Abstract
BACKGROUND
Damage control resuscitation (DCR) has improved outcomes in severely injured patients. In civilian centers, massive transfusion protocols (MTPs) represent the most formal application of DCR principles, ensuring early, accurate delivery of high fixed ratios of blood components. Recent data suggest that DCR may also help address early trauma-induced coagulopathy. Finally, base deficit (BD) is a long-recognized and simple early prognostic marker of survival after injury.
METHODS
Outcomes of patients with admission BD data resuscitated during the DCR era (2007–2010) were compared with previously published data (1995–2003) of patients cared for before the DCR era (pre-DCR). Patients were considered to have no hypoperfusion (BD, >−6), mild (BD, −6 to −14.9), moderate (BD, −15 to −23.9), or severe hypoperfusion (BD, <−24).
RESULTS
Of 6,767 patients, 4,561 were treated in the pre-DCR era and 2,206 in the DCR era. Of the latter, 218 (9.8%) represented activations of the MTP. DCR patients tended to be slightly older, more likely victims of penetrating trauma, and slightly more severely injured as measured by trauma scores and BD. Despite these differences, overall survival was unchanged in the two eras (86.4% vs. 85.7%, p = 0.67), and survival curves stratified by mechanism of injury were nearly identical. Patients with severe BD who were resuscitated using the MTP, however, experienced a substantial increase in survival compared with pre-DCR counterparts.
CONCLUSION
Despite limited adoption of formal DCR, overall survival after injury, stratified by BD, is identical in the modern era. Patients with severely deranged physiology, however, experience better outcomes. BD remains a consistent predictor of mortality after traumatic injury. Predicted survival depends more on the energy level of the injury (stab wound vs. nonstab wound) than the mechanism of injury (blunt vs. penetrating).
LEVEL OF EVIDENCE
IV, therapeutic/prognostic study.
Keywords: Damage control resuscitation, massive transfusion protocol, base deficit
Based on recent US military experience, many civilian trauma centers have adopted the paradigm of damage control resuscitation (DCR) to care for the most severely injured patients. As an extension of damage control surgery, DCR emphasizes limiting crystalloid infusion in favor of the aggressive transfusion of high fixed ratios of fresh frozen plasma (FFP), platelets (PLT), and cryoprecipitate to packed red blood cells (PRBCs).1 In the civilian setting, the principles of DCR are most formally applied in the setting of a massive transfusion protocol (MTP), which allows for early availability of some of these hard to obtain blood components. The advantages of a well-developed MTP in the civilian setting have been documented in many recent series.2–4
Early trauma-induced coagulopathy (ETIC) is seemingly present in up to one-third of trauma patients on admission.5 Recent evidence would suggest that the combination of tissue damage and hypoperfusion may lead to inappropriate activation of protein C with a resultant hyperfibrinolytic state.6–9 Indeed, the one consistent finding across these recent series is that the development of ETIC is a poor prognostic sign10,11 and a successful resuscitation, using the principles of DCR, is designed to mitigate some of these sequelae.7
Admission base deficit (BD) has long been recognized to be an excellent independent predictor of mortality in trauma patients.12 Moreover, a significant admission BD has also been shown to correlate with the presence of ETIC with several studies demonstrating a dose-dependent lengthening of clotting times as BD worsens.9 Therefore, this parameter seems to be a logical marker to consider in the decision to use the principles of DCR. In our institution, we rely heavily on admission BD as a marker of severity of the physiologic impact of injury and have previously published survival data stratified by severity of BD and mechanism of injury.12 Since the time of this publication, our institution, like many other centers, has adopted the paradigm of DCR. Using this paradigm, local experience with BD as an early marker of mortality after trauma was reassessed.
PATIENTS AND METHODS
Patients from two prospectively maintained data bases were identified and cross-referenced. The first, the National Trauma Registry of the American College of Surgeons (TRACS) for Grady Memorial Hospital in Atlanta, GA (a state of Georgia Level I trauma center), contains data on all trauma admissions since 1995. The second database contains information on all MTP activations and is maintained in the Department of Surgery for Emory University at Grady Memorial Hospital. Patients in this database were cared for between February 1, 2007, and January 31, 2010. MTP patients were resuscitated using a previously described protocol, which is based on DCR principles and is described below.13–15 All patients were cared for by a core group of nine surgeons all of whom are double boarded in Surgery and Surgical Critical Care. The MTP represents the most formal application of DCR principles, and all faculty, fellows, and residents have been well versed in this paradigm since the inception of the MTP in 2007. This study was also reviewed and approved by the institutional review board of Emory University School of Medicine.
Massive Transfusion Protocol
The Grady Memorial Hospital MTP was implemented on February 1, 2007. The specific details of protocol operation have been detailed elsewhere; in brief, the blood bank delivers predetermined packages of blood components every 30 minutes with a goal ratio of PRBC:FFP:PLT of 1:1:1.13–15 Cryoprecipitate is also used aggressively, with 10 units administered every hour. After 18 units of PRBCs, a standard 4 mg dose of recombinant Factor VIIa is also available for use at the surgeon’s discretion, with a second dose available upon request 30 minutes later. In general, the MTP has markedly improved the efficiency of our resuscitations in the critically injured patients with these results published elsewhere.13–15
During the initial resuscitation phase, serial BD measurements from arterial blood gases are used as the primary marker of the adequacy of resuscitation and surgical control of bleeding. The decision to deactivate the MTP is left to the treating surgeon. Point of care testing for coagulopathy (thromboelastography) is not readily available at our institution.
Data Collection
Details of data contained within the trauma registry and the MTP database are also detailed elsewhere.15 For all trauma patients, demographic, laboratory, blood product utilization, injury severity, and outcomes data were entered into the study database. Patients cared for in the DCR era (2007–2010) were identified and comprise the “DCR” study group. In addition, data from 284 patients in the DCR group were also contained within the MTP database, indicating that they had been treated with formal application of DCR principles. Two hundred and eighteen of these patients had an admission BD measured and comprise the MTP subgroup.
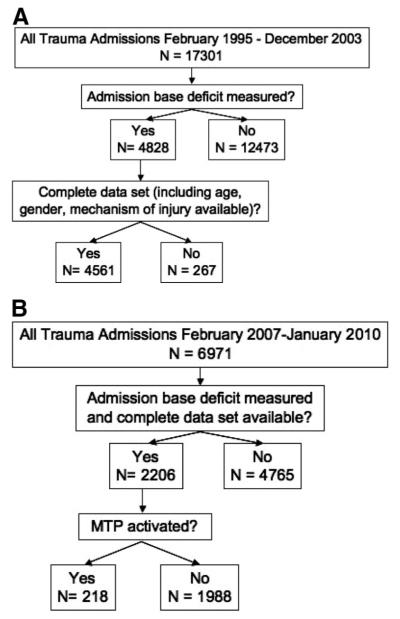
Finally, to develop a pre-DCR era comparison cohort, we queried TRACS and identified all trauma patients admitted from February 1995 through December 2003. These data were cross-referenced with previously published data from our institution to ensure accuracy and is summarized in Figure 1, A and B.12
Figure 1.
(A) Pre-DCR population and (B) DCR era population.
BD values on admission were recorded and analyzed. In animal work done by Brohi et al.,9 a value < −6 was used to define the presence of hypoperfusion. As such, we used the same number to characterize the presence of hypoperfusion. We further subcategorized the hypoperfusion as mild (BD, −6 to 14.9), moderate (−15 to −23.9), and severe (<−24). Cutoff points were chosen prospectively using clinical experience, with adjustments made to allow for the group sizes between periods to be similar.
Statistical Analysis
The primary outcome of interest was survival after traumatic injury. Secondary outcomes included transfusion requirements, number of operative interventions, number of ventilator days, and intensive care unit (ICU) and hospital lengths of stay.
Univariate analysis was performed using JMP Version 9 (SAS Institute Inc., Cary, NC). Continuous data are presented as means ± standard error. Categorical data are presented as proportions. Comparisons between groups for continuous data were done using Student’s t test, and comparisons for categorical data were performed using χ2 analysis. To create survival curves, multivariate logistic regression modeling was performed using SAS version 9.0; stepwise selection was used to determine significant predictors of mortality (SAS Institute Inc., Cary, NC). Significance for all tests was set at p ≤ 0.05.
RESULTS
Demographics and Injury Severity (DCR vs. Pre-DCR)
Of the 17,031 patients admitted in the pre-DCR era, 4,561 (27%) had a BD drawn at the time of admission and a complete data set. Of the 7,010 patients admitted in the DCR era, the same was true for a total of 2,206 (31%) patients. Therefore, further analysis was restricted to these 6,767 patients (Fig. 1, A and B). For both the pre-DCR and DCR era populations, patients who had an admission BD drawn were more severely injured, with a higher average Injury Severity Score (ISS) and lower Trauma Score, were more likely to have sustained a gunshot wound (GSW), received more units of PRBCs, and underwent more operations than those who did not (data not published).
Although gender distribution was similar between DCR and pre-DCR patients (79.6% vs. 78.6% male), DCR patients were slightly older (38.6 years vs. 36.0 years, p < 0.001). In addition, there were a higher percentage of patients who sustained blunt trauma in the DCR group (68.9% vs. 60.1%, p < 0.01). This and other demographic data are summarized in Table 1.
TABLE 1.
Demographics and Outcomes of Pre-DCR vs. DCR Era Patients
| Pre-DCR | DCR | p | |
|---|---|---|---|
| Demographic | n = 4,561 | n = 2,206 | |
| Male (%) | 78.63 | 79.65 | 0.43 |
| Mechanism of injury | <0.001 | ||
| Blunt (%) | 60.12 | 68.86 | |
| GSW (%) | 29.51 | 23.74 | |
| SW (%) | 8.05 | 6.45 | |
| Demographic | Mean ± SD | Mean ± SD | |
| Age | 35.99 ± 14.82 | 38.64 ± 16.25 | <0.001 |
| Base deficit | −5.65 ± 5.61 | −5.15 ± 5.06 | 0.0004 |
| Units PRBCs in 1st 24 h |
3.32 ± 7.64 | 6.65 ± 11.04 | <0.001 |
| SBP in ECC | 126.78 ± 32.35 | 129.66 ± 31.75 | 0.0006 |
| EtOH in ECC (mg/dL) | 75.85 ± 109.30 | 63.38 ± 103.47 | 0.0002 |
| Pulse in ECC | 97.94 ± 25.97 | 99.34 ± 24.69 | 0.036 |
| Resp in ECC | 19.92 ± 8.42 | 15.76 ± 9.90 | <0.0001 |
| TS | 10.77 ± 2.33 | 9.86 ± 3.20 | <0.0001 |
| ISS | 15.63 ± 11.63 | 16.79 ± 11.96 | 0.0002 |
| ISS (blunt only) | 17.11 ± 12.32 | 18.15 ± 12.53 | 0.009 |
| Outcome | Mean ± SD | Mean ± SD | |
| Hospital LOS | 14.92 ± 23.87 | 17.10 ± 28.38 | 0.0009 |
| ICU LOS | 5.95 ± 13.92 | 8.18 ± 12.64 | <0.0001 |
| Ventilator days | 5.04 ± 12.98 | 5.74 ± 11.12 | 0.039 |
| Number of operations | 9.79 ± 9.52 | 11.06 ± 9.31 | <0.0001 |
| Survival (%) | 86.43 | 85.77 | 0.46 |
SD, standard deviation; EtOH, serum alcohol level; Resp: respiratory rate; TS, Trauma Score.
Mean admission physiologic parameters were slightly different between the two eras, although most of these did not seem clinically significant. For instance, while the differences in mean systolic blood pressure (126 mm Hg vs. 129 mm Hg), mean heart rate (97 beats per minute vs. 99 beats per minute), and mean admission ethanol level (75 mg/dL vs. 63 mg/dL) were statistically different, the absolute values were clinically similar.
DCR era patients appeared, on average, more severely injured with a higher mean ISS (16.8 vs. 15.6, p = 0.0002), lower Trauma Score (9.86 vs. 10.77), higher mean transfusion requirement (6.6 units PRBCs vs. 3.3 units PRBCs), and slightly worse mean BD (−5.65 vs. −5.15). The difference in ISS remained significant even when analysis was limited to victims of blunt trauma (18.1 vs. 17.1, p = 0.009; Table 1).
Outcomes (Pre-DCR vs. DCR)
Patients in the DCR group had worse secondary outcomes including a greater number of operations (11.06 vs. 9.79), a greater number of days requiring ventilator support (5.74 vs. 5.04), and increased ICU (8.18 days vs. 5.95 days) and hospital (14.92 days vs. 17.10 days) lengths of stay (Table 1). They also, on average, required twice the number of PRBC units in the first 24 hours (6.65 units vs. 3.32 units, p < 0.0001). This was not surprising given their slight but consistent overall worse injury severity.
Conversely, when examining the primary endpoint of interest, survival to discharge, the two groups had nearly identical outcomes (86.43% vs. 85.77%, p = 0.46). This was true despite the differences in injury severity and initial resuscitation paradigm.
Survival Curves Stratified by BD and Mechanism of Injury (DCR vs. Pre-DCR)
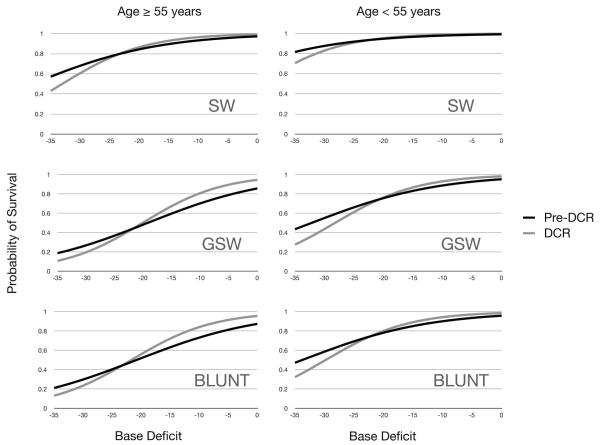
Overall, there was no significant difference in the probability of survival between the DCR and pre-DCR populations (Wald χ2: p = 0.562). Using multivariate logistic regression, we modeled the probability of survival to discharge across BD levels while controlling for the covariates: age (<55 years vs. ≥55 years), mechanism of injury (stab wound [SW], GSW, and blunt), hospital length of stay, ICU length of stay, and injury severity (based on ISS). No significant change in survival was observed between the pre-DCR and DCR eras with BD used as a continuous variable (DCR: pre-DCR odds ratio [OR], 1.059, 95% Wald confidence interval, 0.87–1.29). Victims of SWs in both eras tended to have better survival then victims of either GSWs or blunt trauma (SW:GSW OR, 6.25; p = 0.0038 and SW:blunt OR, 5.0; p = 0.01). This was true across all levels of the BD (Fig. 2). There was no clinically or statistically significant difference in survival between GSWs and blunt trauma (p = 0.313). Of note, patients who had sustained GSWs tended to have worse outcomes for a given Trauma Score than patients who were victims of a blunt or low energy penetrating injury (data not published). Within each BD category, a significantly higher mortality rate was observed than predicted by the Trauma-Injury Severity Score predicted survival rate method16 (Table 2). The only exception was in the severe BD category (BD, <−24), which did not reach significance likely due to the small number of patients included in that sample.
Figure 2.
Survival for all groups is essentially unchanged for all BD values. This is true for both young patients and older patients and regardless of mechanism of injury. Pre-DCR, historic controls; DCR, current era patients.
TABLE 2.
Predicted vs. Observed Mortality Stratified by Admission Base Deficit
| Pre-DCR | n (4,561) | Mean TRISS | MR (%) | p | DCR | n (2,206) | Mean TRISS | MR (%) | p |
|---|---|---|---|---|---|---|---|---|---|
| BD, >−6 | 2844 | 98 ± 10% | 8.6 | <0.001 | BD, > −6 | 1413 | 94 ± 15% | 8.4 | <0.001 |
| BD, −6 to −14.9 | 1515 | 94 ± 16% | 19.5 | <0.001 | BD, −6 to −14.9 | 688 | 87 ± 25% | 22.1 | <0.001 |
| BD, −15 to −23.9 | 146 | 82 ± 32% | 41.4 | <0.001 | BD, −15 to −23.9 | 91 | 69 ± 37% | 48.3 | <0.001 |
| BD, < −24 | 76 | 52 ± 46% | 68.4 | <0.001 | BD, < −24 | 14 | 82 ± 30% | 28.5 | 0.24 |
TRISS, Trauma-Injury Severity Score predicted survival rate; MR, mortality rate.
Use of MTP in DCR Era
MTP patients comprised ~10% of patients cared for in the modern era (218 of 2,206). MTP patients tended to be slightly younger than their non-MTP DCR era counterparts and were 10 times more likely to have sustained a GSW (31% vs. 3%). MTP patients were, not surprisingly, more severely injured, with a much higher ISS (26.52 vs. 15.74), increased mean heart rate (113 beats per minute vs. 97 beats per minute), lower initial systolic blood pressure (102 mm Hg vs. 132 mm Hg), and a more severe admission BD (−12.56 vs. −4.34). Finally, patients undergoing resuscitation under the MTP required an average of 20 units of PRBC in the first 24 hours compared with an average of 1.75 units in the DCR era non-MTP patients (Table 3). One-hundred ninety of the 218 patients actually received >10 units of PRBCs; of the 38 patients who did not receive 10 or more units, 27 survived.
TABLE 3.
Demographics and Outcomes of MTP Patients vs. DCR Era Non-MTP Patients
| MTP | Non-MTP DCR | p | |
|---|---|---|---|
| Demographic | n = 218 | n = 1,988 | |
| Male (%) | 83.94 | 79.18 | 0.088 |
| Mechanism of injury | <0.0001 | ||
| Blunt (%) | 51.38 | 70.78 | |
| GSW (%) | 31.30 | 3.44 | |
| SW (%) | 0.07 | 0.009 | |
| Demographic | Mean ± SD | Mean ± SD | |
| Age | 35.3 ± 14.99 | 39.00 ± 16.34 | 0.0014 |
| Base deficit | −12.56 ± 6.62 | −4.34 ± 4.12 | <0.0001 |
| Units PRBCs in 1st 24 h | 20.87 ± 13.37 | 1.75 ± 2.94 | <0.0001 |
| SBP in ECC | 102.69 ± 35.70 | 132.42 ± 29.99 | <0.0001 |
| EtOH in ECC (mg/ dL) | 46.64 ± 82.97 | 64.45 ± 104.56 | 0.09 |
| Pulse in ECC | 113.45 ± 30.27 | 97.83 ± 23.52 | <0.0001 |
| Resp in ECC | 14.58 ± 13.18 | 15.88 ± 9.50 | 0.076 |
| TS | 8.26 ± 3.82 | 9.99 ± 3.10 | <0.0001 |
| ISS | 26.52 ± 13.58 | 15.74 ± 11.28 | <0.0001 |
| Outcome | Mean ± SD | Mean ± SD | |
| Hospital days | 21.29 ± 31.17 | 16.64 ± 28.03 | 0.02 |
| ICU days | 11.53 ± 14.21 | 7.82 ± 12.40 | <0.0001 |
| Ventilator days | 8.65 ± 11.19 | 5.41 ± 11.07 | <0.0001 |
| Survival (%) | 55.05 | 89.13 | <0.0001 |
SD, standard deviation; EtOH, serum alcohol level; Resp, respiratory rate; TS, trauma score.
As expected, MTP patients had worse outcomes than non-MTP patients. The number of ventilator days (8.65 vs. 5.41), ICU days (11.53 vs. 7.82), and hospital days (21.29 vs. 16.64) were all greater in the non-MTP cohort. Finally, survival was drastically reduced from 89% in the non-MTP group to 55% in the MTP group. These data are summarized in Table 3.
Use of DCR and Outcomes by Severity of Tissue Hypoperfusion
Because the majority of patients who presented in both eras were not hypoperfused and, therefore, had a low overall mortality rate, the two groups were then stratified by severity of initial BD. Indeed, admission BD was an excellent predictor of the need for MTP activation, with only 2% of nonhypoperfused patients (BD, >−6) and 93% of extremely hypoperfused (BD, <−24) patients undergoing resuscitation via the MTP. The 14 MTP patients with the most extreme BD (BD, <−24) had a significant increase in survival compared with pre-DCR patients (n = 76). In this group, patients who sustained a blunt injury had the greatest increase in survival (19.05% vs. 66.67%), although patients with a penetrating injury had improved survival (47.62% vs. 71.43%), as well. Patients with more moderate BDs had similar survival in both eras although these data are less helpful because of the more variable use of the MTP (Table 4).
TABLE 4.
Mortality vs. Resuscitation Strategy Stratified by Admission Base Deficit
| Pre-DCR | N (4,561) | MR (%) | DCR | N (2,206) | MR (%) | p | MTP (%) |
|---|---|---|---|---|---|---|---|
| BD, >−6 | 2,844 | 8.6 | BD, > −6 | 1,413 | 8.4 | 0.80 | 2 |
| BD, −6 to −14.9 | 1,515 | 19.5 | BD, −6 to −14.9 | 688 | 22.1 | 0.16 | 18 |
| BD, −15 to −23.9 | 146 | 41.4 | BD, −15 to −23.9 | 91 | 48.3 | 0.26 | 60 |
| BD, <−24 | 76 | 68.4 | BD, < −24 | 14 | 28.5 | 0.004 | 93 |
DISCUSSION
The principles of damage control surgery have been part of routine practice in civilian trauma centers since the 1980s.17 DCR seems to be a logical extension of this paradigm, as it attempts to directly address the “bloody vicious cycle” during the earliest stages of resuscitation. A multitude of recent military and civilian studies have demonstrated the improvements in survival afforded by DCR, particularly when used as an adjunct to other damage control techniques.18 Protocolized resuscitation has been shown to reduce the incidence of multiple organ dysfunction syndrome, ensure efficient delivery of component therapy in the desired ratios, reduce costs, and reduce overall blood product utilization also.4,14,15,19–22 Finally, given the recent evidence which suggests a central role for hypoperfusion in ETIC,11 it would make sense that BD, a simple and universally available measurement, would be a useful early marker of outcome after traumatic injury. Indeed, much of the animal data which suggests the importance of hypoperfusion in ETIC uses BD itself as an estimate of the degree of hypoperfusion and shows a correlation between acidosis, as measured by a BD <−6 and the presence of ETIC. Moreover, our center has previously published several studies on the prognostic implications of admission BD in the era before the routine application of the DCR paradigm.12 Because the DCR paradigm, which is designed to combat ETIC, has markedly changed the early resuscitation of the trauma patient, it falls to reason that survival curves generated by admission BD might change in the modern era. The reassessment of these curves was the primary purpose of this study.
Of the many interesting findings in this very large data set, the most profound was the overall similarity in the primary outcome between the two time periods. Indeed, there was nearly an identical overall survival rate in the two data sets. It may be that the increased severity of injury that is being seen has offset any improvements in outcome achieved through the use of protocolized resuscitation. Moreover, it is likely that DCR tenets are not as important in less gravely injured patients who, fortunately or unfortunately, comprised 90% or more of our total population. Indeed our MTP, which is by far the most formal application of DCR tenets, was only required in ~10% of the total population. Finally, standard resuscitation techniques, which have long been protocolized in the Advanced Trauma Life Support course sponsored by the American College of Surgeons, have changed little over the past 20 years.23 This likely had a significant effect, resulting in the similar overall survival rates.
When the data based on severity of initial BD were further analyzed, a drastic improvement in survival in the patients with the most extreme acidosis (BD, <−24) was documented. Although this comprised by far the smallest subset of patients, it does represent the subset of patients most likely to benefit from DCR. Indeed, in the DCR era, mortality decreased from ~70% to <30%. This group was almost universally resuscitated with the described MTP and, therefore, they were the most likely to receive a true “damage control resuscitation.” It was interesting to note that patients with evidence of hypoperfusion but more moderate BDs (24 <BD <6) had no improvement in survival in the DCR era. These data, unfortunately, were, as previously noted, less helpful because of the more variable use of the MTP (<25% of this entire group). It may be that more universal application of the MTP or even an extension of the MTP to include more formal application of DCR outside the setting of massive transfusion may be of benefit in this more moderately injured group. In addition, DCR era patients had longer hospital and ICU lengths of stay, more ventilator days, and a higher mean transfusion requirement. This may be related to improved survival (i.e., longer lengths of stay) in the most critically injured patients or just may represent a more severely injured group of patients. Indeed, this phenomenon has been noted previously.24
Previous studies from this institution have shown that admission BD is an independent predictor of mortality in trauma12 and this finding was validated in the current study. In addition, BD was also found to be an excellent predictor of the need for MTP activation, with almost universal activation at the extremes of BD severity and rare activation in patients with minimal deficits. This is concordant with the scoring system developed by Larson et al.25 which was able to predict the need for massive transfusion (MT) with reasonable accuracy based on admission parameters including a BD <−6, tachycardia, and hemoglobin <11 g/dL. In addition, Cotton et al.19 found that both BD and lactate levels were predictive of multiple organ dysfunction syndrome development. Another study, instead, advocated the use of regional tissue oxygen saturation as a measure of hypoperfusion and did not find BD to be an accurate predictor of transfusion requirement.26
The fact that admission BD is a reliable predictor of the need for MT indicates that it may serve as a useful adjunct to aid in making the decision to both initiate transfusion of blood components as well as to activate a MTP. Indeed, it has already been incorporated into the Trauma Associated Severe Hemorrhage score developed by the German Trauma Society to predict the need for massive transfusion.27 Moreover, BD serves as a reliable indicator of the adequacy of resuscitation, and it may prove to be a useful indicator of the appropriate time to deactivate the MTP as well. Future studies are required to determine the precise role of BD measurements in these decisions. Although patients with penetrating trauma have been demonstrated to be more likely to require MT, one previous study from the same authors documented that patients with a blunt mechanism of injury are more likely to benefit from activation of the protocol.13,28
Finally, one of the most interesting findings from this dataset is the difference in mortality between patients with high- versus low-energy injuries. Classically, a distinction has been made between patients who have sustained blunt and penetrating injuries, as this was thought to reflect the risk of hollow viscus or vascular injury and, therefore, the need for operative management. However, based on the findings of this study, it may be that patients should be classified based on the energy level of the mechanism of injury when assessing mortality risk. SWs, which are very low-energy injuries, were found to have lower mortality rates at all admission BD ranges in both the DCR and pre-DCR era. This is likely because SWs typically result in only a single source of hemorrhage which may be easily controlled. In contrast, high-energy injuries, including blunt injuries and GSWs, were found to have similar mortality at all BD levels. This reflects the increased likelihood of more complex injuries that can be caused by high-velocity missiles (e.g., bullets) or the impact of a blunt force.
Limitations of this study include the relatively small sample size of patients who received the MTP as well as the use of historic controls. Moreover, there were a large number of patients in both the DCR and pre-DCR era cohorts who lacked admission BD and these patients had to be excluded. It is also a single institution’s experience with the use of a very familiar and ubiquitous marker of hypoperfusion, and other centers may not have a similar experience. Next, an admission BD represents only a single snap shot in what may be a very prolonged resuscitation and hospital course. Serial BD measurements, as a marker of the effectiveness of the resuscitation, have also been shown to be useful prognostic tools after injury.12 Unfortunately, the inconsistency in timing and number of measurements obtained in such a large set of patients made that analysis difficult to replicate. We have, however, previously published our experience with the DCR paradigm on several occasions and have documented significant improvements in patient outcomes and resuscitation efficiency.13–15 Finally, the two periods studied were noncontiguous. The historic survival curves were created and previously published using a data set ending in 2001. The modern survival curves were created using data beginning in 2007. This gap between time periods was created because we felt that patients treated after the inception of our MTP (February 1, 2007) would most consistently reflect the application of a distinct resuscitation paradigm from the patients treated in the historic time period and show any changes in the prognostic of admission BD that this different paradigm had produced.
Despite adoption of the principles of DCR along with damage control surgery, the overall survival after traumatic injury stratified by BD and mechanism of injury has remained essentially unchanged. Furthermore, admission BD continues to be a remarkably consistent predictor of mortality across the two eras. Moreover, BD is a reliable predictor of the need for massive transfusion and should be used as an aid in clinical decision making for both the initiation and possibly the termination of an MTP. Finally, the extension of formal DCR protocols outside the setting of massive transfusion should be a new area of investigation in the future.
Footnotes
AUTHORSHIP
C.J.D., B.H.S., J.M.N. and E.I.H. conceived and designed study; E.I.H., B.C.M. collected and organized data; E.I.H., M.J.M. analyzed data; E.I.H., B.C.M., M.J.M. and C.J.D. prepared manuscript which was critically reviewed by A.D.W., J.P.S., G.S.R. and D.V.F.
DISCLOSURE
The authors declare no conflicts of interest.
Presented at the 70th Annual Meeting of the American Association for the Surgery of Trauma, September 14–17, 2011, Chicago, Illinois.
DISCUSSION
Dr. Patrick J. Offner (Denver, Colorado): Good morning. With this study the Emory group continues their investigation of the base deficit in trauma resuscitation, particularly with regard to massive transfusion in the context of damage control.
Their stated primary focus was to reexamine the base deficit as an early marker of mortality in the era of damage control resuscitation. The finding that admission base deficit remains a significant predictor of mortality is really not that surprising to me. I wouldn’t have had expected otherwise given that this is a reflection of shock prior to any intervention except for that provided in the prehospital environment.
On the other hand it’s more intuitive that application of a formalized, more efficient, massive transfusion protocol might alter the time course of the resuscitation as well as the outcome.
Did the authors perform serial measurements of base deficit? And if so did the base deficit normalize more quickly or in a greater number of patients at a pre-defined time point in those who received the massive transfusion protocol?
Damage control resuscitation and massive transfusion protocols were developed for severely injured patients in hemorrhagic shock. These patients represent only a fraction of the total number, about 10 percent in this series. As such, these protocols are not necessarily applicable to all patients and, in fact, may be deleterious in some, not unlike what we heard yesterday with damage control surgery.
It’s not surprising, then, that the demonstration of a survival benefit was not present in the general trauma population, as already discussed. It was lost in the noise of all those patients who didn’t require specialized resuscitation.
When restricted to patients in severe shock, the benefit was significant, as demonstrated. Perhaps the most significant potential of this study and the dataset is to help us address what I believe are the two biggest challenges in the continued refinement of damage control and massive resuscitation, namely, when and in whom to initiate these protocols and what comprises the optimal ratio of blood components.
A more robust multivariate analysis of this data might help to further elucidate these issues. Have you done this or are you planning to do this?
Dr. Juan Asensio (Miami, Florida): Chris, congratulations again, to your group for another predictive study. I have a couple of questions:
I’m curious why you did not use Ph. How about lactic acid level? And do you think that perhaps tracking the trend of these endpoints of resuscitation would have allowed you to come up with better data for the moderately hypoperfused group which I think is still at risk? In our examination study based on 548 patients we used Ph and that seemed to be a very good predictor of outcome.
Dr. Christopher J. Dente (Atlanta, Georgia): Thank you, Dr. Offner, Dr. Asensio. To answer your questions in order, serial base deficit measurements are obviously a useful thing and the question is did they normalize quicker with massive transfusion protocol.
I will tell you we do track this in our MTP database and while I don’t have the numbers in front of me I will tell you a couple of things. Number 1, we are finishing a lot more operations than we used to. We’re having to leave less abdomens open.
And we’re also able to close a lot more abdomens. We’ve never had the closure rates at Grady that some people have published with the VAC PAC techniques.
Ours have always been a little bit lower but I will tell you I think our resuscitations are markedly improved and that we are able to get patients out of shock a lot quicker.
In terms of the multivariate analysis, we did not specifically do this for this paper although the survival curves were created using multivariate logistic regression. I think the next step will be to look at serial measurements of base deficit in an effort to figure out exactly how good a marker that is.
Dr. Asensio, in terms of Ph, it’s another marker. It’s easy to use. We’ve always used base deficit. And it’s a marker that we’re very comfortable with. But I think you could also use Ph very similarly. And, again, I sort of talked a little bit about trending the base deficits. We do have this data collected and it is something we are looking at.
Again, I would like to thank the Association for the privilege of the floor.
REFERENCES
- 1.Beekley A. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36:S267–S274. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 2.Nunez T, Young P, Holcomb J, Cotton B. Creation, implementation, and maturation of a massive transfusion protocol for the exsanguinating trauma patient. J Trauma. 2010;68:1498–1505. doi: 10.1097/TA.0b013e3181d3cc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaz B, Dente C, Harris R, MacLeod J, Hillyer C. Transfusion management of trauma patients. Anesth Analg. 2009;108:1760–1768. doi: 10.1213/ane.0b013e3181a0b6c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riskin D, Tsai T, Riskin L, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209:198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Kauvar D, Lefering R, Wade C. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;43(Suppl 6):809–813. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 6.Kashuk J, Moore E, Sawyer M, et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann Surg. 2010;252:434–442. doi: 10.1097/SLA.0b013e3181f09191. [DOI] [PubMed] [Google Scholar]
- 7.Frith D, Brohi K. The acute coagulopathy of trauma shock: clinical relevance. Surgeon. 2010;8:159–163. doi: 10.1016/j.surge.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Hess J, Brohi K, Hutton R, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 9.Brohi K, Cohen M, Ganter M, Matthay M, Mackersie R, Pittet J. Acute traumatic coagulopathy: initiated by hypoperfusion modulated through the protein C pathway? Ann Surg. 2007;245:812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frith D, Goslings J, Gaarder C, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–1925. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 11.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay L, Feliciano D, Rozycki G. Assessment of initial base deficit as a predictor of outcome: mechanism of injury does make a difference. Am Surg. 2002;68:689–694. [PubMed] [Google Scholar]
- 13.Dente C, Shaz B, Nicholas J, et al. Early predictors of massive transfusion in patients sustaining torso gunshot wounds in a civilian level I trauma center. J Trauma. 2010;68:298–304. doi: 10.1097/TA.0b013e3181cf7f2a. [DOI] [PubMed] [Google Scholar]
- 14.Dente C, Shaz B, Nicholas J, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–1624. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 15.Shaz B, Dente C, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50:493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyd CR, Tolson M, Copes W. Evaluating trauma care: the TRISS method. J Trauma. 1987;27:370–3778. [PubMed] [Google Scholar]
- 17.Rotondo M, Schwab C, McGonigal M, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:382–383. [PubMed] [Google Scholar]
- 18.Duchesne J, Kimonis K, Marr A, et al. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma. 2010;69:46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- 19.Cotton B, Brigham K, Nunez T, Gunter O, Robertson A, Young P. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–49. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- 20.Cotton B, Gunter O, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1183. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 21.O’Keeffe T, Refaai M, Tchorz K, Forestner J, Sarode R. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–691. doi: 10.1001/archsurg.143.7.686. [DOI] [PubMed] [Google Scholar]
- 22.Duchesne J, Hunt J, Wahl G, et al. Review of current blood transfusion strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–278. doi: 10.1097/TA.0b013e31817e5166. [DOI] [PubMed] [Google Scholar]
- 23.Trauma ACoSCo . Advanced Trauma Life Support Course Manual. American College of Surgeons; Chicago, IL: 2008. [Google Scholar]
- 24.Dutton RP, Stansbury LG, Leone S, et al. Trauma mortality in mature trauma systems: are we doing better? An analysis of trauma mortality patterns, 1997–2008. J Trauma. 2010;69:620–626. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 25.Larson C, White C, Spinella P, et al. Association of shock, coagulopathy, and initial vital signs with massive transfusion in combat casualties. J Trauma. 2010;69:S26–S32. doi: 10.1097/TA.0b013e3181e423f4. [DOI] [PubMed] [Google Scholar]
- 26.Smith J, Bricher S, Putnam B. Tissue oxygenation saturation predicts the need for early blood transfusion in trauma patients. Am Surg. 2008;74:1006–1011. [PubMed] [Google Scholar]
- 27.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60:1236–1237. doi: 10.1097/01.ta.0000220386.84012.bf. [DOI] [PubMed] [Google Scholar]
- 28.Cotton B, Dossett L, Haut E, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010;69:S33–S39. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]