Abstract
Study Objectives:
To evaluate the association between self-reported sleep quality and levels of brain β-amyloid (Aβ) burden, and to determine the effect of the apolipoprotein E (APOE) ε4 allele on any associations found.
Methods:
This study is a cross-sectional analysis of 184 cognitively healthy men and women aged over 60 y. We measured sleep quality factors: specifically, sleep duration, latency (time taken to fall asleep), disturbances, efficiency, daytime dysfunction, and overall sleep quality, using the Pittsburgh Sleep Quality Index. All participants underwent Aβ positron emission tomography imaging for the quantification of brain Aβ burden and were APOE genotyped. Linear regression analyses were used to evaluate the relationship between sleep quality factors and brain Aβ burden, adjusting for age, body mass index, cardiovascular disease, and symptoms of depression, with APOE ε4 carriage entered as a moderator.
Results:
Of the sleep factors, longer sleep latency was associated with higher levels of brain Aβ (B = 0.003 [standard error = 0.001], P = 0.02). APOE ε4 allele (carrier/noncarrier) did not moderate the relationship between sleep latency and brain Aβ burden.
Conclusions:
Our findings suggest a relationship between brain Aβ burden and sleep latency, independent of APOE ε4 genotype.
Citation:
Brown BM, Rainey-Smith SR, Villemagne VL, Weinborn M, Bucks RS, Sohrabi HR, Laws SM, Taddei K, Macaulay SL, Ames D, Fowler C, Maruff P, Masters CL, Rowe CC, Martins RN, AIBL Research Group. The relationship between sleep quality and brain amyloid burden. SLEEP 2016;39(5):1063–1068.
Keywords: Alzheimer disease, apolipoprotein ε4 allele, beta-amyloid, sleep, sleep latency
Significance.
Sleep disruption increases with advancing age, and has previously been associated with increased Alzheimer disease pathology in numerous animal studies. However, to date, few human studies have evaluated the relationship between sleep and brain beta-amyloid burden (an indicator of Alzheimer disease pathology) in a highly characterised cohort. Thus, in the current study we have evaluated the relationship between self-reported sleep factors and brain beta-amyloid burden in a cohort of 184 cognitively healthy older adults. We report an association between longer sleep latency and higher levels of brain beta-amyloid burden. Previous reports suggest the relationship between sleep and beta-amyloid is likely bi-directional; thus, future longitudinal studies are essential to further understand this relationship.
INTRODUCTION
Sleep disorders become more prevalent with advancing age.1 Previous studies have demonstrated consistently the detrimental effects of suboptimal sleep on cognitive function and increased risk of Alzheimer disease (AD).2–5 Nevertheless, it appears the association between sleep and AD is bidirectional: sleep-wake disturbances are a common comorbidity of AD, and evidence suggests that these detrimental changes to sleep are associated with an increase in the severity of AD pathology.6
Soluble Aβ can be measured in cerebrospinal fluid (CSF) and interstitial fluid (ISF) and correlates with the extent of deposition of insoluble Aβ in the brain. This insoluble Aβ aggregates to form brain extracellular senile plaques, which are one of the neuropathological hallmarks of AD, and can be measured by amyloid positron emission tomography (PET) imaging.7 Previous animal research has established a link between both soluble and plaque Aβ and sleep. For example, Kang and colleagues8 reported acute sleep deprivation increased levels of ISF Aβ, whereas chronic sleep deprivation was associated with increased Aβ plaque formation in amyloid precursor protein transgenic mice. This group also reported that Aβ disrupts the sleep-wake cycle. After plaque formation, the sleep-wake cycle of AD transgenic mice deteriorated and diurnal fluctuations of ISF Aβ decreased.9 Building on this research, Xie and colleagues10 reported a 60% increase in cortical interstitial space in the adult mouse brain during sleep, concluding that the restorative function of sleep may be due to enhanced removal of toxic waste products, such as Aβ, that have accumulated during wakefulness. More recently, studies in human cohorts of older adults have shown that poorer sleep quality and shorter sleep duration are associated with decreased and increased Aβ measurements in the CSF11 and brain12–14 respectively. Nevertheless, research on this topic is still in its infancy, and the nature of this association and the particular parameters of sleep quality that are associated with Aβ deposition require further investigation in well-characterized cognitively healthy cohorts.
Previous studies suggest that the relationship between lifestyle factors (i.e., physical activity and diet) and brain health may be moderated by the carriage of the apolipoprotein (APOE) ε4 allele.15,16 For example, the relationship between increased physical activity and lower brain Aβ is more marked in carriers of the APOE ε4 allele.15,17 Similarly, the relationship between Mediterranean diet adherence and executive function performance was only evident in APOE ε4 carriers.16 Furthermore, the relationship between higher Aβ deposition and sleep disordered breathing severity is more marked in APOE ε4 allele carriers, compared with non-carriers.18 Based on this previous work, we hypothesized that carriers of the APOE ε4 allele reporting poor sleep quality will have the highest levels of Aβ burden. However, to our knowledge, no other studies have investigated the effect of APOE ε4 allele carriage on the association between parameters of sleep quality and brain Aβ levels.
The aim of this study was to evaluate the relationships between self-reported sleep quality factors (including duration, disturbances, latency, efficiency, and daytime dysfunction) and brain Aβ burden in a well characterized cohort of cognitively healthy individuals. Furthermore, we assessed the moderating effect of APOE ε4 allele carriage on the relationship between sleep quality and brain Aβ burden.
METHODS
Participants and Procedures
Participants from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging19 were included. Briefly, the AIBL study comprises men and women aged older than 60 y, without history of severe psychiatric illness, recent cancer, heart attack or other, uncontrolled, chronic illnesses. For inclusion in the current analyses, participants were required to demonstrate normal cognitive function on an array of neuropsychological tests, with these results reviewed by a panel of clinicians, undergo Aβ PET imaging, and complete a sleep questionnaire. From the total number of participants who undertook the AIBL inception cohort 72-mo follow-up or the AIBL enrichment cohort 18-mo follow-up (total n = 791), 193 were excluded due to mild cognitive impairment (MCI) or AD. A further 286 did not undergo neuroimaging, 42 did not complete the sleep questionnaire, and 45 had missing covariate data. Thirty-six individuals reporting the use of sleep medication on most nights of the week and 5 individuals reporting a medical history of stroke were also excluded, leaving data from 184 individuals. Data from the PET scan closest to the participants' most recent AIBL clinical and neuropsychological evaluation are reported. Subjects were given written instructions of the risks and benefits of study participation, and signed informed consent was obtained prior to assessment. Approval was obtained from the following institutions' Human Research Ethics Committees: Austin Health, St Vincent's Health, Edith Cowan University, and Hollywood Private Hospital.
Sleep Questionnaire
All participants completed the Pittsburgh Sleep Quality Index (PSQI)20: a 19-item, self-report measure assessing sleep quality and disturbances over the previous month. The PSQI provides the following factor scores: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleep medication use, and daytime dysfunction, as well as a global score. A global PSQI score > 5 indicates poor sleep. In the current study, we assessed the relationship between brain Aβ burden and sleep latency (reported in minutes), sleep duration (reported in hours), and PSQI-derived measures of sleep disturbance, daytime dysfunction, sleep efficiency, and the global PSQI score.
Amyloid Imaging
Aβ imaging was performed using three different tracers: 11C-Pittsburgh Compound B (PiB), 18F-flutemetamol (FLUTE) and 18F-florbetapir (FBP). Sixty-eight participants underwent imaging with PiB, 58 with FLUTE, and 58 with FBP. PET methodology has previously been described in detail.21–23 Briefly, a 30-min acquisition was performed 40 min postinjection of PiB, whereas a 20-min acquisition was performed 50 min postinjection of FBP and 90 min postinjection of FLUTE. For semiquantitative analysis, a volume of interest template was applied to the summed and spatially normalised PET images in order to calculate a standardized uptake value (SUV). Images were then scaled to the SUV of each tracer's recommended reference region to generate a tissue ratio termed SUV ratio (SUVR). For PiB, the cerebellar cortex was used as the reference region, whereas the whole cerebellum and the pons were used as reference regions for FBP and FLUTE, respectively. Global SUVR was calculated using the mean SUVR in the frontal, superior parietal, lateral temporal, occipital, and anterior and posterior cingulate regions. Previous work, from our group, applied a linear regression transformation to generate FBP and FLUTE SUVR in PiB-like SUVR units. This transformation gave rise to the “Before the Centiloid Kernel Transformation” (BeCKeT) scale, allowing the combination of results obtained with different Aβ tracers to yield a single continuous variable.24
Covariates
Participants provided demographic data and medical history via the completion of a questionnaire at their AIBL study assessment closest to their PET scan. At the study visit, participants also undertook a comprehensive neuropsychological assessment, completed lifestyle questionnaires and provided a fasted blood sample. Data from this visit were used to classify participants into cognitively healthy, MCI and AD participants: data from cognitively healthy participants were used in the current analysis. All participants completed the short-form Geriatric Depression Scale (GDS).25 DNA was isolated from whole blood samples, and APOE genotype was determined through polymerase chain reaction amplification and restriction enzyme digestions, or through TaqMan genotyping as-says (Life Technologies, Carlsbad, CA) for rs7412 (assay ID: C____904973_10) and rs429358 (assay ID: C___3084793_20), as previously described.26
Statistical Analysis
All statistical analyses were performed using SPSS version 22 (IBM Corporation, Armonk, NY). For all analyses, significance is indicated by P < 0.05. To evaluate any differences in demographic and medical history data between APOE ε4 carriers and noncarriers, t-tests (for continuous variables) and chi-square analyses (for categorical variables) were performed.
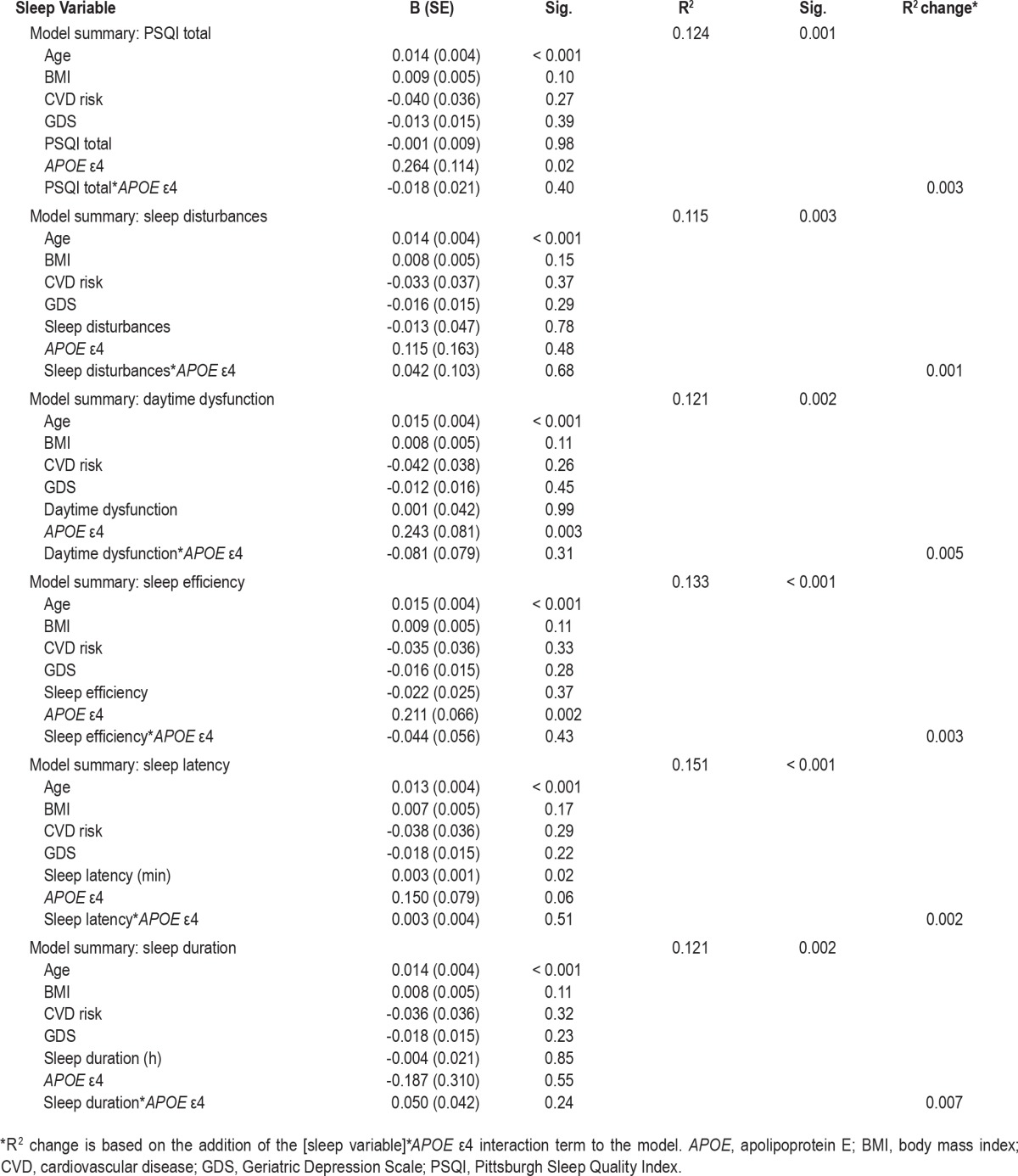
A series of moderation models was used to examine the relationship between sleep factors and brain Aβ burden, and the effect of APOE ε4 allele carriage on these relationships was assessed, using Hayes'27 simple moderation models. Bootstrapping analysis (5,000 bootstrap samples) was used to calculate 95% bias corrected and accelerated confidence intervals. Separate models were used for each sleep variable, with the sleep factor entered as an independent variable and Aβ SUVR as the dependent variable (Table 1). Age, body mass index (BMI), GDS score, and medical history of cardiovascular disease (CVD) were entered into the models as covariates, and APOE ε4 allele carriage (carrier = 1/noncarrier = 0) was entered as the moderator.
Table 1.
Association between sleep variables and beta-amyloid burden (continuous standardized uptake value ratio), results from regression models.
RESULTS
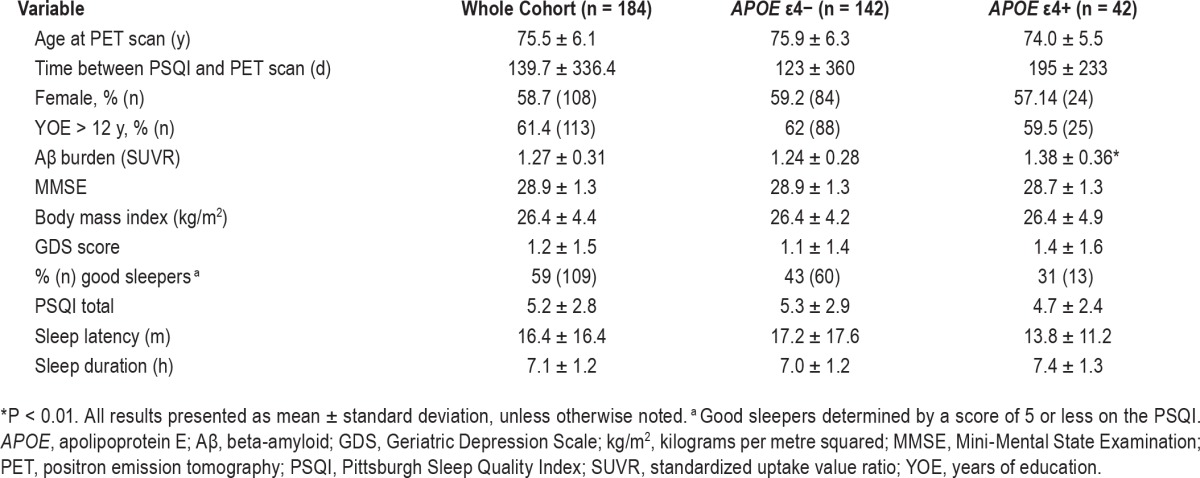
Descriptive statistics of demographic and medical history variables are presented in Table 2. Forty-two participants were APOE ε4 allele carriers; five of whom were homozygous for ε4. APOE ε4 allele carriers had significantly higher levels of brain Aβ than noncarriers, t(182) = 2.72, P < 0.01, d Cohen = 0.47.
Table 2.
Cohort descriptive statistics for the total sample and after stratification by Apolipoprotein E ε4 allele carriage.
Regression analyses (Table 1) revealed that longer sleep latency was associated with higher Aβ burden (B = 0.003 [SE: 0.001], P = 0.02). There were no other significant associations between the sleep quality variables and brain Aβ burden. Furthermore, no significant interactions between APOE ε4 carriage and sleep quality variables were observed.
DISCUSSION
This study examined the cross-sectional relationship between self-reported sleep quality factors and levels of Aβ brain deposition determined by PET. After adjustment for potential confounding variables, longer sleep latency was associated with higher brain Aβ burden. Other factors of sleep quality such as duration, disturbance, efficiency, and daytime dysfunction were not associated with Aβ burden. We also examined the moderating effect of APOE ε4 allele carriage on the relationship between sleep and Aβ deposition; no moderating effects were found.
This study is the first to report an association between sleep latency (time taken to fall asleep) and brain Aβ deposition. Our finding supports those previously reported by Blackwell and colleagues28 in which objectively measured, longer sleep latency was associated with increased risk of cognitive impairment in a group of older women; with a 0.8% decline observed on the Mini-Mental State Examination for every additional 30 min in sleep latency. Nevertheless, our findings are in contrast to a previous study investigating the cross-sectional relationship between sleep and Aβ deposition. Spira and colleagues12 reported no significant association between “trouble falling asleep” and brain Aβ levels. It is possible, however, that differing definitions of sleep latency, between the current study and that of Spira et al., may have contributed to the disparate findings. Ju and colleagues,11 who reported significant associations between CSF Aβ and actigraphy-measured total sleep quality and frequent napping, also collected a measure of sleep latency. However, this latter measure was not included in their final analysis. Similarly, Sprecher et al.14 collected data on sleep latency, but combined this measure with other sleep factors to calculate a sleep disturbance score: thus, the individual contribution of sleep latency to Aβ deposition was not reported. Our cross-sectional findings indicate that an increase in sleep latency of 1 min would be associated with a 0.003 increase in SUVR. To put this into context, estimates of brain Aβ accumulation from Villemagne et al.29 suggest a 0.043 annual increase in brain Aβ in known cognitively healthy “Aβ accumulators.” Thus, our findings suggest a 30-min longer sleep latency would translate to an equivalent of 2 y of brain Aβ accumulation. However, such a finding should be interpreted with caution, particularly when considering our lack of information regarding causal direction. Indeed, a relationship between longer sleep latency and higher levels of brain Aβ burden may provide support to previous animal data suggesting Aβ deposition contributes both to a circadian rhythm delay and decrease in amplitude, as has been shown in APP/PS1 mouse models of AD.30 Nevertheless, recent literature supports a bidirectional relationship between sleep and Aβ,31 and as mentioned previously, the causal direction of our cross-sectional analysis cannot be inferred.
Although we demonstrated an association between sleep latency and brain Aβ, contrary to previous reports, no other sleep quality factor (i.e., disturbances, efficiency, daytime dys-function, and duration) was associated with brain Aβ levels in our cohort. Ju and colleagues11 reported that actigraphy-measured sleep efficiency was poorer and frequent napping was more evident in a group with lower CSF Aβ1-42 (an indicator of higher brain Aβ levels), compared to those with normal Aβ levels. It is important to note that the cohort studied by Ju et al. was significantly younger than the current study (65 y versus 75 y), and different methods of sleep assessment and Aβ measurement were used. Although sleep latency is a contributor to sleep efficiency, it is likely that actigraphy-derived sleep quality measures are different from those calculated using self-report questionnaires. Spira et al.12 reported an association between suboptimal sleep duration and quality and greater Aβ burden. The demographic aspects (age, sex, APOE ε4 frequency, BMI) of the current cohort are very similar to the cohort studied by Spira et al.12; thus, other factors, such as differing questionnaires used for sleep assessment, may have contributed to these discrepant findings. Furthermore, it is possible that our cohort had lower levels of Aβ deposition. Only 17% of our cohort met criteria for “high Aβ,” as defined by an SUVR/ BeCKeT greater than 1.5. By contrast, Spira and colleagues reported that 34% of their cohort had “elevated Aβ levels”: albeit, “cut-off” used to assign individuals to the “elevated Aβ levels” group was not reported. It is conceivable that the proportion of individuals on the AD pathology pathway may explain the differing results of these two studies. A cross-sectional pilot study conducted by the same authors, investigating the association between objectively measured sleep and brain Aβ in older adults with normal cognition or MCI, suggested no observable association between sleep duration or fragmentation and Aβ deposition. Nevertheless, greater sleep disordered breathing severity was associated with greater Aβ load among the MCI participants.18 Finally, sample size is an important consideration when interpreting these results, with only 13 individuals studied (8 “normal”; 5 MCI). As the current and all previous human studies of sleep and Aβ have been cross-sectional, future longitudinal studies are essential to evaluate the nature of this association, and to more confidently identify which sleep factors are affected by and/or are affecting brain Aβ burden.
Previous studies suggest that the effect of lifestyle factors (i.e., physical activity and diet) on brain health may be moderated by carriage of an APOE ε4 allele.15,16 Furthermore, a significant effect of the interaction between APOE ε4 allele carriage and sleep disordered breathing on Aβ deposition has been reported.18,32 To our knowledge, no previous studies have investigated the effect of APOE ε4 allele carriage on the association between sleep quality factors and brain Aβ levels. In the current study, we hypothesized that any observed associations between sleep quality factors and Aβ deposition would be more pronounced in carriers of the APOE ε4 allele. Despite a significant difference between mean SUVR of carriers and noncarriers of the ε4 allele, we found no interaction between any of the investigated sleep quality factors and APOE ε4 carriage on brain Aβ burden. Further, in our cohort there were only five individuals homozygous for the APOE ε4 allele, which thereby precluded investigations of a dose-dependent allelic response. Ideally, studies of larger cohorts should investigate this potential association further.
The current study reports a novel association between sleep latency and brain Aβ. Nevertheless, the study was not without limitations. The Aβ PET imaging and sleep quality assessment were completed on separate days; however, Aβ deposition is a relatively slow process, occurring over many years,29 and sleep habits are usually chronic, particularly in the age group studied. Measurements of sleep quality were evaluated using self-report; the PSQI has demonstrated internal consistent reliability and construct validity,33 however, and studies of the association between PSQI and objective measures of sleep remains equivocal. Thus, future studies should ideally utilize actigraphy and/or polysomnography to determine the relationship between objective sleep measures and Aβ burden in moderate to large sized cohorts. Furthermore, this exploratory cross-sectional study does not allow for the inference of causal relationships. Analyses of longitudinal changes in sleep and Aβ would be required to further understand the nature of this complex association.
In summary, our findings report a novel association between sleep latency and brain Aβ burden that was not moderated by carriage of the APOE ε4 allele. In this cross-sectional study of high-functioning, cognitively healthy older adults it was not possible to determine the validity of the hypothesized bidirectional relationship between sleep and Aβ. Future longitudinal studies utilizing objective measures of sleep are essential in this area to understand the relationship between sleep quality factors and Aβ deposition.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding for the AIBL study is provided by the CSIRO Flagship Collaboration Fund and the Science and Industry Endowment Fund (SIEF) in partnership with Edith Cowan University (ECU), The Florey Institute of Neuroscience and Mental Health, Alzheimer's Australia (AA), National Ageing Research Institute (NARI), Austin Health, CogState Ltd., Hollywood Private Hospital, and Sir Charles Gairdner Hospital. The study also receives funding from the National Health and Medical Research Council (NHMRC), the Dementia Collaborative Research Centres program (DCRC2) and the McCusker Alzheimer's Research Foundation, and Operational Infrastructure Support from the Government of Victoria. Dr. Villemagne has financial interests in GE Healthcare, Piramal Imaging, and Novartis, Dr. Bucks has financial interests in NHMRC, Hogrefe Publisher, Speechmark Publisher, Parkinson's Western Australia: Zrinski Grant and Brain Foundation. Dr. Sohrabi has financial interests in Takeda Pharmaceutical Company and Pfizer Inc. Dr. Macaulay has financial interests in CSIRO and various small holdings. Dr. Ames has financial interests in PranaBio and Eli Lilly. Dr. Masters has financial interests in PranaBio, Eli Lilly, and Actinogen. Dr. Martins has financial interests in Alzhyme. Drs. Brown, Rainey-Smith, Weinborn, Laws, Taddei, Fowler, Maruff, and Rowe have indicated no financial conflicts of interest. The work was performed at the School of Medical Sciences, Edith Cowan University, Joondalup, Western Australia; Sir James McCusker Alzheimer's Disease Research Unit, Hollywood Private Hospital, Nedlands, Western Australia; Austin Health, Department of Nuclear Medicine and Centre for PET, Heidelberg, Victoria, Australia; and the Florey Institute for Neurosciences and Mental Health, University of Melbourne, Victoria, Australia.
REFERENCES
- 1.Wolkove N, Elkholy O, Baltzan M, Palayew M. Sleep and aging: 1. Sleep disorders commonly found in older people. CMAJ. 2007;176:1299–304. doi: 10.1503/cmaj.060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 4.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller MA, Wright H, Ji C, Cappuccio FP. Cross-sectional study of sleep quantity and quality and amnestic and non-amnestic cognitive function in an ageing population: the English Longitudinal Study of Ageing (ELSA) PLoS One. 2014;9:e100991. doi: 10.1371/journal.pone.0100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatfield CF, Herbert J, van Someren EJ, Hodges JR, Hastings MH. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004;127:1061–74. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 7.Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–9. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer's disease pathology. Sci Transl Med. 2012;4:150ra22. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju YE, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mander BA, Marks SM, Vogel JW, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nature Neurosci. 2015;18:1051–-7. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprecher KE, Bendlin BB, Racine AM, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–76. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-beta plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry. 2013;18:875–81. doi: 10.1038/mp.2012.107. [DOI] [PubMed] [Google Scholar]
- 16.Gardener SL, Rainey-Smith SR, Barnes MB, et al. Dietary patterns and cognitive decline in an Australian study of ageing. Mol Psychiatry. 2015;20:860–6. doi: 10.1038/mp.2014.79. [DOI] [PubMed] [Google Scholar]
- 17.Head D, Bugg JM, Goate AM, et al. Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch Neurol. 2012;69:636–43. doi: 10.1001/archneurol.2011.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spira AP, Yager C, Brandt J, et al. Objectively measured sleep and beta-amyloid burden in older adults: A pilot study. SAGE Open Medicine. 2014:2. doi: 10.1177/2050312114546520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis KA, Bush AI, Darby D, et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer's disease. Int Psychogeriatr. 2009:1–16. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Wong DF, Rosenberg PB, Zhou Y, et al. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51:913–20. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–83. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Vandenberghe R, Van Laere K, Ivanoiu A, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68:319–29. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- 24.Villemagne V, Dore V, Yates P, et al. En attendant centiloid. Adv Res. 2014;2:723–9. [Google Scholar]
- 25.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Brown BM, Bourgeat P, Peiffer JJ, et al. Influence of BDNF Val66Met on the relationship between physical activity and brain volume. Neurology. 2014;83:1345–52. doi: 10.1212/WNL.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 27.Hayes AF. New York, NY: Guildford Press; 2013. Introduction to mediation, moderation, and process analysis. [Google Scholar]
- 28.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 29.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 30.Duncan MJ, Smith JT, Franklin KM, et al. Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APPxPS1 knock-in mice, a model for Alzheimer's disease. Exp Neurol. 2012;236:249–58. doi: 10.1016/j.expneurol.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osorio RS, Ayappa I, Mantua J, et al. The interaction between sleep-disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging. 2014;35:1318–24. doi: 10.1016/j.neurobiolaging.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67:433–9. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]


