Abstract
Study Objectives:
To examine the integrity of sleep-promoting neurons of the ventrolateral preoptic nucleus (VLPO) in postmortem brains of narcolepsy type 1 patients.
Methods:
Postmortem examination of five narcolepsy and eight control brains.
Results:
VLPO galanin neuron count did not differ between narcolepsy patients (11,151 ± 3,656) and controls (13,526 ± 9,544).
Conclusions:
A normal number of galanin-immunoreactive VLPO neurons in narcolepsy type 1 brains at autopsy suggests that VLPO cell loss is an unlikely explanation for the sleep fragmentation that often accompanies the disease.
Citation:
Gavrilov YV, Ellison BA, Yamamoto M, Reddy H, Haybaeck J, Mignot E, Baumann CR, Scammell TE, Valko PO. Disrupted sleep in narcolepsy: exploring the integrity of galanin neurons in the ventrolateral preoptic area. SLEEP 2016;39(5):1059–1062.
Keywords: galanin, narcolepsy, sleep fragmentation, ventrolateral preoptic area
Significance.
Sleep fragmentation is common in narcolepsy type 1 but difficult to explain by orexin deficiency alone. As elderly people with fragmented sleep have a loss of sleep-promoting galanin neurons in the ventrolateral preoptic area (VLPO), we examined the VLPO in people with narcolepsy type 1. We found a normal number of galanin VLPO neurons, suggesting that the fragmented sleep of narcolepsy must arise from another cause.
INTRODUCTION
Type 1 narcolepsy is a common and debilitating neuro-degenerative disorder characterized by excessive daytime sleepiness and cataplexy.1 In addition, sleep fragmentation is common in type 1 narcolepsy, and typically develops a few years after the onset of sleepiness and cataplexy.2 Sleep fragmentation can worsen other manifestations of narcolepsy,3 and sodium oxybate consolidates sleep and improves daytime sleepiness and cataplexy.4 The cause of sleep fragmentation in narcolepsy is unknown, but the delayed onset of sleep fragmentation suggests a pathophysiology distinct from that underlying excessive daytime sleepiness and cataplexy.
The loss of hypothalamic neurons producing orexin (hypo-cretin) is regarded as the main cause of narcolepsy, and orexin levels in cerebrospinal fluid (CSF) are already undetectable or very low when symptoms first occur.5–7 The orexin neurons control the activity of wake-promoting and sleep-promoting nuclei, and the components of these two systems have mutually inhibitory connections.8 Several monoaminergic and cholinergic nuclei promote wakefulness. Conversely, neurons of the ventrolateral preoptic nucleus (VLPO) use gamma-amino-butyric acid and galanin to inhibit these arousal regions, and loss of VLPO neurons can cause long-lasting sleep fragmentation.9–11 The loss of orexin neurons leads to behavioral state instability, with poor maintenance of wakefulness and poor consolidation of sleep.8
In postmortem brains of type 1 narcolepsy patients, we recently found a marked increase in histamine-producing neurons of the tuberomammillary nucleus (TMN),12,13 a key arousal-promoting structure densely innervated by the orexin and VLPO neurons.9,14 We speculated that the increase in histaminergic TMN neurons reflects a compensatory response to counterbalance the loss of excitatory drive from orexin neurons, and that stronger or dysregulated histaminergic signaling may subsequently cause fragmented nighttime sleep.12
However, sleep fragmentation could arise if inhibitory inputs from sleep-active VLPO galanin neurons are insufficient to silence neuronal activity in the histaminergic TMN and other arousal-promoting nuclei. To test this hypothesis, we stereologically measured the numbers of VLPO galanin neurons in brains of people with narcolepsy and control individuals, and examined the correlations between galanin, orexin, and histaminergic TMN neurons.
METHODS
Human Subjects
We studied hypothalamic tissue of five patients with type 1 narcolepsy and eight non-neurological controls. Data on orexin and histamine cell counts of the five narcolepsy patients (cases A, C-F) and six controls (cases 2, 3, 6, 9, 11, 12) were published previously.12 Two additional control brains were provided by the Neuropathology Department of Beth Israel Deaconess Medical Center, Boston, Massachusetts. Compared to the six previously reported brains, the two additional brains contained similar numbers of orexin and histamine neurons. The medical records of the control subjects did not contain any history of brain disorders and their routine neuropathological examination was unremarkable. Postmortem delay was slightly shorter in the narcolepsy group than in controls (8.3 ± 6.7 h versus 18.4 ± 6.7 h, P = 0.10), but the difference was insignificant. Fixation time was longer in the narcolepsy group than in the controls (1 w to 2 y versus 2 mo to 11 y, respectively). The local ethics committees of all involved institutes approved the study protocol.
Brain Tissue Processing and Immunohistochemistry
We performed immunostaining for orexin and histidine decarboxylase (HDC) as detailed in our previous paper.12 Because the human VLPO is a much smaller structure than the orexin field or the TMN, we examined VLPO-containing sections in a 1:6 series, as opposed to 1:24 series for orexin and HDC immunostaining. To identify VLPO neurons, we immunostained sections for galanin after antigen retrieval to improve labeling. After the initial reaction with hydrogen peroxide, we put sections in Tris-buffered saline (TBS; pH 11.0) at 95°C for 20 min. After cooling to room temperature, we washed the sections twice in TBS for 10 min, and then continued with the blocking step. Overnight incubation with polyclonal rabbit anti-galanin primary antiserum (1:2000; Peninsula Laboratories, San Carlos, CA LLC; Product# T-4326; Lot# A08910) was followed by incubation with biotinylated donkey anti-rabbit secondary antiserum (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA, Product# 711-065-152; Lot# 101909).
Stereological Cell Counts
To count VLPO galanin neurons, we used the same general stereological techniques as recently described.11 In brief, we placed a 1 × 3 mm rectangle over the VLPO, so that the long axis was parallel to the lateral wall of the third ventricle. This allowed assignment of the VLPO galanin neurons and their reliable separation from galanin neurons of other nuclei in the anterior hypothalamus. To achieve accurate cell counts as determined by Gunderson coefficient of error < 10%, we chose a counting grid of 150 × 150 μm and a counting frame of 50 × 50 μm.
Statistics
Group data are reported as means and standard deviations. We compared cell counts between groups using Student t test and performed correlation analyses using the Pearson test. Statistical significance was accepted at P < 0.05.
RESULTS
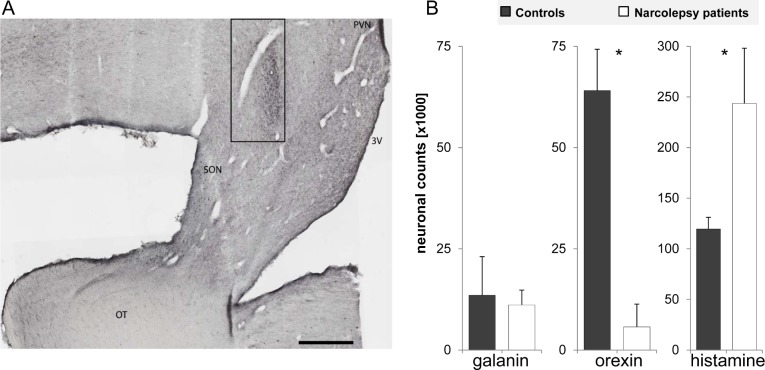
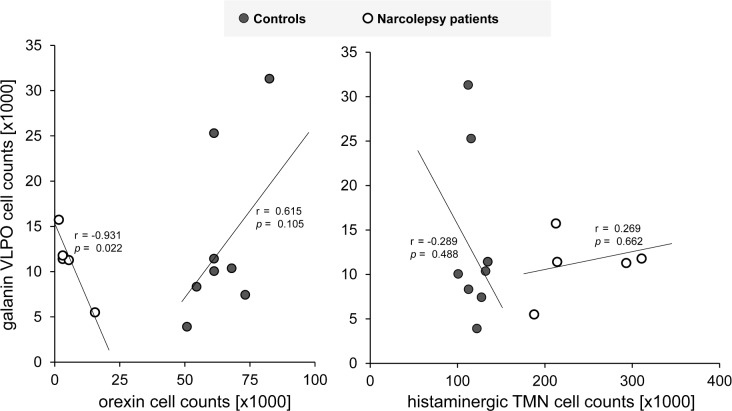
The number of VLPO galanin neurons was 13,526 ± 9,544 in controls, and 11,151 ± 3,656 in narcolepsy patients (P = 0.54) (Figure 1). In narcolepsy patients, the number of galanin neurons inversely correlated with the number of orexin neurons (r = −0.93, P = 0.02), whereas in controls the association tended to go in the opposite direction (r = 0.615, P = 0.10) (Figure 2). The numbers of VLPO galanin and histaminergic TMN neurons did not correlate in narcolepsy patients (r = 0.27, P = 0.66) nor in controls (r = −0.29, P = 0.49).
Figure 1.
Photomicrograph of the ventrolateral preoptic area (VLPO) of a control brain, with neurons immunolabeled for galanin (A). The number of galaninergic VLPO neurons is similar in narcolepsy patients and controls, whereas the number of orexin neurons was significantly reduced and the number of histaminergic TMN neurons was significantly increased in narcolepsy patients compared to controls (B). *P < 0.001. 3V, third ventricle; OT, optic tract; PVN, paraventricular nucleus; SON, supraoptic nucleus. Scale bar, 1 mm.
Figure 2.
In narcolepsy patients (open circles), the numbers of galaninergic VLPO neurons and orexin neurons are inversely correlated. In controls (closed circles), the association of the two systems tends to be opposite. The numbers of galaninergic VLPO neurons and histaminergic TMN neurons are not correlated.
Mean age was similar in narcolepsy patients and controls (71 ± 15 y versus 66 ± 10 y, P = 0.59). Age had no major effect on the number of VLPO galanin neurons in narcolepsy patients (r = −0.443, P = 0.46) nor in controls (r = −0.391, P = 0.34). All narcolepsy patients were male, whereas four controls (50%) were female (P = 0.10). The number of VLPO galanin neurons was similar in female and male controls (13,641 ± 7,956 versus 13,411 ± 12,214, P = 0.98).
DISCUSSION
We found that type 1 narcolepsy patients have normal numbers of galanin-containing VLPO neurons. The number of VLPO neurons correlated inversely with the number of orexin neurons but not with the number of histaminergic TMN neurons.
These experiments have some limitations. First, prior studies described sleep fragmentation in narcolepsy,2,3 but as we did not directly measure sleep disruption, we cannot be certain how cell counts correlate with sleep/wake behavior. Second, we examined a relatively small number of brains, and some observations such as the correlations between cell counts in different brain regions will be better supported with a larger number of subjects. Third, we cannot exclude that sleep-active, nongalaninergic VLPO neurons, such as those producing gamma-aminobutyric acid, are affected in narcolepsy. Nevertheless, more than 80% of sleep-active VLPO neurons express galanin messenger RNA,15 and most sleep-active VLPO neurons that inhibit histaminergic TMN neurons contain galanin.9 Fourth, even though the number of VLPO galanin neurons is normal, it is still possible that dysfunction of VLPO neurons contributes to disrupted sleep. Last, we have not yet examined other brain regions implicated in the production of sleep such as the median preoptic nucleus or parafacial zone,16,17 as these cell groups lack specific markers for the sleep-active neurons.
The first postmortem studies of narcolepsy patients noted that the loss of orexin neurons was highly selective, with preservation of adjacent neurons producing melanin-concentrating hormone,6,12 and researchers speculated that the symptoms of narcolepsy are entirely due to orexin neuron loss. However, the recent discovery of increased numbers of histaminergic TMN neurons challenged this view, and it is now important to examine other sleep-wake regulating nuclei.
The pattern of disrupted nighttime sleep in narcolepsy is clearly different from that seen in typical insomnia. Narcolepsy patients usually fall asleep quickly and often enter stage R sleep in less than 15 min. They also have frequent arousals and brief awakenings, with subsequent reduction of sleep efficiency, more time spent in N1 sleep, and prolonged wake time after sleep onset.2,3,18,19 Last, research in rodents and humans demonstrated that homeostatic and circadian processes are essentially preserved in narcolepsy.20–22
This pattern of sleep disruption has some similarities to that seen with VLPO lesions. During sleep, VLPO galanin neurons likely inhibit histaminergic neurons of the TMN and other arousal regions.9,23–25 Lesions of the VLPO in rats fragment sleep and reduce total sleep amount.10 Recently, Lim et al.11 counted VLPO galanin neurons in 45 participants of the Rush Memory and Aging Project, and compared the neuron numbers with actigraphy data obtained in the years before death. They found that actigraphic measures of sleep fragmentation were associated with lower numbers of VLPO galanin neurons, and this correlation was significant in both participants with and without Alzheimer disease.11 Moreover, patients with Alzheimer disease, a neurodegenerative disorder that typically causes severe sleep disruption, had only half as many VLPO galanin neurons as participants without Alzheimer disease. We used the same immunostaining and stereological techniques, but the numbers of VLPO galanin neurons in our narcolepsy patients were slightly higher than reported by Lim et al. for the elderly participants without Alzheimer disease, but similar to the galanin neuron count in 14 control males of another study.26 Thus, although VLPO neuron loss can fragment sleep, this does not appear to be the cause of disrupted sleep in narcolepsy. Considering its delayed development after the initial attack to the orexin neurons, it is hard to explain fragmented sleep without assuming changes in other cell groups, and based on current evidence, increased histamine signaling is one possible mechanism. In this line, histamine antagonists improve chronic primary insomnia,27 and photo-evoked release of histamine inhibits VLPO neurons in mice.28 Still, other hypotheses such as reduced VLPO activity or changes in other sleep-promoting systems need to be tested as well.
It remains unclear why the number of VLPO galanin neurons is inversely correlated with the number of orexin neurons. Possibly, more severe orexin neuron loss spurs greater compensatory changes in VLPO neurons. If so, then this response must be independent of the TMN as TMN cell counts did not correlate with VLPO counts. In addition, more VLPO neurons may not result in better sleep because people with type 1 narcolepsy probably have more extensive orexin neuron loss than those with type 2 narcolepsy, but they have more sleep disruption,29 lower sleep efficiency, and more awakenings.3 Understanding the functional relationship between the VLPO and orexin neurons will require larger postmortem studies with premortem measurements of sleep architecture.
In conclusion, disrupted nighttime sleep remains a poorly understood yet common problem in narcolepsy. We now find that the VLPO appears intact in narcolepsy, but other potential causes of disrupted sleep need to be evaluated in future studies.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by research grants from the Swiss National Science Foundation (grant No. 32003B-125504) and the Clinical Research Priority Program “Sleep and Health” of the University of Zurich (to C.R.B.), and a research grant from Wake up Narcolepsy (to T.E.S.). Dr. Scammell has received research support from Eisai Pharma and has consulted forMerck, Symphony, Prexa, Jazz, Cereve, Purdue Pharma, Ferrer, Heptares, and Synageva. Dr. Mignot has received research support from Jazz, has consulted for Novo Nordisk and UCB Pharma, and is on the speakers' bureau for Vox Media. Dr. Baumann has consulted for Teva, UCB Pharma, and Otsuka Pharma (Japan). The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Drs G. Rosen and L. Slomianka for use of their Stereo Investigator software.
REFERENCES
- 1.Scammell TE. The Neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154–66. doi: 10.1002/ana.10444. [DOI] [PubMed] [Google Scholar]
- 2.Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9:955–65. doi: 10.5664/jcsm.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harsh J, Peszka J, Hartwig G, Mitler M. Night-time sleep and daytime sleepiness in narcolepsy. J Sleep Res. 2000;9:309–16. doi: 10.1046/j.1365-2869.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 4.Boscolo-Berto R, Viel G, Montagnese S, Raduazzo DI, Ferrara SD, Dauvilliers Y. Narcolepsy and effectiveness of gamma-hydroxybutyrate (GHB): a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2012;16:431–43. doi: 10.1016/j.smrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 6.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 8.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim AS, Ellison BA, Wang JL, et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer's disease. Brain. 2014;137:2847–61. doi: 10.1093/brain/awu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valko PO, Gavrilov YV, Yamamoto M, et al. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74:794–804. doi: 10.1002/ana.24019. [DOI] [PubMed] [Google Scholar]
- 13.John J, Thannickal TC, McGregor R, et al. Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013;74:786–93. doi: 10.1002/ana.23968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci. 2001;21:9273–9. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 16.Alam MA, Kumar S, McGinty D, Alam MN, Szymusiak R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol. 2014;111:287–99. doi: 10.1152/jn.00504.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anaclet C, Ferrari L, Arrigoni E, et al. The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci. 2014;17:1217–24. doi: 10.1038/nn.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montplaisir J, Billiard M, Takahashi S, Bell IF, Guilleminault C, Dement WC. Twenty-four-hour recording in REM-narcoleptics with special reference to nocturnal sleep disruption. Biol Psychiatry. 1978;13:73–89. [PubMed] [Google Scholar]
- 19.Frauscher B, Gschliesser V, Brandauer E, et al. Motor disturbances during non-REM and REM sleep in narcolepsy-cataplexy: a video-polysomnographic analysis. J Sleep Res. 2011;20:514–21. doi: 10.1111/j.1365-2869.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 20.Khatami R, Landolt HP, Achermann P, et al. Insufficient non-REM sleep intensity in narcolepsy-cataplexy. Sleep. 2007;30:980–9. doi: 10.1093/sleep/30.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khatami R, Landolt HP, Achermann P, et al. Challenging sleep homeostasis in narcolepsy-cataplexy: implications for non-REM and REM sleep regulation. Sleep. 2008;31:859–67. doi: 10.1093/sleep/31.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 24.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Falgueras A, Ligtenberg L, Kruijver FP, Swaab DF. Galanin neurons in the intermediate nucleus (InM) of the human hypothalamus in relation to sex, age, and gender identity. J Comp Neurol. 2011;519:3061–84. doi: 10.1002/cne.22666. [DOI] [PubMed] [Google Scholar]
- 27.Krystal AD, Richelson E, Roth T. Review of the histamine system and the clinical effects of H1 antagonists: basis for a new model for understanding the effects of insomnia medications. Sleep Med Rev. 2013;17:263–72. doi: 10.1016/j.smrv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Williams RH, Chee MJ, Kroeger D, et al. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci. 2014;34:6023–9. doi: 10.1523/JNEUROSCI.4838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rye DB, Dihenia B, Weissman JD, Epstein CM, Bliwise DL. Presentation of narcolepsy after 40. Neurology. 1998;50:459–65. doi: 10.1212/wnl.50.2.459. [DOI] [PubMed] [Google Scholar]