Abstract
Study Objectives:
Environmental noise exposure disturbs sleep and impairs recuperation, and may contribute to the increased risk for (cardiovascular) disease. Noise policy and regulation are usually based on average responses despite potentially large inter-individual differences in the effects of traffic noise on sleep. In this analysis, we investigated what percentage of the total variance in noise-induced awakening reactions can be explained by stable inter-individual differences.
Methods:
We investigated 69 healthy subjects polysomnographically (mean ± standard deviation 40 ± 13 years, range 18–68 years, 32 male) in this randomized, balanced, double-blind, repeated measures laboratory study. This study included one adaptation night, 9 nights with exposure to 40, 80, or 120 road, rail, and/or air traffic noise events (including one noise-free control night), and one recovery night.
Results:
Mixed-effects models of variance controlling for reaction probability in noise-free control nights, age, sex, number of noise events, and study night showed that 40.5% of the total variance in awakening probability and 52.0% of the total variance in EEG arousal probability were explained by inter-individual differences. If the data set was restricted to nights (4 exposure nights with 80 noise events per night), 46.7% of the total variance in awakening probability and 57.9% of the total variance in EEG arousal probability were explained by inter-individual differences. The results thus demonstrate that, even in this relatively homogeneous, healthy, adult study population, a considerable amount of the variance observed in noise-induced sleep disturbance can be explained by inter-individual differences that cannot be explained by age, gender, or specific study design aspects.
Conclusions:
It will be important to identify those at higher risk for noise induced sleep disturbance. Furthermore, the custom to base noise policy and legislation on average responses should be re-assessed based on these findings.
Citation:
McGuire S, Müller U, Elmenhorst EM, Basner M. Inter-individual differences in the effects of aircraft noise on sleep fragmentation. SLEEP 2016;39(5):1107–1110.
Keywords: noise, sleep, arousal, awakening, health, trait, ICC
Significance.
Sleep hygiene is a prerequisite for sufficient good quality sleep. Environmental noise is ubiquitous, disturbs sleep and impairs recuperation. This polysomnographic study demonstrates large individual differences in the degree of noise-induced sleep fragmentation despite a relatively homogeneous, healthy, and adult study population. It will thus be important to identify biomarkers of susceptibility for noise-induced sleep disturbance. These markers will help develop mitigation and therapeutic strategies. At the same time, noise policy needs to reflect the large individual differences in the susceptibility to noise-induced sleep disturbance. It may not be enough simply protecting an “average” sleeper.
INTRODUCTION
Environmental noise exposure disturbs sleep, impairs sleep recuperation, and may contribute to long-term negative health consequences.1,2 Noise policy and regulation are usually based on average responses, although anecdotal reports suggest substantial inter-individual differences in the effects of traffic noise on sleep even in relatively homogeneous and healthy populations (Figure 1A).3 Sleep spindles have been identified as a potential biomarker of the susceptibility of the sleeping organism to noise intrusions.4 However, the magnitude of inter-individual differences in susceptibility to noise-induced sleep disturbance relative to within-subject variability observed during repeated exposure to noise has not been systematically studied. In this analysis, we investigated what percentage of the total variance of noise-induced awakenings and EEG arousals can be explained by stable inter-individual differences.
Figure 1.
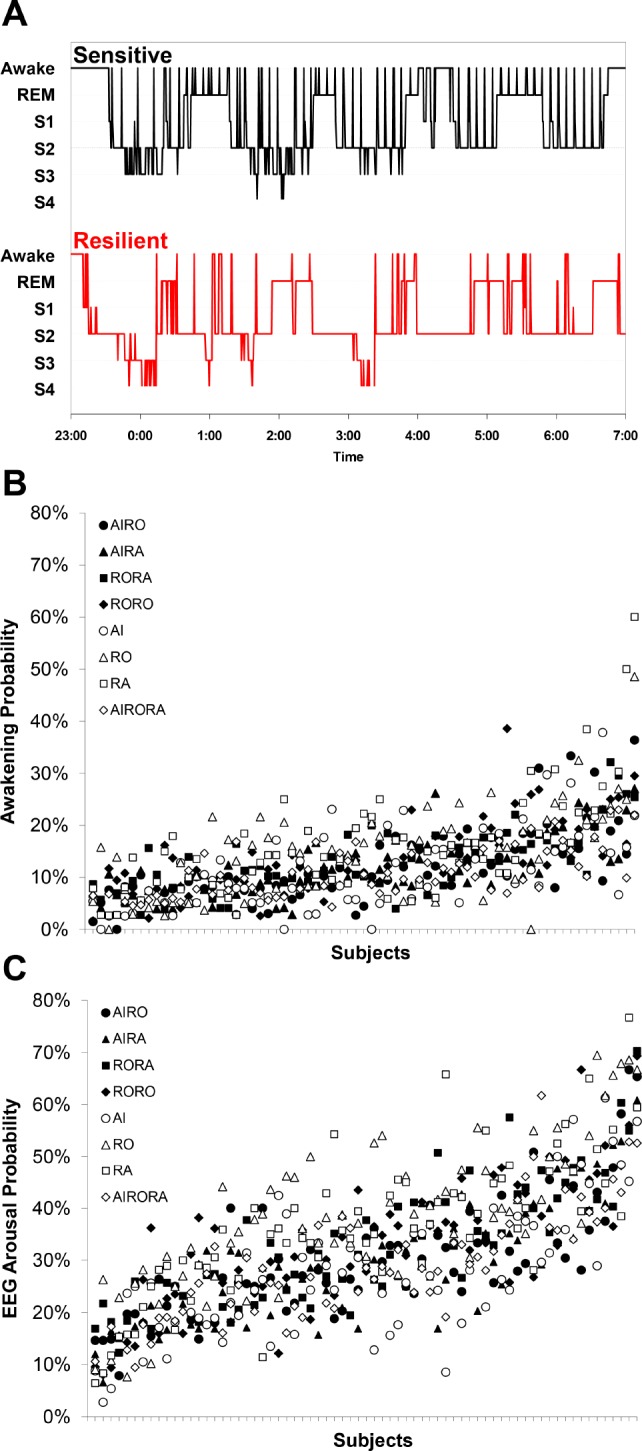
(A) Hypnograms of the most sensitive (black, above) and the most resilient (red, below) subject exposed to 64 aircraft noise events with maximum sound pressure levels of 65 dB(A) are shown. The most sensitive subject woke up with a probability of 88%, whereas the most resilient subject woke only up in 3.3% of aircraft noise events. Adopted from Basner et al.3 (B,C) Average awakening (B) and average EEG arousal (C) probability are shown for each of the 69 subjects and for each of the 8 exposure nights (represented by different symbols; AI, aircraft noise; RO, road traffic noise; RA, rail noise). The subjects are ordered from smallest (left) to largest (right) average reaction probability. The variance explained by stable inter-individual differences varied between 39.1% to 48.4% for awakenings and 50.3% to 65.3% for EEG arousals depending on model and data set.
METHODS
Between 2004 and 2006, we performed a laboratory study on single and combined effects of road, rail, and aircraft noise on sleep in the sleep laboratory of the DLR-Institute of Aerospace Medicine in Cologne, Germany.5 We investigated 69 healthy subjects (mean ± SD 39.7 ± 13.2 years, range 18–68 years, 32 male) for 11 consecutive nights. Subjects were medically screened and without relevant hearing impairment as established via audiometry. Night 1 served as adaptation and night 11 as recovery. Nine different noise scenarios (including one noise-free control night) were played back during exposure nights 2 to 10. Three subjects (3/72 = 4.2%) were excluded from this analysis because they reported pain or nausea during the noise-free control night. Traffic noise events were recorded with class 1 sound level meters in the bedroom of residents living close to a road, railway, or airport. Each sleep cabin was acoustically calibrated to assure accurate playback of recorded noise events. Playback of each noise event started at the beginning of a 30-s sleep epoch. There were 9 different noise scenarios with single, double, and triple exposure nights. The 3 single-exposure nights each consisted of 40 noise events from one traffic mode only, i.e., aircraft (AI), road (RO), or rail (RA). Noise events belonged to 1 of 5 maximum sound pressure level (SPL) categories: 45, 50, 55, 60, or 65 dB. SPLs were A-weighted with the time constant set to slow. Single exposure nights consisted of 8 noise events from each of the SPL categories. There were 3 double exposure nights: Aircraft plus road noise (AIRO), aircraft plus rail noise (AIRA), and road plus rail noise (RORA). Each of the double exposure nights consisted of 40 noise events from both of the respective single exposure nights, i.e., 80 noise events in total. There was one triple exposure night (AIRORA) consisting of all 120 noise events from the single exposure nights.
With this study design, exposures with different traffic modes were comparable according to number and maximum SPL of noise events. Additionally, the equivalent continuous sound levels LA,eq (i.e., average noise levels across the night) of the single exposure nights of aircraft and rail traffic noise were identical. Due to the shorter duration of road traffic noise events, the LA,eq of the road traffic single exposure night was lower. In order to get an equivalent LA,eq, the number of road noise events was doubled in one exposure night (RORO). Additionally, there was one night free of any traffic noise which had an LA,eq of 30 dB(A), caused by the constant sound of the air-conditioning system.
To balance the study design, each exposure was applied in each study night position once, and there were 9 study periods with 8 subjects each. Because sound insulation of sleep cabins was not absolute, in each study period, all 8 subjects received the same noise pattern in the same night. Aside from one noise-free control night, there were no noise-free nights interposed between 2 exposure nights, i.e., there were no washout periods. A more detailed description of the study design can be found in Basner et al.5
Electrophysiological signals included EEG (C3-A2, C4-A1), EOG, submental EMG, ECG, respiratory movements, and finger pulse amplitude. Sleep stages were scored according to the standards of Rechtschaffen et al.6 The study was approved by the local ethics committee. Subjects gave written informed consent prior to study participation and were free to discontinue any time without explanation.
Our outcomes of interest were noise-induced sleep fragmentation, operationalized as awakenings (R&K sleep stage changes from stages S1-S4 and REM to wake or movement time) and EEG arousals (as defined by Bonnet et al.7). A noise event was excluded from the analysis if the subject was already awake in the 30-s epoch preceding noise onset (for awakenings) or if there was an EEG arousal in a 10-s time window preceding noise-onset (for EEG arousals). For eligible noise events, the next 60 s were screened for a sleep stage change to awake or movement time (i.e., an awakening) or for the onset of an EEG arousal. For each exposure night, average noise-induced awakening and EEG arousal probability was calculated by dividing the number of awakenings/ EEG arousals through the number of eligible noise events. Spontaneous awakening and EEG arousal probability was determined in a similar way using the noise-free control nights. For each noise event and each subject, we first determined elapsed time after sleep onset. We then used this time as the onset of a virtual or sham noise event relative to sleep onset in the noise-free control night. Spontaneous awakening and arousal probability was then determined in the same way it was determined in the noise nights (i.e., by screening a 60-s window in the noise-free control night for an awakening and an EEG arousal if the subject was not awake and arousal-free prior to virtual noise onset, respectively). Sixteen of 552 exposure nights (2.9%) were excluded from the analysis because less than half of the 8-h TIB period could be analyzed due to signal loss.
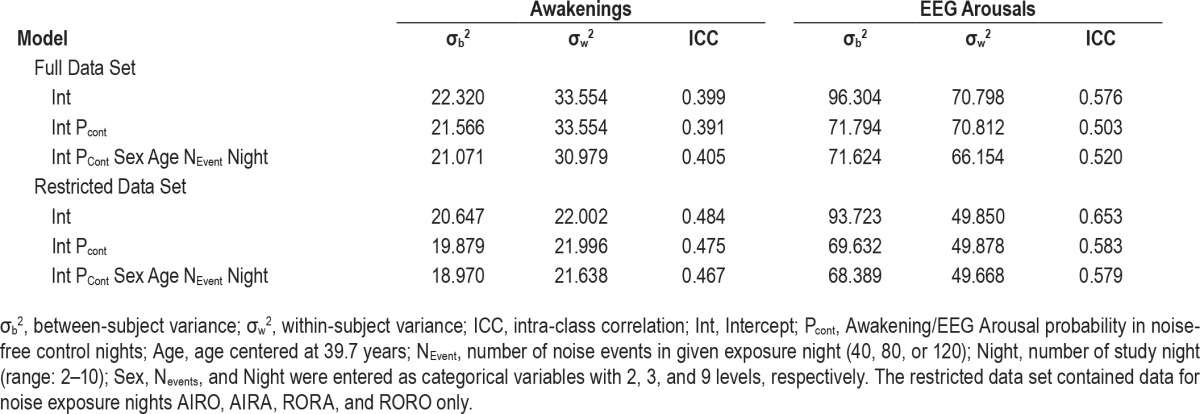
Random subject effect mixed-effect models of variance (variance component covariance structure, uncorrected degrees of freedom) were run with Proc Mixed in SAS (Version 9.3, SAS Institute, Cary, NC). The intra-class correlation (ICC) coefficient was calculated according to Van Dongen et al.8 by dividing estimates of between-subject variance by the sum of between- (σb2) and within-subject (σw2) variance. As subjects with higher spontaneous awakening/EEG arousal probability could also be more susceptible to noise, we also ran a model controlling for spontaneous awakening/EEG arousal probability in noise-free nights. Finally, we investigated the robustness of the ICC to the effects of age and gender (that potentially contribute to between-subject variance) and study night and number of noise events per night (that potentially contribute to within-subject variance) in a third mixed-effect model. In a sensitivity analysis, we re-ran the mixed models for a data set restricted to the 4 nights with exposure to 80 noise events from air, road, and/or rail traffic noise (i.e., AIRO, AIRA, RORA, and RORO). Due to the similarity of these exposure scenarios, we expected a reduction of within-subject variance relative to the analysis with all exposure nights.
RESULTS
Figure 1B shows average awakening probability and Figure 1C shows average EEG arousal probability for each of the 8 exposure nights and for each of the 69 subjects. Subjects were ordered from lowest (left) to highest (right) average reaction probability across exposure nights. Table 1 shows within- and between-subject variance and ICCs for the 3 mixed models stratified by outcome (awakening; EEG arousal) and by data set (full; restricted). ICCs for awakenings ranged between 0.391 and 0.405 in the full data set and between 0.467 and 0.484 in the restricted data set. ICCs for EEG arousals were higher and ranged between 0.503 and 0.576 in the full data set and between 0.579 and 0.653 in the restricted data set. A 10% increase in spontaneous awakening probability was statistically nonsignificantly associated with a 3.9% increase in noise-induced awakening probability (SE 2.3%; P = 0.0849), and accounting for spontaneous awakening probability decreased σb2 only marginally by 3.4% (full model). In contrast, a 10% increase in spontaneous EEG arousal probability was associated with a statistically significant 7.7% increase in noise-induced EEG arousal probability (SE 1.7%; P < 0.0001), and accounting for spontaneous EEG arousal probability decreased σb2 by 25.5% (full model). Additionally, accounting for age, sex, study night, and number of noise events per night caused only minor changes (< 8%) in σb2, σw2, and ICC. If the data set was restricted to nights with exposure to 80 noise events (black symbols in Figures 1B and 1C), σw2 decreased by 30% to 34% for awakenings and by 25% to 30% for EEG arousals.
Table 1.
Intra-class correlations for awakenings and EEG arousals.
DISCUSSION
Traffic noise causes sleep fragmentation through increases in the number of awakenings, shorter EEG arousals, and cardiac activations that have been shown to affect daytime sleepiness.5,9 Importantly, whether a sleeper reacts to a given noise event during the night not only depends on the acoustical properties of the noise event (like sound level rise time or spectral composition), but also on the significance of the noise for the sleeper,10 situational moderators (e.g., elapsed sleep time, current sleep stage), and personal moderators (e.g., sex, age, noise sensitivity). Despite anecdotal reports of large individual differences in noise sensitivity, we are not aware of any studies systematically investigating how much of the total variance in noise-induced sleep fragmentation can be attributed to stable differences between-subjects.
Our results demonstrate marked inter-individual differences in the susceptibility of sleeping subjects to nocturnal traffic noise exposure. A considerable amount of the variance (varying between 39.1% and 65.3% depending on data set, outcome, and model) observed in noise-induced sleep fragmentation was explained by inter-individual differences, indicating “moderate stability” according to conventional standards.11 These inter-individual differences could neither be explained by individual characteristics (i.e., age or gender) nor by study design characteristics (i.e., study night or number of noise events per night). As expected, restricting the data set to exposure nights with 80 noise events per night was associated with lower within-subject variability and higher ICCs compared to the full data set. A floor effect related to relatively low awakening probabilities produces lower inter-individual variability and could explain why ICCs were slightly higher for EEG arousals compared to awakenings.
Importantly, the data were derived in a controlled laboratory setting and in a relatively homogeneous, healthy, adult study population. ICC estimates are therefore likely conservative and expected to be higher in real world situations. Furthermore, although the noise scenarios were similar, they were not identical. If we had used identical noise scenarios, we would expect lower within-subject variance and, again, higher ICCs. This was corroborated by an analysis restricting the data set to nights with more similar noise exposure. Finally, awakening and EEG arousal probability estimates were based on 63 and 61 noise events, respectively, on average (range 21–119). The imprecision associated with this relatively modest sample size likely increased within-subject variability, and lowered ICCs.
In conclusion, our analyses demonstrate non-negligible inter-individual differences in the susceptibility to noise-induced sleep disturbance. It will thus be important to identify those at higher risk for noise-induced sleep disturbance. Furthermore, the custom to base noise policy and legislation on average responses should be re-assessed based on these findings.12
DISCLOSURE STATEMENT
This was not an industry supported study. The AIRORA study was internally funded by the German Aerospace Center (DLR). Dr. Basner is a Deputy Editor for SLEEP. Dr. Basner recuses himself from all decisions related to SLEEP manuscripts on which they have a conflict of interest. The authors have indicated no financial conflicts of interest. Work was performed at the Division of Sleep and Chronobiology, Department of Psychiatry, University of Pennsylvania, Perelman School of Medicine, Philadelphia, Pennsylvania and at the German Aerospace Center (DLR), Cologne, Germany.
ACKNOWLEDGMENTS
Thanks go to the subjects participating in the study and to our colleagues at the German Aerospace Center who helped collect the data during many weekend and night shifts.
REFERENCES
- 1.Basner M, Babisch W, Davis A, et al. Auditory and non-auditory effects of noise on health. Lancet. 2014;383:1325–32. doi: 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muzet A. Environmental noise, sleep and health. Sleep Med Rev. 2007;11:135–42. doi: 10.1016/j.smrv.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Basner M, Buess H, Elmenhorst D, et al. Cologne, Germany: Deutsches Zentrum für Luft- und Raumfahrt (DLR); 2004. Effects of nocturnal aircraft noise (Volume 1): executive summary. Report No.: FB2004-07/E, ISSN 1434-8454. [Google Scholar]
- 4.Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20:R626–R7. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 5.Basner M, Müller U, Elmenhorst E-M. Single and combined effects of air, road, and rail traffic noise on sleep and recuperation. Sleep. 2011;34:11–23. doi: 10.1093/sleep/34.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rechtschaffen A, Kales A, Berger RJ, et al. Washington, DC: Public Health Service, U.S. Government, Printing Office; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 7.Bonnet M, Carley DW, Carskadon MA, et al. EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 8.Van Dongen HP, Olofsen E, Dinges DF, Maislin G. Mixed-model regression analysis and dealing with interindividual differences. Methods Enzymol. 2004;384:139–71. doi: 10.1016/S0076-6879(04)84010-2. [DOI] [PubMed] [Google Scholar]
- 9.Basner M. Nocturnal aircraft noise increases objectively assessed daytime sleepiness. Somnologie. 2008;12:110–7. [Google Scholar]
- 10.Oswald I, Taylor AM, Treisman M. Discriminative responses to stimulation during human sleep. Brain. 1960;83:440–53. doi: 10.1093/brain/83.3.440. [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 12.Basner M. Validity of aircraft noise induced awakening predictions. Noise Contr Eng J. 2009;57:524–35. [Google Scholar]


