Abstract
Study Objectives:
To examine the relationship between hypertension prevalence in individuals with insomnia who have short total sleep duration < 6 h or sleep duration ≥ 6 h, using both objective and subjective measures of total sleep duration.
Methods:
Using a cross-sectional, observational design, 255 adult volunteers (n = 165 women; 64.7%) meeting current diagnostic criteria for insomnia disorder (MAge = 46.2 y, SDAge = 13.7 y) participated in this study at two large university medical centers. Two nights of polysomnography, 2 w of sleep diaries, questionnaires focused on sleep, medical, psychological, and health history, including presence/absence of hypertension were collected. Logistic regressions assessed the odds ratios of hypertension among persons with insomnia with short sleep duration < 6 h compared to persons with insomnia with a sleep duration ≥ 6 h, measured both objectively and subjectively.
Results:
Consistent with previous studies using objective total sleep duration, individuals with insomnia and short sleep duration < 6 h were associated with a 3.59 increased risk of reporting hypertension as a current medical problem as compared to individuals with insomnia with sleep duration ≥ 6 h. Increased risk for hypertension was independent of major confounding factors frequently associated with insomnia or hypertension. No significant risk was observed using subjectively determined total sleep time groups. Receiver operating characteristic curve analysis found that the best balance of sensitivity and specificity using subjective total sleep time was at a 6-h cutoff, but the area under the receiver operating characteristic curve showed low accuracy and did not have good discriminant value.
Conclusions:
Objectively measured short sleep duration increased the odds of reporting hypertension more than threefold after adjusting for potential confounders; this relationship was not significant for subjectively measured sleep duration. This research supports emerging evidence that insomnia with objective short sleep duration is associated with an increased risk of comorbid hypertension.
Citation:
Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. SLEEP 2016;39(5):1037–1045.
Keywords: hypertension, insomnia, polysomnography, prevalence, short sleep
Significance.
Insomnia and hypertension are highly prevalent medical concerns; practitioners should be aware that patients with untreated chronic insomnia may also have comorbid hypertension. Previous research found an association between objective short sleep duration (< 6 h per night) and increased risk for comorbid hypertension; this study replicates and extends these previous findings using a different population. Additionally, we examine differences between using objective and subjective measures of sleep to evaluate associated hypertension risk. The current study found that insomnia with objective short sleep was associated with a 3.59 increased risk of reporting hypertension, regardless of age, sex, race, weight, frequency of sleep medication use, sleep disordered breathing, daytime sleepiness, diabetes, high cholesterol, depression, and consumption of alcohol, caffeine, and tobacco.
INTRODUCTION
A growing body of research has suggested that inadequate sleep, as a result of insomnia or short sleep duration, can have negative effects on individuals' medical health. Insomnia, characterized by difficulty initiating or maintaining sleep at least three times per week for 3 mo with associated daytime impairment,1 affects more than 30% of the population intermittently, and 10% to 15% chronically.2 Previously studies examining chronic insomnia on its own have shown it is associated with increased mortality risk (adjusted hazard ratio [HR] = 1.58, 95% confidence interval [95% CI] = 1.02–2.45, P < 0.05),3 incident depression (odds ratio [OR] = 1.9, P = 0.031),4 and medical disorders associated with adverse outcomes, such as hypertension.5–7 Short sleep duration (< 6 h per night), which is a separate phenomenon, affects approximately 70.1 million US adults.8 As is the case with insomnia, short sleep duration on its own has been correlated with a variety of adverse health outcomes. Both meta-analytic and population based studies have indicated that short sleep is associated with increased risk for mortality (relative risk [RR] = 1.12, 95% CI = 1.06–1.18, P < 0.01),9 incident myocardial infarction in women (HR = 2.98, 95% CI = 1.48–6.03) but not men (HR = 1.13, 95% CI = 0.66–1.92),10 and incident hypertension (RR = 1.21, 95% CI = 1.05–1.40; test for overall effect: Z = 2.61, P = 0.009),7 compared to individuals without short sleep duration (commonly ≥ 7 h). As such, having either insomnia or short sleep alone may have significant negative implications for individuals' health.
Although insomnia and short sleep duration have been independently correlated to increased risk of mortality and morbidity, the combination of insomnia and short sleep duration within the same individual appears especially detrimental. Sivertsen et al.11 reported that individuals with both insomnia and short sleep duration (< 6.5 h) showed nearly a threefold increased risk for mortality compared to those sleeping ≥ 6.5 h; the association between insomnia with normal/greater sleep duration was not associated with mortality.11 Furthermore, Vgontzas et al.12 found that insomnia with objectively measured short sleep duration (< 6 h) was associated with significantly increased mortality rates in men (but not women), after adjusting for diabetes, hypertension, and other confounders.
Perhaps one of the more consistently documented morbidity outcomes linked to insomnia with short sleep duration is its association with an increased risk for hypertension. Hypertension currently affects 26.4% of adults worldwide and is the leading risk factor for mortality.13 The association between hypertension risk and insomnia with short sleep duration has been documented in several cross-sectional observation studies,6,14,15 a recent clinical review,16 and meta-analytic work with prospective adult cohort studies using at least a 1-y follow-up period.7 Only one study showed conflicting results, reporting no associations between insomnia symptoms, short sleep duration, and increased risk for hypertension.17 However, that particular study identified insomnia based solely on subjective reports of symptom frequencies per month (i.e., did not include daytime impairment as part of the insomnia definition), and used subjective reports of sleep duration, which is commonly discordant with objective, polysomnographic (PSG) measures of sleep duration.18,19 As much of the available literature7,16 has suggested, hypertension risk is associated with the combination of insomnia and objective short sleep duration (< 6 h). Furthermore, it appears that objectively measured total sleep duration is a better predictor of associated hypertension risk in insomnia populations compared to subjectively-measured total sleep duration.
To date, most research linking insomnia with objective short sleep duration and hypertension risk has been conducted by Vgontzas and colleagues using the Penn State Cohort, a sample of 1,700 men and women from central Pennsylvania recruited originally to determine the age distribution for sleep disordered breathing.20,21 Key findings from this group have shown that individuals with insomnia who also have objectively measured sleep durations of ≤ 5 h or 5–6 h per night, had a fivefold and 3.5-fold increased odds of having hyper-tension, respectively, compared to a control group of individuals without insomnia complaints sleeping > 6 h.6 Authors also found that after a 7.5-y follow-up period, those having insomnia with short sleep duration (< 6 h) showed 3.75-fold (95% CI = 1.58–8.95, P = 0.012) greater odds for hyperten -sion incidence compared to normal sleepers who slept ≥ 6 h.5 These studies showed that neither individuals with chronic insomnia sleeping ≥ 6 h (using objective PSG measurement) nor a control group of normal sleepers showed elevated odds for either current or incident hypertension.5,6 Based on these findings, Vgontzas et al.22 contend that objective short sleep duration < 6 h in individuals with insomnia is associated with increased morbidity risk (measured via incident hypertension) and has clinical utility for severity assessment and subsequent treatment selection.
Whereas these findings are both significant and compelling, they are primarily based on one research cohort in which sleep duration was defined using 1 PSG night to identify individuals with insomnia who also have short sleep duration. The purpose of this study was to replicate and extend the findings of Vgontzas et al. in a sample other than the Penn State cohort. Specifically, the current study: (1) examined the odds for current hypertension among individuals with insomnia with and without short sleep duration; and (2) tested whether there are differences in associated hypertension risk based on total sleep duration derived objectively from 1 or 2 nights of PSG, or subjectively from 2 w of sleep diaries.
METHODS
Design
This study consisted of a secondary analysis of data from a parent study at Duke University (Durham, NC) and Rush University (Chicago, IL) Medical Centers examining the reliability and validity of insomnia diagnoses.23 This study's protocol was reviewed and approved by the Institutional Review Boards of Duke and Rush University Medical Centers.
Participants
The original sample (n = 352) was recruited between January 2004 and February 2009. Participants included: (1) met Research Diagnostic Criteria for insomnia disorder,24 (2) were older than 18 y, and (3) spoke English fluently. To simulate Insomnia Disorder criteria from the Diagnostic Statistical Manual for Mental Disorders, Fifth Edition25 and the International Classification of Sleep Disorders, Third Edition,1 we selected individuals reporting insomnia for ≥ 3 mo for the current analysis. We also required that participants have complete data for all variables used in planned analyses. Participants excluded were: (1) suffering an unstable or life-threatening medical condition; (2) imminently suicidal; (3) cognitively impaired (i.e., a score of < 24 on the Mini Mental State Examination [MMSE]26); or (4) previously evaluated by any of the study clinicians. Based on the aforementioned criteria, 97 of the original 352 participants were excluded (24 reported having less than 3 mo of insomnia and 73 did not have complete questionnaire data). There were no significant baseline differences between those excluded (n = 97) and our final sample (n = 255) on their objective total sleep time (TST) group assignment, age, sex, body mass index (BMI), frequency of hypnotic medication use, excessive daytime sleepiness (ESS), apnea-/hypopnea index (AHI from PSG), current hypertension, current diabetes, current hypercholesterolemia, depression (Inventory to Diagnose Depression [IDD]), or consumption of alcohol, caffeine, and tobacco. In terms of racial distribution (whites versus non-whites), we found a significantly greater percentage of whites in the excluded group (68/97, 70.1%) compared to whites in our final sample (144/255, 56.47%; P = 0.02), suggesting that our final sample (n = 255; 130 from Duke, 125 from Rush) was more racially diverse than those who were in the excluded group.
Sleep Assessments
Polysomnography
Participants underwent two consecutive nights of in-laboratory PSG with a monitoring montage consisting of electroencephalography (C3-M2, Oz-Cz), chin electromyography channel, electro-oculography (left eye-M1, right eye-M2), airflow (nasal-oral thermistor), respiratory effort (thoracic and abdominal impedance), pulse oximetry, anterior tibialis electromyography (right and left legs), and body position monitoring. Participants followed their customary bed and rising times on PSG nights. Those with occasional hypnotic use underwent PSGs off of such medications; those using hypnotics ≥ 3 nights per week or taking antidepressants/anxiolytics underwent PSGs while on these medications. PSGs were scored using traditional scoring criteria for sleep stages, apneas/hypopneas, periodic limb movements in sleep (PLMS), and related or idiopathic arousals.27–29
Electronic Sleep Diary
Participants maintained 2-w records of their sleep (bedtime, sleep onset latency, nocturnal wakefulness, rising time, etc.) using a personal digital assistant. Those who had difficulty using this device completed paper diaries. Diary entries were downloaded (or hand-entered for paper diaries) into a computer database for subsequent analyses. The database contained information about each participant's time in bed and total wake time from which nightly TST was calculated.
Questionnaire Instruments
Participants completed a sleep history questionnaire (SHQ), Epworth Sleepiness Scale (ESS),30,31 and IDD.32 The 10-page SHQ contained questions about demographic information, current and past sleep complaints, sleep schedule, bedtime routines, and use of hypnotic agents, caffeine, alcohol, and tobacco. It also contained a health problem checklist containing 54 medical (e.g., hypertension, hypercholesterolemia, etc.) and mental (e.g., depression, anxiety, etc.) disorders allowing participants to check all current and past health problems. The eight-item ESS questionnaire is designed to assess daytime sleepiness using a four-point rating scale (0 = “would never doze” to 3 = “high chance of dozing”) with the sum of all items comprising the participant's ESS score. The 22-item, well-validated IDD was used to measure mood status.
Procedures
Study candidates had an initial visit with a project coordinator (PC), who described study procedures, obtained informed consent, and assessed the candidate's cognitive status using the MMSE.26 Candidates with MMSE scores < 24 were excluded and referred for further cognitive evaluation. The PC also administered insomnia diagnostic questions from the Duke Sleep Structured Interview for Sleep Disorders,33 to confirm candidates met Research Diagnostic Criteria (RDC) for insomnia disorder.24 Those who had a MMSE score ≥ 24 and met Research Diagnostic Criteria for insomnia disorder were enrolled. However, to simulate Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition and International Classification of Sleep Disorders, Third Edition diagnostic criteria, only individuals who reported having insomnia for at least 3 mo were selected for the current study.
Enrolled participants completed study questionnaires, 2 w of sleep diaries, and 2 consecutive nights of PSG. Values of TST and the AHI were computed for each PSG night. Each participant's objective TST was determined by using the TST observed solely on the first night, and then computing the mean TST from 2 PSG nights. We regarded the latter of these estimates to be more reliable, as it was based on repeated observations, whereas the former was a more pragmatic index, as clinicians often have just 1 night of recorded PSG for their patients.
Participants were classified as having short objective sleep duration < 6 h (n = 130, based on 2 nights of PSG; n = 136, using first night PSG only) or objective sleep duration ≥ 6 h (n = 125, based on 2 nights of PSG; n = 119, using first night of PSG only). A cutoff of < 6 h was used because it has proven optimal for predicting morbidity and mortality among individuals with insomnia.5,6,12 Participants were also classified into groups based on their subjective total sleep duration (i.e., mean TST across 2 w of sleep diaries), resulting in those with short sleep duration < 6 h (n = 123) and sleep duration ≥ 6 h (n = 132).
The presence of hypertension was discerned from participants' self-report of this condition on the SHQ's health problem checklist. This process was repeated for diabetes and hypercholesterolemia. The total depression score was obtained from IDD and daytime sleepiness score from the ESS. Participants' age, sex, race, height, weight, and daily consumption of alcohol (number of drinks/day), caffeine (number of servings/ day), hypnotic agents and tobacco (number of cigarettes/day) were obtained from the SHQ. BMI was calculated using self-reported height and weight.
Statistical Analyses
Correlation and paired t-tests were used to determine if we should use sleep duration groups derived from the PSG TST from night 1 only, night 2 only, or an average across both nights.
Chi-square tests determined if there were significant proportional differences for categorical variables by study site and sleep duration group (defined by 2 PSG nights). One-way analyses of variance determined if there were significant mean differences among continuous variables by study site, sleep duration group, or for the study site × sleep duration group interaction. Logistic regression models assessed the association between hypertension prevalence and insomnia with objective and subjective short sleep duration. Each analysis adjusted for various covariates including age, sex, race (white vs. nonwhite), BMI, frequency of hypnotic medication use, daytime sleepiness (ESS), apnea-hypopnea index (AHI from PSG), current diabetes, current hypercholesterolemia, depression (IDD), and consumption of alcohol, caffeine, and tobacco. OR with 95% CI were calculated for three models. Model 1 used objective sleep data from 2 consecutive nights of PSG, model 2 used objective sleep data from the first PSG night only, and model 3 used subjective sleep data from 2 w of sleep diaries. To reduce type 1 error, we applied a Bonferroni correction for the multiple logistic regression analyses conducted; our adjusted α = 0.016 (i.e., 0.05 ÷ 3).
Chi-square tests were used to examine the sensitivity and specificity of data derived from (1) the first night of PSG only and (2) 2 w of sleep diary monitoring for correctly determining the objective sleep duration classification (i.e., short < 6 h versus ≥ 6 h) based on their mean TST across 2 PSG nights. Although a cutoff of < 6 h using PSG has been shown to be optimal for predicting morbidity and mortality among individuals with insomnia,5,6,12 it is unclear whether this cutoff also applies to subjective reports of TST. A receiver-operating characteristic (ROC) curve was used to graphically depict the relationship between the sensitivity and specificity of hyper-tension detection in individuals with insomnia using average TST from 2 w of sleep diaries.
RESULTS
Participants were mostly female (64.7%), white (56.5%), well educated (Meducation = 15.2 ± 3.1 y), middle-aged (MAge = 46.2 ± 13.7 y), and most were single (40.8%) or married/living as married (39.6%). One quarter of our sample reported hypertension (25.5%). Objective TST from PSG night 1 and PSG night 2 were significantly correlated (r = 0.46, P < 0.0001), and there were no significant differences between TST on night 1 (M1 = 346.35 min, SD1 = 92.57) and night 2 (M2 = 357.46, SD2 = 93.19), t = −1.62, P = 0.11. Therefore, we choose to conduct analyses that looked at objective TST group based on the average across both nights of PSG, as well as the first night of PSG only.
Using the TST duration average across 2 nights of PSG, participants were evenly divided between the objective short sleep duration group < 6 h (n = 130, 51%) and objective normal sleep duration group ≥ 6 h (n = 125, 49%). When using the TST duration of night 1 only, participants fell into similar groupings, with n = 136 (53.3%) being in the objective short sleep duration group < 6 h, and n = 119 (46.7%) being in the objective normal sleep duration group ≥ 6 h.
Compared to the Rush sample, Duke participants were significantly older (MDuke = 49.6 years, SD = 13.6; MRush = 42.6 y, SD = 13.0; F(3,251) = 12.93, P < 0.001), more likely to be married/living as married, χ2 = 25.22 (2, n = 255), P < 0.001, more likely to report diabetes, χ2 = 6.26 (1, n = 255), P < 0.05, and less ethnically diverse, χ2 = 12.02 (4, n = 255), P < 0.05. No other significant differences were observed between study sites. Table 1 provides demographic frequencies, means, and values for chi-square and analysis of variance analyses.
Table 1.
Demographic and health characteristics of insomnia participants.
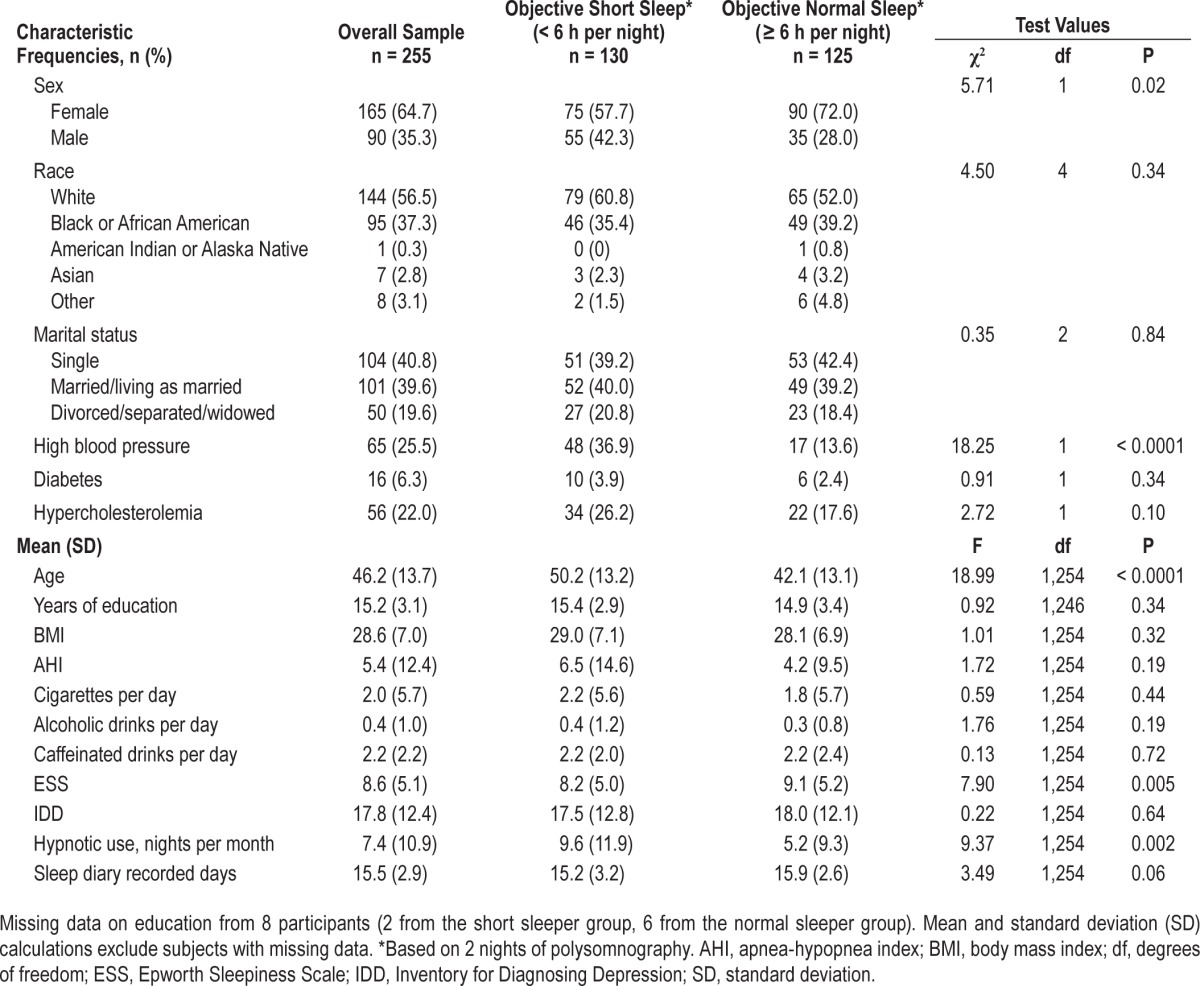
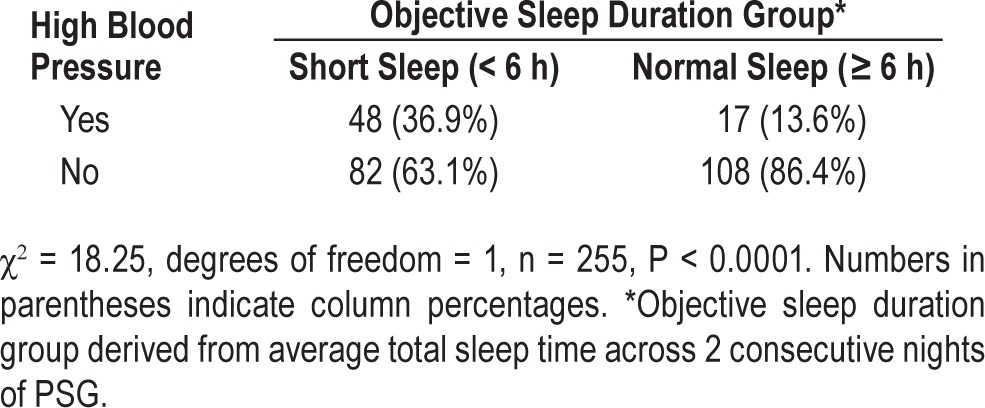
There were more females than males in the sample but there was a significantly larger proportion of males in the objective short sleep duration < 6 h group (42.3%) compared to the objective normal sleep duration ≥ 6 h group (28%). Participants in the objective short sleep group were also significantly older, had a higher frequency of nightly hypnotic medication use over the past month, reported lower ESS scores, and were more likely to report high blood pressure as a current medical concern. Table 2 shows chi-square results for hypertension prevalence by objective sleep duration group based on 2 nights of PSG. No significant differences were observed between objective short sleep < 6 h and normal ≥ 6 h sleep duration groups on race, education, marital status, BMI, insomnia duration, number of sleep diary days completed, AHI, diabetes, hyper-cholesterolemia, depressive symptoms, and daily consumption of alcohol, caffeine, and tobacco.
Table 2.
Results of chi-square test and descriptive statistics for hypertension prevalence by objective sleep duration group.
More participants with objective short sleep < 6 h were from Duke (59.2%) than Rush (40.1%), χ2 (1, n = 255) = 7.22, P < 0.01. However, this is likely a result of Duke being a significantly older sample, as TST tends to decrease with age.34 No signifi-cant interactions between study site and objective sleep duration group were observed by age, education, BMI, daytime sleepiness, AHI, frequency of hypnotic medication use, diabetes, hypercholesterolemia, depressive symptoms, and daily consumption of alcohol, caffeine, and tobacco.
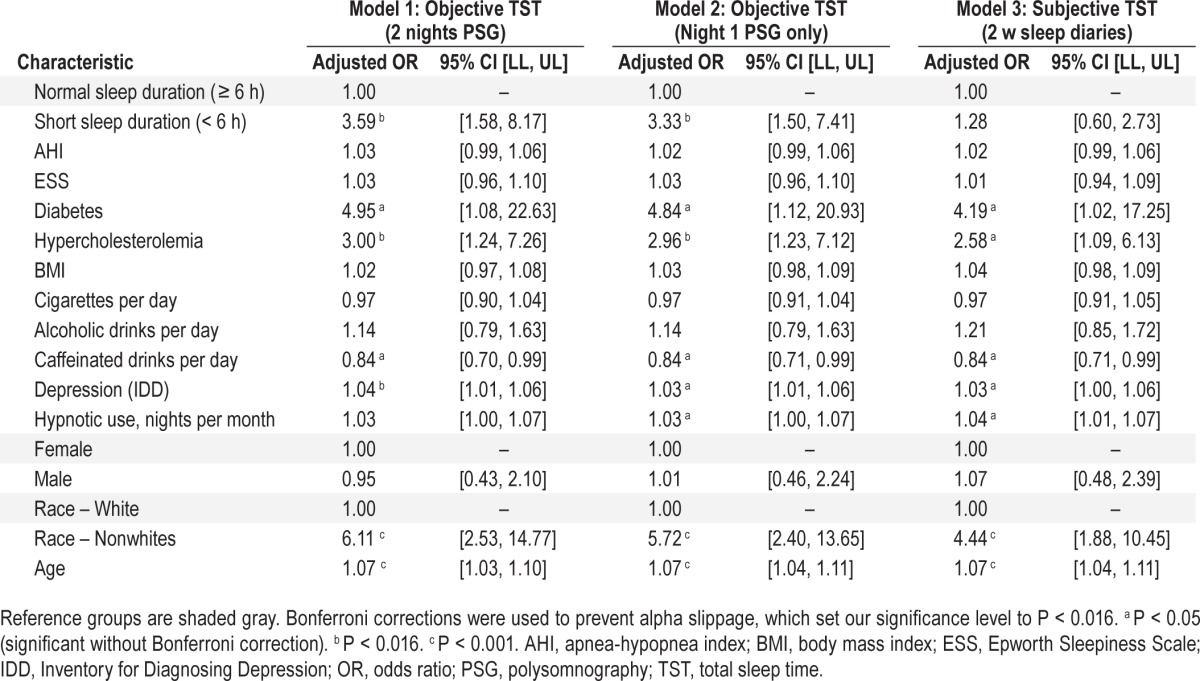
Table 3 shows results of logistic regression analyses. In model 1, insomnia with objectively short sleep < 6 h, based on 2 PSG nights, was associated with a significantly greater risk for hypertension after adjusting for covariates (OR = 3.59, 95% CI = 1.58–8.17, P = 0.002), as compared to insomnia with objective sleep duration ≥ 6 h. In model 2, objective short sleep duration < 6 h (first PSG night only) was associated with increased risk for hypertension after adjusting for covariates (OR = 3.33, 95% CI = 1.50–7.41, P = 0.003), as compared to insomnia with objective sleep duration ≥ 6 h. In model 3, no significant differences were found between groups having insomnia with subjective (2 w of sleep diaries) short sleep < 6 h and subjective longer sleep duration ≥ 6 h in their hypertension risks after adjusting for covariates.
Table 3.
Multivariable adjusted odds ratios of hypertension associated with insomnia and objective sleep duration.
A chi-square analysis was used to test the level of agreement between sleep duration classification based on only the first night of PSG and such classification based on the mean TST from 2 consecutive nights of PSG. Results of this analysis show a high rate of agreement between classifications based on the first PSG night and those based on the mean TST of 2 PSG nights (χ2 = 109.46, degrees of freedom = 1, n = 255, P < 0.0001). In fact the classification based on the first PSG night only showed a high sensitivity (85%) and specificity (80%) for the classification results based on the 2 PSG nights. Therefore, if only 1 night of PSG is available, which may often be the case for clinicians, using the TST gathered from that night will still be clinically useful. In contrast, using means data from 2 w of subjective sleep diary reports demonstrated both lower sensitivity (60%) and lower specificity (64%) of classifying persons into the sleep duration groups derived from using 2 consecutive nights of PSG (χ2 = 14.70, degrees of freedom = 1, n = 255, P < 0.0001).
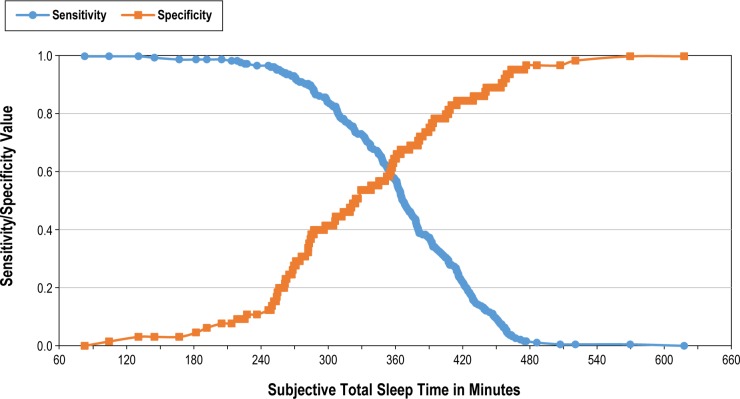
Given the systemic differences between measuring TST using PSG versus self-report, it is plausible that the TST cutoff point that dictates “short sleep” might be different for diary and PSG measures. To test this assumption we conducted an ROC curve analysis to examine the association between hypertension risk and the subjective sleep time reports of those in our study sample (see Figure 1). The ROC curve is plotted for all TST values; the further the ROC curve lies above the diagonal reference line, the more accurate the test.35 Another index of accuracy is the area under the curve (AUC); this is the probability that a test result for a randomly chosen positive case will exceed the result for a negative case. Swets36 has suggested that AUC values under 0.7 have “low” accuracy, values between 0.7 and 0.9 have “moderate” accuracy, and values greater than 0.9 have “high” accuracy. Our model had low accuracy (AUC = 0.639), which suggests that subjective TST is not able to reliably predict the probability of reporting hypertension. Figure 2 shows a plot of the sensitivity (i.e., percentage of those with reported hyper-tension included) and specificity (i.e., percentage of those without reported hypertension included) when TST data from sleep diaries are used to discriminate those with and without hypertension. Assuming it is desirable to obtain a balance between correct identification of true hypertension cases and correct exclusion of false positives (i.e., people without hypertension that are classified as having hypertension), the figure shows that as we approach 6 h (360 min), we achieve an optimal balance of sensitivity and specificity. However, as stated previously, the AUC for this test has low accuracy, which supports our logistic regression finding that subjective TST duration group was not a significant predictor of increased hypertension risk.
Figure 1.
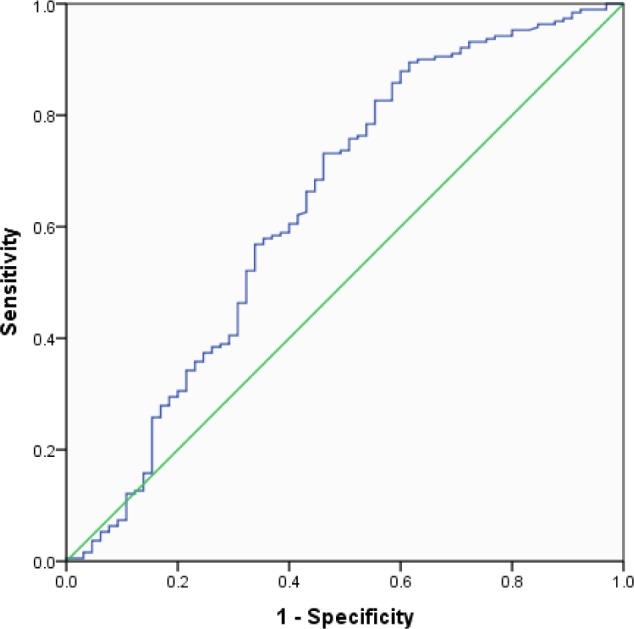
Receiver operating characteristic curve for detecting hyper-tension using average total sleep time derived from a 2-w sleep diary. Diagonal segments are produced by ties.
Figure 2.
Sensitivity and specificity of detecting hypertension by average total sleep time derived from a 2-w sleep diary.
DISCUSSION
This is one of the first studies outside of those with the Penn State Cohort demonstrating that insomnia with objective short sleep duration < 6 h is associated with increased risk for hypertension prevalence, independent of major confounding factors including age, sex, race, BMI, frequency of hypnotic medication use, AHI, daytime sleepiness, diabetes, hypercholesterolemia, depression, or alcohol, caffeine, and tobacco consumption. Consistent with previous literature,5,6 we found that individuals with the combination of insomnia with objective short sleep duration < 6 h resulted in increased OR of reporting hypertension as a current problem compared to individuals with insomnia and objective sleep duration ≥ 6 h. This finding was also significant when using data from solely the first PSG night, which provided higher sensitivity and specificity of correctly classifying individuals into their respective sleep duration group compared to using 2 w of sleep diary reports. Our findings also align with two large cohort samples that did not specifically select for insomnia patients. The Coronary Artery Risk Development in Young Adults (CARDIA) Study,15 which included a large cohort of African American and white adults, and the Sleep Heart Health Study,14 which enrolled a large community cohort to examine cardiovascular consequences of obstructive sleep apnea, found an association between objectively short sleep and increased hypertension incidence and prevalence, respectively.
There were some significant baseline differences in our objective short sleep < 6 h and ≥ 6 h sleep duration groups, such that the short sleepers were more likely to report lower ESS scores and were more likely to report high blood pressure as a current medical concern. Lower daytime sleepiness scores in individuals with insomnia and short sleep duration might indicate a higher level of physiological hyperarousal, which may make such individuals more vulnerable to medical comorbidities. A recent study37 on persons with insomnia using a Multiple Sleep Latency Test (MSLT) supports this conjecture. Results of that study showed that those with insomnia and physiological hyperarousal, defined as the 75th percentile of the sample's mean MSLT value (> 17 min), showed increased odds of having hypertension (OR = 4.33; 95% CI = 1.48–12.68) as compared to normal sleepers with MSLT ≤ 14 min.37 The lower ESS scores in our short sleep duration group might also reflect a likelihood of physiological hyperarousal.
This study provides further support for the use of objective sleep measures to determine sleep duration, rather than subjective measures, when attempting to detect comorbidity risk associated with insomnia. Subjective sleep duration appears to be a less reliable index, with recent evidence suggesting that there is no significant association between insomnia with subjectively short sleep and hypertension risk.17 Consistent with this previous finding, we found that subjectively determined short sleep was not associated with increased risk of hypertension; a significant association was only found when using objective sleep duration. Although our study found that the a subjective TST cutoff point of 6 hours maximized sensitivity and specificity of predicting the probability of reporting hypertension as a current medical problem, this cutoff was of little use since the model exhibited such low accuracy (AUC = 0.639). The accuracy of sleep time perceptions within our sample varied greatly; more than one-third of participants with insomnia misperceived what objective total sleep group they would be in based on their sleep diary reports. Forty percent of the objective short sleep duration < 6 h group reported sleeping more than 6 h, whereas 36% of the ≥ 6 h sleep duration group reported sleeping less than 6 h. It appears that when people are asked to report their nightly sleep using a sleep diary, their subjective reports do not always reflect what the objective PSG measures record. This discrepancy between subjective and objective TST is similar to previous work our group has done with individuals with insomnia, which found that only 36.5% accurately predicted their TST; most underestimated their TST (52%), but some consistently overestimated TST (11.5%).19 Sleep time misperceptions among individuals with insomnia seem to explain, in part, the discrepancy in findings between our objectively and subjectively determined TST groups and their association with an increased risk of hypertension.
Hypertension in this study's insomnia population was ascertained by self-report and found to have a slightly lower prevalence (25.5%) compared a national, age-stratified average of individuals with and without insomnia at the time of data collection (30.4%, years 2009–2010).38 Similarly, our sample's diabetes prevalence was lower (6.3%) than the Centers for Disease Control and Prevention's (CDC's) diabetes prevalence rate among 45–64 y olds in 2009 (12.2%).39 The prevalence differences observed could reflect differences in the way hyper-tension was ascertained (subjectively measured in our sample versus objectively measured in the CDC data report). It might indicate that our sample of insomnia participants was slightly healthier than the sample in the CDC report, which was a more general US adult population consisting of individuals with and without insomnia.
When considering the association between insomnia with short sleep and hypertension, studies vary on whether they relied on a self-reported hypertension, or a combination of self-report and objective blood pressure measurement. A meta-analysis of 11 prospective studies found that approximately half of the studies (45%) measured incident hypertension by self-reported diagnosis or treatment only.7 Although short sleep duration seemed more strongly associated with incident hyper-tension ascertained with objective blood pressure measurement compared to self-report, the difference between the groups was not significant.7 Having an objective measure of blood pressure would likely have increased the number of individuals classified as having hypertension, which might positively affect the association between insomnia with short sleep and hypertension.
Admittedly, this study had several limitations. The sample was relatively large and diverse, but included mainly research volunteers. Results may have differed for clinical patients or individuals with insomnia randomly selected from the community. Given our cross-sectional design, we were unable to infer causality between insomnia with short sleep and hypertension. However, based on the large amount of evidence documenting the connection between insomnia and physiologic hyper-arousal,40–43 it appears likely that physiologic hyperarousal may be a driving factor of insomnia with short sleep, which may lead to increased adverse outcomes, such as hypertension. Because our entire sample consisted of individuals with insomnia, we lacked a control group of normal sleepers without insomnia, which has often been used in previous research studies examining insomnia, short sleep duration, and hypertension.5,6,12,37 Additionally, our insomnia sample consisted largely of individuals with chronic insomnia symptoms for one year or longer. Although this is consistent with most other studies examining insomnia, short sleep, and hypertension, future longitudinal studies might want to measure blood pressure at multiple points to help determine how long it takes hypertension to surface after experiencing insomnia. Despite these limitations, we think we were able to minimize experimenter bias because participants enrolled in the study were not originally chosen for their sleep duration characteristics. Additionally, having subjective and objective sleep duration measurements in the same dataset provided us the opportunity to compare the relative usefulness of each in determining hypertension risk. Thus, our results fill a notable void in the literature and provide guidance for future research on potential insomnia phenotyping using objective short sleep as an indicator.
In conclusion, our results indicate a positive association between insomnia, objective short sleep duration, and hyper-tension prevalence. Objective but not subjective short sleep increased the odds of reporting hypertension more than threefold after adjusting for potentially important covariates. Despite practice guidelines dissuading the use of PSG for routine differential diagnosis, or severity assessment of insomnia,44 our research supports emerging evidence that objective measures of sleep can be useful in determining morbidity risks associated with insomnia.22 Future research might focus on whether extending TST in short sleepers with insomnia can help improve health by reducing associated hypertension risk.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was supported by the National Institute of Mental Health Grant # R01 MH67057. The funding agency did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The views expressed herein are those of the authors and do not necessarily represent the views of the National Institute of Mental Health, National Jewish Health, Duke University Medical Center, or Rush University Medical Center. Dr. Bathgate has received research support from Merck and Philips Respironics. Dr. Edinger has received grant and research support from Merck and Philips Respironics. Dr. Wyatt has received research support from and is a consultant to Philips Respironics. Dr. Krystal has received grant, research support, and/or is a consultant for: Abbott, Actelion, Arena, Astellas, AstraZeneca, Axiom, BMS, Cephalon, Eli Lilly, Evotec, GlaxoSmithKline, Jazz, Johnson and Johnson, King, Kingsdown Inc., Merck, Neurocrine, Neurogen, Neuronetics, NIH, Novartis, Organon, Ortho-McNeil-Janssen, Pfizer, Philips Respironics, Roche, Sanofi-Aventis, Sepracor, Somaxon, Takeda, and Transcept. A partial report of this study using the same data was presented at the Sleep 2013 Annual Meeting on June 4, 2013, in Baltimore, MD.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- AUC
area under curve
- BMI
body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CI
confidence interval
- df
degrees of freedom
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ESS
Epworth Sleepiness Scale
- h
hour(s)
- HR
hazard ratio
- ICSD
International Classification of Sleep Disorders
- IDD
inventory to diagnose depression
- M
mean
- MI
myocardial infarction
- MMSE
Mini-Mental Status Examination
- MSLT
Multiple Sleep Latency Test
- n
number
- ns
non-significant
- OR
odds ratio
- PC
project coordinator
- PLMS
periodic limb movements in sleep
- RDC
research diagnostic criteria (for insomnia disorder)
- ROC
receiver-operating characteristic
- RR
risk ratio
- SD
standard deviation
- SHQ
Sleep History Questionnaire
- TST
total sleep time
- χ2
chi-square ratio
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128:268–75 e2. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015;24:390–8. doi: 10.1111/jsr.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–35. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–7. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta-analysis of prospective cohort studies. Hypertens Res. 2013;36:985–95. doi: 10.1038/hr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Cunningham TJ, Croft JB. Trends in Self-Reported Sleep Duration among US Adults from 1985 to 2012. Sleep. 2015;38:829–32. doi: 10.5665/sleep.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–7. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivertsen B, Pallesen S, Glozier N, et al. Midlife insomnia and subsequent mortality: the Hordaland health study. BMC Public Health. 2014;14:720. doi: 10.1186/1471-2458-14-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33:1159–64. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 15.Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–61. doi: 10.1001/archinternmed.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev. 2014;18:509–19. doi: 10.1016/j.smrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Vozoris NT. Insomnia symptom frequency and hypertension risk: a population-based study. J Clin Psychiatry. 2014;75:616–23. doi: 10.4088/JCP.13m08818. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Zhou J, Li Z, Lei F, Tang X. Sleep perception and the multiple sleep latency test in patients with primary insomnia. J Sleep Res. 2012;21:684–92. doi: 10.1111/j.1365-2869.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- 19.Means MK, Edinger JD, Glenn DM, Fins AI. Accuracy of sleep perceptions among insomnia sufferers and normal sleepers. Sleep Med. 2003;4:285–96. doi: 10.1016/s1389-9457(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 20.Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 21.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Fernandez-Mendoza J. Insomnia with short sleep duration: nosological, diagnostic, and treatment implications. Sleep Med Clin. 2013;8:309–22. doi: 10.1016/j.jsmc.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edinger JD, Wyatt JK, Stepanski EJ, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Arch Gen Psychiatry. 2011;68:992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- 24.Edinger JD, Bonnet M, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report on an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Kales A, Rechtschaffen A. Bethesda, MD: US National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. University of California Los Angeles, Brain Information Service, NINDB Neurological Information Network. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Allan Rechtschaffen and Anthony Kales, editors. [Google Scholar]
- 28.Coleman R. Menlo Park, CA: Addison-Wesley; 1982. Periodic movements in sleep (nocturnal myoclonus) and restless legs syndrome. [Google Scholar]
- 29.EEG arousals: scoring rules and examples: a preliminary report from the sleep disroders atlas task force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 30.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman M. Minneapolis, MN: National Computer Systems, Inc; 1994. The inventory to diagnose depression manual. [Google Scholar]
- 33.Edinger JD, Wyatt JK, Olsen MK, et al. Reliability and validity of insomnia diagnoses derived from the Duke Structured Interview for Sleep Disorders. Sleep. 2009:32. [Google Scholar]
- 34.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 35.Mossman D, Somoza E. ROC curves, test accuracy, and the description of diagnostic tests. J Neuropsychiatry Clin Neurosci. 1991;3:330–3. doi: 10.1176/jnp.3.3.330. [DOI] [PubMed] [Google Scholar]
- 36.Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Vgontzas AN, Fernandez-Mendoza J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension. 2015;65:644–50. doi: 10.1161/HYPERTENSIONAHA.114.04604. [DOI] [PubMed] [Google Scholar]
- 38.Yoon SS, Burt V, Louis T, Carroll MD. Hyattsville, MD: National Center for Health Statistics; 2012. Hypertension Among Adults in the United States, 2009-2010. NCHS data brief, no 107. [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health Interview Statistics. Diabetes Public Health Resource, Data from the National Health Interview Survey. [Accessed February 1, 2016]. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm.
- 40.Basta M, Chrousos GP, Vela-Bueno A, Vgontzas AN. Chronic insomnia and the stress system. Sleep Med Clin. 2007;2:279–91. doi: 10.1016/j.jsmc.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiegelhalder K, Riemann D. Hyperarousal and Insomnia. Sleep Med Clin. 2013;8:299–307. [Google Scholar]
- 42.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Bonnet MH. Hyperarousal and insomnia. Sleep Med Rev. 2010;14:33. doi: 10.1016/j.smrv.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]