Abstract
Study Objectives:
To examine whether insomnia is associated with spectral electroencephalographic (EEG) dynamics in the beta (15–35Hz) range during sleep in an adolescent general population sample.
Methods:
A case-control sample of 44 adolescents from the Penn State Child Cohort underwent a 9-h polysomnography, clinical history and physical examination. We examined low-beta (15–25 Hz) and high-beta (25–35 Hz) relative power at central EEG derivations during sleep onset latency (SOL), sleep onset (SO), non-rapid eye movement (NREM) sleep, and wake after sleep onset (WASO).
Results:
Compared to controls (n = 21), individuals with insomnia (n = 23) showed increased SOL and WASO and decreased sleep duration and efficiency, while no differences in sleep architecture were found. Insomniacs showed increased low-beta and high-beta relative power during SOL, SO, and NREM sleep as compared to controls. High-beta relative power was greater during all sleep and wake states in insomniacs with short sleep duration as compared to individuals with insomnia with normal sleep duration.
Conclusions:
Adolescent insomnia is associated with increased beta EEG power during sleep, which suggests that cortical hyperarousal is present in individuals with insomnia as early as adolescence. Interestingly, cortical hyperarousal is greatest in individuals with insomnia with short sleep duration and may explain the sleep complaints of those with normal sleep duration. Disturbed cortical networks may be a shared mechanism putting individuals with insomnia at risk of psychiatric disorders.
Citation:
Fernandez-Mendoza J, Li Y, Vgontzas AN, Fang J, Gaines J, Calhoun SL, Liao D, Bixler EO. Insomnia is associated with cortical hyperarousal as early as adolescence. SLEEP 2016;39(5):1029–1036.
Keywords: adolescence, beta, EEG, hyperarousal, insomnia
Significance.
This is the first study to show that adolescents who complain of insomnia have increased fast frequencies in the electroencephalogram while trying to fall asleep or while asleep. Beta frequencies are regarded as a marker of cortical arousal and this study showed that they were elevated in adolescent insomniacs with normal sleep duration and highest in those with short sleep duration. Thus, even when asleep, the brain of adolescents with insomnia continues to process information and remains more alert than that of good sleepers. This is an important finding because this type of brain activation has been found in individuals with psychiatric disorders, insomnia usually precedes the onset of such disorders, and adolescence is a critical developmental period.
INTRODUCTION
Insomnia is the most prevalent sleep disorder and is associated with increased risk of psychiatric and cardiometabolic morbidity.1,2 Data from multiple systems, such as cognitive-emotional, stress, cardiovascular, metabolic, and immune, support that insomnia is a disorder of 24-h hyperarousal.3–5 Insomnia in adults has been associated with anxious-ruminative traits, increased cortisol secretion, impaired heart rate variability, increased whole-body and brain metabolic rate, and a shift in the secretion of pro-inflammatory cytokines.3–5 As shown in Table 1,6–20 spectral electroencephalographic (EEG) studies in adults have shown that insomnia is associated with cortical hyperarousal as measured by high-frequency EEG dynamics, particularly in the 15–35 Hz range, during sleep onset latency (SOL) and non-rapid eye movement (NREM) sleep,6–13,15–17,20 whereas less consistent differences have been found in other frequency bands (e.g., 8–12 Hz) or sleep states (e.g., REM sleep).6,9–12,14,15,17–20 These findings are important because cortical hyperarousal has been suggested to contribute to the pathophysiology of insomnia and its associated psychiatric risk.3–5,21,22
Table 1.
Studies on spectral electroencephalographic dynamics in adults with insomnia as compared to good sleeping adult controls.
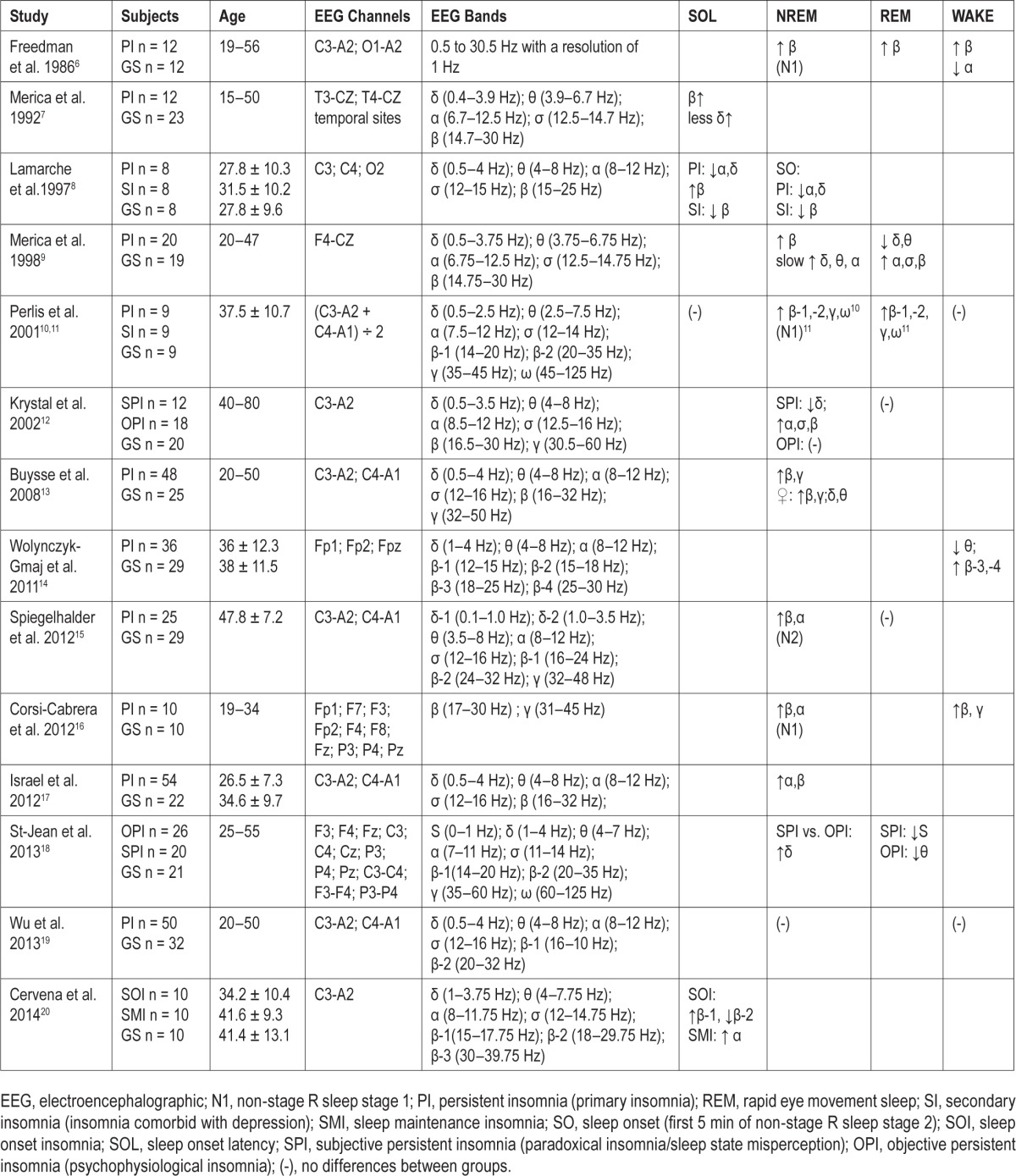
Despite the association of insomnia with behavioral functioning in adolescents,23 little is known about its pathophysiology. Adolescent insomnia, particularly difficulty falling asleep, is typically conceptualized as a result of sleep-incompatible behaviors (i.e., poor sleep habits) and/or a circadian misalignment (i.e., delayed circadian sleep phase).23 However, no study to date has examined whether insomnia is associated with EEG markers of cortical hyperarousal in adolescents. Thus, the aim of this study was to examine the association of insomnia with spectral EEG high-frequency dynamics during sleep in an adolescent sample of the general population.
METHODS
Sample
In order to examine the independent association of insomnia with spectral EEG dynamics, we studied a randomly selected, case-control subsample of adolescents (16.6 ± 2.0 y) who participated in the Penn State Child Cohort (PSCC), a population-based random sample of 421 adolescents.24 The insomnia group fulfilled the following criteria: (1) presence of insomnia, defined as a self-report of difficulty falling (DFA) and/or staying (DSA) asleep, (2) absence of overweight or obesity, defined as a body mass index percentile (BMI) < 85%, (3) absence of sleep-disordered breathing (SDB), defined as an apnea-hypopnea index (AHI) < 1.5 events/h of sleep using polysomnography (PSG), and (4) age younger than 21 y. The good sleeping control group met the following criteria: (1) absence of insomnia, as defined above, (2) absence of over-weight or obesity, as defined above, (3) absence of SDB, as defined above, (4) age younger than 21 y, and (5) presence of subjectively reported and objectively measured normal sleep, defined as a self-reported SOL below the median of the entire population, i.e., < 25 min, and PSG-measured SOL and sleep efficiency (SE) below and above the median, i.e., < 21 min and ≥ 85% respectively, of the entire population. Finally, none of the subjects reported a current use of legal or illegal substances/drugs, except habitual use of morning caffeine. A total of 21 controls and 23 cases of insomnia were selected using the random procedure of sample cases in SPPS version 21. All participants or legal guardians provided informed written consent and the study protocol was approved by Penn State Hershey Institutional Review Board.
Key Measurements
All subjects underwent an in-laboratory, 9-h PSG, clinical history, and physical examination and completed self-reported questionnaires. Upon clinical history, sex, race, and age (date of birth) were collected by self-report. Height and weight were measured during the physical examination; overweight or obesity was defined as a BMI percentile ≥ 85% and scored accordingly using CDC tables.25 Standardized self-reported questionnaires were used to assess Tanner stage,26 internalizing and externalizing behaviors,27 circadian preference (i.e., eveningness),28 and insomnia; the presence of insomnia was defined as a self-report of DFA (“do you have difficulty falling asleep?”) and/or DSA (“do you have difficulty staying asleep?”) based on a modified self-report version of the Pediatric Sleep Questionnaire.29 Sleep was recorded on 14-channel GrassPSG for 9 h from “lights out” (22:00) until “lights on” (07:00). All recordings included EEG (e.g., C3-A2; C4-A1), EOG (horizontal and vertical) and electromyography (EMG; limbs and submental), and were scored visually by experienced raters in 30-sec periods according to standard criteria.30 All participants were screened for apneas and periodic leg movements by monitoring abdominal and thoracic effort, nasal airflow, oximetry, and bilateral tibialis anterior EMG. Respiration was monitored with nasal pressure, thermocouple, and thoracic and abdominal strain gauges and hemoglobin oxygen saturation (SpO2) was obtained from the finger. The sleep records were subsequently scored independently according to standardized criteria by a registered polysomnography technician (RPSGT).30 An apnea was defined as a cessation of airflow with a minimum duration of 5 sec for age younger than 16 y and 10 sec for age 16 y or older with an out-of-phase strain gauge movement. A hypopnea was defined as a reduction of airflow of approximately 50% with an associated decrease in oxygen saturation (SpO2) of at least 3% or an associated arousal. AHI was calculated as the number of apneas and hypopneas summed per hour of sleep. Sleep records were evaluated for the following parameters of sleep continuity: SOL defined as time from lights out until the first epoch of NREM sleep stage 2; total sleep time (TST); SE (ratio of TST to time in bed × 100); and wake after sleep onset (WASO) defined as difference between sleep period time (SPT; time from sleep onset until final awakening). Sleep architecture parameters included the amount of stages 1, 2, slow wave sleep (SWS) and rapid eye movement (REM) sleep as percentage of SPT. As per further spectral analyses, sleep onset (SO) was defined as the first 5 min of NREM sleep after SOL (by definition, any segment with wake was not included in the analyses), NREM sleep was defined as any period of at least 15 min of stages 2 or SWS, and WASO as any period of at least 5 min of wake after sleep onset.
Spectral Analyses
Based on the findings of previous studies in adults (Table 1), we focused our spectral analyses on central EEG derivations (averaged C3-A2 and C4-A1 channels), beta frequencies (15–35 Hz), and four sleep/wake states (SOL, SO, NREM sleep, and WASO). Continuous nighttime EEG was amplified with a band pass at 0.5–70 Hz (−3 dB), digitized at 200 Hz and stored for off-line analysis. An all-night spectral analysis was performed following Spiegelhalder et al.15 procedures with adaptations32 as described below, on the same 30-sec epochs for which sleep stages had been determined. Within each epoch, spectral power was calculated using the fast Fourier transform (FFT) algorithm from 23 windows32 (512 points each) overlapping by approximately half (261 overlapping samples), resulting in a spectral resolution of 0.39 Hz. Within each FFT window, a Hanning window32 was applied before calculating the FFT. The 23 spectral power estimates within each epoch were averaged to increase the stability of the estimate. The goal of further analysis was to minimize the effects of confounding variables on the spectra averaged across epochs, such as the number of movements or arousals and other sleep parameters that can be analyzed separately.15 This was done by two techniques: (1) arousals and myoclonias, including periodic leg movements (i.e., defined as four leg movements within 90 sec, at least 0.5 sec in duration and 5 sec apart), were visually marked during staging and epochs including any such events were excluded from the analysis; (2) a fully automatic exclusion of “deviant” epochs from the average was performed.15 Deviant epochs were those containing movements or arousals as determined during staging; furthermore, the total (0.39–100 Hz) and gamma-band (36– 100 Hz) power of each epoch was related to the corresponding median-filtered value (the median of values in the 5 min preceding and 5 min following the epoch) and an epoch was excluded if the deviation was larger than the difference between the median and the first quartile of all median-filtered values across the night.15 In this way, artifacts mainly restricted to low frequencies (such as electrooculographic events) as well as those occurring mainly in higher frequencies (such as EMG contamination) were eliminated in a data-driven way.15 The relative power in low-beta (15–25 Hz) and high-beta (25–35 Hz) in the total power (up to 100 Hz excluding 0.0 Hz) were further calculated for each epoch. All-night spectral power averages were obtained across all artifact-free epochs of wake during SOL and WASO and sleep during SO and NREM. The averaged C3-A2 and C4-A1 relative power was calculated as [(number of segments at C3 * C3 relative power) / number of segments at C3 + (number of segments at C4 * C4 relative power) / number of segments at C4) / 2]. Spectral analyses were performed with SleepFFT software (Biosoft Studio, Hershey, PA).32
Statistical Analyses
Descriptive presentation of the data includes mean values and standard deviations. The logarithmic (base e) spectra for artifact-free epochs were averaged and NREM sleep was restricted to stage 2 and SWS in order to eliminate the influence of different NREM sleep stage distributions (i.e., stage 1 which has greater wake intrusions) across subjects; logarithmic spectral band power was calculated after adding the spectral power values of FFT bins with center frequency within the low-beta (15–25 Hz) and high-beta (25–35 Hz) frequency bands.15 Initial exploration of the raw data indicated that minimal levels of AHI were significantly associated with our primary outcome of high-beta power (r = 0.488, P = 0.025; P for group × AHI interaction = 0.044), most likely as a result of visually undetected arousals; consistently, we conducted our analyses adjusting for the potential confounding effect of AHI and its group interaction on relative power data. Mean differences in relative power of low-beta and high-beta spectral bands between good sleeping controls and individuals with insomnia were analyzed using multivariate analyses of covariance (MANCOVA) to control for experiment-wise error rate (type I error); the level of significance was set at P < 0.05 (two-tailed). Based on previous studies in adults suggesting differences in the association of insomnia with “normal” and “short” sleep duration with medical morbidity3–5 and hyperarousal, including cortical hyperarousal,4,18 we further examined group differences in relative power of high-frequency spectral bands by subtyping the insomnia group into those with “normal” (SE ≥ 85%) and “short” (SE < 85%) sleep duration. Thus, the insomnia with “normal” sleep duration group slept for 8.1 ± 0.2 h with a range of 7.8–8.4 h, whereas the insomnia with “short” sleep duration slept for 6.7 ± 0.7 h with a range of 5.4–7.6 h. Good sleeping controls had, by definition, normal sleep duration (SE ≥ 85%) and slept for 8.2 ± 0.2 h with a range of 7.7–8.7 h. Statistical analyses were performed using SPSS version 21.
RESULTS
Characteristics of the Sample
As shown in Table 2, the insomnia and control groups were not significantly different in terms of key potential confounders such as age, Tanner stage, sex, race, BMI percentile, eveningness, internalizing or externalizing behaviors. Furthermore, self-reported time to go to bed (22:30 ± 1:10 versus 22:45 ± 0:54, P = 0.434) or to get out of bed (6:52 ± 1:18 versus 7:23 ± 1:58, P = 0.309) on weekdays as well as time to go to bed (23:52 ± 1:29 versus 24:13 ± 1:18, P = 0.416) or to get out of bed (9:11 ± 1:34 versus 9:52 ± 1:41, P = 0.168) on weekends were not significantly different between controls and individuals with insomnia. Similarly, habitual caffeine use was not significantly different between controls (95.2%) and individuals with insomnia (82.6%, P = 0.348). Upon PSG assessment, individuals with insomnia showed significantly increased SOL and WASO and decreased sleep duration and SE as compared to controls, whereas no differences in sleep architecture were found (Table 2).
Table 2.
Demographic, behavioral and sleep characteristics of the study groups.

Beta Frequencies during Difficulty Initiating or Resuming Sleep and NREM Sleep
Table 3 presents the relative power in low-beta and high-beta frequency bands at central EEG derivations for the control and insomnia groups during different sleep and wake states. Overall, individuals with insomnia showed significantly increased low-beta and high-beta relative power during SOL (P = 0.020 and P = 0.021, respectively), SO (P = 0.004 and P = 0.016, respectively), and NREM sleep (P = 0.007 and P = 0.007, respectively). Marginally significant differences in low-beta and high-beta relative power were found during WASO (P = 0.061 and P = 0.068, respectively).
Table 3.
Low-beta and high-beta power in adolescents with and without insomnia.

In order to test whether insomnia subtypes based on PSG-measured sleep duration were differentially associated with cortical hyperarousal, we examined differences in beta frequency bands between individuals with insomnia with “normal” and “short” sleep duration as compared to controls. Table 3 presents the relative power in low-beta and high-beta frequency bands at central EEG derivations for the “normal” and “short” sleep duration insomnia subgroups during different sleep and wake states. As shown in Figure 1, individuals with insomnia with “short” sleep duration showed significantly increased and highest relative power in low-beta and high-beta frequencies during all sleep and wake states (P values reported in Table 3). In contrast, individuals with insomnia with “normal” sleep duration showed significantly increased low-beta and high-beta relative power during SOL (P = 0.021 and P = 0.016, respectively) and marginally increased low-beta relative power during SO (P = 0.057) and NREM sleep (P = 0.067) as compared to controls, but did not show significantly increased low-beta relative power during WASO (P = 0.159) or high-beta relative power during SO (P = 0.198), NREM (P = 0.116), or WASO (P = 0.389). Furthermore, sig -nificant differences between insomniacs with “short” sleep duration and insomniacs with “normal” sleep duration were found in high-beta relative power during all sleep and wake states (P = 0.034 –0.001), whereas differences between the two insomnia subtypes in low-beta relative power were marginal to nonsignificant (P = 0.063–0.495) for all sleep and wake states, except WASO (P = 0.008).
Figure 1.
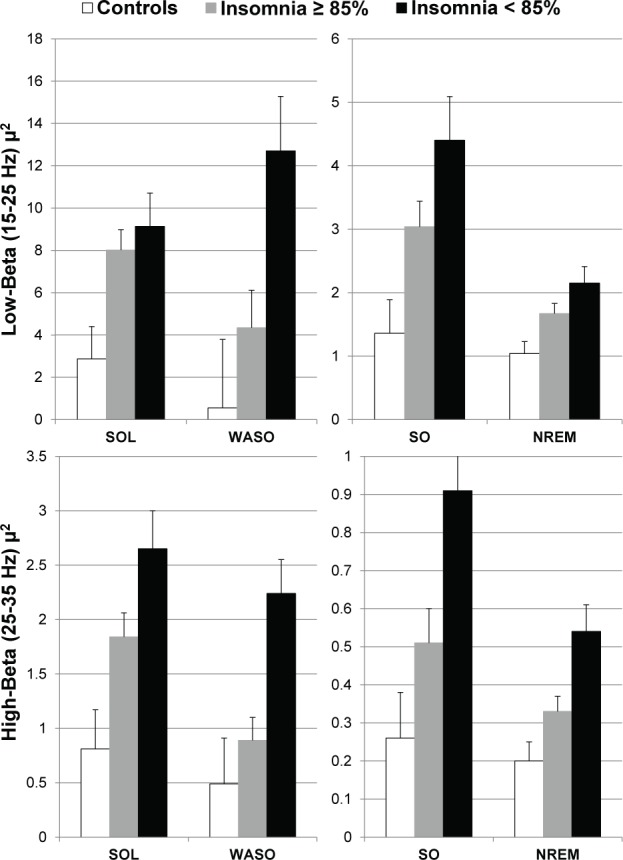
Low-beta and high-beta relative power during wake and sleep states in controls and insomnia phenotypes. Left panels depict differences during wake states (i.e., SOL and WASO), whereas right panels depict differences during sleep states (i.e., SO and NREM sleep) between controls and adolescents with insomnia with “normal” (sleep efficiency ≥ 85%) and “short” (sleep efficiency < 85%) sleep duration. NREM, non-REM sleep; SO, sleep onset; SOL, sleep onset latency; WASO, wake after sleep onset.
Other Sleep States and Frequency Bands
As mentioned previously, we tested the hypothesis that cortical hyperarousal during the sleep period, as measured by beta (15–35 Hz) frequencies during difficulty falling (SOL) or staying (WASO) asleep and NREM sleep, is associated with insomnia in adolescents based on the findings of previous studies in adults (Table 1). In secondary analyses, we examined whether cortical hyperarousal was present during REM sleep (defined as any period of at least 5 min of stage R) or whether relative power in other frequency bands differed between the control and insomnia groups. Consistent with previous studies in adults, relative power in low-beta and high-beta frequency bands during REM sleep was not significantly different between the control and insomnia groups (P = 0.178–0.236). Furthermore, no differences in relative power in delta (0.39–3.91 Hz), theta (4.11–7.91 Hz), or alpha (8.20–11.72 Hz) frequency bands were found between the control and insomnia groups during any sleep/wake state (P = 0.121– 0.968). Significant and marginally significant differences in frequency bands close to the beta frequency spectrum were found between the control and insomnia groups; specifically, relative power in the sigma (12.11–14.84 Hz) frequency band was increased in individuals with insomnia as compared to controls during SOL (P = 0.086), SO (P = 0.015), NREM sleep (P = 0.003), and WASO (P = 0.017) but not during REM sleep (P = 0.535), whereas relative power in the gamma (35.55–99.61 Hz) frequency band was increased in individuals with insomnia as compared to controls during SO (P = 0.091) and NREM sleep (P = 0.008), but not during SOL (P = 0.287), WASO (P = 0.592) or REM sleep (P = 0.535).
DISCUSSION
This is the first study to demonstrate that adolescent insomnia is associated with increased beta EEG frequencies when attempting to fall asleep and during NREM sleep, which indicates that cortical hyperarousal is present in insomnia patients as early as adolescence independent of behavioral problems (i.e., poor sleep habits, internalizing/externalizing behaviors) and circadian misalignment (i.e., delayed sleep phase/eveningness). Furthermore, this study found that the degree and pattern of cortical hyperarousal varies across insomnia subtypes based on objective sleep duration.
Our findings of increased beta (15–35 Hz) frequencies during wake trying to fall asleep, wake in the middle of the night, and NREM sleep in adolescents with insomnia from the general population are consistent with previous studies in adult clinical patients or research volunteers with insomnia (Table 1).6–13,15–18,20,33 Most of these previous studies have interpreted the presence of increased beta EEG frequencies during sleep in adults with insomnia as a sign of disruption of cortical networks involved in information processing (i.e., cognitive arousal).21,34 Given the strong evidence supporting the role of cognitive arousal (i.e., worry, rumination, attentional bias) in insomnia,3,35 it is indeed very likely that increased beta EEG frequencies are a sign of disrupted cortical networks36 that do not dearouse and continue to process information when attempting to initiate or resume sleep and during NREM sleep in individuals with insomnia.21,34
More recently, increased beta EEG frequencies during NREM sleep have been interpreted as a sign of simultaneous activation of sleep-promoting and wake-promoting mechanisms,15,37 particularly when simultaneous increases in sigma and beta frequencies have been found in adults with insomnia.15 Consistent with this hypothesis are studies in other systems, particularly those showing significant activation of the hypothalamic-pituitary adrenal (HPA) and sympatho-adrenal-medullary (SAM) axes of the stress system in adults with insomnia.3,4 In this view, increased beta EEG frequencies are one of many signs of “physiologic hyperarousal” during the nighttime period. This pattern of simultaneous activation of sleep-promoting [i.e., ventrolateral preoptic nucleus (VLPO)] and wake-promoting [i.e., locus coeruleus (LC) and tuberomammillary (TMN) nuclei] mechanisms as well as the central autonomic control system, including the corticotrophin releasing hormone system [e.g., paraventricular hypothalamic nucleus (PVH), ventrolateral periaqueductal gray matter (vlPAG)], has been observed in a rat model of stress-induced insomnia.37 Under normal conditions, the reciprocal inhibition between the sleep system (VLPO) and the arousal system (LC and TMN) would prevent coactivation; however, during the period of stress-induced insomnia in rats, homeostatic sleep pressure keeps the VLPO activated, whereas stress (e.g., PVH, vlPAG) activates the arousal system, resulting in increased fast EEG frequencies and overt sleep disturbance (e.g., objective short sleep duration).37 In humans, however, the paradox of individuals who complain of insomnia and cognitive arousal (i.e., a “racing mind while trying to fall asleep” or “awareness while asleep”) and present with increased beta EEG frequencies despite normal sleep duration does exist, which raises the question whether there is a dissociation between cortical activation and “physiologic hyperarousal” (i.e., arousal system and HPA/ SAM axes activation) in this insomnia phenotype.
In the current study, we found a dose-response association between the severity of insomnia, defined by objective short sleep duration, and degree of cortical arousal. Overall, adolescents with insomnia with short sleep duration showed greatest power in beta (15–35 Hz) frequencies as compared to controls and, particularly, in high-beta (25–35 Hz) frequencies as compared to individuals with insomnia with normal sleep duration. In contrast, individuals with insomnia with normal sleep duration were associated with increased low-beta (15–25 Hz) power during SOL, SO, and NREM sleep, whereas high-beta (25–35 Hz) power was significantly increased only during SOL in this insomnia phenotype. These findings are consistent with some, but not all, previous studies in adults with insomnia. For example, Krystal et al.12 showed that adults with insomnia and “normal” sleep duration showed greater beta (16.5–30.0 Hz) power than adults with insomnia and “short” sleep duration, whereas Spiegelhalder et al.15 reported no significant differences between these two insomnia subtypes in low-beta (16–24 Hz) or high-beta (24–32 Hz) power. How can we interpret and integrate our and others' data on cortical hyperarousal in light of the reported differences of the proposed insomnia phenotypes (i.e., insomnia with short versus normal sleep duration) and their underlying pathophysiology?
Our data clearly show that cortical hyperarousal is present in both adolescent insomnia phenotypes, although with differences in terms of degree and pattern. This is consistent with the finding that both insomnia phenotypes are associated with cognitive-emotional arousal and increased risk of psychiatric disorders, such as depression.2,38 In addition, cortical hyperarousal appears to be associated with deficient sleep-promoting mechanisms, as evidenced by overt objective sleep disturbance, as well as with activation of major physiologic systems, such as HPA and SAM axes, in the insomnia with short sleep duration phenotype.39–41 In fact, the latter “physiologic hyperarousal” has been proposed as the main pathophysiologic mechanism of this insomnia phenotype's adverse effect on physical health in adults (i.e., hypertension, diabetes, cognitive impairment, and mortality).5,42–46 In contrast, cortical hyperarousal in the insomnia with normal sleep duration phenotype does not appear to be associated with activation of wake-promoting mechanisms or of the HPA/SAM axes, because in this subtype objective sleep is not different from good sleeping controls39–41 and it is not associated with adverse effects on physical health.5,42–46 Thus, from a neurophysiologic/ neuroanatomic standpoint, it appears that insomnia with short sleep duration is associated with cortical hyperarousal plus simultaneous activation of the stress system and brainstem and hypothalamic structures involved in the regulation of sleep and arousal, whereas insomnia with normal sleep duration appears to be associated with cortical hyperarousal without any activation of the aforementioned brain structures, that would otherwise cause overt sleep disturbance. Future studies that include simultaneous EEG and functional neuroimaging techniques15,47 and detailed physiologic assessments, including challenge tests of the stress and other biological systems, are needed to further establish and delineate these two insomnia phenotypes as early as childhood40 and adolescence.
Several limitations need to be taken into account when interpreting the results of this study. First, this study was based on a case-control subsample of the PSCC that may not be entirely representative of the adolescent general or clinical population (e.g., those with circadian misalignment). However, the PSCC has the highest response rate (65%) of all child sleep cohorts, and the characteristics of this subsample were not different from those of the unselected individuals. Future studies should examine spectral EEG dynamics in the entirety of the PSCC (n = 421). Second, we analyzed only 1 night of PSG that might have been affected by the first night effect or may have enhanced the differences between insomniacs and good sleepers. Although previous studies showed significant night-to-night variability in PSG measures of sleep continuity, particularly SOL,1 recent studies have shown that measures of sleep duration, such as TST and SE, in fixed-time recordings48 and of spectral EEG dynamics17,49 are relatively stable across multiple night's recordings. Nevertheless, future studies conducting EEG spectral analyses in insomnia should use a multiple nights design. Third, the presence of insomnia was based on self-reported difficulties falling and/or staying asleep and did not include any duration, frequency, or daytime functioning criteria, which captures individuals with either insomnia disorder or poor sleep (i.e., insomnia symptoms). Future studies should use diagnostic criteria of insomnia disorder in adolescents because it may provide even stronger associations. Fourth, the absence of a control group without sleep complaints but with objective short sleep duration did not allow us to examine whether differences in spectral EEG existed within controls and between short sleeping controls and their counterparts with insomnia. Finally, it is possible that statistically adjusting for AHI may not have been sufficient to correct its potential confounding effect on beta EEG relative power. Therefore, we re-ran our analyses by excluding those adolescents with an AHI ≥ 1 (n = 11). As shown in Table S1 (supplemental material), the results remained similar and in the same direction despite decreased statistical power as a result of smaller sample size. Future studies should examine potential differences in beta EEG power in adolescents with insomnia and SDB.
In summary, insomnia in adolescence is associated with increased beta EEG power when attempting to initiate or resume sleep and during NREM sleep, which suggests that cortical hyperarousal is present in insomnia patients as early as adolescence. It appears that cortical hyperarousal, although of different degree and pattern across insomnia phenotypes, is present in all individuals with insomnia, whereas physiologic hyperarousal (e.g., HPA/SAM axes activation) is present only in the insomnia with short sleep duration phenotype.4,5 Consistently, cortical hyperarousal is highest in individuals with insomnia with short sleep duration and may explain the sleep complaints of insomniacs with normal sleep duration. Given the known association of insomnia and cortical hyperarousal with psychiatric disorders, particularly depression,21 it is likely that disturbed cortical networks may be a shared mechanism putting individuals with insomnia at risk of psychiatric disorders.2,3,38
DISCLOSURE STATEMENT
This was not an industry supported study. National Institutes of Health R01 HL63772, R01 HL97165, UL1 RR033184, C06 RR16499. Dr. Fang disclosed ownership of Biosoft Studio (Hershey, PA). The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Buysse DJ. Sleep and psychiatric disorders: a revisit and reconceptualization. Can J Psychiatry. 2010;55:401–2. doi: 10.1177/070674371005500701. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15:418. doi: 10.1007/s11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63:408–13. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 7.Merica H, Gaillard JM. The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav. 1992;52:199–204. doi: 10.1016/0031-9384(92)90258-4. [DOI] [PubMed] [Google Scholar]
- 8.Lamarche CH, Ogilvie RD. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20:724–33. [PubMed] [Google Scholar]
- 9.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 10.Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Perlis ML, Kehr EL, Smith MT, Andrews PJ, Orff H, Giles DE. Temporal and stagewise distribution of high frequency EEG activity in patients with primary and secondary insomnia and in good sleeper controls. J Sleep Res. 2001;10:93–104. doi: 10.1046/j.1365-2869.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 12.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630–40. [PubMed] [Google Scholar]
- 13.Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31:1673–82. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolynczyk-Gmaj D, Szelenberger W. Waking EEG in primary insomnia. Acta Neurobiol Exp (Wars) 2011;71:387–92. doi: 10.55782/ane-2011-1860. [DOI] [PubMed] [Google Scholar]
- 15.Spiegelhalder K, Regen W, Feige B, et al. Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol. 2012;91:329–33. doi: 10.1016/j.biopsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Corsi-Cabrera M, Figueredo-Rodríguez P, del Río-Portilla Y, Sánchez-Romero J, Galán L, Bosch-Bayard J. Enhanced frontoparietal synchronized activation during the wake-sleep transition in patients with primary insomnia. Sleep. 2012;35:501–11. doi: 10.5665/sleep.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israel B, Buysse DJ, Krafty RT, Begley A, Miewald J, Hall M. Short-term stability of sleep and heart rate variability in good sleepers and patients with insomnia: for some measures, one night is enough. Sleep. 2012;35:1285–91. doi: 10.5665/sleep.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St-Jean G, Turcotte I, Pérusse AD, Bastien CH. REM and NREM power spectral analysis on two consecutive nights in psychophysiological and paradoxical insomnia sufferers. Int J Psychophysiol. 2013;89:181–94. doi: 10.1016/j.ijpsycho.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Wu YM, Pietrone R, Cashmere JD, et al. EEG power during waking and NREM sleep in primary insomnia. J Clin Sleep Med. 2013;9:1031–7. doi: 10.5664/jcsm.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cervena K, Espa F, Perogamvros L, Perrig S, Merica H, Ibanez V. Spectral analysis of the sleep onset period in primary insomnia. Clin Neurophysiol. 2014;125:979–87. doi: 10.1016/j.clinph.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Med Rev. 2001;5:363–74. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- 22.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt RE, Van der Linden M. The Relations between sleep, personality, behavioral problems, and school performance in adolescents. Sleep Med Clin. 2015;10:117–23. doi: 10.1016/j.jsmc.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Colón SM, He F, Bixler EO, et al. Sleep variability and cardiac autonomic modulation in adolescents - Penn State Child Cohort (PSCC) study. Sleep Med. 2015;16:67–72. doi: 10.1016/j.sleep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 26.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–5. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach TM. Burlington, VT: University of Vermont Department of Psychiatry; 1991. Integrative guide for the 1991 CBCL/4-8, YSR, and TRF Profiles. [Google Scholar]
- 28.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 29.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1:21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 30.Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office; 1968. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. NIMH Publication 204. [Google Scholar]
- 31.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Pejovic S, Zoumakis E, et al. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–61. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 33.Jobert M, Wilson FJ, Roth T, et al. Guidelines for the recording and evaluation of pharmaco-sleep studies in man: the International Pharmaco-EEG Society (IPEG) Neuropsychobiology. 2013;67:127–67. doi: 10.1159/000343449. [DOI] [PubMed] [Google Scholar]
- 34.Bastien CH, St-Jean G, Turcotte I, et al. Spontaneous K-complexes in chronic psychophysiological insomnia. J Psychosom Res. 2009;67:117–25. doi: 10.1016/j.jpsychores.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Harvey AG, Tang NK, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25:593–611. doi: 10.1016/j.cpr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28:10167–84. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24:390–8. doi: 10.1111/jsr.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and stress system. Sleep Med Clin. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-Mendoza J, Vgontzas AN, Calhoun SL, et al. Insomnia symptoms, objective sleep duration and hypothalamic-pituitary-adrenal activity in children. Eur J Clin Invest. 2014;44:493–500. doi: 10.1111/eci.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vgontzas AN, Tsigos C, Bixler EO, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez-Mendoza J, Calhoun S, Bixler EO, et al. Insomnia with objective short sleep duration is associated with deficits in neuropsychological performance: a general population study. Sleep. 2010;33:459–65. doi: 10.1093/sleep/33.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–5. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feige B, Baglioni C, Spiegelhalder K, Hirscher V, Nissen C, Riemann D. The microstructure of sleep in primary insomnia: an overview and extension. Int J Psychophysiol. 2013;89:171–80. doi: 10.1016/j.ijpsycho.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Gaines J, Vgontzas AN, Fernandez-Mendoza J, et al. Short- and Long-Term Sleep Stability in Insomniacs and Healthy Controls. Sleep. 2015;38:1727–3. doi: 10.5665/sleep.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curcio G, Ferrara M, Piergianni A, Fratello F, De Gennaro L. Paradoxes of the first-night effect: a quantitative analysis of antero-posterior EEG topography. Clin Neurophysiol. 2004;115:1178–88. doi: 10.1016/j.clinph.2003.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


