Abstract
Study Objectives:
Physiological adaptation to high altitude hypoxia may be impaired in Andeans with significant European ancestry. The respiratory ‘burden’ of sleep may challenge adaptation, leading to relative nocturnal hypoxia. Developmental aspects of sleep-related breathing in high-altitude native children have not previously been reported. We aimed to determine the influence of development on diurnal-nocturnal oxyhemoglobin differences in children living at high altitude.
Methods:
This was a cross-sectional, observational study. Seventy-five healthy Bolivian children aged 6 mo to 17 y, native to low altitude (500 m), moderate high altitude (2,500 m), and high altitude (3,700 m) were recruited. Daytime resting pulse oximetry was compared to overnight recordings using Masimo radical oximeters. Genetic ancestry was determined from DNA samples.
Results:
Children had mixed European/Amerindian ancestry, with no significant differences between altitudes. Sixty-two participants had ≥ 5 h of nocturnal, artifact-free data. As predicted, diurnal mean oxyhemoglobin saturation decreased across altitudes (infants and children, both P < 0.001), with lowest diurnal values at high altitude in infants. At high altitude, there was a greater drop in nocturnal mean oxyhemoglobin saturation (infants, P < 0.001; children, P = 0.039) and an increase in variability (all P ≤ 0.001) compared to low altitude. Importantly, diurnal to nocturnal altitude differences diminished (P = 0.036), from infancy to childhood, with no further change during adolescence.
Conclusions:
Physiological adaptation to high-altitude living in native Andeans is unlikely to compensate for the significant differences we observed between diurnal and nocturnal oxyhemoglobin saturation, most marked in infancy. This vulnerability to sleep-related hypoxia in early childhood has potential lifespan implications. Future studies should characterize the sleep- related respiratory physiology underpinning our observations.
Citation:
Hill CM, Baya A, Gavlak J, Carroll A, Heathcote K, Dimitriou D, L'Esperance V, Webster R, Holloway J, Virues-Ortega J, Kirkham FJ, Bucks RS, Hogan AM. Adaptation to life in the high andes: nocturnal oxyhemoglobin saturation in early development. SLEEP 2016;39(5):1001–1008.
Keywords: altitude, central apnea, sleep disordered breathing, respiratory control, intermittent hypoxia
Significance.
This is the first study to report significant differences between diurnal and nocturnal oxyhaemoglobin in infants and children resident at high altitude. These differences are dramatic in infants, where oxyhaemoglobin saturation is also unstable, but improve in childhood. Urban Andean high altitude dwelling populations have significant European ancestry and sleep physiology may challenge an already constrained system. Whether reduction in tissue oxygen delivery is clinically significant requires further study. Intermittent nocturnal hypoxia in obstructive sleep apnoea in children living at sea-level, has long-term consequences for cognitive performance and cardiovascular health. We hypothesise that exposure to low nocturnal oxyhaemoglobin may contribute to the subtle psychomotor slowing we have demonstrated in Andean high altitude dwelling children.
INTRODUCTION
Physiological adaptation to high-altitude (HA) hypoxia is critical for the 140 million people who live 2,500 m or more above sea level.1 As altitude increases, atmospheric pressure drops, resulting in a fall in inspired oxygen, such that populations living above 4,000 m breathe air containing only ∼60% of the oxygen found at sea level (Figure 1).
Figure 1.

Partial pressure of inspired oxygen (PiO2) as a percentage of sea-level values at increasing altitudes. Altitudes at which participants were recruited in this study (500 m, 2,500 m, > 3,650 m) and in other studies (A: Burg et al.22, at 1,600 m; B: Coote et al.19, Spicuzza et al.20, at 4,380 m) are shown. The solid line represents inspired oxygen in mmHg (left hand y axis) and the dashed line represents percent of inspired oxygen compared to sea-level values (right hand y axis). Figure adapted from: Beall et al.7 Copyright (2007) National Academy of Sciences, USA.
The ancestors of native Andeans settled on HA plains approximately 11,000 years ago. Over this time their physiology evolved to compensate for HA hypoxia, principally through erythrocytosis and an increase in pulmonary artery pressure, diffusion capacity, and total lung capacity.2 Spanish colonization, half a century ago, diluted the Amerindian gene pool and threatened this adaptation. This may explain the 15% prevalence of chronic mountain sickness2 (a triad of extreme polycythaemia, hypoxia, and impaired right heart function) and reduced life expectancy3 in this population.
Research into native HA respiratory physiology has largely overlooked the challenge that the sleep state presents to an already constrained system, specifically through decreased minute ventilation, increased upper airway resistance, and reduction in the slopes of the ventilatory response curves to hypoxemia and hypercapnia. This is particularly important in children who sleep more than adults and in whom early exposure to hypoxia may affect life-long cardiovascular status.4 Furthermore, developmental studies of HA natives5,6 have consistently shown that mean daytime oxyhemoglobin saturation (SpO2) increases across the first decade of life. Bolivian adolescents at 3,900–4,000 m had daytime SpO2 values of 92.7% (females) and 93.3% (males)7 compared to only 87.3% in children younger than 5 y. We, therefore, hypothesized that nocturnal SpO2 would also reflect developmental adaptation in HA compared to low-altitude (LA) native children.
METHODS
Data were collected across two consecutive research expeditions examining cognitive function at high altitude, and included 547 infants, children, and adolescents. Study 1 recruited children aged 6–10 y from: Santa Cruz (LA), at 500 m; Cochabamba, moderate altitude (MA) at 2,500 m, and La Paz (HA), at 3,700 m.8 Study 2 recruited participants aged 6 mo to 17 y from LA and HA only.9 Children were sampled from three discrete age groups: infancy, mid-childhood (4–10 y) and adolescence (13–16 y). The physiological measures of interest reported here are ancillary oximetry studies involving a subset of the total study sample. Ethics committee approval was granted by the University of Southampton, UK and Univalle, Bolivia (Study 1), and by Universidad Privada Abierta Latinoamericana, Bolivia and the University of Western Australia (Study 2). Parents provided written informed consent for their child's participation.
Inclusion criteria were consistent across studies.8,9 Participants were healthy with no developmental disorders and had been born at, and lived continuously at, the altitude location for at least the year prior to study. In both studies, all children attended a research center during the day, and parents provided information on maternal education, and their child's medical and developmental history. Physical examination was over-seen by physicians and included anthropometry (height and weight), clinical cardiorespiratory examination, and resting blood pressure (Microlife, Zurich). Weight for length centiles (infants) and body mass index centiles (children and adolescents) were derived from standard US growth charts. Systolic blood pressure centiles were computed for children and adolescents.10 Proportion of Native American, European, and African genetic ancestry was determined from DNA samples as previously described.8,9
Diurnal Measures
Resting pulse oximetry was sampled using a Masimo Radical oximeter (Masimo Corp, Irvine, CA). End-tidal carbon dioxide (ETCO2; nasal cannulae) was measured in children and adolescents only (TIDALWAVE: Philips Respironics, Murrysville, PA, USA). Participants were required to relax and breathe steadily at rest for 3 min to achieve steady baseline ETCO2 values, and readings were only made from the device if an expiratory plateau was present.
Nocturnal Measures
Pulse oximetry was sampled for 1 night at home using a Masimo Radical oximeter with a 2-sec averaging time. Parents were trained in use of the device at the research center and detailed step-by-step instructions, supplemented with images, were provided. Parents were instructed to start the recording when the child had settled to sleep and stop recording when the child finally woke in the morning. Data were analyzed using Download 2001 software (Stowood Scientific, Oxford, UK). Poor perfusion, low signal quality, and movement artefact data were rejected, as were recordings < 5 h duration.11 Variables generated included mean SpO2; delta-12-sec index (the absolute difference between successive 12-sec interval recordings); desaturation indices of > 3%/h; and time in minutes with SpO2 below 90% and 80%.
Analysis
Data were analyzed in SPSS version 22 (IBM). Given that data were not available at all age groups and all altitudes, analyses were conducted within altitudes, by age-group and time-of-day, or within age-groups, by altitude and time-of-day. For infants and children, in whom we had diurnal and nocturnal measurements at low and high altitude, we conducted a full-factorial, three-way analysis. Simple age-group or altitude differences were explored using Kruskal-Wallis or Jonkheere trend tests (where ordered differences were predicted) given the non-normality of some variables. For analyses exploring interaction terms, for which there is no non-parametric alternative, analysis of variance (ANOVA, univariate or repeated measures) were conducted and then non-parametric comparisons were run to confirm significant effects. Given the exploratory nature of these analyses, adjustments to P values for post hoc analyses were not made, to reduce the risk of a type 2 error.12,13 Partial eta-squared effect sizes (η2p) were computed for all ANOVA. Categorical group differences were explored using χ2 (Fisher exact) test.
RESULTS
Seventy-five overnight studies were performed (24 LA, 12 MA, and 39 HA), of which 62 (68.9%) had ≥ 5 h of artefact-free data (21 LA, 9 MA, and 32 HA).
Demographic Variables
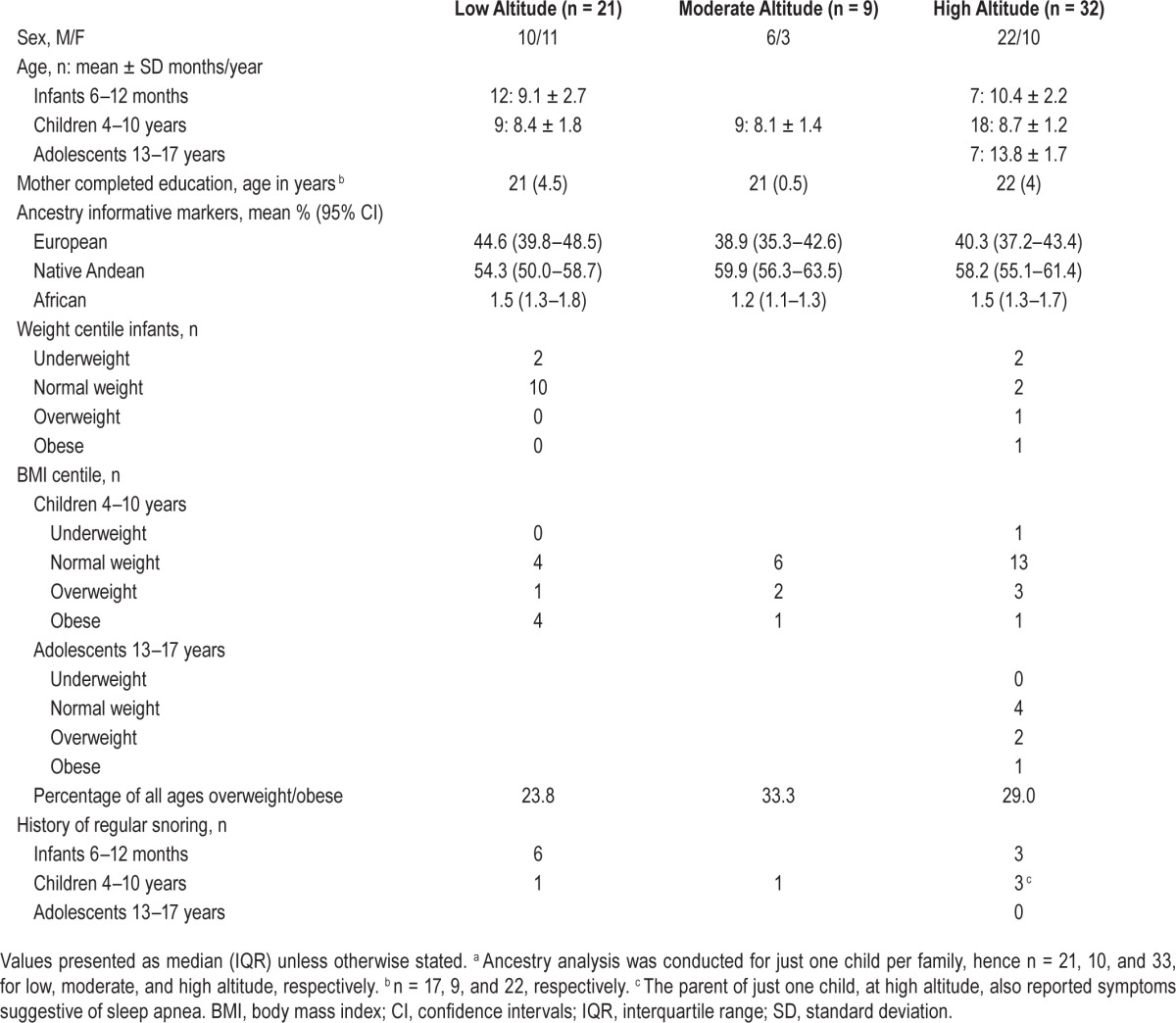
There were no sex by altitude differences overall, Fisher exact test, P = 0.299, nor altitude differences within age groups. Two HA babies were born at 36 w and one baby at LA at 32 w gestation; chronological age was adjusted in these infants. All children were healthy, developing typically and from middle-to-high social strata, with no altitude differences in maternal educational level. Ancestry informative markers revealed no altitude differences, F < 1, and a significant proportion of European ancestry (Table 1). This analysis was repeated within age groups, and revealed no altitude differences in genetic admixture, all P > 0.34.
Table 1.
Demographic and clinical data by altitude.
Clinical Measures
Parents reported asthma in two children at LA and one adolescent at HA. There were no significant differences in resting daytime ETCO2 across altitudes. Regular snoring was reported in 21% of children (Table 1), with apnea symptoms in one HA child. One LA and 2 HA children had systolic blood pressure readings at, or above, the 95th centile, but there were no altitude-related differences in the distribution of children across the systolic blood pressure centiles, Fisher exact test, P = 0.614. Cardiorespiratory examination was normal in all participants. Eleven percent of children were obese, but this was not influenced by altitude location, P = 0.339.
Diurnal Pulse Oximetry Measures
As predicted, there were statistically significant trends of lower median diurnal SpO2 with increasing altitude in infants (from LA to HA), TJT = 18.0, z = −5.6, P < 0.001, and in children (from LA, to MA, to HA), TJT = 0, z = −3.5, P < 0.001, confirmed by significant pairwise comparisons, all P ≤ 0.001 (Table 2).
Table 2.
Oximetry and end-tidal carbon dioxide data.
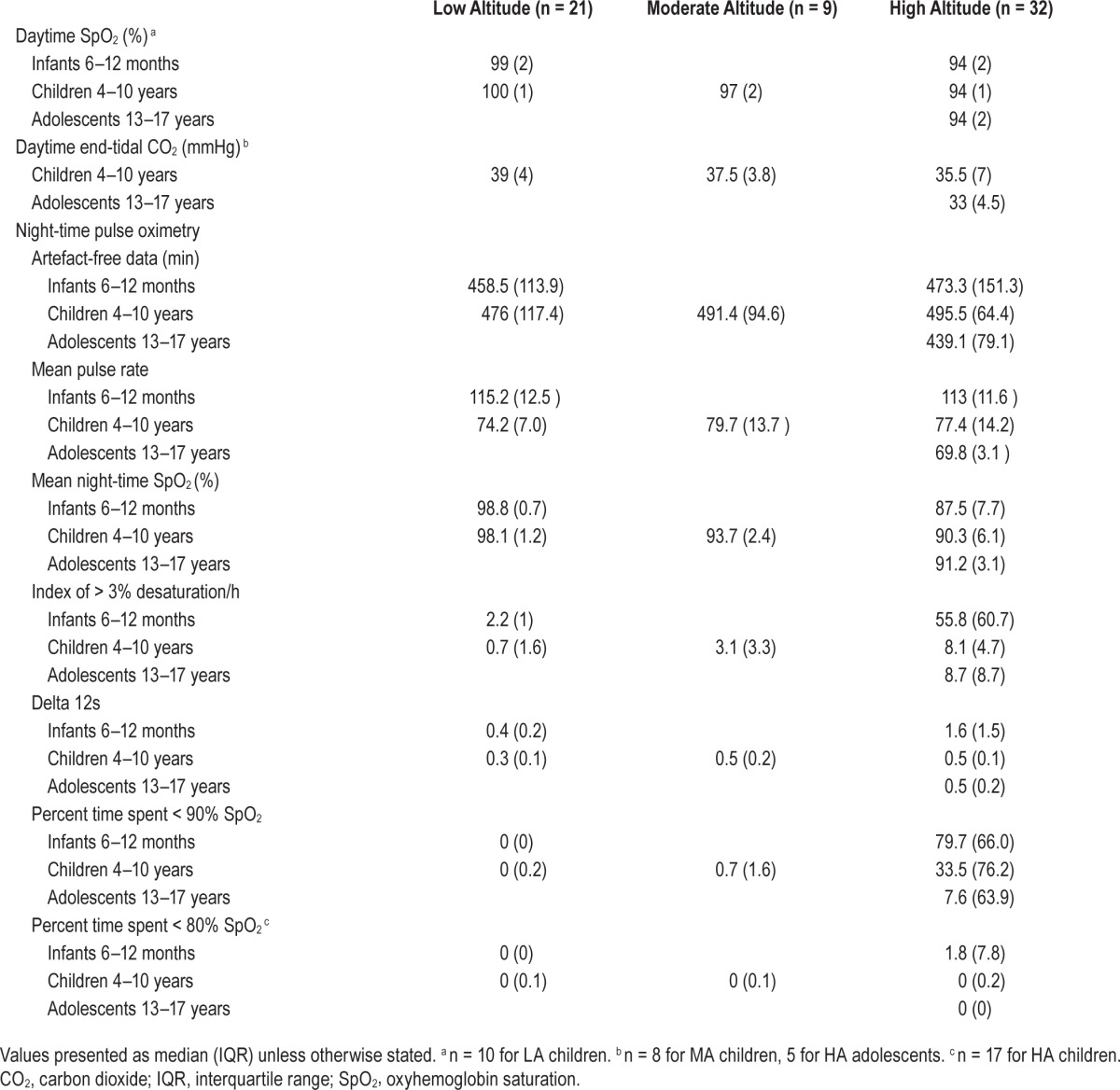
Within altitude, there were no developmental differences, in that diurnal SpO2 did not vary with age at LA, TJT = 62.5, z = 1.7, P = 0.097, or at HA, TJT = 177.5, z = 1.1, P = 0.291.
Nocturnal Pulse Oximetry Recordings
For overnight studies, the mean duration of data analyzed did not differ between age or altitude groups. Pulse rate as measured by the SpO2 probe did not differ across altitudes, either in infants or in children, both χ2 < 1 (Table 2).
Mean SpO2 Data
Figure 2 illustrates differences between mean diurnal and nocturnal SpO2 measurements by age and altitude.
Figure 2.
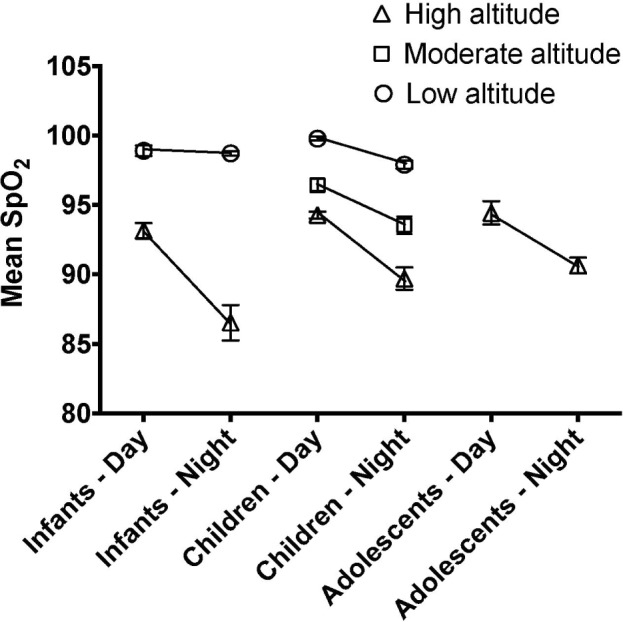
Developmental trajectory of diurnal and nocturnal oxyhemoglobin saturation (SpO2) values. Difference between diurnal and nocturnal mean oxyghemoglobin saturation at three different altitudes by age group (infants, 6–12 mo old; children, 4–10 years old; adolescents, 13–17 years old). Altitude values are: high > 3,650 m, moderate 2,500 m, and low 500 m. Error bars are standard errors.
Within-Altitude Comparisons, by Time of Day and Age Group
At LA, there was a significant effect of time of day (diurnal, nocturnal), F(1,17) = 15.23, P = 0.001, η2p = 0.47, and a time of day by age group (infants, children) interaction, F(1,17) = 7.61, P = 0.013, η2p = 0.31, such that nocturnal measures were consistently lower than diurnal measures and this difference was slightly larger in children than in infants.
In comparison, at HA, as well as a main effect of time of day (diurnal, nocturnal), F(1,29) = 62.84, P < 0.001, η2p = 0.68, with nocturnal consistently lower than diurnal measures in all age groups, there was also a significant effect of age group (infants, children, adolescents), F(2,29) = 4.80, P = 0.016, η2p = 0.25 with infants showing a higher nocturnal drop in SpO2 than children or adolescents, who did not differ, but no interaction, F < 1.5.
Within Age Group Comparisons by Time of Day and Altitude
In infants, there was an effect of time of day (diurnal, nocturnal), F(1,15) = 35.95, P < 0.001, η2p = 0.71, with lower nocturnal oximetry, and of altitude (LA, HA), F(1,15) = 178.08, P < 0.001, η 2p = 0.92, in which SpO2 was lower at HA. The significant time of day by altitude interaction, F(1,15) = 29.59, P < 0.001, η2p = 0.66 was driven by a larger drop in nocturnal SpO2 in HA relative to LA infants. In children, the same effects were found across three altitudes: time of day (diurnal, nocturnal), F(1,33) = 47.00, P < 0.001, η2p = 0.59, altitude (LA, MA, HA), F(2,33) = 61.41, P < 0.001, η2p = 0.79, with SpO2 lowest at HA, highest at LA, and with MA intermediate. The significant time of day by altitude interaction, F(1,33) = 3.58, P = 0.039, η2p = 0.18, mirrored that seen in infants in that the differential between diurnal and nocturnal SpO2 increased with increasing altitude.
Three-Way Comparisons of Altitude, Age Group, and Time of Day
Importantly, a three-way ANOVA of age by altitude (LA, HA) by time of day in infants and children (no adolescent data available for LA) demonstrated that altitude-related differences in diurnal and nocturnal mean SpO2 decrease with age; that is, they are reduced in children compared to infants, suggesting developmental adaptation: three-way interaction, F(1,40) = 4,69, P = 0.036, η2p = 0.11. As expected, there were also interactions between time of day and altitude, F(1,40) = 29.43, P < 0.001, η2p = 0.42, with a bigger drop at night as altitude increases, and between age group and altitude, F(1,40) = 4.51, P = 0.040, η2p = 0.10, with altitude decreases in SpO2 greater in infants than in children.
Differences in diurnal and nocturnal mean SpO2 values could not be explained by history of snoring, overweight, or obesity, because there were no age group or altitude differences in the proportion of participants with these problems, all P > 0.05.
Measures of Nocturnal SpO2 Stability
Within-Altitude Comparisons, by Age Group
There were notable developmental differences in nocturnal SpO2 stability, measured by the delta (12s) and 3% desaturation indices (Table 2). As previously observed, at LA, developmental differences were evident between infants and children: 3% dips, TJT = 21.0, z = −2.3, P = 0.019, and delta 12s index, T JT = 7.0, z = −3.3, P = 0.001. For both measures, there was a reduction in these indices with age. However, this developmental difference was more striking at HA: 3% dips, TJT = 73.0, z = −2.7, P = 0.008; and delta 12s, TJT = 49.0, z = −3.7, P < 0.001, where very highly significant differences were noted between infants and both children and adolescents, but not between children and adolescents.
Within Age Group Comparisons by Altitude
Within age groups, altitude effects were apparent, with increasing variability noted in both infants: 3% dips, LA versus HA: TJT = 84.0, z = 3.6, P < 0.001; delta 12s, TJT = 82.0, z = 3.4, P = 0.001, and within children, LA versus MA versus HA: 3% dips, TJT = 342.0, z = 5.2, P < 0.001,; delta 12, TJT = 375.0, z = 5.1, P < 0.001, where variability increased linearly with altitude.
Two-Way Comparisons of Altitude by Age Group
This was confirmed by a very strong age group (infants, children) by altitude (LA, HA) interaction in both 3% desaturation F(1, 41) = 35.91, P < 0.001, η2p = 0.47 and delta 12s indices, F(1, 41) = 24.58, P < 0.001, η2p = 0.38.
Exposure to Low Nocturnal SpO2
Within Age Group, Comparisons by Altitude
Within infants, at HA a greater percentage of time was spent with SpO2 < 90% and < 80% saturation, relative to LA, T JT = 84.0 and 78.0, both z = 3.7, P < 0.001 (Table 2). Within children, at HA a greater percentage of time was spent at SpO2 < 90% saturation, TJT = 351.0, z = 4.4, P < 0.001, but not SpO2 < 80%, TJT = 207.5.0, z = 0.5, P = 0.631, with pairwise comparisons confirming increasing time spent below threshold from LA to MA to HA. Of note, at HA, two infants and one child spent > 30 min with SpO2 < 80%. Diurnal SpO2 values in these three participants were indistinguishable from age and altitude peers (92%, 94%, and 94%, respectively), yet they experienced 37.1 (7.9%), 98.7 (19.2%), and 37.5 (8.3%) min, respectively, with SpO2 < 80% at night.
Within Altitude, Comparisons by Age Group
Within HA participants, time spent at SpO2 < 80% saturation, T JT = 91.0 z = −2.2, P = 0.030, and < 90%, TJT = 64.0, z = −3.2, P = 0.001, was significantly different, driven by significantly greater time spent below 90% in HA and MA children than in LA (where HA and MA did not differ), and significantly greater time spent below 80% in a linear fashion across LA, MA, and HA. Comparisons within low altitude participants were not conducted, given very low levels of exposure to low nocturnal SpO2 (see Table 2), consistent with previous literature.11
DISCUSSION
Consistent with previous studies, we report a decrease in diurnal SpO2 with increasing altitude in infants and children. We also report novel findings of developmental maturation of nocturnal oxyhemoglobin saturation from infancy to childhood in HA Andean natives. Of particular note, the degree of diurnal to nocturnal SpO2 difference at HA is greatest in infancy and decreases with progression to childhood, with no further gains into adolescence.
Tissue oxygenation at HA is determined by total oxygen carrying capacity. Oxygen carrying capacity is determined by the following equation:
where HC is hemoglobin concentration. Hemoglobin carries 98% of oxygen in the blood, with the remainder dissolved in plasma. Andean HA residents increase their oxygen-carrying capacity through erythropoiesis which, along with increased tissue perfusion and 2-3–diphosphoglycerate (DPG) levels, increases tissue oxygen delivery. Could these adaptive mechanisms fully compensate for the degree of diurnal- nocturnal difference in SpO2 observed in this study? Healthy adults take up to 4 days to increase 2-3-DPG levels when exposed to hypobaric hypoxia at 3,400 m, making this an unlikely source of nocturnal compensation.14 Similarly, hemoglobin concentration does not fluctuate over 24 h. Our data, therefore, suggest a genuine decrease in oxygen- carrying capacity in HA children at night. Lower metabolic demand in sleep may offset the effect of this reduction in oxygen carriage, or tissue oxygen delivery may be maintained through other mechanisms. However, the fact that both nocturnal mean SpO2 and indices of stability of SpO2 improve with advancing age at HA suggests two possible hypotheses.
First, our SpO2 findings may be a marker of maturation of ventilatory control at HA. Periodic breathing (PB) is commonly reported in healthy, adult, altitude sojourners and causes significant oscillations of nocturnal SpO2 and an increasing divergence of diurnal and nocturnal SpO2.15 Similar findings in our data, most striking in infants at HA, suggest PB as a possible explanation. In the first 2 w of life, 79% of healthy term infants at sea level have PB. By 39 to 52 w of age (the age of infants in our study) only 29% retain PB, typically for less than 1% of total sleep time.16 Thus, HA infants may have delayed maturation of respiratory control, with persistent PB into late infancy. We did not measure daytime naps, but lower diurnal SpO2 values in napping versus awake infants at HA have previously been reported.6 Infants spend more than half of their lives asleep, increasing the potential for hypoxic exposure. It is feasible that such exposure could recalibrate the carotid body,17,18 and predispose Andeans to later ventilatory instability. This hypothesis is supported by findings in adult, Andean, HA natives. Coote et al. reported PB with marked, arterial oxygen desaturation, in 8 healthy, young men living at 4,380 m,19 later confirmed by Spicuzza and colleagues20 in 20 men (mean age 38 y) at the same altitude (Figure 1B). In contrast, other authors have noted only minor sleep abnormalities in these populations.21 Such contradictory findings may reflect sampling from different ancestry populations, highlighting the importance of reporting genetic ancestry.
Another plausible explanation relates to the affinity of hemoglobin for oxygen according to the partial pressure of arterial oxygen (PaO2). The steep slope of the oxygen dissociation curve at lower SpO2 predicts that small perturbations in PaO2, due to sleep-related fluctuations in ventilation, would lead to larger drops in SpO2. This provides a possible explanation for the higher delta 12s index and 3% desaturation indices seen at all ages at HA. Other authors have noted SpO2 instability in children resident at higher altitude (1,600 m) that was not explained by PB,22 although this location was below the 2,500-m threshold used to define HA (Figure 1A). According to this hypothesis, the lower diurnal baseline in infants would be predicted to predispose to greater instability at night. Lower baseline SpO2 in infants, in turn, could be explained by persistently increased pulmonary arterial pressure as previously described at HA in Andean infants, albeit at higher altitudes (> 4,000 m) than the current sample.23
The implications of relative nocturnal hypoxia in HA children deserve consideration. The developing brain is dependent on a continuous supply of oxygen.24 Alterations in nocturnal oxygen-carrying capacity at altitude may impair neurocognitive development, as evidenced by the extensive literature on the neurocognitive cost of chronic and intermittent hypoxia in childhood.25 Conversely, nocturnal respiratory instability could serve as adaptive preparation for HA survival through the calibration of physiological equilibrium between the up-regulation of hypoxia-protective pathways (e.g., hypoxia inducible factors) and the reduction of potential harm from excessive erythropoiesis.26 Indeed, we have reported relatively subtle cognitive impairments in this population of HA children,8,9 suggesting the presence of compensatory mechanisms. Such mechanisms could be challenged in children with additional constraints, such as those with congenital or acquired brain injury or underlying cardiorespiratory disease, and this merits further study.
Limitations of This Study
The lack of measures of respiratory effort and airflow to identify apnea make the interpretation of our findings speculative. However, in a healthy child, SpO2 is a marker of tissue oxygen availability independent of body size and, importantly, hemoglobin does not have the response kinetics to accommodate the day-night variation we have described.
We studied nocturnal SpO2 in the absence of objective sleep measures and, therefore, differences between diurnal and nocturnal measures are inferred to be related to sleep. However, these differences are consistent with participants sleeping during the recordings in the absence of any other viable explanation. Indeed, failure to extract periods of wake from the data, particularly in infants who may have awakened for feeds at night, will have under-estimated diurnal-nocturnal differences, thus strengthening our findings.
The high prevalence of reported snoring compared to European samples could indicate sampling bias. However, our data are consistent with a Chilean study which reported habitual snoring in 18% of children aged 7–17 y.27. Furthermore, the lack of altitude-related differences in snoring history suggests that HA findings cannot be attributed to snoring.
Our original aim was to measure oximetry across all ages. Delays in importing equipment limited assessment at LA to infants and children. However, there was no a priori expectation of developmental differences in sleep related SpO2 or ventilatory control between children and adolescents at LA. Importantly, data were collected for all ages at HA. Sample sizes in each age and altitude subgroup were also smaller than intended; nonetheless, they were sufficient to demonstrate significant age and altitude effects between infants and children.
Notwithstanding these limitations, our findings are not only of academic interest to HA researchers but are also clinically relevant to pediatric critical care medicine. Children with respiratory disease may experience profound nocturnal oxy-hemoglobin desaturation at HA, compromising an already constrained system. Andean children with sleep-disordered breathing living at only 2,240 m who experienced > 43% of the night with SpO2 < 90% had pulmonary arterial hypertension on echocardiography.28 In our study, at HA, infants spent a median of 79.7% of the night with SpO2 < 90% compared to 33.5% of children and 7.6% of adolescents. Based on studies of diurnal SpO2 measurements, previous authors have recommended that oxygen therapy should be initiated for SpO2 < 85% in HA hospital pediatric settings.29 Our data indicate that healthy infants living at HA will often experience oxyhemoglobin desaturation in sleep below this threshold. Thus, these guidelines may not apply to Andean populations.
These novel findings indicate potentially significant nocturnal oxyhemoglobin desaturation in healthy Andean HA dwelling children with mixed European and Amerindian ancestry. Increased SpO2 variability at HA could be explained by a number of mechanisms and appears to stabilize across childhood. Further studies could usefully replicate our findings and establish normative, sleep-related SpO2 data in childhood at HA, alongside characterization of respiratory patterns with polysomnography.
DISCLOSURE STATEMENT
This was not an industry supported study. The work reported in this article was funded by the British Academy, the London Law Trust, the Gerald Kerkut Trust and the World Universities network. Equipment was loaned by Masimo Inc. Dr. Kirkham has consulted for Eisai and has participated in speaking engagements for Shire. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are indebted to Univalle (Cochabamba, La Paz), USPA (Santa Cruz), Universidad Privada Abierta Latinoamericana (Santa Cruz), Universidad de La Salle (La Paz), and to their student volunteers who supported these studies. The authors also gratefully acknowledge the assistance of the Australian Medical Bioinformatics Resource, a National Health and Medical Research Council of Australia Medical Bioinformatics Genomics Proteomics Program.
REFERENCES
- 1.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;(Suppl 27):25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation. 2007;115:1132–46. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 3.Virués-Ortega J, Hogan AM, Baya-Botti A, et al. on behalf of the Bolivian Children Living at Altitude Project (BoCLA 2006) Survival and mortality in older adults living at high altitude in Bolivia: a preliminary report. J Am Geriatr Soc. 2009;57:1955–6. doi: 10.1111/j.1532-5415.2009.02468.x. [DOI] [PubMed] [Google Scholar]
- 4.Giussani DA, Davidge ST. Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis. 2013;4:328–37. doi: 10.1017/S204017441300010X. [DOI] [PubMed] [Google Scholar]
- 5.Beall CM. Oxygen saturation increases during childhood and decreases during adulthood among high altitude native Tibetans residing at 3,800-4,200 m. High Alt Med Biol. 2000;1:25–32. doi: 10.1089/152702900320658. [DOI] [PubMed] [Google Scholar]
- 6.Gamponia MJ, Babaali H, Yugar F, Gilman RH. Reference values for pulse oximetry at high altitude. Arch Dis Child. 1998;78:461–5. doi: 10.1136/adc.78.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beall CM, Almasy LA, Blangero J, et al. Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3,900-4,000 m. Am J Phys Anthropol. 1999;108:41–51. doi: 10.1002/(SICI)1096-8644(199901)108:1<41::AID-AJPA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Hogan AM, Virues-Ortega JV, Baya Botti A, et al. Development of aptitude at altitude. Dev Sci. 2010;13:533–44. doi: 10.1111/j.1467-7687.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- 9.Hill CM, Annaz D, Baya A, et al. Cognitive performance in high altitude Andean residents compared to low altitude populations: from childhood to older age. Neuropsychology. 2014;28:752–60. doi: 10.1037/neu0000065. [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung and Blood Institute. Fourth report of the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Blood pressure tables for children and adolescents. 2004. [Accessed 03/03/2015]. Available from http://www.nhlbi.nih.gov/health-pro/guidelines/current/hypertension-pediatric-jnc-4/blood-pressure-tables.htm.
- 11.Urschitz MS, Wolff J, Von Einem V, Urschitz-Duprat PM, Schlaud M, Poets CF. Reference values for nocturnal home pulse oximetry during sleep in primary school children. Chest. 2003;123:96–101. doi: 10.1378/chest.123.1.96. [DOI] [PubMed] [Google Scholar]
- 12.Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 14.Moore LG, Brewer G. Biochemical mechanisms of red blood cell 2,3-diphosphoglycerate increase at high altitude. Am J Phys Anthropology. 1980;53:ll–8. doi: 10.1002/ajpa.1330530104. [DOI] [PubMed] [Google Scholar]
- 15.Anholm JD, Powles AC, Downey R, 3rd, et al. Operation Everest II: arterial oxygen saturation and sleep at extreme simulated altitude. Am Rev Respir Dis. 1992;145:817–26. doi: 10.1164/ajrccm/145.4_Pt_1.817. [DOI] [PubMed] [Google Scholar]
- 16.Kelly DH, Stellwagen LM, Kaitz E, Shannon DC. Apnea and periodic breathing in normal full-term infants during the first twelve months. Pediatr Pulmonol. 1985;1:215–9. doi: 10.1002/ppul.1950010409. [DOI] [PubMed] [Google Scholar]
- 17.Reeves SR, Gozal D. Developmental plasticity of respiratory control following intermittent hypoxia. Respir Physiol Neurobiol. 2005;149:301–11. doi: 10.1016/j.resp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Carroll JL, Kim I. Carotid chemoreceptor “resetting” revisited. Respir Physiol Neurobiol. 2013;185:30–43. doi: 10.1016/j.resp.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coote JH, Tsang G, Baker A, Stone B. Respiratory changes and structure of sleep in young high-altitude dwellers in the Andes of Peru. Eur J Appl Physiol Occup Physiol. 1993;66:249–53. doi: 10.1007/BF00235102. [DOI] [PubMed] [Google Scholar]
- 20.Spicuzza L, Casiraghi N, Gamboa A, et al. Sleep-related hypoxaemia and excessive erythrocytosis in Andean high-altitude natives. Eur Respir J. 2004;23:41–6. doi: 10.1183/09031936.03.00000703. [DOI] [PubMed] [Google Scholar]
- 21.Bernardi L, Roach RC, Keyl C, et al. Ventilation, autonomic function, sleep and erythropoietin. Chronic mountain sickness of Andean natives. Adv Exp Med Biol. 2003;543:161–75. [PubMed] [Google Scholar]
- 22.Burg CJ, Montgomery-Downs HE, Mettler P, Gozal D, Halbower AC. Respiratory and polysomnographic values in 3- to 5-year-old normal children at higher altitude. Sleep. 2013;36:1707–14. doi: 10.5665/sleep.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huico L. Postnatal cardiopulmonary adaptations to high altitude. Respir Physiol Neurobiol. 2007;158:190–203. doi: 10.1016/j.resp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Hogan AM, De Haan M, Datta A, Kirkham FJ. Hypoxia: an acute, intermittent and chronic challenge to cognitive development. Dev Sci. 2006;9:335–7. [Google Scholar]
- 25.Bass JL, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114:805–16. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 26.Höpfl G, Ogunshola O, Gassmann M. Hypoxia and high altitude. The molecular response. Adv Exp Med Biol. 2003;543:89–115. doi: 10.1007/978-1-4419-8997-0_7. [DOI] [PubMed] [Google Scholar]
- 27.Brockmann PE, Bertrand P, Pardo T, Cerda J, Reyes B, Holmgren NL. Prevalence of habitual snoring and associated neurocognitive consequences among Chilean school aged children. Int J Pediatr Otorhinolaryngol. 2012;76:1327–31. doi: 10.1016/j.ijporl.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Vázquez JC, Montes FM, Rivera CA, Vargas SM, Pérez-Padilla R. Clinical predictors of sleep disordered breathing in children at moderate altitude. Arch Med Res. 2004;35:525–31. doi: 10.1016/j.arcmed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Subhi R, Smith K, Duke T. When should oxygen be given to children at altitude? A systematic review to define altitude-specific hypoxaemia. Arch Dis Child. 2009;94:6–10. doi: 10.1136/adc.2008.138362. [DOI] [PubMed] [Google Scholar]


