref | 57 (13.9) | Ref | - | ||
| Male | 65 (13.7) | 14 (21.5) | 1.60 (0.83–3.07) | 0.157 | 28 (43.1) | 1.00 (0.59–1.69) | 0.989 | 6 (9.2) | 0.63 (0.26–1.53) | 0.306 | |
| Race Ethnicity | White, Non Hispanic | 166 (35.4) | 29 (17.5) | Ref | - | 64 (38.6) | Ref | - | 12 (7.2) | Ref | - |
| Hispanic | 228 (47.6) | 33 (14.5) | 0.70 (0.41–1.21) | 0.203 | 103 (45.1) | 1.36 (0.91–2.04) | 0.138 | 37 (16.2) | 2.54 (1.28–5.04) | 0.008 | |
| Black | 19 (4.0) | 3 (15.8) | 0.83 (0.23–3.02) | 0.776 | 12 (63.1) | 2.79 (1.04–7.44) | 0.041 | 7 (36.8) | 7.58 (2.52–22.8) | 0.000 | |
| Asian | 55 (11.6) | 8 (14.5) | 0.75 (0.32–1.75) | 0.509 | 23 (41.8) | 1.17 (0.63–2.17) | 0.623 | 5 (9.1) | 1.30 (0.44–3.87) | 0.637 | |
| More than one | 7 (1.5) | 1 (14.3) | 0.74 (0.09–6.34) | 0.781 | 3 (42.9) | 1.22 (0.26–5.62) | 0.800 | 2 (28.6) | 5.20 (0.91–29.68) | 0.064 | |
| Cancer History | No | 107 (22.7) | 7 (6.5) | Ref | - | 45 (42.0) | Ref | - | 15 (14.0) | Ref | - |
| Yes | 368 (77.5) | 67 (18.2) | 3.13 (1.39–7.06) | 0.006 | 160 (43.5) | 1.03 (0.67–1.61) | 0.868 | 49 (13.3) | 0.91 (0.49–1.69) | 0.760 |
The study population consisted of individuals from diverse ethnic backgrounds. Forty-eight percent of the cohort self-identified as Hispanic, 35.4% as non-Hispanic White, 11.6% as Asian, and 4.0% as Black. The majority of the 226 self-identified Hispanics were of Mexican ancestry (n=116, 71.2%); 21.2% (n=48) were of Central American ancestry (El Salvador, n=23; Guatemala, n=18; Other, n=8), and the remainder were South American (n=6), Caribbean, (n=4), or Spanish and Hispanic, ancestry (n=7). Amongst the 55 Asian patients, 30.9% were Filipino, 30.9% were Chinese, 18.2% were Vietnamese, and the remaining 20.0% were Korean, Japanese Bangladeshi, Pakistani, or Indonesian. Most of the 19 Black patients (94.7%), identified as Black, non-Hispanic including one Jamaican and one Belizean patient, and one Black, Hispanic patient from Panama. The sample also consisted of 29 patients (6.1%) reporting Jewish ancestry who were among the White, non-Hispanic (n=17) or Hispanic participants (n=2).
Genetic Test Results
From the 475 next-generation cancer panels performed, 74 (15.6%) patients were found to carry deleterious mutations or suspected deleterious mutations for a total of 79 mutations identified, with five patients having two mutations (Table 1). The positivity rate was 18.2% for patients with a cancer diagnosis. This was significantly higher than the 6.5% positivity rate seen in individuals with no personal history of cancer, tested based on family history. (OR=3.13 (1.39–7.06), p=0.006) Among the 475 panels, 205 (43.2%) had at least one VUS and 63 (13.3%) had at least two VUSs identified.
Relative to non-Hispanic Whites, the odds of detecting a deleterious mutation was slightly lower among Hispanics (OR=0.70 (0.41–1.21), p=0.203), Asians (OR=0.75 (0.32–1.75), p=0.509), and Blacks (OR=0.83 (0.23–3.02), p=0.776). These differences were not statistically significant (Table 1). However, relative to non-Hispanic Whites, the odds of detecting one or more VUSs was higher in Hispanics (OR=1.36 (0.91–2.04), p=0.138), Asians (OR=1.17 (0.63–2.17), p=0.623), and Blacks (OR=2.79 (1.04–7.44), p=0.041). Additionally, both Hispanics and Blacks were both significantly more likely than non-Hispanic Whites to have two or more VUSs (OR=2.54 (1.28–5.04), p=0.008 and OR=7.58 (2.52–22.8), p<0.001, respectively). (Table 1)
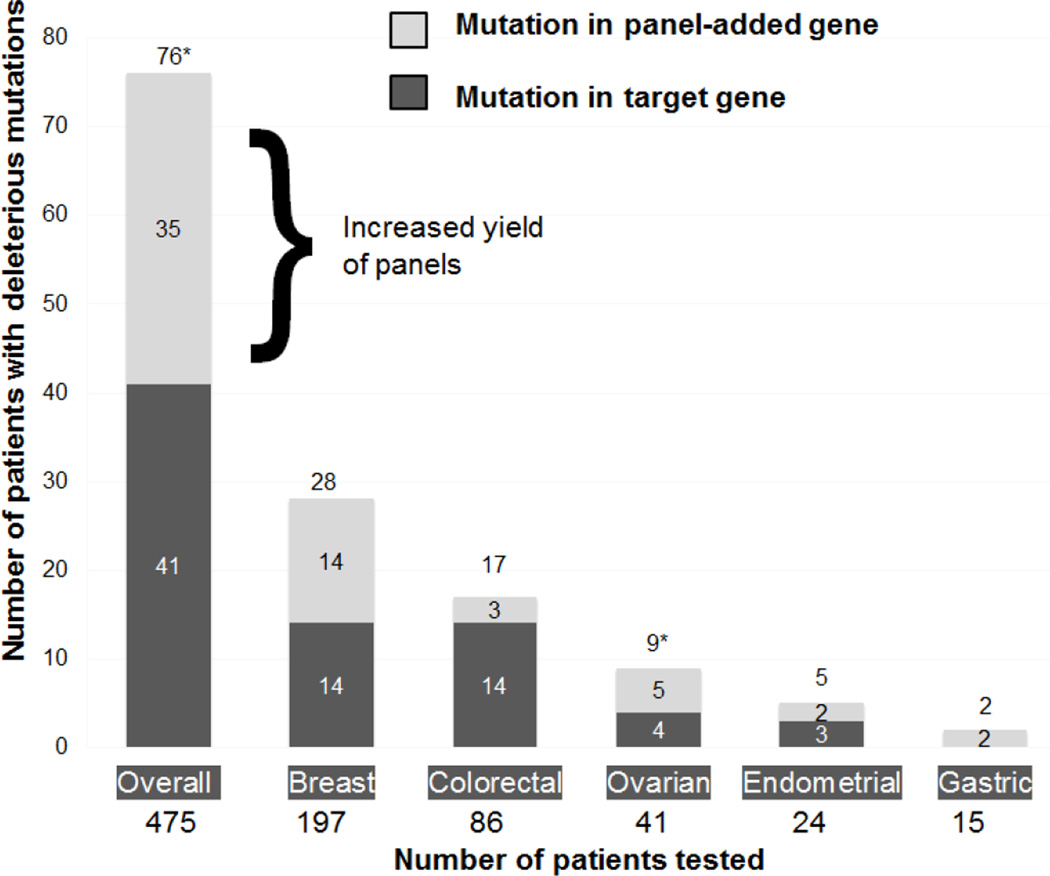
Amongst the 74 individuals who tested positive, over half (55.5%, 41/74) carried a target-gene mutation and almost half (47.3%, 35/74) carried a mutation in a panel-added gene (Figure 1). The clinical characteristics of mutation carriers are shown in Table 2.
Figure 1.
Mutations detected in 475 patients, overall and by cancer site, illustrating yield of panels
*Patient(s) with both a target mutation and panel-detected mutation are included in both categories
Table 2.
Mutations (N=79) identified in 475 patients’ panels performed by Myriad, Ambry, Fulgent, and other labs, with clinical information provided
| Gene | Number of observations |
Patient(s) cancer history (bold if gene would not have been tested in a target gene approach) |
|---|---|---|
| APC | 5 | 5 colorectal |
| ATM | 8 | 3 ovariana, 1 thyroid, 1 gastric, 1 bile duct, 1 GISTb, 1 fam hx breast |
| BARD1 | 1 | 1 breastc |
| BRCA1 | 11 | 7 breast, 2 ovarian, 2 fam hx breast and ovarian |
| BRCA2 | 11 | 6 breast, 2 ovariana, 1 pancreatic, 1 duodenal with fam hx breast, 1 gastric |
| BRIP1 | 2 | 2 fam hx ovarian |
| CDH1 | 4 | 3 breast, 1 breast with fam hx gastric |
| CHEK2 | 3 | 3 breast |
| EPCAM | 1 | 1 fam hx colond |
| EXO1 | 1 | 1 renal cell |
| FLCN | 1 | 1 renal cell |
| KIT | 1 | 1 GISTb |
| MLH1 | 4 | 3 colorectal, 1 appendiceal |
| MSH2 | 3 | 1 fam hx colond, 1 endometrial, 1 endometrial + colon, 1 GIST + thyroid |
| MSH6 | 1 | 1 endometrial |
| MUTYH | 10 | 2 colorectale, 1 endometrial + colon, 3 breast, 2 ovarian, 1 fam hx breast |
| NF1 | 1 | 1 clinical NF + ampullary cancer + fam hx breast |
| PALB2 | 4 | 2 breastc, 1 colorectal + renal, 1 endometrial |
| PMS2 | 2 | 2 colorectal |
| RAD50 | 2 | 1 colorectal, 1 breast |
| RAD51C | 1 | 1 colorectal |
| RAD51D | 1 | 1 breast + endometrial |
| TP53 | 1 | 1 breast |
| Total | 79 |
One patient with ovarian cancer had both ATM and BRCA2 mutations
Patient with GIST had ATM and KIT mutations
Patient with breast cancer had BARD1 and PALB2 mutations
Patient with family history of colon with MSH2 and EPCAM mutations, likely representing a contiguous gene deletion
One colorectal cancer patient with biallelic MUTYH mutations
Mutations detected, by cancer site
The deleterious mutations identified among individuals of each type of cancer are shown in Table 3. Among 197 patients with breast cancer, 28 patients (14.2%) were positive for one or more mutation. An analyses of the type of mutations among breast cancer patients found that 7.1% (14/197) carried a targeted BRCA1, BRCA2, or CDH1 mutation, and 7.1% (14/197) carried at least one mutation in genes that would not have been clinically tested without a panel including BARD1, CDH1, CHEK2, MUTYH, PALB2, RAD50, RAD51D, or TP53. Among 86 patients with colorectal cancer, 16.3% (14/86) had a mutation in APC, EPCAM, MLH1, MSH2, MUTYH, or PMS2, and 3.5% (3/86) had a mutation in the panel added genes, RAD50 or RAD51C. If the percentage of panel added-gene mutations is considered the “increased yield” of panels, similar analyses for other cancer sites found the increased yield of panels was 12.2% for ovarian cancer (5/41), 8.3% for endometrial cancer (2/24) and 12.5% for gastric cancer (2/16). (Figure 1)
Table 3.
Deleterious mutations by cancer site *
| Cancer Site |
Patients with Mutations, Overall N (%) |
Patients with Target Mutations N (%) |
Target Genes Identified (N observations) |
Patients with Panel Added Mutations, N (%) |
Panel-added gene mutations identified (N observations) |
|---|---|---|---|---|---|
| Breast N=197 |
28 (14.2%) | 14 (7.1%) | BRCA1 (7) BRCA2 (6) CDH1 (1) | 14 (7.1%) |
BARD1 (1)c
CDH1 (3) CHEK2 (3) MUTYH (3) PALB2 (2)c RAD50 (1) RAD51D (1) TP53 (1) |
| Colorectal N=86 |
17 (19.8%) | 14 (16.3%) |
APC (5) EPCAM (1)d
MLH1 (3) MSH2 (1)d MUTYH (3)e PMS2 (2) |
3 (3.5%) | PALB2 (1) RAD50 (1) RAD51C (1) |
| Ovarian N=41 |
8 (19.5%) | 4 (9.8%) | BRCA1 (2) BRCA2 (2)a | 5 (12.2%) | ATM (3)a MUTYH (2) |
| Endometrial N=24 |
5 (20.8%) | 3 (12.5%) | MSH2 (1) MSH6 (1) MUTYH (1) | 2 (8.3%) | PALB2 (1) RAD51D (1) |
| Gastric N=16 |
2 (12.5%) | 0 (0%) | 2 (12.5%) | ATM (1) BRCA2 (1) | |
| Other N=58 |
14 (24.1%) | 7 (12.1%) |
APC (1) BRCA2 (3) KIT (1)b MLH1 (1) NF1 (1) |
8 (13.7%) |
ATM (3)b
EXO1 (1) FLCN (1) MSH2 (1) MUTYH (1) PALB2 (1) |
Patients with more than one type of cancer are included in more than one category
One patient with ovarian cancer had both ATM and BRCA2 mutations
Patient with GIST had ATM and KIT mutations
Patient with breast cancer had BARD1 and PALB2 mutations
Patient with family history of colon with MSH2 and EPCAM mutations, likely representing a contiguous gene deletion
One colorectal cancer patient with biallelic MUTYH mutations
Management of patients with panel-added mutations
Pedigrees and medical records of the 35 individuals identified to carry a panel-added mutation were reviewed to assess the impact of the particular mutation on management for the proband and family members. This analysis, detailed below, found that the panel-added mutation influenced clinical recommendations in 68.6% (24/35) of patients and led to genetic testing recommendations for the first-degree relatives of 91% (32/35) of these patients.
Well-defined cancer syndromes
Seven panel-added mutations were identified in high-risk cancer genes associated with known cancer predisposition syndromes (BRCA2, CDH1, FLCN, MSH2, TP53). These individuals represented 20.0% of the patients with panel-added gene mutations, and established cancer prevention guidelines were recommended for all, except the two with metastatic disease. Of note, prophylactic gastrectomy was discussed with all three CDH1 mutation carriers. None of these individuals had family or personal history suggestive of hereditary diffuse gastric cancer syndrome; one underwent prophylactic gastrectomy and was identified to have an early-stage gastric cancer. All seven of the high-risk gene carriers had first degree relatives for whom genetic testing was clinically indicated.
Moderate risk breast cancer genes
Over 40% (15/35) of the patients with a panel-added mutation carried a gene associated with moderately increased breast cancer risk (ATM, CHEK2, PALB2), although one carried a BRCA2 mutation in addition to an ATM mutation. Breast MRI was recommended for the eight individuals who were female, had at-risk breast tissue, and did not have metastatic disease. Four of these individuals met ACS guidelines for MRI based on family history alone, but the mutation provided additional support. In addition, the option of a contralateral risk-reducing mastectomy was discussed for women with these mutations identified in the context of breast cancer surgical decisions. Two CHEK2 mutation carriers were identified at the time of breast cancer diagnosis. Risk-reducing mastectomy was presented as an option and both ultimately chose to undergo bilateral mastectomy. However, none of the 8 ATM carriers identified had breast cancer and none of the PALB2 patients had at-risk breast tissue or were in good health, therefore risk-reducing mastectomy was not offered to those individuals. Regarding the first degree relatives of these fifteen individuals with moderate risk breast cancer gene mutations, fourteen had first degree relatives for whom genetic testing was recommended, including three patients whose relatives already met ACS guidelines for MRI screening but for whom testing was discussed to support enhanced surveillance.
Only 8.6% (3/35) of the panel added gene mutation carriers had a BARD1 or RAD50 mutation, which are also considered moderate risk breast cancer genes, with more limited data. While breast MRI would have been considered, it was not appropriate, based on the patients’ clinical status due to lack of breast tissue or metastatic disease. However, a 39 year old female with Stage IV colon cancer in a full clinical remission (confirm, but this is what I think you meant) had a RAD50 mutation and was recommended to undergo a mammogram. The other RAD50 mutation carrier had already undergone bilateral mastectomy at the time of her breast cancer diagnosis, prior to identification of her mutation. However, she also had a family history of ovarian cancer and elected a risk-reducing bilateral salpingo-oophorectomy after learning her results; the possibility that RAD50 can increase ovarian cancer risk lent support to her decision. Genetic testing was recommended for the first degree relatives of the three BARD1 or RAD50 mutation carriers.
Moderate risk ovarian cancer genes
Eleven percent (4/35) of the panel-added mutations were in genes associated with moderate ovarian cancer risk (BRIP1, RAD51C, RAD51D). Bilateral salpingooophorectomy (BSO), upon completion of childbearing, was discussed with both BRIP1 carriers and the one RAD51C carriers who had ovaries intact; the RAD51D carrier had already undergone BSO. Both of the BRIP1 carriers had significant family history of ovarian cancer. A 44-year old woman undergoing a hysterectomy for benign indications was not inclined towards a BSO, despite her mother’s and maternal grandmother’s ovarian cancer diagnoses. However, upon documentation of her BRIP1 mutation she elected for BSO at the time of her hysterectomy. Serial dissection of the ovaries and fallopian tubes demonstrated revealed no lesions or malignancy. We recommended genetic testing for risk assessment for the female first-degree relatives of all four mutation carriers in this category.
MUTYH
The most common panel-added findings were monoallelic MUTYH mutations, constituting 17.1% (6/35) of the patients with panel-added mutations. None of these carriers had a family history of colon cancer and all were over age 50. We discussed initiation or continuation of colonoscopy screening with all carriers, at 5-year intervals, equivalent to that of individuals with a first-degree relative with colorectal cancer. Genetic testing was recommended for the first degree relatives of all six MUTYH carriers.
Other genes
A panel-added mutation in the EXO1 gene was identified in one patient. This finding was not used for any management or family member testing as the clinical significance of such a mutation is unknown and did not meet our clinical threshold to alter medical management.
Panel size analysis
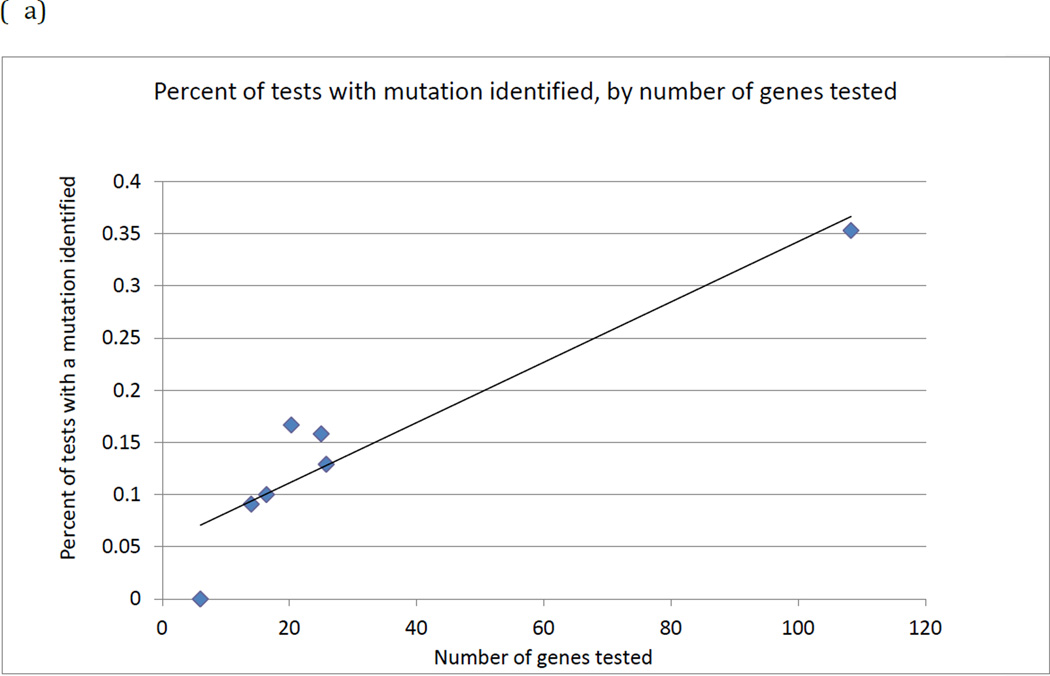
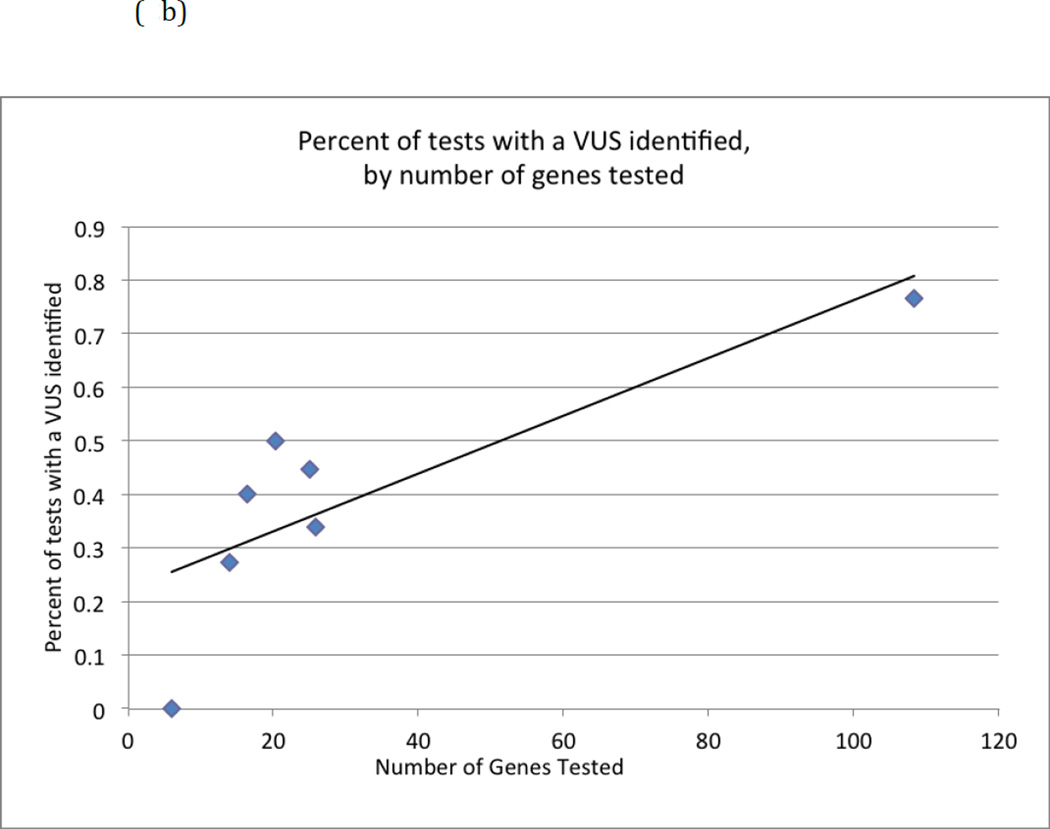
We observed evidence that as the number of genes on a panel increased, there was a higher proportion of tests identifying a mutation (linear trend R2 =0.87) or a VUS (R2=0.65). (Figures 2a and 2b) These trends are also apparent (dashed lines) even if the Fulgent panels (upper point in each figure) were excluded, both for identifying a mutation (R2 = 0.80) or a VUS (R2=0.65). These figures also suggest some evidence for a nonlinear relationship, although the sample size is too limited to determine the specific relationship.
Figure 2.
(a) Percentage of tests with a mutation identified as the number of genes on a panel test increases.
(b) Percentage of tests with a variant of uncertain significance identified as the number of genes on a panel test increases.
DISCUSSION
This study reports on the largest multi-ethnic population to undergo hereditary cancer multi-gene panel testing published to date. Deleterious mutations were identified in 15.6% (74/475) of tested patients on a variety of multi-gene hereditary cancer panels. If a targeted gene-by-gene approach had been used, only 8.6% (41/475) would have had a mutation detected. Thus, the panel approach nearly doubled the detection of mutations.
We found a higher mutation rate utilizing multi-gene panels than the 6.6–10.0% mutation rate previously report in other clinical cohorts. (5–7) These other studies utilized multi-gene panels in a selected high-risk subset (n=60–100) of their clinic populations. Our study suggests that when more broadly applied across larger patient cohorts, multi-gene panels markedly increase yield. This may be driven by the identification of individuals and families with atypical presentations of known, high penetrant syndromes, as well as those with mutations in moderate risk genes.
In previous reports of multi-gene panel studies, race/ethnicity was either not reported or was reported as majority non-Hispanic white. (5–8,10) Two clinical registry based cohorts of previously tested BRCA-negative breast cancer patients included ethnic minority participants. In Kurian et al (9), 20% were Asian (n=28) and 24% (n=66) of a similarly ascertained cohort report by Maxwell et al (11) were Black. As seen in our cohort, there was no significant difference in mutation rates by race and ethnic groups in either study. However, we did see a statistically significant difference in the proportions of VUSs identified by racial/ethnic group. Yorczyk et al (7) also found a higher proportion of VUSs among Blacks which represented 16% (n=17) of their cohort.
Almost two-thirds of our study population was from ethnic and racial groups that are underrepresented in previous reports. Our study population is reflective of the diversity of both the Los Angeles area and the large number of participants included from the safety-net hospital setting. While not significant, there was a general trend of fewer deleterious mutations identified in Hispanic, Black and Asian participants. There were a statistically significant higher VUS rates among Hispanics and Blacks. The trend of fewer deleterious mutations and more VUSs identified in race/ethnic minorities may reflect the presence of VUSs that will eventually be reclassified as deleterious mutations. This requires further exploration as well as improvements in VUS classification.
VUS rates in BRCA1 and BRCA2 testing are known to be higher among ethnic minorities(13) due to the lower testing volume and less data available about genetic variants from diverse populations. For example, African populations are characterized by greater levels of genetic diversity, leading to higher proportions of rare single nucleotide polymorphisms (14). Rare variants with allele frequencies of less than 1% can be challenging to classify for molecular diagnostic labs. As ethnic and racial minority patients have less access to testing, they are underrepresented in clinical laboratory databases, leading to a greater wait time for reclassification. Increased clinical access and insurance coverage as well as research focused on these specific populations can aid in accelerating the reclassification process.
Regardless of ethnicity, multi-gene panel testing inherently results in identification of more VUSs. As larger panels are developed, clinicians can expect to manage a plethora of VUS data. With the identification of VUSs in a larger proportion of patients, the number of families for whom variant tracking may be considered may grow. Protocols for the communication of reclassifications to patients and providers are needed, as well as appropriate clinical documentation and management of VUS misunderstanding.
For our analysis, deleterious mutations identified were classified as either being in “target” or “panel-added” genes. Over half of patients who tested positive carried a target-gene mutation and almost half carried a mutation in a panel-added gene. Thus, using a panel approach nearly doubled the mutations identified. While not yet studied and beyond the scope of the current analysis, panel testing may be particularly cost-effective in the long term, due to the cost savings of testing for multiple syndromes with one analysis. Additionally, patients seen in the safety-net hospital setting will often continue their care within such a system. Therefore, the cost-benefit of preventing second primary cancers or detecting them at earlier stages offers the potential to minimize costs to the health systems. Further studies are necessary to expand understanding of implementation and utilization of genetic advances across diverse clinical settings, as well as quantification of costs and benefits.
Panels increased the yield of testing for all cancer sites analyzed in the study, including breast, colorectal, ovarian, uterine and gastric cancer. However, this finding was most striking in the 197 individuals with breast cancer in which the use of a multi-gene panel increased the mutations identified from 14 (7.1%) to 28 (14.2%). Panel-detected mutations were identified in both high and moderate-risk genes including BARD1, CDH1, CHEK2, MUTYH, PALB2, RAD50, RAD51D, and TP53. Our results are comparable to previous registry-based panel testing studies of BRCA negative cohorts(9–11) that have found that 3.7% – 11.4% of individuals have mutations in genes other than BRCA1 and BRCA2. A recent study of a clinical cohort also found that panel testing doubles the mutation detection rate for patients undergoing testing for hereditary breast cancer (15). Of note, for colorectal cancer cases, the additional yield of the panels used in this study was lower than for patients with other types of cancer. This likely reflects the composition of the cancer gene panels that were clinically available during the study period.
Since the inception of panel testing in the hereditary cancer setting our understanding of these genes and their phenotypes has evolved. For example, a study of PALB2-mutation carriers reported a 35% breast cancer risk by age 70, a risk which overlaps the risk associated with BRCA2 mutations(16). New NCCN Guidelines@ (Genetic/Familial High-Risk Assessment: Breast Ovarian Version 1.2015) have included recommendations to discuss risk-reducing mastectomy for CDH1 mutation carriers as well as to offer breast magnetic resonance imaging (MRI) screening for ATM, CHEK2, and PALB2 mutation carriers. Despite this, there are still clinical questions to answer concerning the risk for other cancers, penetrance, and attributable risk. For example, insufficient evidence exists regarding MRI screening for BRIP1 and BARD1 carriers, per the same guidelines. Further ongoing, collaborative studies of mutation carriers will be integral to answering these questions and have immediate clinical impact for the growing numbers of carriers identified by multi-gene panels(17).
In most cases, identifying panel-added mutations led to enhanced surveillance and risk-reduction interventions for patients and family members. Another recent study reached a similar conclusion among BRCA negative individuals in which panel-detected mutations were found and most patients were offered additional disease-specific screening or preventive measures(18).
The identification of panel-added mutations represents an opportunity for prevention, as demonstrated by the clinical recommendations made on the basis of the mutation for 69% of patients with such mutations. This may underrepresent the potential impact of panel-added mutation, as we excluded individuals who were metastatic. The impact of panel-added mutations is well illustrated by an asymptomatic CDH1 mutation carrier who underwent prophylactic gastrectomy and was identified to have an early stage gastric cancer. Surgical decisions were also influenced by the mutation in two CHEK2 mutation carriers who underwent RRM at the time of a breast cancer diagnosis and one RAD50 mutation carrier and one BRIP1 mutation carrier who each decided on BSO due to their family history in combination with the mutation.
Some individuals identified on a panel to have a moderate risk breast gene such as ATM or CHEK2 may have already met ACS guidelines for MRI screening on the basis of family history. However, the mutation lends motivation to adhere to guideline on the part of the patient, her providers, and potentially her insurer, and therefore the mutation became an important part of her preventive care. The same rationale applied to the recommendation for testing first degree family members for moderate risk breast genes even among female family members already meeting ACS guidelines for MRI screening.
Clinicians utilizing multi-gene panel testing need to be prepared to identify clinically actionable mutations which may not account for the primary testing indication but can impact medical management. Careful interpretation of moderate risk or lower penetrance genes is important, as further testing in other family members may be required to rule-out other etiologies. The impact of a negative or a positive test result for family members is often not as informative as it is with the highly penetrant genes since the attributable risk of cancer in mutation carriers is substantially lower for moderate and low-penetrance genes. In addition, a significant number of the breast cancer genes have implications for specific pediatric conditions when inherited in the homozygous state (i.e. ataxia telangiectasia and Fanconi anemia). This adds another dimension to the counseling of families with mutations in these genes, especially for individuals of reproductive age.
As expected, individuals with cancer were more likely to be identified to have a deleterious mutation than those referred for family history only. However, there were also clinically actionable mutations identified in unaffected individuals that led to preventive cancer screening strategies. For families without informative living family members or with those family members in countries with limited access to genetic testing, a multi-gene panel provides the ability to more fully assess cancer risk.
This study is representative of a clinical population with diverse cancer histories and a variety of insurance plans. For this reason, several different laboratories and various were utilized. Using many different panels allowed us to note a correlation between the number of genes on a panel and the proportion of tests with a mutation or VUS identified.
Our results would likely have differed if all the patients were tested by the same lab and for the same genes. Even panels with similar genes offered by different labs utilized distinct methodologies and technologies, as well as specific variant classification practices, which impacts the result provided to the clinician.
This study provides a unique snapshot of the evolving clinical practice of cancer genetics. It represents the largest clinically ascertained cohort of racially and ethnically diverse patients undergoing multi-gene cancer testing with several CLIA-approved laboratories. Our study demonstrates that these panels are relevant across racial and ethnic groups and can increase the yield of clinically actionable mutations. However, it also highlights the need for a greater understanding of the underlying genetic variation in all populations to minimize the clinical uncertainty that accompanies VUSs. In conclusion, multi-gene panel testing increases the yield of mutation detection and adds to the capability of providing individualized cancer risk assessment.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Cancer Institute at the National Institutes of Health, USC Norris Cancer Center Core Grant (P30CA014089); American Cancer Society (RSGT 1020301); Avon Foundation (052011057); The Anton B. Burg Foundation; and the Lynne Cohen Foundation
Dr. Gregory Idos and Stephen Gruber receive research funding from Myriad Genetics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The other co-authors do not have any potential conflicts of interest to disclose.
REFERENCES
- 1.Laduca H, Stuenkel aJ, Dolinsky JS, et al. Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genet Med. 2014;16:1–8. doi: 10.1038/gim.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cragun D, Radford C, Dolinsky JS, Caldwell M, Chao E, Pal T. Panel-based testing for inherited colorectal cancer: a descriptive study of clinical testing performed by a US laboratory. Clin Genet. 2014 doi: 10.1111/cge.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121:25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 4.Yurgelun MB, Allen B, Kaldate RR, et al. Identification of a Variety of Mutations in Cancer Predisposition Genes in Patients with Suspected Lynch Syndrome. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mauer CB, Pirzadeh-Miller SM, Robinson LD, Euhus DM. The integration of next-generation sequencing panels in the clinical cancer genetics practice: an institutional experience. Genet Med. 2014;16:407–412. doi: 10.1038/gim.2013.160. [DOI] [PubMed] [Google Scholar]
- 6.Selkirk CG, Vogel KJ, Newlin AC, Weissman SM, Weiss SM, Wang C-H, Hulick PJ. Cancer genetic testing panels for inherited cancer susceptibility: the clinical experience of a large adult genetics practice. Fam Cancer. 2014 doi: 10.1007/s10689-014-9741-4. [DOI] [PubMed] [Google Scholar]
- 7.Yorczyk A, Robinson LS, Ross TS. Use of panel tests in place of single gene tests in the cancer genetics clinic. Clin Genet. 2014 doi: 10.1111/cge.12488. [DOI] [PubMed] [Google Scholar]
- 8.Doherty J, Bonadies DC, Matloff ET. Testing for Hereditary Breast Cancer: Panel or Targeted Testing? Experience from a Clinical Cancer Genetics Practice. J Genet Couns. 2014 doi: 10.1007/s10897-014-9796-2. [DOI] [PubMed] [Google Scholar]
- 9.Kurian AW, Hare EE, Mills Ma, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32:2001–2009. doi: 10.1200/JCO.2013.53.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2014:1–9. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell KN, Wubbenhorst B, D’Andrea K, et al. Prevalence of mutations in a panel of breast cancer susceptibility genes in BRCA1/2-negative patients with early-onset breast cancer. Genet Med. 2014 doi: 10.1038/gim.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couch FJ, Hart SN, Sharma P, et al. Inherited Mutations in 17 Breast Cancer Susceptibility Genes Among a Large Triple-Negative Breast Cancer Cohort Unselected for Family History of Breast Cancer. J Clin Oncol. 2014;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eggington JM, Bowles KR, Moyes K, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet. 2013:229–237. doi: 10.1111/cge.12315. [DOI] [PubMed] [Google Scholar]
- 14.Campbell MC, Tishkoff Sa. AFRICAN GENETIC DIVERSITY: Implications for Human Demographic History, Modern Human Origins, and Complex Disease Mapping. Annu Rev Genomics Hum Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor NS, Curcio LD, Blakemore Ca, Bremner AK, McFarland RE, West JG, Banks KC. Multigene Panel Testing Detects Equal Rates of Pathogenic BRCA1/2 Mutations and has a Higher Diagnostic Yield Compared to Limited BRCA1/2 Analysis Alone in Patients at Risk for Hereditary Breast Cancer. Ann Surg Oncol. 2015:1–7. doi: 10.1245/s10434-015-4754-2. [DOI] [PubMed] [Google Scholar]
- 16.Antoniou AC, Casadei S, Heikkinen T, et al. Breast-Cancer Risk in Families with Mutations in PALB2. N Engl J Med. 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easton, Douglas F, PhD, Pharoah PDPP, Antoniou ACP, et al. Gene-Panel Sequencing and the Prediction of Breast-Cancer Risk. N Engl J Med. 2015;372:2243–2257. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmond A, Kurian AW, Gabree M, et al. Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA Oncol. 2015;02114:1–9. doi: 10.1001/jamaoncol.2015.2690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.