Abstract
Study Objectives:
Obesity hypoventilation and obstructive sleep apnea are common complications of obesity linked to defects in respiratory pump and upper airway neural control. Leptin-deficient ob/ob mice have impaired ventilatory control and inspiratory flow limitation during sleep, which are both reversed with leptin. We aimed to localize central nervous system (CNS) site(s) of leptin action on respiratory and upper airway neuroventilatory control.
Methods:
We localized the effect of leptin to medulla versus hypothalamus by administering intracerbroventricular leptin (10 μg/2 μL) versus vehicle to the lateral (n = 14) versus fourth ventricle (n = 11) of ob/ob mice followed by polysomnographic recording. Analyses were stratified for effects on respiratory (nonflow-limited breaths) and upper airway (inspiratory flow limitation) functions. CNS loci were identified by (1) leptin-induced signal transducer and activator of transcription 3 (STAT3) phosphorylation and (2) projections of respiratory and upper airway motoneurons with a retrograde transsynaptic tracer (pseudorabies virus).
Results:
Both routes of leptin administration increased minute ventilation during nonflow-limited breathing in sleep. Phrenic motoneurons were synaptically coupled to the nucleus of the solitary tract, which also showed STAT3 phosphorylation, but not to the hypothalamus. Inspiratory flow limitation and obstructive hypopneas were attenuated by leptin administration to the lateral but not to the fourth cerebral ventricle. Upper airway motoneurons were synaptically coupled with the dorsomedial hypothalamus, which exhibited STAT3 phosphorylation.
Conclusions:
Leptin relieves upper airway obstruction in sleep apnea by activating the forebrain, possibly in the dorsomedial hypothalamus. In contrast, leptin upregulates ventilatory control through hindbrain sites of action, possibly in the nucleus of the solitary tract.
Citation:
Yao Q, Pho H, Kirkness J, Ladenheim EE, Bi S, Moran TH, Fuller DD, Schwartz AR, Polotsky VY. Localizing effects of leptin on upper airway and respiratory control during sleep. SLEEP 2016;39(5):1097–1106.
Keywords: dorsomedial hypothalamus, nucleus of the solitary tract, ob/ob mouse, obesity hypoventilation syndrome, sleep apnea syndrome
Significance.
We demonstrated that recurrent hypopneas with oxyhemoglobin desaturations develop in ob/ob mice during sleep, similar to human obstructive sleep apnea. We also showed that (1) leptin administration to the lateral cerebral ventricle reversed upper airway obstruction during sleep, whereas leptin administration to the fourth ventricle had no effect; and (2) upper airway motoneurons projected to the dorsomedial hypothalamus, which showed robust leptin receptor signaling. These data provide the first evidence that leptin may relieve obstructive sleep apnea acting in the hypothalamus. Additional studies are needed to determine the precise hypothalamic localization of the leptin action on the upper airway. Our study suggests that leptin signaling in the hypothalamus should be examined as a therapeutic target in sleep apnea.
INTRODUCTION
Sleep-disordered breathing is a recognized complication of obesity,1 and includes obesity hypoventilation syndrome (OHS) and obstructive sleep apnea (OSA). These disorders are caused by respiratory loads on the diaphragm and upper airway structures and disturbances in neuromotor control of the respiratory pump and upper airway musculature.1 Studies performed in our laboratory suggested that the adipocyte-produced hormone, leptin, can activate central nervous system (CNS) mechanisms that compensate for adipose loads on respiratory and upper airway structures.2 In leptin deficient ob/ob mice, elevations in partial pressure of carbon dioxide (PaCO2) and reductions in hypercapnic ventilatory responses mimicked findings in OHS,3 and were reversed by subcutaneous administration of leptin.2 Similarly, anesthetized ob/ob mice demonstrated increases in upper airway collapsibility that resulted from defects in both upper airway structure and neuromuscular control. Subcutaneous leptin administration restored protective neuromuscular reflexes that maintain pharyngeal patency, suggesting that leptin signaling defects can play a vital role in OSA pathogenesis.4 We have recently shown that in ob/ob mice, cardinal manifestations of sleep-disordered breathing develop, including inspiratory flow limitation (IFL) and hypoventilation during sleep, which were relieved by sub-cutaneous leptin administration.5 Thus, in the ob/ob mouse, disturbances in neuromuscular control of upper airway and respiratory pump muscles can be attributed to leptin deficiency rather than obesity per se.
CNS site(s) of leptin action on respiratory and upper airway neuroventilatory control have not been well characterized. Leptin signaling in the brain occurs via the long isoform of leptin receptor ObRb.6 Leptin binding to ObRb receptor activates the Janus kinase/signal transducer and activator of transcription 3(JAK/STAT3) pathway,6,7 which regulates food intake and metabolic rate in the hypothalamus and medullary centers.8,9 Direct administration of leptin into the fourth ventricle and the nucleus of the tractus solitarius (NTS) increases the hypercapnic ventilatory response.10,11 The exact loci of leptin control of respiratory drive to the diaphragm and the upper airway structures during sleep remain unknown.
The major goal of this study was to localize CNS effects of leptin on upper airway and ventilatory control during sleep. We took advantage of the unidirectional rostral-caudal flow of cerebrospinal fluid12 to localize the effect of leptin to medulla versus hypothalamus by administering leptin intracerbroventricular (ICV) to the lateral versus fourth ventricle. We hypothesized that leptin regulates both ventilatory and upper airway control through medullary centers, and predicted that leptin would be equally effective in treating hypoventilation and upper airway obstruction during sleep when it is administered to both lateral and fourth ventricles. Potential CNS loci were further identified by characterizing (1) markers of leptin signaling in the hypothalamus and medulla (STAT3 phosphor-ylation) and (2) projections of respiratory and upper airway motoneurons with a retrograde tracer pseudorabies virus (PRV).
METHODS
Animals
Twenty-five male C57BL/6J-Lepob (ob-/ob-) leptin-deficient mice from Jackson Laboratory (Bar Harbor, ME), 19–21 w of age (at the time of surgery) were used in this study. All mice had free access to food and water and were housed in the standard laboratory environment, at 22–23°C with the 12-h light/ dark cycle (09:00–21:00 on/21:00–09:00 lights off). Mice were housed individually, maintained on a regular chow diet; their caloric intake and weight were monitored daily at 10:00. For all surgical procedures, anesthesia was induced with 1–2% isoflurane administered through a facemask. The study was approved by the Johns Hopkins University Animal Use and Care Committee and complied with the American Physiological Society Guidelines for Animal Studies.
Cannulation of the Lateral and Fourth Cerebral Ventricles and EEG/EMG Electrodes Implantation
Mice were implanted with permanent stainless steel cannula into the right lateral ventricle or the fourth ventricle of the brain. Coordinates for the cannulation were: the lateral ventricle: 0.6 mm from bregma,1.2 mm from midline (to the right side), and 3.0 mm dorsoventral (from skull surface); the fourth ventricle: 6.0 mm from bregma, 0 mm from midline, and 4.5 mm dorsoventral (from skull surface). During the same surgery, electroencephalography (EEG) and electromyography (EMG) electrodes were implanted with an EEG/EMG Head-mount (Pinnacle Technology, Lawrence, KS) as previously described13 with modifications. In the lateral ventricle cannulation experiments, three EEG electrodes were implanted in the left and right frontal regions and left parietal regions. In the fourth ventricle cannulation experiments, four EEG electrodes were implanted in the left and right frontal regions and left and right parietal regions. The headmount EMG leads were tunneled subcutaneously and placed over the nuchal muscles posterior to the skull. Following surgical recovery period (2 w), animals were tested for proper cannula placement. For the lateral ventricle cannula, animals were injected with angiotensin II (100 ng/μL).14 Animals showing water drinking behavior within 5 min after injection were used in the experiments. For the fourth ventricle cannula, animals were injected with 50 μg 5-Thio-D glucose.15 Animals showing increased blood glucose greater than 100% of baseline were used. In addition, cannula placement was confirmed postmortem upon completion of the experiments.
Experimental Design
The ICV treatment was designed as a crossover study. Mice recovered from the surgery for 2 w. Then they were treated with vehicle (2 μL of 5 mM Tris-HCl, pH 8.0) administered to the right lateral cerebral ventricle (n = 14) or the fourth cerebral ventricle (n = 11) and polysomnographic recordings were performed. After 7 days washout, the same animals were injected with leptin (10 μg/2 μL in 5 mM Tris HCl pH 8.0). The dose of leptin was selected according to Bassi et al.10 On the injection day, previously acclimated animals were placed in the whole body plethysmography (WBP) chamber at 10:00, injected with vehicle/leptin at 11:00, and the recordings were performed for 6 h after the injection. After the second recording, animals were sacrificed with exception of six mice cannulated into the lateral ventricle. The latter animals recovered for 1 w, infected with PRV, and then, 72 h after infection, injected with leptin (10 μg/2 μL) into the lateral or fourth ventricle and sacrificed in 45 min for immunohistochemistry.
Mouse Polysomnography
WBP recordings (mouse WBP, Buxco, Wilmington, NC) were performed as previously described.13 In brief, the chamber was designed to record tidal airflow, respiratory effort, and sleep-wake state continuously. We generated a steady biased flow through the mouse chamber by placing mass flow controllers at inflow and outflow ports between positive and negative pressure sources. A slow leak was created to maintain atmospheric chamber pressure. High resistances were placed in series at inflow and outflow ports to make the chamber nearly air-tight, which was required to maintain high fidelity of tidal volume and airflow signals. Respiratory effort was transduced from an air bladder upon which the mouse lay and referenced to an identical bladder below the supporting platform. It was modified to increase the diameter of a port on the top of the chamber to accommodate the passage of EEG/EMG leads to the outside. EEG and EMG signals were acquired by connecting electrodes to a head-stage preamplifier before passing leads through a sealed port in the roof of the chamber.
During full polysomnographic recording sessions, the chamber was humidified to 90% relative humidity, and the mouse was allowed 30 min to acclimate to the chamber before recordings were initiated. All signals were digitized at 1,000 Hz (sampling frequency per channel) and recorded in Lab-Chart 7 Pro (Version 7.2, ADInstruments, Dunedin, NZ). Each mouse recording was evaluated with RemLogic 1.3 (Embla, Ontario, Canada) to determine sleep-wake, ventilatory parameters, and sleep-disordered breathing indices. Respiratory signals were analyzed from all rapid eye movement (REM) sleep periods and from periods of nonrapid eye movement (NREM) sleep sampled periodically throughout the recording. Custom software was used to demarcate the start and end of inspiration and expiration for subsequent calculations of timing and amplitude parameters for each respiratory cycle. Pulse oximetry was monitored throughout the polysomnography recordings with a mouse collar clip (Starr Life Science, Oakmont, PA). Body temperature was measured by a rectal probe.
Sleep-wake state was scored visually in 5-sec epochs from 10:00 until 16:00. Standard criteria were employed to score sleep-wake state based on EEG and EMG frequency content and amplitude, as previously described.16 Wakefulness was characterized by low-amplitude, high-frequency (∼10 to 20 Hz) EEG waves and high levels of EMG activity compared with the sleep states. NREM sleep was characterized by high-amplitude, low frequency (∼2 to 5 Hz) EEG waves with EMG activity considerably less than during wakefulness. REM sleep was characterized by low-amplitude, mixed frequency (∼5 to 10 Hz) EEG waves with EMG amplitude either below or equal to that during NREM sleep.
The instantaneous respiratory rate (RR, breaths/min) was calculated as the reciprocal of the respiratory period, and the instantaneous minute ventilation (VE, mL/min) was the product of the respiratory rate and tidal volume for each breath. The severity of airflow obstruction was defined by the level of maximal inspiratory flow (VImax) at the onset of flow limitation, measured at the point of peak inspiratory airflow.4 We then utilized the airflow and respiratory effort signals to develop an algorithm for detecting upper airway obstruction during sleep. Obstruction was characterized by the development of inspira-tory airflow limitation (IFL), which is the cardinal feature in humans who snore and have OSA.17 IFL was marked by an inspiratory flow plateau at a maximal level, despite continued increases in breathing effort.
The algorithm for detecting IFL was developed based on airflow timing, and amplitude parameters for each respiratory cycle haves been previously described.5 Briefly, IFL was recognized by the presence of a plateau in midrespiratory flow as defined by one of the following criteria: (1) breaths with an early peak of inspiratory flow followed by a plateau or decrease in flow thereafter, (2) a mid-inspiratory plateau of sufficient duration (≥ 20% of inspiratory time between early and late inspiratory peaks in flow), or (3) when the late inspiratory airflow peak exceeded the early peak, these peaks demarcated a change in flow contour from outward to inward convexity. In addition to the IFL, sleep- disordered breathing was characterized by the oxygen desaturation index (ODI). Desaturation events were defined as discernable decreases in oxyhemoglobin saturation by ≥ 3%, 4%, or 5% from the baseline with return to the baseline. The percent of sleep time with oxyhemoglobin desaturation less than 90% (T90) was also measured.
PRV Infection
One week after completion of sleep recordings, mice cannulated into the lateral ventricle were infected with the Bartha strain of PRV (a gift from Drs. Paul J. Reiter and David C. Bloom, University of Florida) for retrograde labeling of respiratory neurons either in the diaphragm (n = 3) or in the genioglossus muscle (n = 3). For diaphragm infection, mice were anesthetized with 1–2% isoflurane, a midline incision of the abdominal wall was performed, and PRV (8.0–9.9 × 108 pfu/ mL, 20 μL) was topically applied to the inferior surface of the right diaphragm as previously described.18 A total volume of 20 μL was applied only to the right side of the diaphragm. The abdominal muscles were sutured and the skin was closed. For genioglossus infection, mice were also anesthetized with 1–2% isoflurane, the genioglossus muscle was exposed unilaterally (the right side) with a ventral approach carefully avoiding all overlying muscles and injected with four to five 100 nL boluses of PRV (3.55 × l07 pfu/mL).19
Immunostaining
Seventy hours after PRV injection, mice were injected with ICV via the lateral ventricle cannula with leptin (10 μg/2 μL). Forty-five minutes after leptin injection, mice were anesthetized and rapidly perfused with ice-cold 4% paraformaldehyde in phosphate buffered saline (PBS). The brains were carefully removed, postfixed in 4% paraformaldehyde for 24 h at 4°C and cryoprotected in 20% sucrose in PBS overnight at 4°C. The next morning, brains were frozen on dry ice and cut into 30-μm-thick coronal sections on a sliding microtome and stored in antifreeze solution at −20°C until further use. The sections were performed via the entire hypothalamus and medulla. Leptin signaling was assessed by immunostaining for phosphorylated signal transducer and activator of transcription 3 (pSTAT3). For pSTAT3 staining, the tissue was pretreated with 1% NaOH and 1% H2O2 in potassium phosphate-buffered saline (KPBS) for 20 min, 0.3% glycine for 10 min, and 0.03% sodium dodecyl sulfate for 10 min. After that, sections were blocked for 2 h with 4% goat serum in KPBS/0.4% TritonX-100. The pSTAT3 antibody was then added (1:100, Cell Signaling Technology, Danvers, MA) and incubated overnight at 4°C. The next day sections were washed, incubated with biotinylated secondary goat antirabbit antibody (Vector, Novi, MI) for 2 h, and then treated with avidin-biotin complex solution for 1 h. Finally, the signal was developed with diaminobenzidine solution, giving a brown precipitate. For PRV staining, brain sections were blocked with 10% goat serum, incubated with anti PRV primary antibody (a gift from Dr. Lynn Enquist, Princeton University, 1:10,000) overnight at 4°C. On the next day, sections were washed at room temperature, incubated with Alexa Fluor 488 goat antirabbit antibody (Invitrogen, Grand Island, NY) for 2 h (1:200). The signals were then checked under confocal microscope (Leica Microsystems CMS GmbH, Mannheim, Germany).
Statistical Analysis
Statistical analysis was designed to test the hypothesis that ICV leptin administered in the lateral and fourth cerebral ventricles would be equally effective in abolishing the upper airway obstruction (IFL breathing) and increasing minute ventilation in nonflow-limited breathing. The analyses in this crossover study were structured to examine responses in mouse characteristics, sleep architecture, and ventilatory parameters as a function of treatment (leptin versus vehicle) within the same animal. The analysis of ventilatory parameters was stratified for separate outcomes for respiratory pump muscles (nonflow-limited breaths) and the upper airway (IFL). The effects of leptin versus vehicle administered via different routes (lateral versus fourth cerebral ventricles) were analyzed with two-way analysis of variance using Minitab 16 (State College, PA) with the Tukey post hoc test for multiple comparisons. The differential effects of leptin administered via different routes were also analyzed with two-way analysis of variance. The differences of between vehicle and leptin treatments were analyzed within the same mice in this crossover study by paired t-test. In all cases, a value of P < 0.05 was considered significant. All data presented in the text, tables, and figures represent mean ± standard error of the mean.
RESULTS
Basic Characteristics
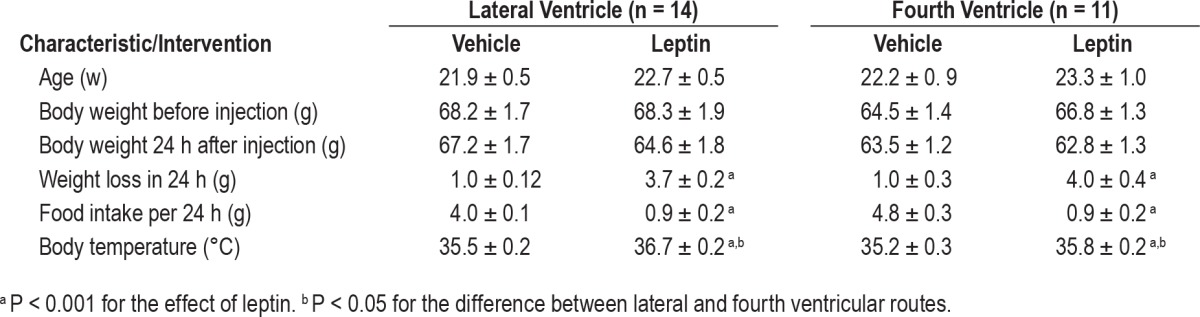
Ob/ob mice had similar body weight and temperature in all treatment groups at baseline (Table 1). Leptin administration ICV suppressed food intake, raised body temperature by 1–1.4°C and induced acute weight loss of 3.5–4 g (P < 0.001, Table 1) compared to the vehicle group, and the effects of leptin were identical for both lateral and fourth ventricle injections.
Table 1.
Basic characteristics of ob/ob mice treated with intracerebroventricular injections of leptin or vehicle to lateral or fourth ventricles.
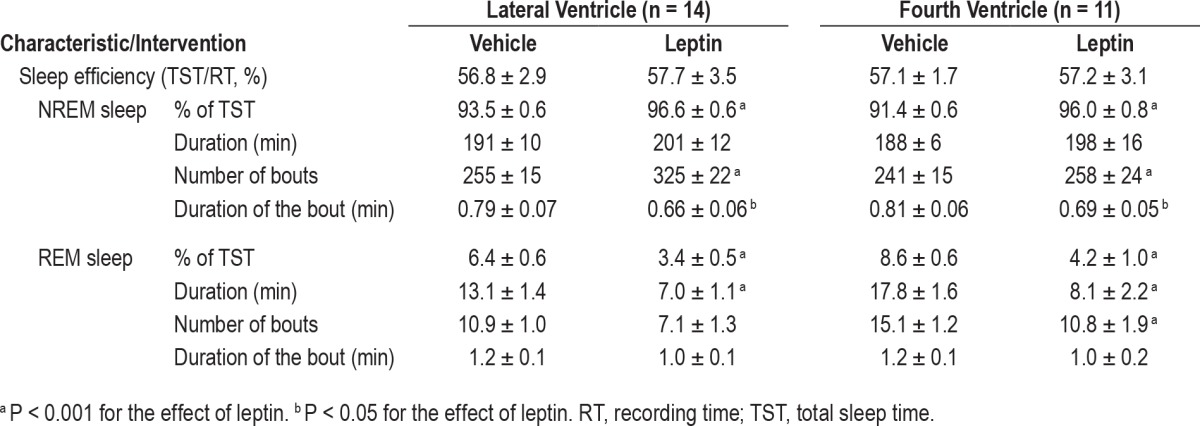
Effects of ICV Leptin on Sleep Architecture
Leptin treatment did not affect sleep efficiency which averaged between 56% and 58% in all treatment groups (Table 2). Leptin significantly increased the percent of time spent in NREM sleep for both routes of administration (Table 2). The hormone increased the total number of NREM sleep bouts, especially in the lateral ventricle treatment group, whereas the duration of NREM bouts was reduced. Leptin ICV decreased REM sleep time, reducing the duration of REM sleep bouts for both routes and decreasing the number of REM sleep bouts in the fourth ventricle treatment group (Table 2).
Table 2.
Sleep parameters of ob/ob mice treated with intracerebroventricular injections of leptin or vehicle to lateral or fourth ventricles and recorded for 6 h of the light phase (10:00–16:00).
Effects of ICV Leptin on Nonflow-Limited Breathing in Sleeping Mice
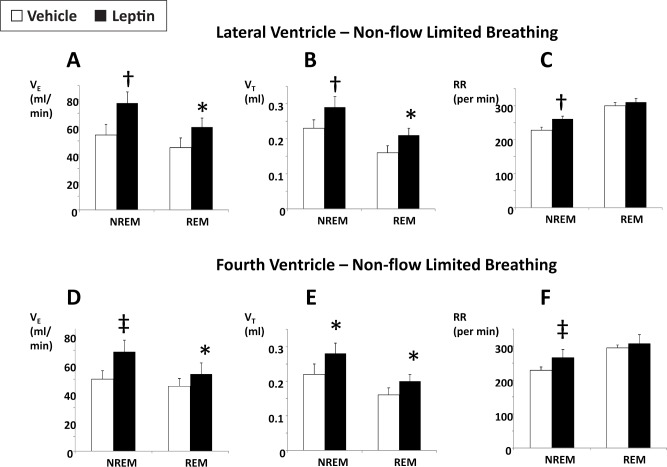
Figure 1 illustrates the effect of leptin on nonflow-limited breathing in NREM and REM sleep, summarizing the data over the 6-h observation period. Leptin increased minute ventilation both during NREM and REM sleep, increasing tidal volumes during both sleep stages, whereas respiratory rate was increased during NREM but not REM sleep. The effects of leptin on nonflow- limited breathing were identical for the lateral and fourth ventricle routes of administration (Figure 1).
Figure 1.
The effects of intracerebroventricular administration of leptin (10 μg/2 μL in 5 mM Tris HCl) or vehicle (2 μL of 5 mM Tris HCl) on minute ventilation (VE), tidal volume (VT), and respiratory rate (RR) during nonflow-limited breathing in nonrapid eye movement (NREM) and rapid eye movement (REM) sleep. (A–C) Lateral ventricle administration. (D–F) Fourth ventricle administration. *, †, and ‡, P < 0.05, < 0.01, and < 0.001, respectively, for the effect of leptin.
Effects of ICV Leptin on Flow-Limited Breathing in Sleeping Mice
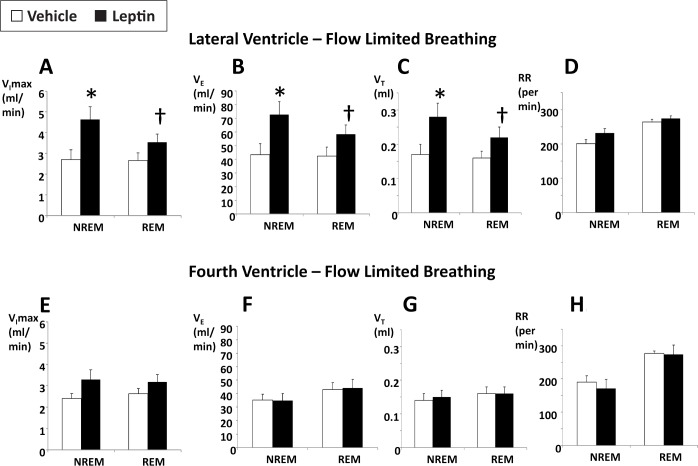
IFL was infrequently observed in NREM sleep with only 3.7 ± 1.5% and 2.8 ± 1.8% breaths being flow limited in the lateral ventricle-vehicle and fourth ventricle-vehicle groups, respectively. The frequency of flow limitation was much higher in REM sleep, 36.1 ± 6.4% and 32.4 ± 5.6% breaths in the lateral ventricle-vehicle and fourth ventricle-vehicle groups, respectively. Leptin treatment did not influence the frequency of IFL regardless of the administration route (not shown). However, the severity of flow limitation depended on the route of leptin administration (P < 0.05 for the effect of the route and the route-flow limitation interaction). Leptin administration to the lateral ventricle significantly increased the maximal inspiratory flow at the onset of flow limitation (VImax), both in NREM and REM sleep (Figure 2A), compared to the vehicle treatment within the same animal, indicating reductions in flow limitation severity and improvements in upper airway patency. Leptin administration to the lateral ventricle also increased minute ventilation during IFL, from 43.4 ± 8.2 mL/ min in vehicle-treated mice to 72.7 ± 9.7 mL/min in NREM sleep (P < 0.05) and from 42.5 ± 6.6 mL/min in vehicle-treated mice to 58.4 ± 6.7 mL/min in REM sleep (P < 0.01, Figure 2B). Its effect on ventilation during flow-limited breathing was entirely related to increases in tidal volume rather than respiratory rate, which was consistent with alleviation of upper airway obstruction (Figure 2C and 2D). Leptin's effects on minute ventilation during flow- limited breathing differed significantly between lateral and fourth ventricle routes (P < 0.001 in NREM sleep and P < 0.01 in REM sleep). Leptin administration to the fourth ventricle had no effect on VImax (Figure 2E), indicating no change in the severity of IFL, and had no effect on minute ventilation or tidal volumes during IFL (Figure 2F–2H).
Figure 2.
The effects of intracerebroventricular administration of leptin (10 μg/2 μL in 5 mM Tris HCl) or vehicle (2 μL of 5 mM Tris HCl) on maximal inspiratory flow (VImax), minute ventilation (VE), tidal volume (VT), and respiratory rate (RR) during flow-limited breathing in nonrapid eye movement (NREM) and rapid eye movement REM sleep. (A–D) Lateral ventricle administration. (E–H) Fourth ventricle administration. * and †, P < 0.05 and < 0.01, respectively, for the effect of leptin.
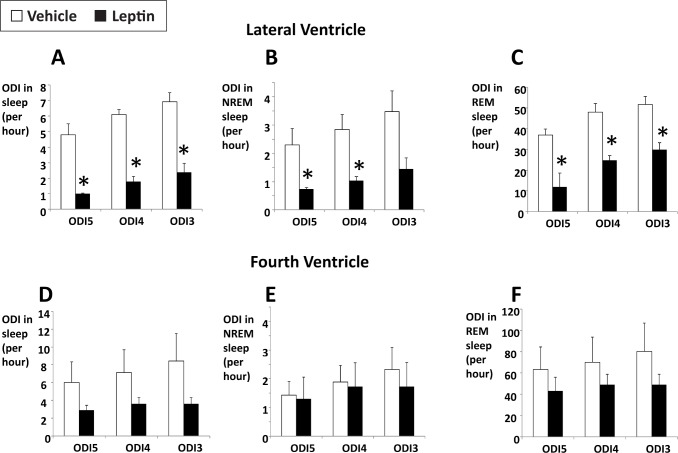
Severe IFL resulted in recurrent obstructive hypopneas, particularly in REM sleep (Figure 3). A representative polysomnography (Figure 3) demonstrates inspiratory flow limitation (left panel) with early plateaus in inspiratory airflow, reductions in tidal volume, and increased respiratory effort, which were not present during nonflow-limited breaths. IFL breathing was associated with recurrent severe oxyhemoglobin desaturations (Figure 3, right panel). Of note, the baseline saturation during REM sleep was also low, which was likely attributable to obesity hypoventilation previously reported in ob/ob mice.2 In vehicle-treated mice, the percentage of time spent with oxygen saturation (SpO2) < 90% (T90) was 16.0 ± 6.3%. Recurrent oxyhemoglobin desaturations were observed in association with IFL in vehicle-treated mice, resulting in an ODI of 6.9 ± 0.7, 6.1 ± 0.6, and 4.8 ± 0.7/h of total sleep time for desatu-rations ≥ 3%, ≥ 4%, and ≥ 5%, respectively (Figure 4A). These events were relatively uncommon in NREM sleep (Figure 4B). In contrast, the desaturations were frequent in REM sleep with an ODI of 51.7 ± 3.9, 48.1 ± 4.2, and 36.9 ± 3.0/h for desatu-rations ≥ 3%, ≥ 4%, and ≥ 5%, respectively (Figure 4C). As observed for flow- limited breathing (Figure 2), leptin administration to the lateral cerebral ventricle abolished intermittent hypoxemia in NREM sleep and significantly attenuated intermittent hypoxemia in REM sleep (Figure 4A–4C). In contrast, leptin administration to the fourth ventricle did not decrease the ODI (Figure 4D–4F). T90 was not affected by any route of leptin administration (not shown).
Figure 3.
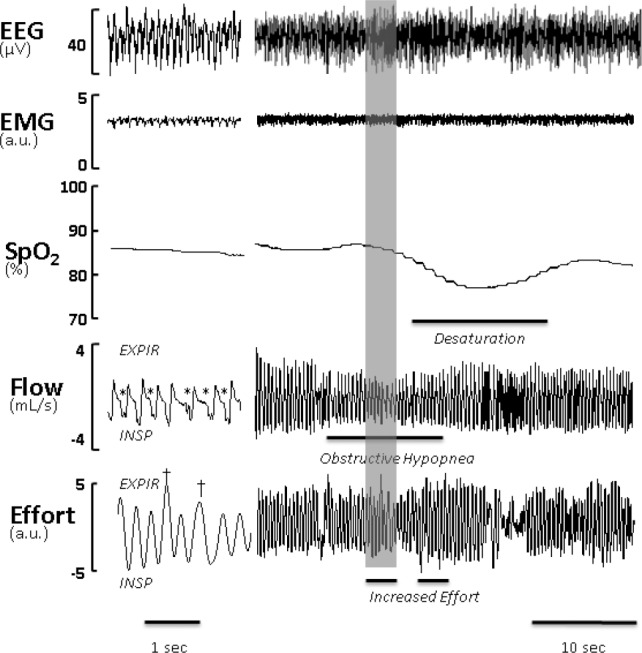
A representative trace of rapid eye movement sleep in the ob/ob mouse treated with vehicle (2 μl of 5 mM Tris-HCl, pH 8.0). Right panel, Compressed recording of electroencephalogram (EEG), nuchal electromyogram (EMG), neck pulse oximetry (SpO2), respiratory flow and effort are shown. Obstructive hypopnea was defined as a discrete period of reduced airflow due to inspiratory limitation flanked by nonflow-limited breaths accompanied by increased effort and oxyhemoglobin desaturation > 3%. Left panel, The shaded area is decompressed. EXPIR denotes expiration and INSP denotes inspiration. Flow limited breaths are denoted with *, and increased effort is labeled with †. a.u., arbitrary units.
Figure 4.
The effects of intracerebroventricular administration of leptin (10 μg/2 μL in 5 mM Tris HCl) or vehicle (2 μL of 5 mM Tris HCl) on the oxygen desaturation index (ODI) with oxyhemoglobin desaturations of ≥ 3%, 4%, and 5% (ODI3, ODI4, and ODI5 respectively) during total sleep time, nonrapid eye movement (NREM), and rapid eye movement (REM) sleep. (A–C) Lateral ventricle administration. (D–F) Fourth ventricle administration. *P < 0.05 for the effect of leptin.
Leptin Signaling in the Brain and Respiratory Motoneurons
Leptin signaling via the ObRb receptor was examined in the medulla and hypothalamus by phosphorylation of STAT3 in response to leptin administration to the lateral ventricle. Numerous positive pSTAT3 nuclei were noted in the hypothalamus, especially in the arcuate nucleus and dorsomedial hypothalamus (DMH, Figure 5A). In the medulla, the most abundant pSTAT3 positive nuclei were located in the nucleus of the solitary tract (NTS, Figure 5B), occasional pSTAT3 positive nuclei were noted in the pre-Bötzinger complex and the retrotrapezoid nucleus (not shown), whereas no positive staining was detected in the hypoglossal nucleus (XII, Figure 5B).
Figure 5.
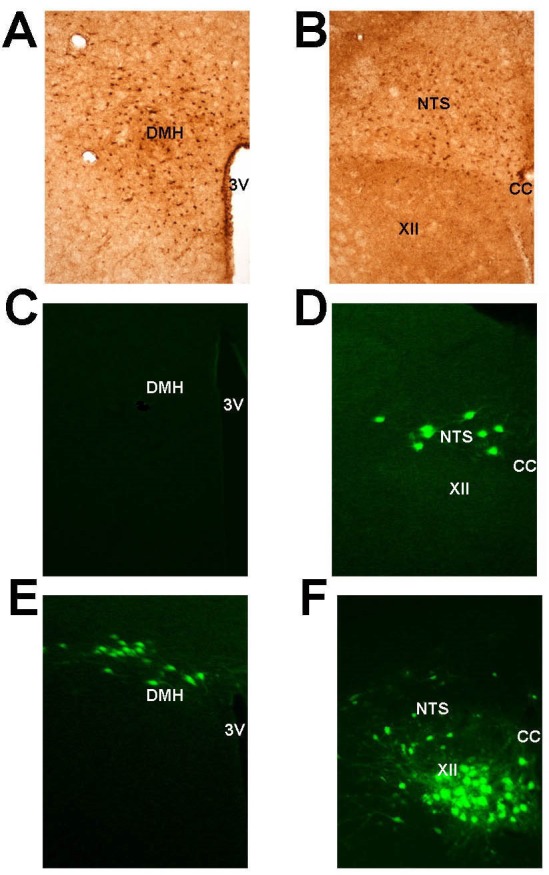
Relationships between leptin signaling and respiratory neurons in the brain. The dorsomedial hypothalamus (DMH) (A) and the nucleus of the tractus solitarius (NTS) (B) show positive leptin signaling determined by dark brown staining of the neuron nuclei for phosphorylated signal transducer and activator of transcription 3 (STAT3) 40 min after leptin administration to the lateral ventricle. The Bartha strain of pseudorabies virus (PRV) was topically applied to the inferior surface of the diaphragm (C,D) or injected in the genioglossus muscle (E,F) and neurons involved in respiratory control or the upper airway function were detected in the DMH (C,E) or the medulla (D,F) by positive immunofluorescence of PRV 72 h after injection. CC, corpus callosum; XII, hypoglossal nucleus; 3V, third ventricle.
PRV was applied to either the diaphragm or genioglossus muscle in order to track CNS projections of respiratory “pump” or upper airway motoneurons, respectively. After PRV treatment mice rapidly recovered, demonstrating normal food intake and grooming behavior prior to sacrifice. Diaphragmatic application did not result in any significant positive PRV staining in any part of the hypothalamus (Figure 5C), but did show multiple PRV-infected neurons in the NTS (Figure 5D). As expected, genioglossus infection resulted in numerous PRV positive neurons in the hypoglossal nucleus (XII nerve) and scattered neuronal staining in the NTS. However, in contrast to inoculation of the diaphragm, multiple positively stained neurons were also noted in the hypothalamus, specifically DMH (Figure 5E and 5F).
DISCUSSION
The goal of our study was to examine CNS effects of leptin on OSA and OHS in a mouse model. We improved our polysomnographic recording methods with the addition of continuous pulse oximetry and demonstrated that recurrent hypopneas with oxyhemoglobin desaturations develop in leptin-deficient obese mice during sleep, which indicated that the ob/ob mouse models human OSA in addition to OHS. Capitalizing on unidirectional rostral-caudal cerebrospinal fluid flow, we localized the effects of leptin on ventilatory and upper airway motor control. Our main novel finding was that leptin decreased the severity of upper airway obstruction during sleep through its effect on hypothalamic rather than medullary centers. This conclusion is based on the findings that (1) leptin administration to the lateral cerebral ventricle reversed upper airway obstruction during sleep, whereas leptin administration to the fourth ventricle had no effect; and (2) upper airway motoneurons projected to the DMH, which showed robust leptin receptor signaling. In contrast, leptin stimulated ventilation through medullary centers, because (1) leptin administration to both lateral and fourth cerebral ventricles was effective in treating obesity hypoventilation when upper airway obstruction was absent; and (2) respiratory motoneurons projected to regions of the NTS expressing robust leptin receptor signaling, but not to the hypothalamus. In the discussion in the next paragraphs, we elaborated on the mechanisms by which leptin impacts respiratory control, as well as the clinical implications of our work.
Leptin Mechanisms of Action Differs Between Flow-Limited and Nonflow-Limited Breathing During Sleep
OSA with oxyhemoglobin desaturations have been previously described in the English bulldog20 and obese Yucatan mini-pigs.21 However, these animal models are expensive and have the diverse genetic background lacking advantages of rodent models with the well-characterized genotype and availability of inbred and transgenic strains. Previous work in rodent models showed that obese Zucker rats and New Zealand obese mice exhibit adiposity of the upper airway structures22,23 similar to patients with OSA. We have invented a novel system for characterizing sleep and breathing patterns in mice13 and demonstrated that, similar to human OSA, obese ob/ob mice exhibit two types of respiratory dynamics during asleep, IFL, and nonflow-limited breathing (Figure 3).5 During IFL, dynamic collapse of the upper airway resulted in the inspiratory flow plateau (see * breaths, Figure 3) as effort continued to increase, and tracheal pressure swings were observed.13 Our data demonstrated that leptin-deficient obese mice exhibit classic features of OSA, which include recurrent episodes of inspira-tory flow limitation, increased effort and intermittent oxyhemoglobin desaturations (Figure 3).
Neuromuscular reflexes play a protective role in preventing IFL in obese humans and in mice. We have previously reported that leptin modulates these neuromuscular reflexes attenuating IFL in anesthetized and sleeping mice.4,5 As in our previous study,5 the current data on ob/ob mice showed that (1) IFL was predominant during REM sleep when muscular tone was decreased; (2) IFL was absent during wakefulness; and (3) leptin relieved upper airway obstruction (increased VImax); but (4) leptin repletion did not affect the percent of IFL breaths or degree of oxyhemoglobin desaturation (T90). These findings suggested that passive upper airway properties in severely obese mice predisposed to IFL and obstructive hypopneas, and that leptin increased airway patency acting on neuromuscular control rather than anatomic characteristics (Figures 2A–2D and 4A–4C).5
In contrast to IFL, nonflow-limited breathing is a function of the metabolic rate and ventilatory control. We had previously shown that ob/ob mice had defects in hypercapnic sensitivity and ventilatory drive during sleep,2,5 similar to that observed in patients with OHS, and that systemic leptin replacement corrected these defects augmenting minute ventilation during NREM and REM sleep.5 In the current study, we demonstrated that nonflow-limited breathing was augmented similarly by acute ICV administration of leptin into the lateral and fourth ventricle (Figure 1), whereas IFL and OSA were treated effectively only by administration of the hormone to the lateral cerebral ventricle (Figures 2 and 4). These data suggest that leptin acts in the CNS to alleviate obesity hypoventilation and OSA, and that medullary and hypothalamic centers mediate leptin responses to ventilatory control and upper airway dys-function, respectively.
Effects of Leptin on the Upper Airway
Acute ICV administration of leptin to the lateral ventricle significantly increased maximal inspiratory flow at the onset of flow limitation (Figure 2A), and this suggests that leptin protects the upper airway patency during sleep, preventing the inspiratory collapse.4,24 The sites of leptin action on the upper airway are located rostral to the medulla, because fourth ventricle injections of leptin had no effect on VImax (Figure 2E). We measured leptin ObRb receptor signaling by determining STAT3 phosphorylation in response to leptin. Our experiments demonstrated the highest ObRb receptor activity in the arcuate nucleus of the hypothalamus and in the dorsomedial hypothalamus (Figure 5A), which was consistent with findings of other investigators.25,26 We examined relationships between motoneurons innervating pharyngeal muscles and leptin signaling in the same mice by infecting the genioglossal muscle with PRV, which spreads retrogradely between synaptically connected neurons.18,27 The only PRV positive neurons rostral to the medulla were in the DMH (Figure 5E). Our findings may indicate that leptin maintains upper away patency during sleep by acting in the DMH.
Several neurotransmitters could mediate leptin's action on upper airway control in the DMH.
Leptin receptor positive neurons in DMH control energy expenditure by releasing prolactin-releasing peptide,28 cocaine- and amphetamine-regulated transcript, and galanin.29 Of note, DMH ObRb receptor positive neurons do not contain other metabolically active peptides as neuropeptide Y, melanin-concentrating hormone, orexin, or neurotensin, which are regulated by leptin in other brain nuclei.8,29,30 DMH stimulation is known to increase respiratory drive,31,32 but, to our knowledge, we have provided the first evidence that leptin signaling in DMH may be involved in controlling upper airway function during sleep. Leptin-signaling neurons in DMH may project to the XII nucleus releasing specific mediators affecting hypoglossal motoneurons, such as galanin, which is widely distributed in the hypoglossal nucleus.33,34 Overall, our data suggest that leptin relieves OSA through specific ObRb positive neurons in the forebrain, possibly in the DMH, but additional studies targeting specific populations of ObRb positive neurons in the hypothalamus are needed for precise localization of the leptin's effect on the upper airway.
Effects of Leptin on Ventilatory Control
Our current data demonstrated that leptin injections to lateral and fourth cerebral ventricles were equally effective in augmenting minute ventilation during sleep in the absence of the upper airway obstruction (Figure 1), which suggested that leptin could modulate ventilation through effects on both hypothalamic and medullary centers. Leptin ObRb receptor-mediated pSTAT3 signaling was abundantly present in both the hypothalamus and the NTS35,36 (Figure 5A and 5B). However, respiratory motoneurons innervating the diaphragm projected to the NTS, but not to the hypothalamus or any other areas in the forebrain (Figure 5C and 5D). Given that leptin administration to the NTS is known to increase minute ventilation dramatically,11 our data suggest that the NTS is a putative site of leptin action on ventilatory control during sleep, possibly by triggering proopiomelanocortin pathways.15,37
Limitations
Our study had several limitations. First, sleep and breathing were recorded during a relatively short period of time. Our primary goal was to explore acute effects of leptin on sleep-disordered breathing rather than the sleep/wake cycle and sleep architecture, and short duration of recording was adequate to achieve sufficient NREM and REM sleep to fulfill our goal. Second, our control mice showed relatively low sleep efficiency for the light phase and reduced REM sleep.38 Mice were chronically implanted with head mounts and ICV cannulas and treated with 2 μL of solution ICV, all of which could disrupt sleep. However, our placebo-controlled crossover design allowed us to identify independent effects of leptin. Third, we did not randomize mice; vehicle was always injected first and leptin second. This was done because leptin induced significant weight loss and effects of leptin lingered for several days. Nevertheless, dramatic differences in responses to leptin during flow-limited breathing between different routes of administration indicate that the effects were specific to leptin rather than the order of administration. Fourth, leptin suppressed REM sleep. This effect of leptin has been previously described in rats.39 Effects of leptin on sleep- disordered breathing were determined separately for NREM and REM sleep and could not be influenced by the stage duration. Fifth, neurons mediating leptin's effects on the upper airway function and ventilatory control have not been precisely localized. Nevertheless, we identified putative sites of leptin action, the DMH for the upper airway, and the NTS for ventilatory control. Specific interventions can be designed in the near future to inactivate or overexpress leptin receptors in these areas using Cre-recombinase technology and viral vector delivery.
Implications and Conclusions
Our study has important clinical implications for the understanding and treatment of sleep- disordered breathing. Although human obesity is predominantly characterized by leptin resistance rather than leptin deficiency,40 leptin resistance is associated with reduced leptin permeability of the blood-brain barrier,41,42 leading to relative CNS leptin deficiency in obese humans.42 Our data suggest that leptin can be tested for treatment of OHS and OSA in the population of obese patients with relative leptin deficiency.43,44 Targeting leptin resistance and pathways downstream of leptin in the DMH and NTS represents a novel approach to treat OHS and OSA in leptin-resistant patients.
In summary, our findings suggest that leptin exerts effects on upper airway and diaphragmatic control at distinct CNS loci. Leptin relieves upper airway obstruction in sleep apnea acting in the forebrain, possibly in the dorsomedial hypothalamus. In contrast, leptin's effect on respiratory pump muscles is mediated primarily in the hindbrain, possibly in the NTS, reversing hypoventilation in human and murine obesity.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by the following grants: NIH R01 HL080105, R01 HL050381, and R01 HL128970. Dr. Moran has consulted for Novo Nordisk and Healthways. Dr. Kirkness has received research support from ResMed, has consulted for Insleep Technologies, and reports intellectual property rights for respEQ Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Paul J. Reiter and Dr. David C. Bloom (University of Florida, Gainesville, FL) for their generous gift of the Bartha strain of pseudorabies virus (PRV) and to Dr. Lynn Enquist (Princeton University) for his gift of anti-PRV antibody.
REFERENCES
- 1.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5:185–92. doi: 10.1513/pats.200708-137MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Donnell CP, Schaub CD, Haines AS, et al. Leptin prevents respiratory depression in obesity. Am J Resp and Crit Care Med. 1998;159:1477–84. doi: 10.1164/ajrccm.159.5.9809025. [DOI] [PubMed] [Google Scholar]
- 3.Tankersley C, Kleeberger S, Russ B, Schwartz A, Smith P. Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol. 1996;81:716–23. doi: 10.1152/jappl.1996.81.2.716. [DOI] [PubMed] [Google Scholar]
- 4.Polotsky M, Elsayed-Ahmed AS, Pichard L, et al. Effects of leptin and obesity on the upper airway function. J Appl Physiol. 2012;112:1637–43. doi: 10.1152/japplphysiol.01222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pho H, Hernandez AB, Arias RS, et al. The effect of leptin replacement on sleep disordered breathing in the leptin-deficient ob/ob mouse. J Appl Physiol. 2016;120:78–86. doi: 10.1152/japplphysiol.00494.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wauman J, Tavernier J. Leptin receptor signaling: pathways to leptin resistance. Front Biosci. 2011;16:2771–93. doi: 10.2741/3885. [DOI] [PubMed] [Google Scholar]
- 7.Hileman SM, Pierroz DD, Masuzaki H, et al. Characterizaton of short isoforms of the leptin receptor in rat cerebral microvessels and of brain uptake of leptin in mouse models of obesity. Endocrinology. 2002;143:775–83. doi: 10.1210/endo.143.3.8669. [DOI] [PubMed] [Google Scholar]
- 8.Leinninger GM, Jo YH, Leshan RL, et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–7. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 10.Bassi M, Giusti H, Leite CM, et al. Central leptin replacement enhances chemorespiratory responses in leptin-deficient mice independent of changes in body weight. Pflugers Arch. 2012;464:145–53. doi: 10.1007/s00424-012-1111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inyushkina EM, Merkulova NA, Inyushkin AN. Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol. 2010;407:707–13. doi: 10.1007/s11055-010-9316-2. [DOI] [PubMed] [Google Scholar]
- 12.Boulton M, Young A, Hay J, et al. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol. 1996;22:325–33. doi: 10.1111/j.1365-2990.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez AB, Kirkness JP, Smith PL, et al. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol. 2012;112:671–80. doi: 10.1152/japplphysiol.00818.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews EC, Rowland NE. Role of angiotensin in body fluid homeostasis of mice: effect of losartan on water and NaCl intakes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R638–44. doi: 10.1152/ajpregu.00525.2004. [DOI] [PubMed] [Google Scholar]
- 15.De Jonghe BC, Hayes MR, Zimmer DJ, Kanoski SE, Grill HJ, Bence KK. Food intake reductions and increases in energetic responses by hindbrain leptin and melanotan II are enhanced in mice with POMC-specific PTP1B deficiency. Am J Physiol Endocrinol Metab. 2012;303:E644–51. doi: 10.1152/ajpendo.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagaito Y, Polotsky VY, Campen MJ, et al. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol. 2001;9:2758–66. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- 17.Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991;143:1300–3. doi: 10.1164/ajrccm/143.6.1300. [DOI] [PubMed] [Google Scholar]
- 18.Qiu K, Lane MA, Lee KZ, Reier PJ, Fuller DD. The phrenic motor nucleus in the adult mouse. Exp Neurol. 2010;226:254–8. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mack SO, Wu M, Kc P, Haxhiu MA. Stimulation of the hypothalamic paraventricular nucleus modulates cardiorespiratory responses via oxytocinergic innervation of neurons in pre-Botzinger complex. J Appl Physiol. 2007;102:189–99. doi: 10.1152/japplphysiol.00522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in English bulldogs. Am Rev Respir Dis. 1993;148:185–94. doi: 10.1164/ajrccm/148.1.185. [DOI] [PubMed] [Google Scholar]
- 21.Lonergan RP, Ware JC, Atkinson RL, Winter WC, Suratt PM. Sleep apnea in obese miniature pigs. J Appl Physiol. 1998;84:531–6. doi: 10.1152/jappl.1998.84.2.531. [DOI] [PubMed] [Google Scholar]
- 22.Brennick MJ, Pack AI, Ko K, et al. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med. 2009;179:158–69. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennick MJ, Delikatny J, Pack AI, et al. Tongue fat infiltration in obese versus lean zucker rats. Sleep. 2014;37:1095–102. doi: 10.5665/sleep.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polotsky M, Elsayed-Ahmed AS, Pichard L, et al. Effect of age and weight on upper airway function in a mouse model. J Appl Physiol. 2011;111:696–703. doi: 10.1152/japplphysiol.00123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson CM, Leshan RL, Jones JC, Myers MG., Jr Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28. doi: 10.1016/j.brainres.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–47. [PubMed] [Google Scholar]
- 27.Ugolini G. Advances in viral transneuronal tracing. J Neurosci Methods. 2010;194:2–20. doi: 10.1016/j.jneumeth.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Dodd GT, Worth AA, Nunn N, et al. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 2014;20:639–49. doi: 10.1016/j.cmet.2014.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laque A, Zhang Y, Gettys S, et al. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am J Physiol Endocrinol Metab. 2013;304:E999–1011. doi: 10.1152/ajpendo.00643.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi S. Dorsomedial hypothalamic NPY modulation of adiposity and thermogenesis. Physiol Behav. 2013;121:56–60. doi: 10.1016/j.physbeh.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horiuchi J, McDowall LM, Dampney RA. Vasomotor and respiratory responses evoked from the dorsolateral periaqueductal grey are mediated by the dorsomedial hypothalamus. J Physiol. 2009;587:5149–62. doi: 10.1113/jphysiol.2009.179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDowall LM, Horiuchi J, Dampney RA. Effects of disinhibition of neurons in the dorsomedial hypothalamus on central respiratory drive. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1728–35. doi: 10.1152/ajpregu.00503.2007. [DOI] [PubMed] [Google Scholar]
- 33.Perez SE, Wynick D, Steiner RA, Mufson EJ. Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol. 2001;434:158–85. doi: 10.1002/cne.1171. [DOI] [PubMed] [Google Scholar]
- 34.Palkovits M, Horvath S. Galanin immunoreactive neurons in the medulla oblongata of rats. Acta Biol Hung. 1994;45:399–417. [PubMed] [Google Scholar]
- 35.Ciriello J, Moreau JM. Leptin signaling in the nucleus of the solitary tract alters the cardiovascular responses to activation of the chemoreceptor reflex. Am J Physiol Regul Integr Comp Physiol. 2012;303:R727–36. doi: 10.1152/ajpregu.00068.2012. [DOI] [PubMed] [Google Scholar]
- 36.Mercer JG, Moar KM, Hoggard N. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology. 1998;139:29–34. doi: 10.1210/endo.139.1.5685. [DOI] [PubMed] [Google Scholar]
- 37.Bassi M, Nakamura NB, Furuya WI, et al. Activation of the brain melanocortin system is required for leptin-induced modulation of chemorespiratory function. Acta Physiol (Oxf) 2015;213:893–901. doi: 10.1111/apha.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polotsky VY, Rubin AE, Balbir A, et al. Intermittent hypoxia causes REM sleep deficits and decreases EEG delta power in NREM sleep in the C57BL/6J mouse. Sleep Med. 2006;7:7–16. doi: 10.1016/j.sleep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. J Sleep Res. 1999;8:197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 40.Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–500. doi: 10.1152/ajpregu.90669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. Am J Physiol Endocrinol Metab. 2003;285:E10–5. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte DJ. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–93. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 43.Farooqi IS, Keogh JM, Kamath S, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro SD, Chin CH, Kirkness JP, et al. Leptin and the control of pharyngeal patency during sleep in severe obesity. J Appl Physiol. 2014;116:1334–41. doi: 10.1152/japplphysiol.00958.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]