Abstract
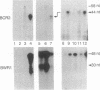
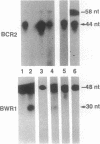
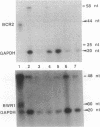
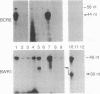
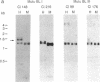
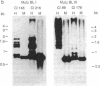
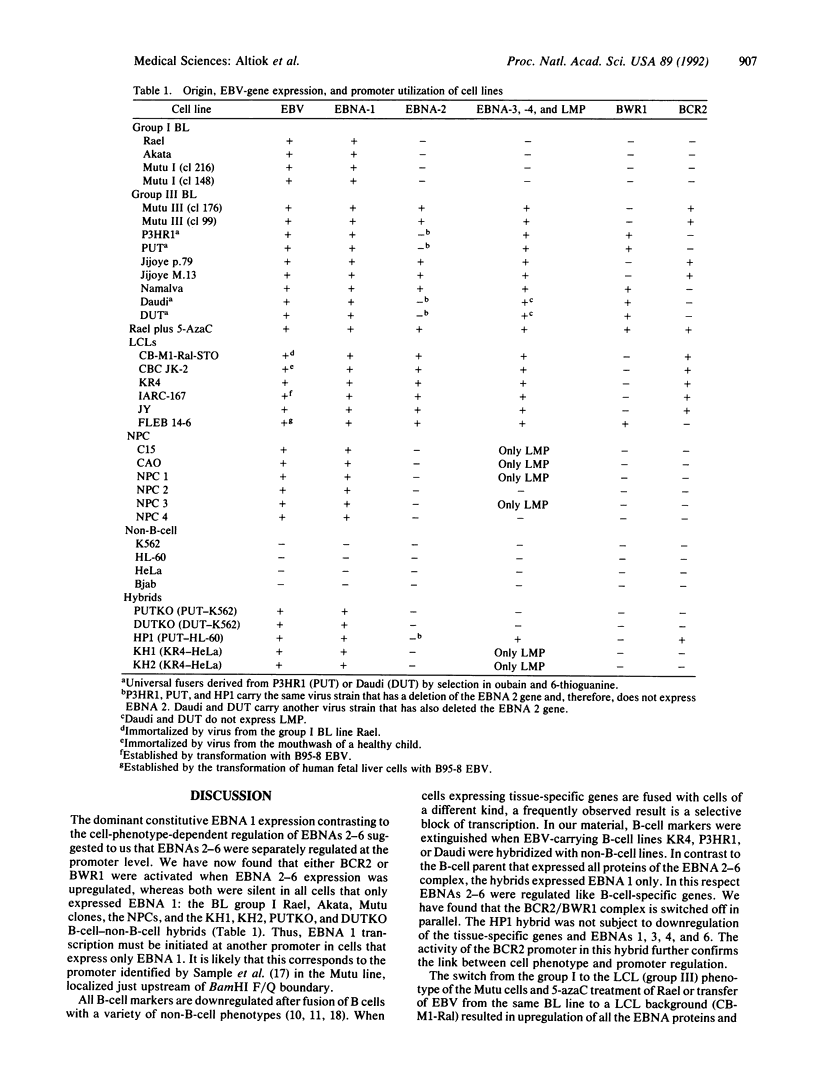
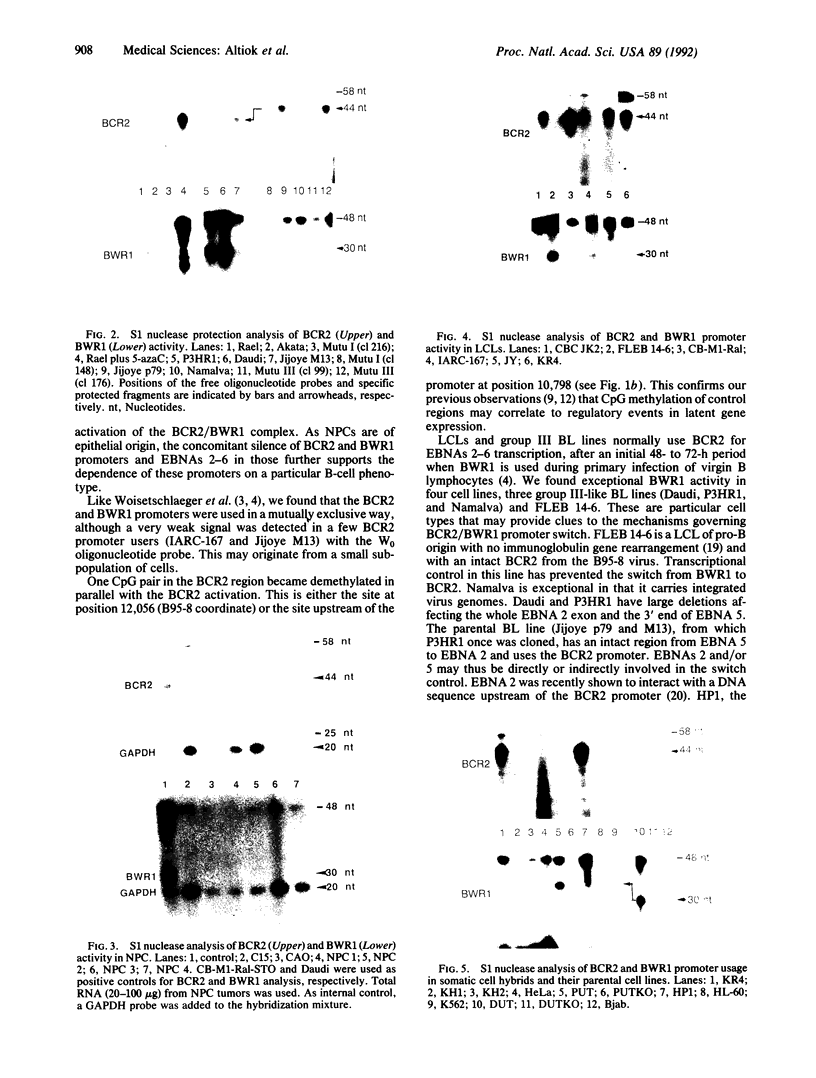
Epstein-Barr virus nuclear antigens (EBNAs) are expressed in a cell-phenotype-dependent manner. EBNA 1 is regularly expressed in all Epstein-Barr virus-carrying cells, whereas EBNAs 2-6 are only expressed in Epstein-Barr virus-carrying cells with a lymphoblastoid phenotype including group III Burkitt lymphoma (BL) lines positive for B-cell activation markers. Transcripts are initiated at the BCR2 or exceptionally at one BWR1 promoter in lymphoblastoid cell lines and group III BL lines. In group I BL lines, nasopharyngeal carcinoma, and the somatic cell hybrids, where EBNAs 2-6 are downregulated, the BCR2/BWR1 promoter complex is inactive or switched off. Upregulation of EBNAs 2-6 in group III BL cells and in 5-azacytidine-treated group I BL cells accompanies the activation of the silent BCR2/BWR1 promoters. Activation of BCR2 parallels demethylation of at least one CpG pair in the same promoter region. The activity of BCR2/BWR1 promoter complex depends on a particular B-cell phenotype. EBNA 1 transcription must be initiated at another promoter in cells that express only EBNA 1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. W., Trach K. A., Hoch J. A. Identification of the 37-kDa protein displaying a variable interaction with the erythroid cell membrane as glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1987 Jan 15;262(2):649–653. [PubMed] [Google Scholar]
- Altiok E., Klein G., Zech L., Uno M., Henriksson B. E., Battat S., Ono Y., Ernberg I. Epstein-Barr virus-transformed pro-B cells are prone to illegitimate recombination between the switch region of the mu chain gene and other chromosomes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6333–6337. doi: 10.1073/pnas.86.16.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Salazar B., Klein G., Masucci M. G. Host cell-dependent regulation of growth transformation-associated Epstein-Barr virus antigens in somatic cell hybrids. J Virol. 1989 Jun;63(6):2768–2772. doi: 10.1128/jvi.63.6.2768-2772.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillner J., Kallin B. The Epstein-Barr virus proteins. Adv Cancer Res. 1988;50:95–158. doi: 10.1016/s0065-230x(08)60436-4. [DOI] [PubMed] [Google Scholar]
- Ernberg I., Falk K., Minarovits J., Busson P., Tursz T., Masucci M. G., Klein G. The role of methylation in the phenotype-dependent modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J Gen Virol. 1989 Nov;70(Pt 11):2989–3002. doi: 10.1099/0022-1317-70-11-2989. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory C. D., Rowe M., Rickinson A. B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990 Jul;71(Pt 7):1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Hu L. F., Minarovits J., Cao S. L., Contreras-Salazar B., Rymo L., Falk K., Klein G., Ernberg I. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5'-flanking region. J Virol. 1991 Mar;65(3):1558–1567. doi: 10.1128/jvi.65.3.1558-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker S., Pedersen S., Schreiber E., Matthias P. Extinction of an immunoglobulin kappa promoter in cell hybrids is mediated by the octamer motif and correlates with suppression of Oct-2 expression. Cell. 1990 May 4;61(3):467–474. doi: 10.1016/0092-8674(90)90528-m. [DOI] [PubMed] [Google Scholar]
- Masucci M. G., Contreras-Salazar B., Ragnar E., Falk K., Minarovits J., Ernberg I., Klein G. 5-Azacytidine up regulates the expression of Epstein-Barr virus nuclear antigen 2 (EBNA-2) through EBNA-6 and latent membrane protein in the Burkitt's lymphoma line rael. J Virol. 1989 Jul;63(7):3135–3141. doi: 10.1128/jvi.63.7.3135-3141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minarovits J., Minarovits-Kormuta S., Ehlin-Henriksson B., Falk K., Klein G., Ernberg I. Host cell phenotype-dependent methylation patterns of Epstein-Barr virus DNA. J Gen Virol. 1991 Jul;72(Pt 7):1591–1599. doi: 10.1099/0022-1317-72-7-1591. [DOI] [PubMed] [Google Scholar]
- Sample J., Brooks L., Sample C., Young L., Rowe M., Gregory C., Rickinson A., Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6343–6347. doi: 10.1073/pnas.88.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls D., Perricaudet M. Novel downstream elements upregulate transcription initiated from an Epstein-Barr virus latent promoter. EMBO J. 1991 Jan;10(1):143–151. doi: 10.1002/j.1460-2075.1991.tb07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M., Jin X. W., Yandava C. N., Furmanski L. A., Strominger J. L., Speck S. H. Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3942–3946. doi: 10.1073/pnas.88.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M., Strominger J. L., Speck S. H. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6498–6502. doi: 10.1073/pnas.86.17.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woisetschlaeger M., Yandava C. N., Furmanski L. A., Strominger J. L., Speck S. H. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1725–1729. doi: 10.1073/pnas.87.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimber-Strobl U., Suentzenich K. O., Laux G., Eick D., Cordier M., Calender A., Billaud M., Lenoir G. M., Bornkamm G. W. Epstein-Barr virus nuclear antigen 2 activates transcription of the terminal protein gene. J Virol. 1991 Jan;65(1):415–423. doi: 10.1128/jvi.65.1.415-423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]