Abstract
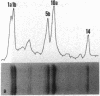
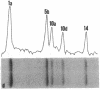
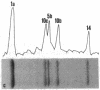
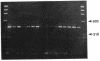
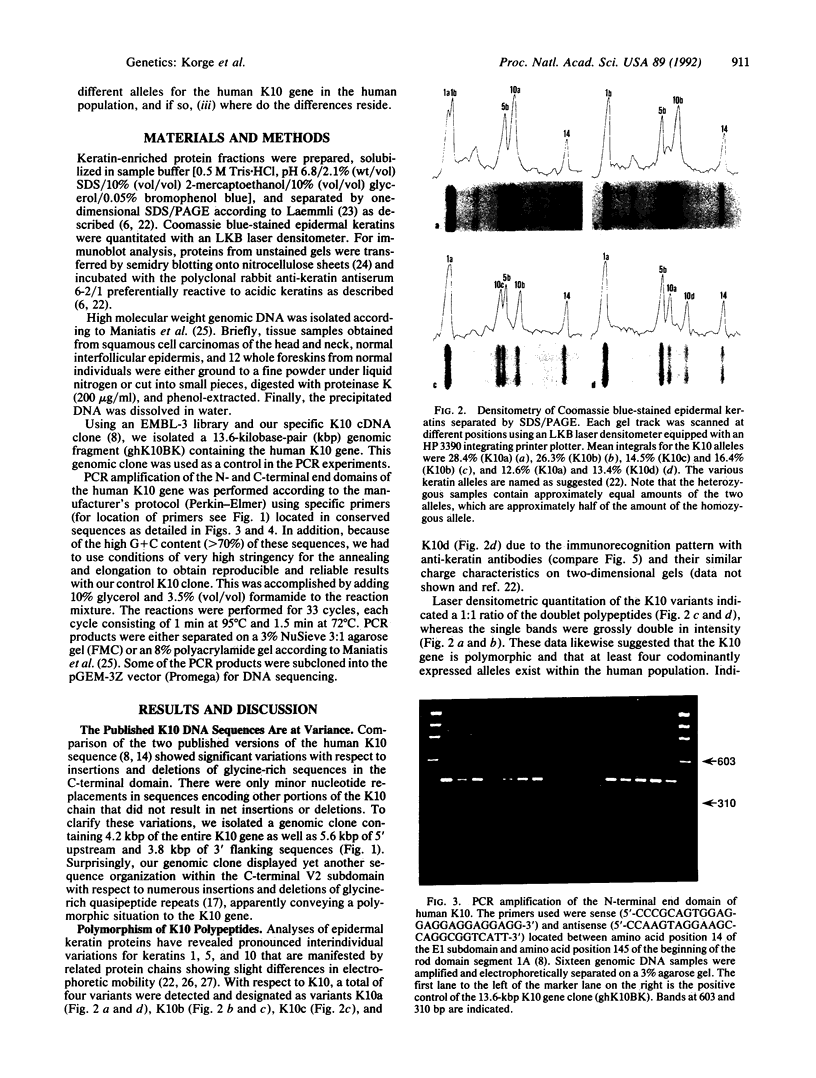
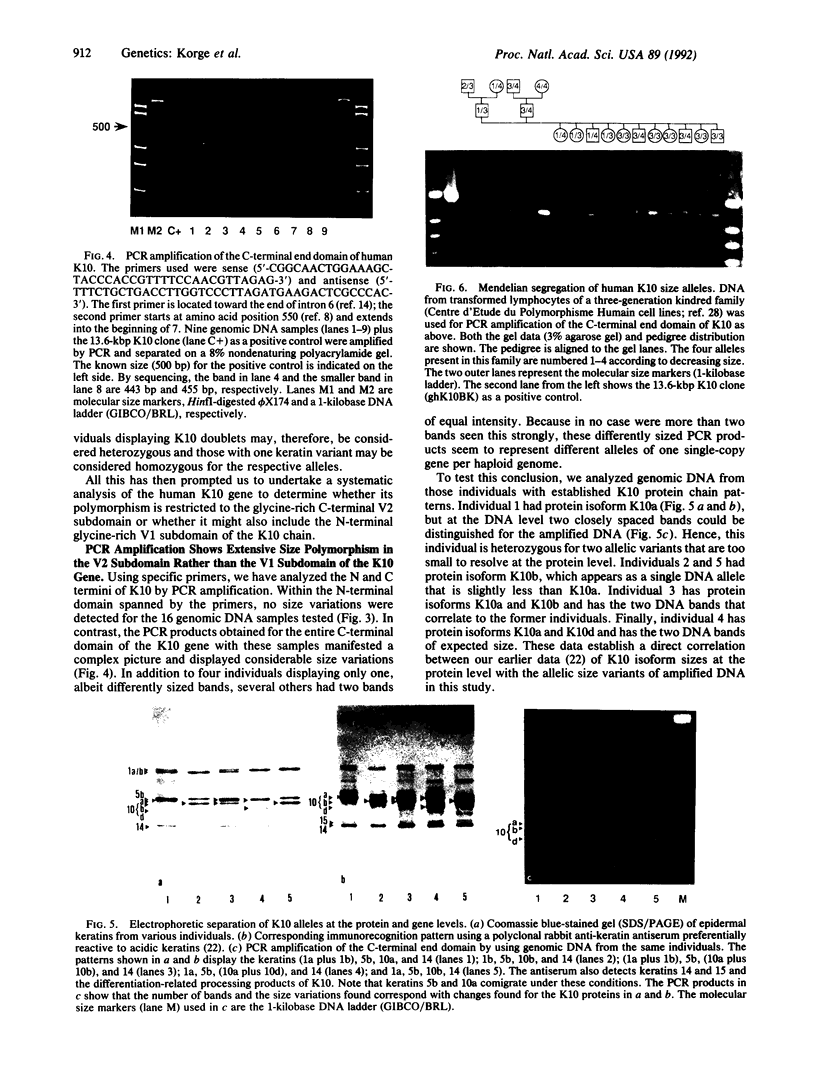
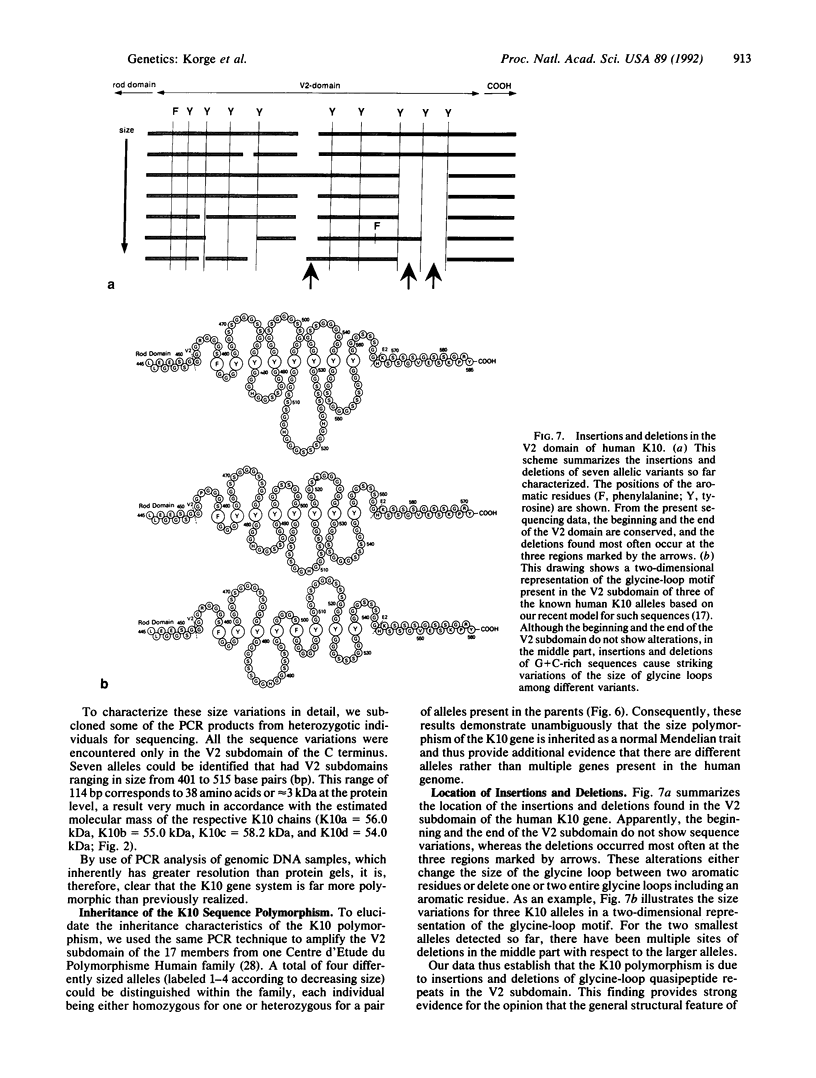
Existing data suggest that the human keratin 10 intermediate filament protein is polymorphic in amino acid sequence and in size. To precisely define the nature of the polymorphism, we have used PCR amplification and sequence analyses on DNA from several individuals including five with documented size variations of the keratin 10 protein. We found no variation in the N-terminal or rod domain sequences. However, we observed many variations in the V2 subdomain near the C terminus in glycine-rich sequences with a variation of as much as 114 base pairs (38 amino acids), but all individuals had either one or two variants. Our results show that (i) the keratin 10 system is far more polymorphic than previously realized, (ii) the polymorphism is restricted to insertions and deletions of the glycine-rich quasipeptide repeats that form the glycine-loop motif in the C-terminal domain, (iii) the polymorphism can be accounted for by simple allelic variations that segregate by normal Mendelian mechanisms, and (iv) the differently sized PCR products most likely represent different alleles of a single-copy gene per haploid genome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K., Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987 Aug;105(2):791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J. F., Parry D. A. Structural features in the heptad substructure and longer range repeats of two-stranded alpha-fibrous proteins. Int J Biol Macromol. 1990 Oct;12(5):328–334. doi: 10.1016/0141-8130(90)90023-4. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Kimball J. R., Hoff M., Sun T. T. Expression of epidermal keratins and filaggrin during human fetal skin development. J Cell Biol. 1985 Oct;101(4):1257–1269. doi: 10.1083/jcb.101.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M., Weber K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol. 1990 Apr;110(4):1199–1210. doi: 10.1083/jcb.110.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl D., Mehrel T., Lichti U., Turner M. L., Roop D. R., Steinert P. M. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991 Apr 5;266(10):6626–6636. [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcano J. L., Rieger M., Franz J. K., Schiller D. L., Moll R., Franke W. W. Identification of two types of keratin polypeptides within the acidic cytokeratin subfamily I. J Mol Biol. 1984 Oct 25;179(2):257–281. doi: 10.1016/0022-2836(84)90468-6. [DOI] [PubMed] [Google Scholar]
- Korge B., Stadler R., Mischke D. Effect of retinoids on hyperproliferation-associated keratins K6 and K16 in cultured human keratinocytes: a quantitative analysis. J Invest Dermatol. 1990 Oct;95(4):450–455. doi: 10.1111/1523-1747.ep12555613. [DOI] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Krieg T. M., Schafer M. P., Cheng C. K., Filpula D., Flaherty P., Steinert P. M., Roop D. R. Organization of a type I keratin gene. Evidence for evolution of intermediate filaments from a common ancestral gene. J Biol Chem. 1985 May 25;260(10):5867–5870. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lessin S. R., Huebner K., Isobe M., Croce C. M., Steinert P. M. Chromosomal mapping of human keratin genes: evidence of non-linkage. J Invest Dermatol. 1988 Dec;91(6):572–578. doi: 10.1111/1523-1747.ep12477087. [DOI] [PubMed] [Google Scholar]
- Lu X., Lane E. B. Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: specific domain functions in keratin stabilization and filament formation. Cell. 1990 Aug 24;62(4):681–696. doi: 10.1016/0092-8674(90)90114-t. [DOI] [PubMed] [Google Scholar]
- Mack J. W., Torchia D. A., Steinert P. M. Solid-state NMR studies of the dynamics and structure of mouse keratin intermediate filaments. Biochemistry. 1988 Jul 26;27(15):5418–5426. doi: 10.1021/bi00415a006. [DOI] [PubMed] [Google Scholar]
- Miller R. K., Vikstrom K., Goldman R. D. Keratin incorporation into intermediate filament networks is a rapid process. J Cell Biol. 1991 May;113(4):843–855. doi: 10.1083/jcb.113.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischke D., Wild G. Polymorphic keratins in human epidermis. J Invest Dermatol. 1987 Feb;88(2):191–197. doi: 10.1111/1523-1747.ep12525329. [DOI] [PubMed] [Google Scholar]
- Mischke D., Wille G., Wild A. G. Allele frequencies and segregation of human polymorphic keratins K4 and K5. Am J Hum Genet. 1990 Mar;46(3):548–552. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Rieger M., Franke W. W. Identification of an orthologous mammalian cytokeratin gene. High degree of intron sequence conservation during evolution of human cytokeratin 10. J Mol Biol. 1988 Dec 20;204(4):841–856. doi: 10.1016/0022-2836(88)90045-9. [DOI] [PubMed] [Google Scholar]
- Rieger M., Jorcano J. L., Franke W. W. Complete sequence of a bovine type I cytokeratin gene: conserved and variable intron positions in genes of polypeptides of the same cytokeratin subfamily. EMBO J. 1985 Sep;4(9):2261–2267. doi: 10.1002/j.1460-2075.1985.tb03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Mack J. W., Korge B. P., Gan S. Q., Haynes S. R., Steven A. C. Glycine loops in proteins: their occurrence in certain intermediate filament chains, loricrins and single-stranded RNA binding proteins. Int J Biol Macromol. 1991 Jun;13(3):130–139. doi: 10.1016/0141-8130(91)90037-u. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A., Idler W. W., Johnson L. D., Steven A. C., Roop D. R. Amino acid sequences of mouse and human epidermal type II keratins of Mr 67,000 provide a systematic basis for the structural and functional diversity of the end domains of keratin intermediate filament subunits. J Biol Chem. 1985 Jun 10;260(11):7142–7149. [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J Biol Chem. 1990 May 25;265(15):8766–8774. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom K. L., Borisy G. G., Goldman R. D. Dynamic aspects of intermediate filament networks in BHK-21 cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):549–553. doi: 10.1073/pnas.86.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Lalouel J. M., Nakamura Y., Donis-Keller H., Green P., Bowden D. W., Mathew C. G., Easton D. F., Robson E. B., Morton N. E. The CEPH consortium primary linkage map of human chromosome 10. Genomics. 1990 Mar;6(3):393–412. doi: 10.1016/0888-7543(90)90469-b. [DOI] [PubMed] [Google Scholar]
- Wild G. A., Mischke D. Variation and frequency of cytokeratin polypeptide patterns in human squamous non-keratinizing epithelium. Exp Cell Res. 1986 Jan;162(1):114–126. doi: 10.1016/0014-4827(86)90430-1. [DOI] [PubMed] [Google Scholar]
- Zhou X. M., Idler W. W., Steven A. C., Roop D. R., Steinert P. M. The complete sequence of the human intermediate filament chain keratin 10. Subdomainal divisions and model for folding of end domain sequences. J Biol Chem. 1988 Oct 25;263(30):15584–15589. [PubMed] [Google Scholar]