Abstract
Study Objectives:
An increased risk of narcolepsy has been observed in children following ASO3-adjuvanted pandemic A/H1N1 2009 (Pandemrix) vaccine. We investigated whether this risk extends to adults in England.
Methods:
Six adult sleep centers in England were visited between November 2012 and February 2014 and vaccination/clinical histories obtained from general practitioners. Suspected narcolepsy cases aged older than 17 y were selected. The risk of narcolepsy following Pandemrix was calculated using cases diagnosed by the time of the center visits and those with a diagnosis by November 30, 2011 after which there was increased awareness of the risk in children. The odds of vaccination in cases and in matched population data were compared using a case-coverage design.
Results:
Of 1,446 possible cases identified, most had onset before 2009 or were clearly not narcolepsy. Of the 60 remaining cases, 20 were excluded after expert review, leaving 40 cases with narcolepsy; 5 had received Pandemrix between 3 and 18 mo before onset. All the vaccinated cases had cataplexy, two received a diagnosis by November 2011 and two were aged 40 y or older. The odds ratio for vaccination in cases compared to the population was 4.24 (95% confidence interval 1.45–12.38) using all cases and 9.06 (1.90–43.17) using cases with a diagnosis by November 2011, giving an attributable risk of 0.59 cases per 100,000 doses.
Conclusions:
We found a significantly increased risk of narcolepsy in adults following Pandemrix vaccination in England. The risk was lower than that seen in children using a similar study design.
Citation:
Stowe J, Andrews N, Kosky C, Dennis G, Eriksson S, Hall A, Leschziner G, Reading P, Shneerson JM, Donegan K, Miller E. Risk of narcolepsy after AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in adults: a case-coverage study in England. SLEEP 2016;39(5):1051–1057.
Keywords: adult, case-coverage, narcolepsy, Pandemrix, vaccination
Significance.
Our study shows that the causal association between narcolepsy and the oil-in-water adjuvanted pandemic H1N1 influenza vaccine is not, as previously thought, confined to children and adolescents and will add further impetus to the research into the etiology of this condition. While possession of the DQB1*06:02 gene is clearly implicated, environmental or other triggers appear to be necessary to instigate the onset in susceptible individuals. Further surveillance of populations who have received pandemic strain vaccines is needed in order to document whether the association is seen with other products and to provide insights into the likely auto-immune pathway by which the oil-in-water adjuvant and/or the viral antigens in the HIN1 pandemic strain trigger the pathological process that results in loss of orexin-producing neurons.
INTRODUCTION
Narcolepsy is a disabling and chronic sleep disorder characterized by excessive daytime sleepiness, hypnagogic hallucinations, sleep paralysis, and cataplexy. Narcolepsy is divided into narcolepsy with cataplexy (type 1) and narcolepsy without cataplexy (type 2).1 Cataplexy is a unique symptom in which there is transient loss of skeletal muscle tone, with preservation of consciousness that is triggered by emotions such as laughter or anger.
The prevalence of narcolepsy with cataplexy is between 25 and 50 per 100,000 people with an incidence of around 0.74 per 100,000 person-years.2 Onset usually occurs between 15 and 40 y of age and symptoms develop gradually, so time from onset to diagnosis can be many years. Both environmental and genetic factors play a role in its etiology. There is a strong association with the HLA DQB1*06:02 genotype, but this alone is not sufficient for the disease to develop. Narcolepsy is associated with specific loss of cells producing the neuropeptide hypocretin, resulting in low levels of hypocretin in the cerebrospinal fluid.
An H1N1 ASO3-adjuvanted pandemic vaccine (Pandemrix, GlaxoSmithKline
Biologicals, Wavre, Belgium) was used in the United Kingdom (UK) from October 2009, initially for people comprising a seasonal influenza vaccine risk group3 or health or social care workers, followed by children younger than 5 y from November 2009 onward.4 Approximately 5.5 million people in the UK were vaccinated with Pandemrix.5 It was the predominant H1N1 vaccine used within the European Union.6 In August 2010 concerns were raised in Finland and Sweden about a possible association between narcolepsy and Pandemrix. A cohort study in Finland reported a 13-fold increased risk of narcolepsy following Pandemrix in children aged 4 to 19 y.7 This was confirmed by a study in sleep centers in England, which identified a 14-fold increased risk in those aged 4–18 y.8 Other studies subsequently published from Ireland and Norway also indicated an increased risk of narcolepsy in children who received Pandemrix.9,10
The initial signal in the Scandinavian countries was in children but more recently adult cases have been reported. A small case-control study in 25 adults in France suggested an elevated risk11 as did a follow-up study in Finland published as an online report.12 A record linkage cohort study in Sweden found no overall increased risk in adults, although there was a marginally elevated risk in those aged 21–30 y.13 Using the same published methodology as the childhood study in England,8 we investigated whether there was an increased risk of narcolepsy in adults who received Pandemrix.
METHODS
Case Ascertainment and Validation
The sleep centers in England where the largest numbers of cases of narcolepsy are diagnosed were identified through the Hospital Episode Statistic (HES) database.14 HES episodes in those age 16 y and older with an ICD 10 code of G474 in any diagnosis field were extracted for the period January 2009 to December 2012. Six sleep centers were identified as being the major centers that together covered 33% of the narcolepsy coded episodes in HES during this period. We estimated that within these centers approximately 30 cases may be seen with onsets from 2010 which should give sufficient power to detect at least a fivefold increased risk (80% power, 5% significance level, 5% vaccine uptake).
The six centers were visited between November 2009 and February 2010 (Table S1, supplemental material) and all those aged 16 y and older at the time of diagnosis were ascertained with the aim to include those aged 18 y and olderon September 1, 2009. These cases were found by searching local databases and electronic clinic letters for the keyword *narco* or searching for multiple sleep latency test (MSLT) reports for a diagnosis of narcolepsy. The cases from HES and those identified from the local searches were then merged and deduplicated using National Health Service (NHS) number or surname and date of birth. These potential cases were reviewed using medical records to establish symptom onset details, clinical history, and sleep study results. If any information was missing from the electronic records, the case notes were reviewed to identify the relevant information.
Details of the anonymized cases collated at center visits were evaluated by a review panel (authors GL, JShn, AH, SE) who were blinded to vaccination status. To expedite the review, cases with a clear history of excessive daytime sleepiness (EDS) and cataplexy or EDS with a positive MSLT or cerebrospinal fluid positive for narcolepsy were not all sent to the panel for review; rather, a few examples of these cases were first shown to the panel for their agreement. The four sleep center consultants on the review panel categorized each case as definite narcolepsy with cataplexy; definite narcolepsy without cataplexy; probable narcolepsy and insufficient evidence to confirm a diagnosis of narcolepsy. The panel based their diagnosis on the International Classification of Sleep Disorders, Second Edition (ICSD-2) criteria.15 A diagnosis based on the consensus view of three of the four panel members was taken, with remaining cases discussed by teleconference.
Pandemrix vaccination histories for cases with definite or probable narcolepsy were obtained from the patient's general practitioner (GP) who was asked for date and batch number of any pandemic vaccine given, the date of first symptoms and/or first consultation for narcolepsy symptoms, presenting symptoms, history of pandemic influenza illness, and whether the patient was in a clinical risk group for which pandemic strain H1N1 vaccine was recommended.
Index Dates: Definitions
The date of symptom onset was defined as the earliest date of EDS or cataplexy as given by the GP or recorded in the sleep center notes or referral letters. When the exact date was not available we used the midpoint of the month of the approximate date and also approximated an earliest and latest date of onset for sensitivity analysis. The date of first known health care contact was the earliest recorded consultation for a sleep related problem as reported by the GP or in the center notes. The date of diagnosis was the date when there was either a clinical history and sleep study confirming narcolepsy or sufficient clinical information to diagnose probable narcolepsy.
Statistical Analysis
We assessed the association between vaccination and narcolepsy using the case coverage method16 in which the odds of vaccination in cases is compared to the odds of vaccination in matched population data. The analysis is by logistic regression with the outcome as vaccinated (yes/no) in the cases and with an offset for the log odds of the matched coverage. Population vaccine coverage was calculated from the Clinical Practice Research Datalink (CPRD).17 We used patient-level data to derive cumulative coverage stratified by exact date (from September 2009 to March 2011), age on January 1, 2010 (categorized as 18, 19, 20–24, 25–29, …, ≥ 80 years) and, when matching by risk group, being in a vaccine target clinical risk group. This was then used to look up the appropriate matched coverage for each narcolepsy case based on their age, risk group status (if matching on risk group) and narcolepsy index date (e.g. date of onset). To determine vaccine coverage within 6 mo of an index date, the coverage 6 mo earlier was subtracted from the matched coverage on the index date. Patients were categorized as being in a risk group if there was any clinical code denoting chronic heart disease, chronic kidney disease, chronic obstructive pulmonary disease, diabetes, chronic liver disease, immunological disorders, multiple sclerosis, or stroke/ transient ischemic attack in the 5 y prior to September 2009 for the 2009–2010 vaccination season and September 2010 for the 2010–2011 season. We used similar criteria for allocating narcolepsy cases to a risk group based on the information provided by the GP on clinical conditions considered high risk for influenza.
The primary analysis was restricted to cases diagnosed by November 30, 2011 after which there was increased awareness of the risk seen in children with the potential for accelerated diagnosis in vaccinated cases. It also used first symptoms as the index date and the odds of vaccination at any time before onset. Additional analyses were performed using first health care contact and diagnosis as the index date, all cases diagnosed by the center visit date, not matching coverage by risk group status and calculating the odds of vaccination within 6 mo of the index date. Stratification by age younger than 30 y and age 30 y and older on September 1, 2009 was also done. Sensitivity analyses in which population coverage was increased or decreased by a relative 20% (for example, 10% coverage decreasing to 8% or increasing to 12%) and using the earliest and latest estimated onset dates were also conducted. These analyses were documented in a statistical analysis plan prior to receipt of the data by the statistician (NA) for analysis. Analysis was done using Stata version 13 (StataCorp, College Station, TX).
RESULTS
Vaccine Coverage
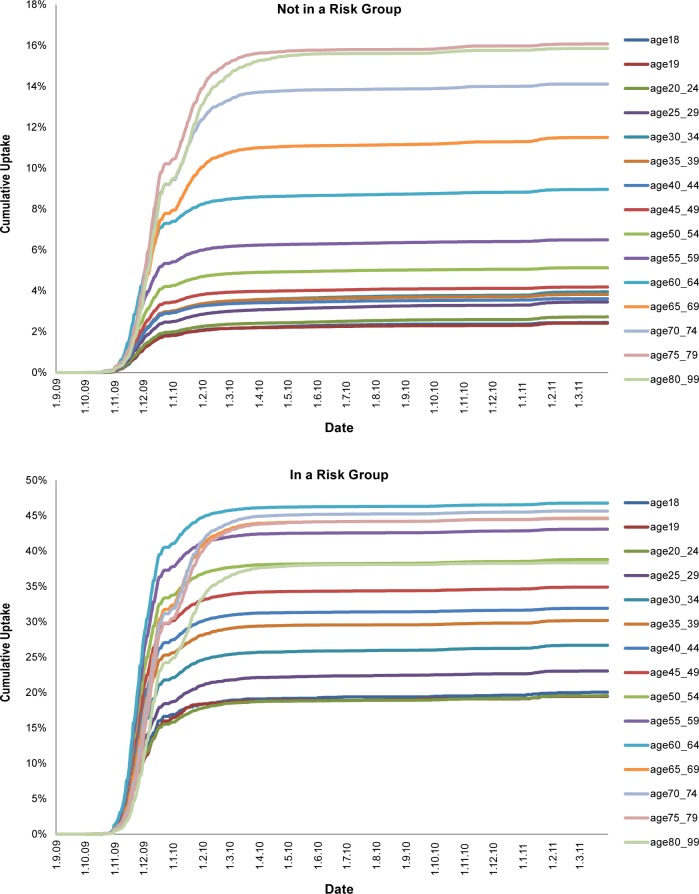
Coverage data were obtained from approximately 3.5 million patients aged 18–99 y registered in the CPRD practices on September 1, 2009. Vaccination coverage was low in healthy young adults and increased with age. As expected for those in a risk group, uptake was higher and also increased with age (Figure 1). Most vaccination was during 2009–2010 with only small increases in 2010–2011, which is in agreement with other data.8
Figure 1.
Vaccine uptake by age, risk group, and period from the Clinical Practice Research Datalink (CPRD) by clinical risk group status.
Study Cases
A total of 2,554 potential patients were identified through the different search strategies and data sources. When cross referenced and de-duplicated 1,446 patients remained and were taken forward for case note review (Table S1). The majority, 926, had symptom onset before 2009 and 441 clearly did not have narcolepsy when the notes were reviewed; these 1,367 cases were excluded. The case notes of 10 could not be traced and one person was seen in two centres. Of the remaining 68 patients 30 were considered definite cases after reviewing the available information and 38 were sent to the panel for review. The panel members were in initial agreement on 28, with agreement reached after teleconference for the remaining 10. Twenty cases were categorized as not narcolepsy/insufficient evidence and excluded with the remaining 18 cases added to the 30 definite cases. Of the 48 cases, 8 were not included in the final analysis because although age 18 y or older at diagnosis they were younger than 18 y on September 1, 2009. This left a total of 40 adults with narcolepsy of whom 28 were categorized as definite narcolepsy with cataplexy, 8 as definite narcolepsy without cataplexy, and 4 probable narcolepsy.
Four individuals were reported to have an influenza-like illness prior to first symptoms, although only one within 3 mo of symptoms; none of these four cases was vaccinated.
Vaccination History
We obtained vaccination history on all 40 cases and risk group status for 38 (Table 1). Five patients had received Pandemrix prior to first symptoms of whom three were in a clinical risk group recommended for vaccination; all five had cataplexy. One had onset within 3 mo, two within 3 to 6 mo, and two between 7 and 18 mo after vaccination; two had a confirmed human leukocyte antigen (HLA) DQB1*06:02 genotyping, with the other three not tested.
Table 1.
Demographic features and clinical features of 40 patients with narcolepsy according to ASO3 adjuvanted pandemic A/H1N1 2009 vaccination.
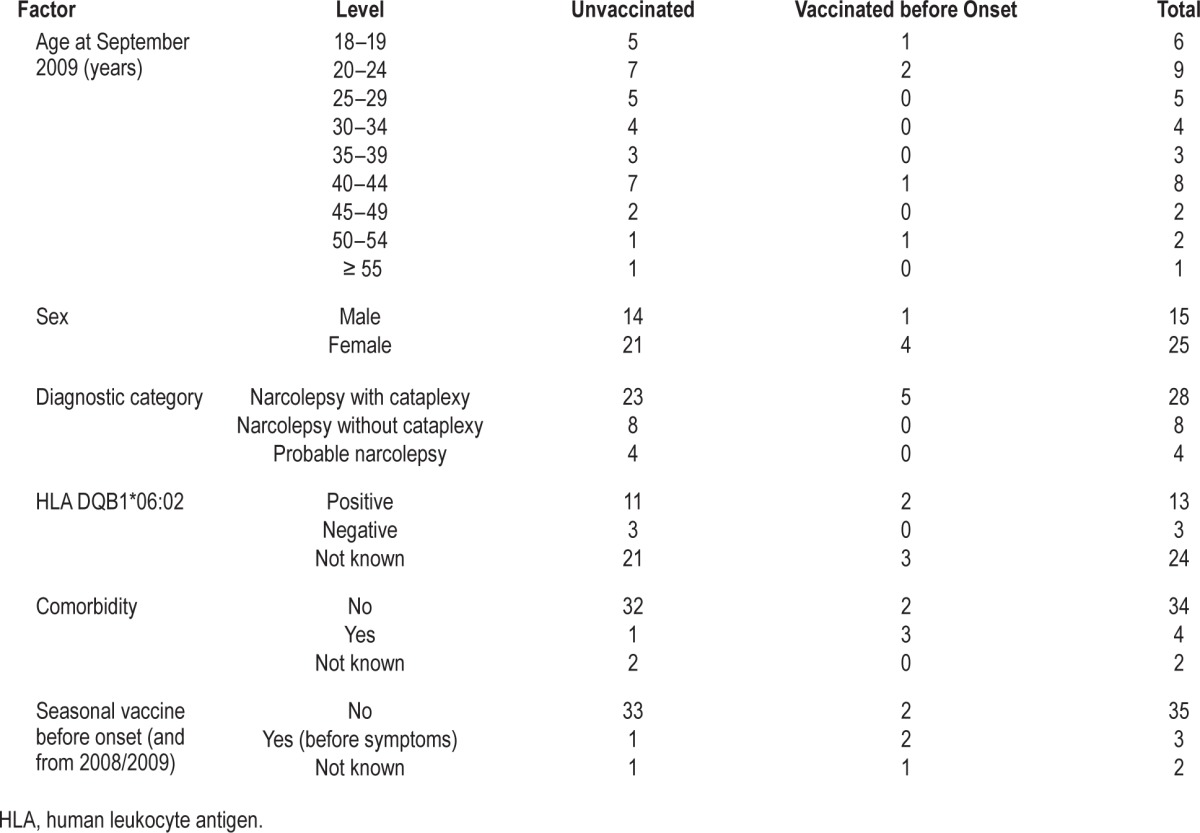
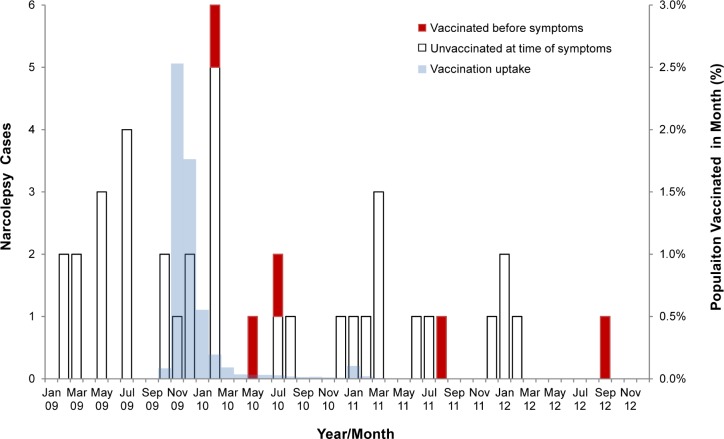
Figure 2 shows the timing of onset for the 40 adult narcolepsy cases by vaccination status and monthly vaccine uptake in the age-matched population. The first vaccinated case had onset in early 2010 and the latest in 2012 after receiving Pandemrix in 2011 when residual stocks were used instead of seasonal vaccine.18 Mean time from onset to diagnosis using cases with onset in 2009–2011 and diagnosis within 30 mo was 493 days in four vaccinated cases and 434 in 28 nonvaccinated cases (P = 0.69, Kruskal-Wallis test).
Figure 2.
Timing of onset for the 40 adult narcolepsy cases by vaccination status and monthly vaccine uptake in the age matched population.
Case Coverage Analysis
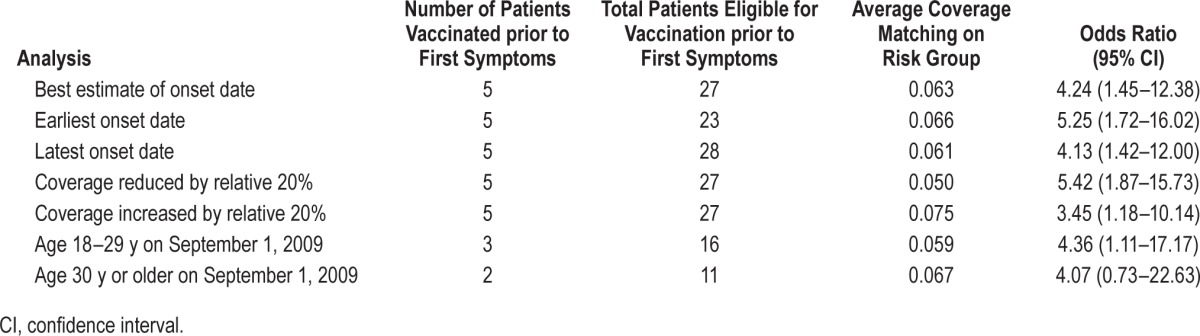
The primary analysis, which used symptom onset, cases with a diagnosis by November 30, 2011 and matching on risk group, only included two of the five vaccinated cases but showed an elevated odds ratio of 9.06 (1.90–43.17) (Table 2). When including all cases ascertained by the date of the centre visit (five vaccinated cases) the odds ratio was lower but still significant at 4.24 (1.45–12.38). Higher odds ratios (but fewer vaccinated cases) were seen when including only cases with onset within 6 mo of vaccination. When other outcome dates were used such as date of first healthcare contact or date of diagnosis, the odds ratios reduced and some became nonsignificant (Table 2). The sensitivity analyses and age stratification were based on all cases diagnosed by the center visit date to increase power (Table 3). Results were similar when allowing for uncertainty in the onset date and remained significant when increasing coverage by a relative 20%. Odds ratios were similar for those younger than 30 y and older individuals, but the number of cases in each age group was small.
Table 2.
Case coverage analysis in patients with narcolepsy showing odds ratios for receipt of ASO3 adjuvanted pandemic A/H1N1 2009 vaccine before narcolepsy onset using different index dates, follow-up periods, and risk intervals.
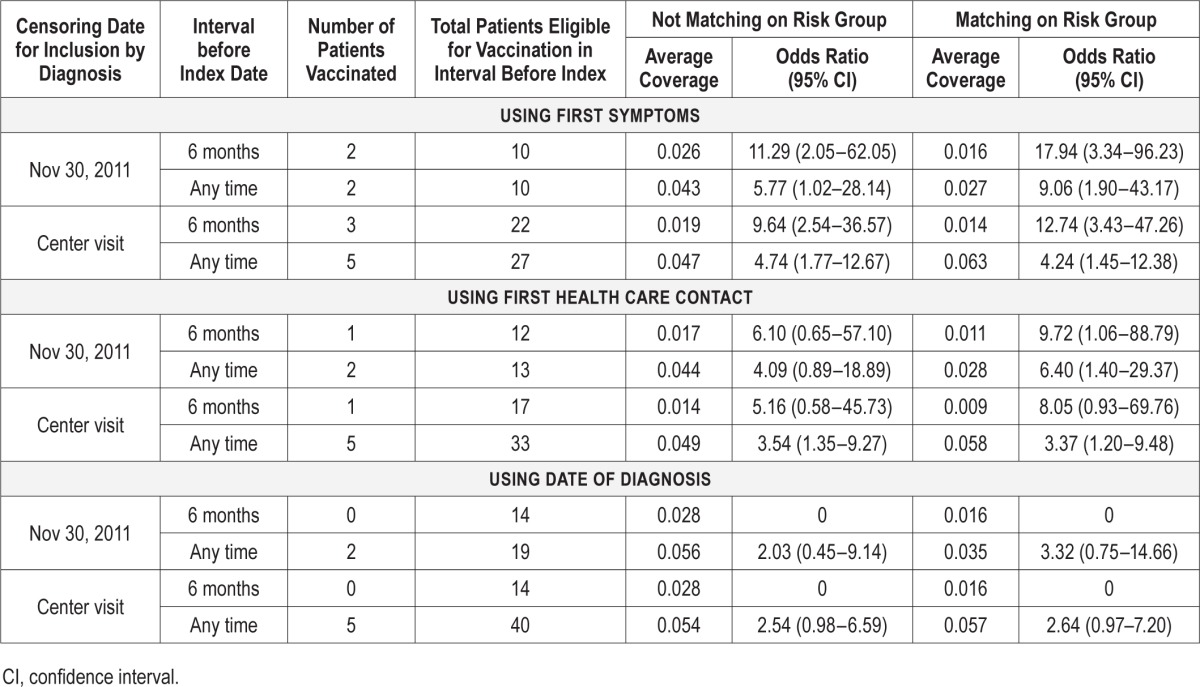
Table 3.
Sensitivity analysis and age stratification using vaccination at any time prior to first symptoms and all cases diagnosed by the center visit date.
Attributable Risk
The calculation for the vaccine-attributable risk used the odds ratio of 4.24 based on symptom onset at any time (Table 2). Using the odds ratio to approximate relative risk (RR), the attributable fraction ((RR−1)/RR)) is (3.24/4.24), which applied to the five vaccinated patients in the analysis gives an estimate of 3.82 attributable cases. HES data indicate that the sleep centers visited provided a diagnosis for approximately 33% of the narcolepsy cases in England in the study period, giving an estimated total of 3.82/0.33 = 11.6 attributable cases in England. Counting pandemic vaccine doses administered to those aged 18–59 y gives a total of 1,975,000 based on the final cumulative uptake and the Office for National Statistics population data for England in 2009.19 The attributable risk is therefore 11.6/1,975,000 = 0.59 per 100,000 doses
DISCUSSION
We found a significantly increased risk of narcolepsy in adults following AS03 adjuvanted pandemic strain vaccine in England. The odds ratio in adults was 9.06 (1.90–43.17) in the primary analysis and 4.24 (1.45–12.38) using all cases with a diagnosis by the date of the sleep centre visit, with an estimated attributable risk 0.59 per 100,000 doses. This risk is lower than we found in children where the comparable odds ratios were 14.4 (4.3 to 48.5) and 8.3 (3.1 to 22.3) respectively, and attributable risk of 1.74 cases per 100,000 doses.8 As in the Finnish adult study,12 the risk was highest within 6 mo of vaccination with an odds ratio of 12.74 (3.43–47.26).
The mechanism by which narcolepsy with cataplexy is associated with Pandemrix is not known. HLA DQB1*06:02 is present in 95% of patients with narcolepsy with cataplexy (type 1).20,21 In this study, all five vaccinated narcolepsy patients developed narcolepsy with cataplexy. The two tested patients were positive for HLA DQB1*06:02. It is possible that Pandemrix provides a second hit in those patients with a genetic vulnerability to the development of narcolepsy with cataplexy. Pandemrix may result in the development or augmentation of autoantibodies to hypocretin-producing cells and the destruction of these cells results in the development of narcolepsy with cataplexy. Others have speculated on autoimmunity as a mechanism to explain the link between narcolepsy and Pandemrix.21 As with the pediatric study in England,8 there was no evidence that prior swine influenza infection was a risk factor, with only one study case reporting influenza-like-illness in the 3 mo prior to their narcolepsy symptoms. Recent research, however, suggests that vaccine-induced narcolepsy may be associated with the induction of antibodies to the H1N1 nucleoprotein of the Pandemrix strain that cross-react with hypocretin receptors.22,23
Our odds ratio for the primary analysis is lower than found in the French case control study which reported an odds ratio of 16.8 (1.9–149.1) for cases aged 18 years and over using symptom onset as the index date.11 In that study 28% of eligible cases declined to participate and onset date was based on patient recall, allowing the potential for participation and recall bias which would likely lead to an overestimate of the association. In the Swedish record linkage study, which failed to find an elevated risk in those aged 20 y and older,13 the narcolepsy diagnosis was not verified and the index date was date of diagnosis, which would likely underestimate the association. In our study, cases were verified by an expert panel according to ICSD-2 diagnostic criteria, and onset date was independently obtained from referral letters, hospital notes, and GP records. Based on this information, we defined the earliest and latest possible date of first symptoms; odds ratios generated with these extreme dates were similar to the odds ratio using the most likely onset date.
To ensure as complete case ascertainment as possible, cases were identified by actively searching local electronic patient records and databases and cross-checking with cases in the national hospital database. This approach should avoid selection bias arising from differential ascertainment of diagnosed cases in vaccinated and unvaccinated individuals, as might occur if reliant on clinician recall. In the primary analysis, data were censored to only include cases diagnosed by November 30, 2011 to limit potential bias from accelerated diagnosis in patients in whom an association with vaccination was suspected once the association had generated media interest in December 2011.8 We found that the odds ratio using cases diagnosed by the center visit date was lower than that using cases diagnosed by November 30, 2011 rather than higher, which might have occurred if there was a tendency for more rapid diagnosis of vaccinated cases after the association was publicized.
Our case-coverage approach relies on the representativeness of the coverage data used. In this study we used information from the CPRD, a different GP dataset than we used in the pediatric narcolepsy study.8 It was reassuring that age- and risk group–specific coverage estimates were similar in both GP datasets (data not shown) and were comparable to national coverage data.17 The sensitivity analysis showed that even if we have underestimated coverage by as much as a relative 20% (for example, due to vaccination given outside of general practice not getting on the record) the association would still be significant, odds ratio 3.45 (1.18–10.14) for vaccinated at any time before onset.
In conclusion, we found evidence of an increased risk of narcolepsy in adults following AS03 adjuvanted pandemic strain vaccine in England. We were unable to define how the risk varied with age due to the relatively small numbers of cases. However, the data do not suggest a threshold age above which the risk is zero as vaccine-associated cases were identified across the age range studied. Further studies in collaboration with other European countries that used Pandemrix may help to more accurately define the age-specific risk in adults.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Eriksson and Dr. Dennis have participated in speaking engagements for UCB Pharma and Eisai Pharma. The other authors have indicated no financial conflicts of interest. Ethics approval: PHE has approval for England from the National Information Governance Board for Health and Social Care (NIGB) (PIAG ref: PIAG 03-(c)/2001), which allows us access to patient identifiable information for vaccine safety monitoring purposes.
ACKNOWLEDGMENTS
The authors thank Professor Stephen Evans, London School of Hygiene and Tropical Medicine for review of the statistical analysis plan. The authors gratefully acknowledge Joanne Williamson, Anne Brooks, Rebecca Chadwick, and Anne Yendley for their assistance at each of the study centres. We also acknowledge Paul Gringras for his help in setting up the study. We thank the general practitioners who assisted with the follow up. The HES data are re-used with permission of the Health and Social Care Information Centre. All rights reserved. Authors' contributions; EM, NA, JS, and CK developed the study protocol. Authors CK, JShn, AH, SE, PR, and GD designed data collection tools and monitored data collection at their individual centres. JS extracted clinical information from the centre notes. JS extracted the HES cases and conducted the GP follow up. KD extracted the CPRD data. Authors GL, JShn, AH, SE formed the review panel. NA conducted the statistical analysis and JS wrote the first draft of the paper. All authors contributed to subsequent revisions and approved the final version.
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Longstreth WT, Jr, Koepsell TD, Ton TG, Audrey F, Hendrickson AF, van Belle G. The epidemiology of narcolepsy. Sleep. 2007;30:13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 3.Public Health England. Immunisation against infectious disease. Influenza: the green book, chapter 19. [Accessed January 22, 2016]. Available from: https://www.gov.uk/government/collections/immunisation-against-infectious-disease-the-green-book.
- 4.Joint Committee on Vaccination and Immunisation. Advice on the H1N1v vaccination programme. Friday 8th January 2010. [Accessed January 22, 2016]. Available from: http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@ab/documents/digitalasset/dh_112665.pdf.
- 5.Sethi M, Pebody R. Pandemic H1N1 (Swine) Influenza Vaccine Uptake amongst Patient Groups in Primary Care in England 2009/10. [Accessed January 22, 2016]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215977/dh_121014.pdf.
- 6.European Medicine Agency. Assessment report: Pandemrix. [Accessed January 22, 2016]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000832/WC500118056.pdf.
- 7.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PloS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller E, Andrews N, Stellitano L, et al. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 9.O'Flanagan D, Barret AS, Foley M, et al. Investigation of an association between onset of narcolepsy and vaccination with pandemic influenza vaccine, Ireland April 2009-December 2010. Euro Surveill. 2014 May 1;19:15–25. [PubMed] [Google Scholar]
- 10.Heier MS, Gautvik KM, Wannag E, et al. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013;14:867–71. doi: 10.1016/j.sleep.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Dauvilliers Y, Arnulf I, Lecendreux M, et al. Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136(Pt 8):2486–96. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 12.Jokinen J, Nohynekm H, Honkanenm J, et al. Working paper: Association between the pandemic vaccine and narcolepsy in adults. [Accessed January 22, 2016]. Available from: http://urn.fi/URN:ISBN:978-952-245-921-3.
- 13.Persson I, Granath F, Askling J, Ludvigsson JF, Olsson T, Feltelius N. Risks of neurological and immune-related diseases, including narcolepsy, after vaccination with Pandemrix: a population- and registry-based cohort study with over 2 years of follow-up. J Intern Med. 2014;275:172–90. doi: 10.1111/joim.12150. [DOI] [PubMed] [Google Scholar]
- 14.Health & Social Care Information Centre. Hospital Episode Statistics. [Accessed January 22, 2016]. Available from: http://www.hscic.gov.uk/hes.
- 15.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2005. International Classification of Sleep Disorders, 2nd ed. Diagnostic and coding manual. [Google Scholar]
- 16.Farrington CP. Control without separate controls: evaluation of vaccine safety using case-only methods. Vaccine. 2004;22:2064–70. doi: 10.1016/j.vaccine.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 17.The Clinical Practice Research Datalink (CPRD) [Accessed January 22, 2016]. http://www.cprd.com.
- 18.Begum F, Pebody R. Seasonal influenza vaccine uptake amongst GP patient groups in England, Winter season 2010-11. [Accessed January 22, 2016]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216393/dh_129856.pdf.
- 19.Office for National Statistics. Population Estimates Unit. Estimated resident population mid-2009 by single year. [Accessed January 22, 2016]. http://www.ons.gov.uk.
- 20.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59:1553–62. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 21.Thebault S, Vincent A, Gringras P. Narcolepsy and H1N1 vaccination: a link? Curr Opin Pulm Med. 2013;19:587–93. doi: 10.1097/MCP.0b013e328365af97. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed SS, Volkmuth W, Duca J, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7:294ra105. doi: 10.1126/scitranslmed.aab2354. [DOI] [PubMed] [Google Scholar]
- 23.Vaarala O, Vuorela A, Partinen M, et al. Antigenic Differences between AS03 Adjuvanted Influenza A (H1N1) Pandemic Vaccines: Implications for Pandemrix-Associated Narcolepsy Risk. PLoS One. 2014;9:e114361. doi: 10.1371/journal.pone.0114361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.