Abstract
Background
Travel-related risks for infectious diseases vary depending on travel patterns such as purpose, destination, and duration. In this study, we describe the patterns of travel and prescription of vaccines as well as malaria prophylaxis medication (MPM) at a travel clinic in South Korea to identify the gaps to fill for the optimization of pre-travel consultation.
Materials and Methods
A cohort of travel clinic visitors in 2011 was constructed and early one-third of the visitors of each month were reviewed. During the study period, 10,009 visited the travel clinic and a retrospective chart review was performed for 3,332 cases for analysis of travel patterns and prescriptions.
Results
People receiving yellow fever vaccine (YFV) (n = 2,933) were traveling more frequently for business and tourism and less frequently for providing non-medical service or research/education compared to the 399 people who did not receive the YFV. Overall, most people were traveling to Eastern Africa, South America, and Western Africa, while South-Eastern Asia was the most common destination for the non-YFV group. Besides YFV, the typhoid vaccine was the most commonly prescribed (54.2%), while hepatitis A presented the highest coverage (74.7%) considering the natural immunity, prior and current vaccination history. Additionally, 402 (82.5%) individuals received a prescription for MPM among the 487 individuals travelling to areas with high-risk of malaria infection. Age over 55 was independently associated with receiving MPM prescription, while purpose of providing service and travel duration over 10 days were associated with no MPM prescription, despite travelling to high-risk areas.
Conclusion
Eastern Africa and South America were common travel destinations among the visitors to a travel clinic for YFV, and most of them were travelling for tourism and business. For the individuals who are traveling to areas with high-risk for malaria, more proactive approach might be required in case of younger age travelers, longer duration, and travel purpose of providing service to minimize the risk of malaria infection.
Keywords: Travel medicine, Yellow fever, Vaccine, Malaria, Antibiotic prophylaxis
Introduction
The major goal of travel medicine is to prevent illness occurring during and after travel by providing health advice, vaccination, and other prophylactic measures to travelers [1]. However, the risk of becoming ill or injured during international travel depends on many factors, such as trip destination, traveler's age and health status, length of the trip, and diversity of planned activities [2]. Therefore, pre-travel consultation is very important to minimize the travel related health risk. While western travelers visiting high-risk areas were more likely to get pre-travel consultation over 70% [3,4], those of Asian travelers were usually less than 50% [5,6,7]. Yoo et al. reported that only 24% had sought pre-travel health information among the Korean travelers departing for India from Incheon International Airport [8].
According to the Korea Tourism Organization, the Republic of Korea accounted for 12 millions of international departures and 89,089 million passenger-kilometre (PKM) of international operations in 2011, ranking as the 8th country worldwide by the International Civil Aviation Organization. However, few data are available about the pattern of travel among individuals departing from South Korea or demographic characteristics [8,9]. In addition, the number of imported malaria case is increasing recently [10], what implies that there are gaps to be filled in the aspect of preventive travel measures in South Korea.
One previous study investigated 37,542 travelers from all over the world who became sick during an international trip and found that 1.5% of those illness were vaccine preventable diseases (VPD) [11] and that hospitalization rate for VPD was higher than hospitalization for other reasons not associated with VPD. Also, yellow fever is the only disease with mandatory vaccine policy for individuals traveling to and from epidemiologically at-risk countries to prevent importation and indigenous transmission of yellow fever. Therefore, yellow fever vaccine (YFV) is a very important service in travel clinic practice. In the Republic of Korea, YFV is offered by selected government operated hospitals and quarantine offices in airports and harbors. In 2011, the National Medical Center was the only site offering YFV in Seoul for over ten million residents and in this period over 8,500 people received YFV at the National Medical Center. Besides VPD, malaria is one of the most relevant diseases for international travelers, as it is the most frequent cause of systemic febrile illness among returning travelers and one of the main causes of death despite available effective prevention measures [2,12].
This study was designed to retrospectively collect data about prescription of vaccination (focusing on yellow fever vaccine) and malaria prophylaxis medication (MPM) from a large number of individuals visiting our travel clinic, which is one of the few clinics specialized in yellow fever vaccine administration in South Korea. These data were interpreted in relation to available demographics, information about travel destination, purpose and duration of travel to present the comparison between YFV and non-YFV groups. In addition, we aimed to identify risk groups of individuals, who did not receive optimal preventive measures in order to improve pre-travel consultation strategies.
Materials and Methods
1. Study population
Data were retrospectively collected between January and December 2011 from the out-patient travel clinic in Seoul, South Korea. We first selected all visitors who received a prescription for one or more vaccines including YFV, hepatitis A, tetanus/diphtheria booster, typhoid fever, and/or MPM with atovaquone/proguanil (AT/PGU) or mefloquine (MQ). In case of multiple visits of the same person, only the first visit was considered. Subjects who received prescriptions for any reasons other than prevention of travel-related illness were excluded (such as trauma, chronic viral hepatitis, immune suppression [human immunodeficiency virus infection, asplenism, and hematologic malignancy], or active malaria infection). Because more than 10,000 individuals visited our travel clinic during the study period, we selected cases for the detail review. The required sample size was 1,541 individuals with the following assumptions; a two-sided test with the conventional minimum effect size 0.1, type I error (alpha) 5% and power 90% to see the difference between YFV group and non-YFV group (G*Power version 3.1). We determined that 30% is appropriate value for detail review and selected those who attended our travel clinic between the first and the 10th day of each month in an effort to reduce selection bias and minimize seasonal variation.
2. Data collection
A more detailed clinical chart review was performed and relevant data were collected from a specialized travel clinic record form including history of prior vaccinations and travel itinerary information (destination, duration, and purpose). Regarding travel destination, we collected information about all destinations mentioned by the visitors. Destination continent was selected according to the United Nations Statistics Division rules (http://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm#ftna, accessed November 2012). Cases were excluded if their visiting continent information was unavailable or uncategorizable. People referred to our clinic for YFV administration only were excluded from the analysis except for the comparison between YFV group and non-YFV group, since a brief record form was used for this group which did not include relevant information like prior vaccination history or prescriptions from other medical institution (but only travel destination and purpose). Cases were excluded from the destination continent analysis if participants visited multiple continents during a single trip.
Detailed information about prescription of vaccines and anti-malarial drugs were collected and categorized by the visiting continent information. If documentation of valid prior vaccination, proven immunity, or prescription of MPM at other institution was available from the travel clinic medical record, this information was included as part of the prevention coverage assessment. According to the guidelines for adult immunization by the Korean Society of Infectious Diseases at the time of this study (2011), hepatitis A vaccination was not recommended for individuals over 40 years of age as a consequence of the high seropositivity acquired by natural infection [13,14]. Unless otherwise documented in the medical records, we assumed this group of individuals to be immune against viral hepatitis A. Similarly, individuals of age under 19 were considered immune against tetanus/diphtheria by virtue of governmental national childhood immunization program. Tetanus/diphtheria/acellular pertussis vaccine was not differentiated from tetanus/diphtheria vaccine.
To investigate the prescription of MPM, travel pattern information and demographic factors were analyzed among the individuals travelling to high or moderate malaria infection risk areas. The relative risk of malaria infection during travelling was defined according to the Center for Disease Control and Prevention guidelines (http://www.cdc.gov/malaria/travelers/country_table/a.html Accessed in October 2014). Countries with infection risk limited to certain areas were excluded (3 among 26 high risk and 11 among 22 moderate risk) to minimize ambiguity. A list of 34 countries was selected for the MPM analysis (Supplement 1). To improve statistical accuracy during MPM analysis, travel purposes were categorized as: (i) business/professional activities (including business, large activity/event, and research/education), (ii) service activities (including non-medical and medical service, and missionary), and (iii) leisure (including tourism and visiting friends/relatives). Individuals were excluded from the MPM analysis if they visited multiple countries because the duration information for each country was not available.
3. Statistical analysis
Statistical analyses were performed by SPSS version 16 (IBM Corporation, Armonk, NY, USA). We compared the medians for continuous variables using the Mann-Whitney Test. For the proportions of categorical variables, we used Pearson's chi-square test or Fisher's exact test (for sparse data). Travel purpose and destination continent analysis was done by comparisons between a specific category and the others. Binary logistic regression was used for multivariate analysis. The Hosmer-Lemeshow (HL) test was used to assess the fitness of logistic regression model. All tests were two-tailed, and a P value of <0.05 was considered significant. This study was approved by institutional review board.
Results
1. General characteristics of study population
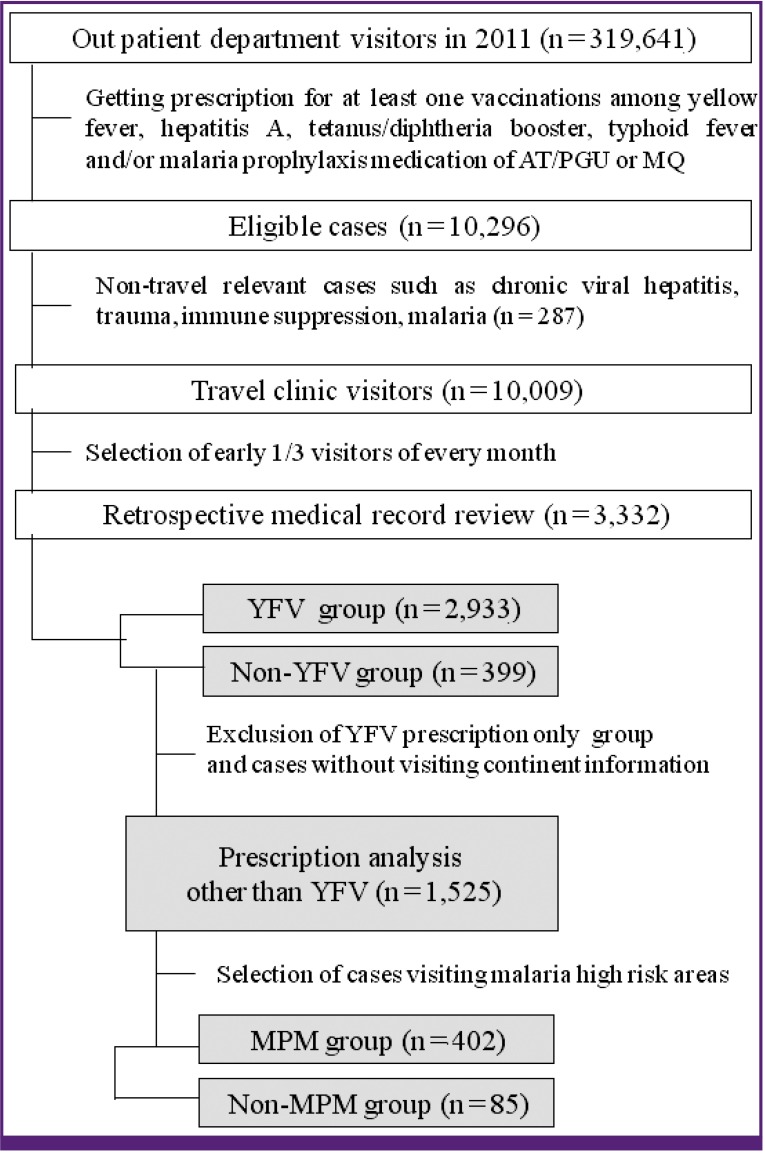
During 2011, 10,009 visited our travel clinic and got prescription for vaccination and MPM as described in the method section (Fig. 1). Among them, 264 individuals were excluded whose visiting continent information was unavailable or uncategorizable and 3,332 cases were selected for retrospective medical record review about the travel destination, purpose, duration, and vaccine/MPM prescriptions (Table 1). The male to female ratio was 58:42, and the median age was 33 years overall (36 for men and 29 for women, P < 0.001). About half of the subjects were referred for YFV only (1,594, 47.8%), while 1,339 (40.2%) visitors received YFV in combination with other prescriptions. A subset of 399 (12.0%) participants received prescriptions other than YFV.
Figure 1. Selection of eligible cases for analysis on yellow fever vaccination and other prescriptions.
AT/PGU, atovaquone/proguanil; MQ, mefloquine; YFV, yellow fever vaccination; MPM, malaria prophylaxis medication.
Table 1. Travel characteristics regarding yellow fever vaccination.
| Characteristicsa | YFV (n = 2,933) |
P-value (YFV only vs. YFV in combination) |
Non-YFV (n = 399) |
P-value (YFV vs. Non-YFV) |
||
|---|---|---|---|---|---|---|
| YFV onlyb (n = 1,594) |
YFV in combination (n = 1,339) |
|||||
| Sex | 0.005 | 0.187 | ||||
| Male | 897 (46.3) | 822 (42.4) | 220 (11.3) | |||
| Female | 697 (50.0) | 517 (37.1) | 179 (12.8) | |||
| Age, year; median (IQR) | 32 (25-46) | 35 (27-47) | 0.001 | 30 (24-40) | <0.001 | |
| Duration, day; median (IQR)c | 11 (7-21) | 15 (11-34.75) | <0.001 | |||
| 1-7 | 291 (84.6) | 53 (15.4) | ||||
| 8-14 | 277 (79.1) | 73 (20.9) | ||||
| 15-30 | 213 (64.2) | 119 (35.8) | ||||
| 31-360 | 106 (74.1) | 37 (25.9) | ||||
| 361- | 71 (59.7) | 48 (40.3) | ||||
| Purposec | ||||||
| Business | 492 (44.0) | 558 (50.0) | <0.001 | 67 (6.0) | 0.001 | |
| Providing non-medical service | 166 (39.1) | 171 (40.2) | 0.034 | 88 (20.7) | <0.001 | |
| Tourism | 656 (59.3) | 413 (37.3) | <0.001 | 38 (3.4) | <0.001 | |
| Research/education | 38 (43.7) | 23 (26.4) | 0.226 | 26 (29.9) | <0.001 | |
| Visiting friends and relatives | 36 (51.4) | 24 (34.3) | 0.401 | 10 (14.3) | 0.064 | |
| Providing medical service | 47 (72.3) | 11 (16.9) | <0.001 | 7 (10.8) | 0.458 | |
| Large activity and event | 21 (30.0) | 45 (64.3) | <0.001 | 4 (5.7) | 0.434 | |
| Missionary | 59 (67.8) | 24 (27.6) | 0.002 | 4 (4.6) | 0.208 | |
| Etc. | 39 (54.9) | 20 (28.2) | 0.074 | 12 (16.9) | 0.007 | |
| Destination countryc,d | ||||||
| Kenya | 298 (18.7) | 272 (20.3) | India | 53 (15.6) | ||
| Tanzania | 213 (13.4) | 237 (17.7) | Peru | 53 (15.6) | ||
| Brazil | 162 (10.2) | 61 (4.6) | Sri Lanka | 28 (8.3) | ||
| Bolivia | 170 (10.7) | 51 (3.8) | Vietnam | 25 (7.4) | ||
| S. Africa | 93 (5.8) | 122 (9.1) | Cambodia | 20 (5.9) | ||
| Ethiopia | 94 (5.9) | 108 (8.1) | U.S.A. | 19 (5.6) | ||
| Ghana | 99 (6.2) | 83 (6.2) | Philippines | 18 (5.3) | ||
| Peru | 131 (8.2) | 39 (2.9) | Indonesia | 15 (4.4) | ||
YFV, yellow fever vaccination; IQR, interquartile range; S. Africa, South Africa; U.S.A., The United States of America.
aValue is number of cases and those in parenthesis represent % unless otherwise identified.
bTravel duration was not available in YFV-only group because only a brief note of destination and purpose was used in this group.
cValues were not available in all cases.
dMost commonly referred eight countries in each group were described.
2. Yellow fever vaccine and travel pattern description
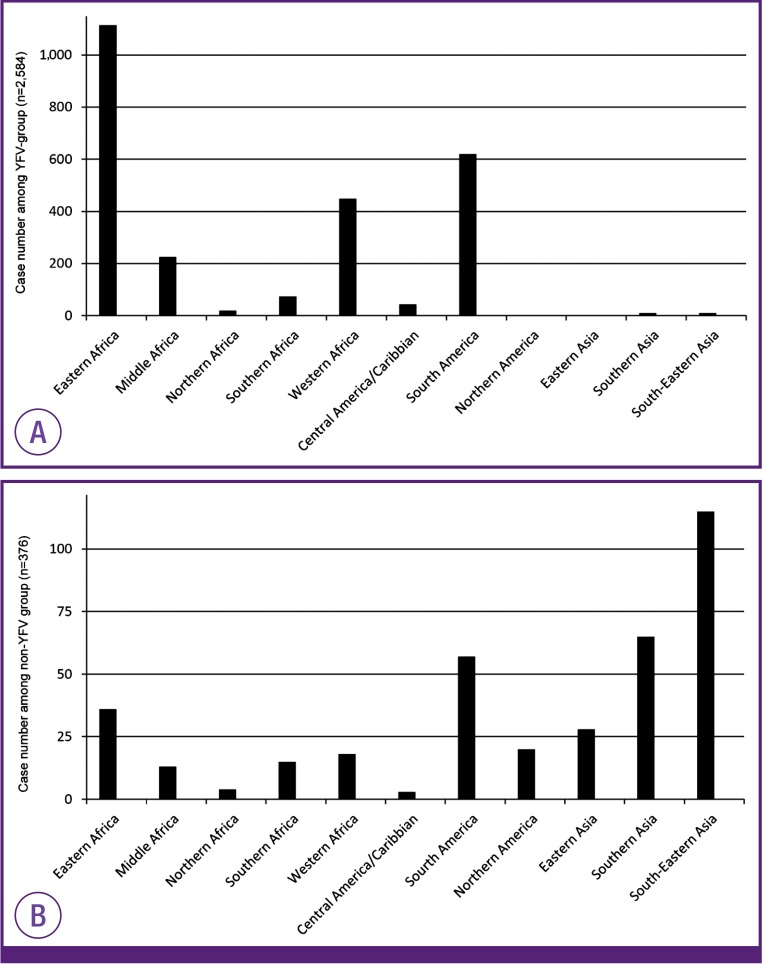
We first investigated differences between people receiving the YFV as part of their travel consultation visit (N = 2,933) and those without YFV (N = 399). When compared with non-YFV group, YFV group revealed older median age and shorter travel duration. Regarding purpose of travel, business (N = 1,117) and tourism (N = 1,107) were most commonly reported overall; however, providing non-medical service was the most common travel purpose among the non-YFV group followed by business. When comparing the YFV and non-YFV group, business (P = 0.001) and tourism (P < 0.001) were more common among the YFV group while providing non-medical service (P < 0.001) and research or education (P < 0.001) were more common among the non-YFV group. As mentioned before, nearly half of our travel clinic visitors got only YFV. When YFV only group was compared with the group with other prescription besides YFV (YFV in combination), female was more common in YFV only group (P = 0.005) and median age was younger in YFV only group. Regarding the travel purpose, people for business (P < 0.001), providing non-medical service (P = 0.034), and large activity/event (P < 0.001) were more common among YFV in combination group, while tourism (P < 0.001), providing medical service (P < 0.001), and missionary (P = 0.002) were more common purposes among YFV only group. The most frequent travel destinations were Kenya (19.4 %), Tanzania (15.3%) and Brazil (7.6%) in the YFV group while India (15.6%), Peru (15.6%), and Sri Lanka (8.3%) were common in non-YFV group. Regarding destination continent analysis, 2,960 cases were reviewed after excluding 372 cases with multiple continents. In the YFV group, East Africa (43.4%), South America (24.1%), and Western Africa (17.0%) were frequently noted as the visiting continent (Fig. 2). Among non-YFV group, South-Eastern Asia (35.9%), Southern Asia (17.3%), and South America (15.2%) were common destination continents.
Figure 2. Number of travelers by the visiting continents (A) Yellow fever vaccination group. (B) Non-yellow fever vaccination group. Continents with proportion of less than 1% were not visualized.
YFV, yellow fever vaccination.
3. Sub-analysis of prescription of other vaccines (excluding YFV) and MPM
We also performed an analysis for other vaccines and MPM including 1,525 cases (Table 2) after excluding 1,435 cases that were referred for YFV only. The typhoid vaccine was the most commonly prescribed among the eligible travel clinic visitors (827/1,525, 54.2%). However, the coverage for hepatitis A was higher (74.7%) than typhoid (55.5%) considering the natural immunity (age >40, n = 529) and prior vaccination history (n = 62) as well as vaccination at our clinic (n = 548) in group of age under 40. Besides hepatitis A and typhoid/diphtheria/tetanus booster, frequently prescribed vaccines among travel clinic visitors were hepatitis B booster (vaccination prescription, [n = 19]/history of valid one, [n = 94]), seasonal influenza (prescription: 38/history: 50), tetravalent meningococcal vaccine (prescription: 12/history: 26), Japanese encephalitis B booster (prescription: 10/history: 1), polio booster (prescription: 7/history: 0), rabies (prescription: 6/history: 0) and measles/mumps/rubella booster (prescription: 5/history: 2).
Table 2. Prescription of major vaccines and anti-malarial drugs other than yellow fever vaccination.
| Destination | Hepatitis A | Tetanus/diphtheria booster | Typhoid | Anti-malarial drug | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinationa | Coverageb (%) | Vaccinationc | Coverage (%) | Vaccination | Coverage (%) | AT/PGU | MQ | Others | Coverage (%) | ||
| Eastern Africa | 174 (248) | 75.1 | 104 (50) | 27.1 | 261 (9) | 48.0 | 333 | 105 | 26 | 82.6 | 562 |
| Middle Africa | 22 (75) | 63.0 | 31 (37) | 41.7 | 59 (0) | 38.3 | 105 | 31 | 1 | 89.0 | 154 |
| Northern Africa | 5 (8) | 68.4 | 5 (1) | 41.2 | 16 (0) | 84.2 | 7 | 0 | 0 | 36.8 | 19 |
| Southern Africa | 21 (16) | 84.1 | 10 (4) | 28.8 | 32 (2) | 77.3 | 8 | 12 | 2 | 50.0 | 44 |
| Western Africa | 86 (109) | 78.3 | 48 (15) | 24.5 | 139 (0) | 55.8 | 143 | 48 | 12 | 81.5 | 249 |
| Central America/Mexico/Caribbean | 14 (6) | 95.2 | 5 (1) | 25.9 | 17 (0) | 81.0 | 5 | 7 | 1 | 61.9 | 21 |
| South America | 131 (54) | 78.1 | 32 (17) | 21.7 | 164 (3) | 70.5 | 42 | 40 | 6 | 37.1 | 237 |
| Northern America | 11 (0) | 52.4 | 0 (5) | 32.0 | 1 (0) | 4.8 | 0 | 0 | 0 | 0.0 | 21 |
| Eastern Asia | 6 (1) | 77.8 | 4 (0) | 41.7 | 7 (0) | 77.8 | 0 | 1 | 0 | 11.1 | 9 |
| Southern Asia | 26 (22) | 67.6 | 12 (15) | 39.2 | 43 (2) | 63.4 | 29 | 31 | 6 | 93.0 | 71 |
| South-Eastern Asia | 52 (52) | 75.4 | 31 (15) | 35.4 | 88 (4) | 66.7 | 61 | 22 | 3 | 62.3 | 138 |
| Total | 548 (591) | 74.7 | 282 (160) | 28.9 | 827 (20) | 55.5 | 733 | 297 | 57d | 71.3 | 1,525 |
AT/PGU, atovaquone/proguanil; MQ, mefloquine.
aNumber in parenthesis represents the cases with history of valid vaccination or known immunity. Individuals of age over 40 were regards as naturally immune against hepatitis A and vaccination count was limited to the group of age under 40 in hepatitis A.
bCoverage was calculated as the proportion of individuals who got prescription of vaccination or with the history of valid vaccination/immunity.
cIndividuals of age under 19 were regards as immune against tetanus/diphtheria by virtue of the national immunization program in their childhood. Vaccination count was limited to the group of age over 19 in tetanus/diphtheria.
d48 individuals got prescription from other institutions and 7 individuals got prescription for both AT/PGU and MQ. Two individuals got prescription for doxycycline and hydroxychlorquine.
4. High-risk areas with/without MPM
Overall, MPM was prescribed to 1,087 (71.3%) among the selected 1,525 cases. AT/PGU was the most commonly prescribed MPM (n = 733) followed by MQ (n = 297). Among them, total of 610 individuals were traveling to 28 predefined high-risk countries and 487 were selected for this additional MPM analysis (123 were excluded for reporting visiting of multiple countries). Overall, Ghana was the most common destination (64, 13.1%), followed by Senegal and Cameroon (57, 11.7%, respectively), Nigeria (51, 10.5%), and Zambia (30, 6.2%). Among this group, 402 individuals (82.5%) received a prescription for MPM. Compared to non-MPM, male gender was more common in MPM group (67.7 % vs. 51.8 %, P = 0.006) and group of age under 40 was less likely to get MPM prescription than older groups (Odds ratio, OR 2.294, P = 0.006 for age 41-55 and OR 6.700, P = 0.010 for age ≥56, Table 3). Also, travel duration over ten days were less likely to get MPM prescription than the group with shorter duration less than 10 days (OR 0.289, P < 0.001). Regarding the travel purpose, individuals travelling for business/professional activity received MPM more frequently compared to the group for leisure (OR 3.729, P = 0.001), while it was less common among those traveling for providing service (OR 0.162, P < 0.001). Travel destination of Middle Africa were related with more frequent prescription of MPM when compared with other continents, as Western Africa and Eastern Africa (OR 1.890, P = 0.029). In multivariate analysis, group of age over 55 received MPM prescription more frequently (OR 8.923, P = 0.041, compared to the group of age under 40) while those with travel purpose of providing service (OR 0.136, P < 0.001, compared to leisure) and travel duration of more than 10 days (OR 0.407, P = 0.015, compared to the group of travel duration ≤10 days) received MPM prescription less frequently.
Table 3. Binary logistic regression model for prescription of malaria prophylaxis drug among travelers with high infection risk.
| Selection variable | Non-MPM group (n = 85)a |
MPM group (n = 402) |
Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-valueb | OR | 95% CI | P-valuea | |||
| Sex | ||||||||
| Female | 41 (24.0) | 130 (76.0) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| Male | 44 (13.9) | 272 (86.1) | 1.950 | 1.214-3.132 | 0.006 | 1.115 | 0.528-2.353 | 0.775 |
| Age, year | ||||||||
| ≤40 | 67 (22.6) | 230 (77.4) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| 41-55 | 16 (11.3) | 126 (88.7) | 2.294 | 1.275-4.126 | 0.006 | 1.889 | 0.770-4.634 | 0.165 |
| ≥56 | 2 (4.2) | 46 (95.8) | 6.700 | 1.585-28.325 | 0.010 | 8.923 | 1.093-72.844 | 0.041 |
| Travel duration, day | ||||||||
| ≤10 | 19 (8.3) | 209 (91.7) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| ≥11 | 46 (24.0) | 146 (76.0) | 0.289 | 0.162-0.513 | <0.001 | 0.407 | 0.198-0.873 | 0.015 |
| Destination | ||||||||
| Other continentsc | 68 (19.9) | 273 (80.1) | 1(Ref.) | - | - | 1 (Ref.) | - | - |
| Middle Africa | 17 (11.6) | 129 (88.4) | 1.890 | 1.068-3.346 | 0.029 | 1.309 | 0.512-3.347 | 0.575 |
| Purposed | ||||||||
| Leisure | 12 (18.5) | 53 (81.5) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| Business/professional activity | 17 (5.7) | 280 (94.3) | 3.729 | 1.684-8.259 | 0.001 | 2.516 | 0.944-6.707 | 0.065 |
| Providing service | 42 (58.3) | 30 (41.7) | 0.162 | 0.074-0.354 | <0.001 | 0.136 | 0.051-0.365 | <0.001 |
MPM, malaria prophylaxis medication; OR, odds ratio; CI, confidence interval.
aValue is number of cases and those in parenthesis represent % unless otherwise identified.
bP-values of the Hosmer-Lemeshow test statistics is 0.352. Therefore, this logistics model is well fit for the data.
cThis includes Western Africa (n = 217), East Africa (n = 111), and etc. group (n = 4).
dLeisure, Business/professional activity, and Providing service represent tourism/visiting friends·relatives, business/large activity·event/research·education, and providing non-medical service/providing medical service/missionary, respectively. 53 cases were excluded because travel purpose information was not available or uncategorizable.
Discussion
In this study, we aimed to understand the difference in demographics and travel patterns between group of YFV and those without YFV at a travel clinic specialized in YFV in Seoul, South Korea. In addition, we also aimed to identify the risk groups who are likely not to receive optimal preventive measures especially for malaria. There have been several reports on travel related illnesses and pre-travel survey among travelers departing from East Asia region [5,8,15,16,17,18]. However, few data are available about detailed travel patterns and pre-travel prescriptions in this region compared to western countries [1,19,20,21]. Therefore, we believe that our data will contribute toward improving future prevention strategies.
Since our clinic is specialized in providing YFV, the proportion of visitors receiving YFV is extremely high (86.1%) [21] compared to some other studies (8.9-38%) [1,20] and the travel destinations were more frequently Africa and South America since YFV is a mandatory preventive measure in some areas in these continents. Interestingly, Peru was included in the frequent destinations among YFV group while YFV is not mandatory but only recommended for travelers who are visiting specific areas such as Amazon regions. It may be explained by the fact that most people visiting Peru also visit Bolivia where YFV is required if traveling from a country with risk of YFV transmission which includes Peru. Actually among the 171 individuals visiting Peru in YFV group, about two thirds also visited Bolivia. About half of the individuals (47.8%) who visited our clinic received only YFV prescription. The most likely explanation for this observation is that many of them received prescriptions for other standard vaccines (except for YFV) and MPM at other medical institutions. Alternatively, some people with minimal risk exposure (such as for example those traveling to cities or just transitioning through airports) or other personal reasons, opted to receive only the mandatory YFV to visit specific areas. However, more systematic approach is required for this population considering that areas of yellow fever epidemic are also vulnerable to other major travel related morbidities such as malaria. Our travel clinic has revised the internal rule to utilize official travel clinic record form for all visitors including YFV only group since 2014.
In our primary analysis, we compared the characteristics of people with YFV administration to those of people who did not receive YFV. Providing non-medical service was the most common purpose among the non-YFV group while business and tourism were the most common travel purposes overall. Proportion of people traveling for providing volunteering services was as high as 13.7% (425/3,099) in our study. In the United Nations World Tourism Organization report, this category was insignificant and thus uncategorized. Because volunteering services are usually provided in developing countries, inclusive rural areas, this particular category of travelers is more likely to visit specialized travel clinics independently of YFV administration, as observed in another similar study [22].
Besides YFV, typhoid vaccination was prescribed most commonly (52.1%) and vaccination rate reached 82.0% (237/289) when we analyzed the subgroup of traveling to developing countries with travel duration of over one month considering the vaccination recommendation (Data not shown. Categorization of developing region was done according to the United Nations Statistics Division Rules). While typhoid vaccination rate was comparable with other studies [21], hepatitis A vaccination revealed different pattern. Hepatitis A vaccination is recommended to the all individuals traveling to endemic area regardless of purpose, frequency and duration [23] and some studies revealed vaccination or immunity of more than 90% among travel clinic visitors [1,20]. In South Korea, seroprevalence of hepatitis A IgG is over 90% among people of age > 40 before 2010 and vaccination was not recommended in this group when our study was done [14]. Considering this epidemiologic feature, 76.1% was considered immune to hepatitis A overall, including the vaccination provided to individuals <40 years of age. However, only 59.4% of 1,009 eligible individuals <40 years of age traveling to developing regions received hepatitis A vaccination in our study population (Data not shown). These observations suggest that a more proactive approach is required for hepatitis A vaccination, considering that the median age of travel clinic visitors was 33 (IQR 26-46) and the proportion of individuals with hepatitis A natural immunity is decreasing in South Korea. Meningococcal vaccination was officially introduced in 2012 after our study period and rabies vaccination are rarely prescribed to travelers due to the complex injection schedule and high cost as well as limited access as it is provided as an orphan drug. Considering this situation, information regarding post exposure prophylasix against rabies should be provided to the individuals who are planning to visit the rabies endemic areas.
Regarding MPM, only 82.5% of individuals visiting high-risk areas received a prescription for MPM. According to the recent report [10], most common areas where Korean travelers have contracted Plasmodium falciparum malaria were Ghana, Nigeria, and Uganda, which are ranked as 4th, 1st and 8th among the destinations of individuals who were included in our MPM analysis. In multivariate analysis, individuals with travel purpose of providing service were less likely to receive MPM prescription when compared with the leisure group. This might be because volunteers are less likely to consider their health as the top priority issue compared to other individuals traveling for business or leisure and the pre-travel medical expense is not reimbursed by medical insurance in South Korea. MPM should be emphasized to this group considering that volunteering services are usually provided in developing countries, especially rural areas. In the aspect of cost issue, doxycyline could be a good alternative to AT/PGU or MQ.
On the other hand, companies or institution most likely reimburse individuals traveling for business/professional activity for the vaccine or MPM prescriptions, which might explains why this group received MPM prescription more frequently compared with the leisure group in our study (P = 0.065). In contrast to our findings, previous studies reported business travel as a negative factor for malaria preventive measures. This difference might be related to different study population and categorization of travel purposes [19,24,25]. For the MPM prescription analysis we re-categorized travel purposes into three groups for better statistical analysis. For example, we considered people traveling for visiting friends/relatives and for tourism into the category of leisure. Usually, travelers with purpose of visiting friends/relatives have increased risk of travel related health problems, so called immigrant visiting friends/relatives [26]. However, in our study, 90% of people reporting the purpose as visiting friends/relatives were Korean and their travel related risk is likely comparable to general tourists for leisure rather than immigrant visiting friends/relatives who are traveling to the developing countries of their origin.
It is intuitive that longer travel duration is a barrier to MPM not only for the compliance during the travel [27] but also for the access itself due to the cost issue. In a prior study, duration more than six weeks was negatively related with chemoprophylaxis in a group of people visiting friends and relatives to Western Africa [25]. In our study, categorization of travel duration using a cutoff of 10 days resulted in significantly different rates of MPM (90.3% vs. 64.1%, P < 0.001), revealing that less MPM prescription in group of longer duration and it was consistent in multivariate analysis after accounting for other factors such as age, sex, destination continent, and purpose. Of note, this difference was not significant when a cutoff of 20 or 30 days was applied (P = 0.646 and P = 0.483, respectively). The lack of MPM prescription in people traveling for longer duration might be problematic, since the incidence of malaria increases proportionally with the length of stay and one study reported median duration of stay as 32 days (IQR 21-62) among 21,888 imported Plasmodium falciparum malaria cases [28]. If cost is the limiting factor for long term travelers, MQ or doxycylcine could be prescribed as lower-costs alternatives to AT/PGU [29] and non-pharmacologic preventive measures such as insect repellant, wearing long sleeves/trousers, or bed-net should be emphasized, especially if MPM is not available. MQ and AT/PGU were about 99% among MPM prescriptions in our study. It has been known that several factors influence choosing MPM such as effectiveness, adverse effect concerns, dosing convenience, previous experiences, and cost [30]. In one report, AT/PGU and doxycycline were well tolerated compared with MQ [31], and travelers who chose MQ and AT/PGU revealed a tendency to be older than those selected doxycycline in another report (43.3 ± 14.5, 37.6 ± 11.2, and 33.8 ± 10.7, respectively) [30]. However, Senn et al. reported that when objective written information was provided, travelers most frequently chose MQ for chemoprophylaxis and suggested that evidence-based information weighs more heavily than negative publicity [29]. In our analysis about the high risk travelers, MQ and AT/PGU were prescribed for 96 and 288 travelers, respectively. When epidemiologic and clinical factors were analyzed, shorter duration and purpose of business were associated with AT/PGU while MQ was more frequently prescribed to the travelers for visiting friends/relatives. However, only longer duration was independently associated with MQ preference (Data not shown). Regarding the MQ resistance issue in South-Eastern Asia, travelers to this area were not included in our high malaria risk travelers analysis because MPM is not recommended to the usual travelers who are visiting the urban areas or resorts in this region. When the target population was expanded to all travelers who got prescriptions for MPM (N = 1,226), the proportion of AT/PGU was not different between South-Eastern regions and other regions (74.7% and 72.6%, respectively). This may be related with the fact that few people visit the border areas in South-Eastern Asia countries where the MQ resistance issue actually matters. In our clinic, we explain merits and faults of every MPM and let the visitors to choose their own. In the case of visitor's request for the doctor's opinion, MQ is recommended rather than AT/PGU for travel of more than two weeks' duration, if there is no medical or non-medical limitation in MPD selection. Another factor that was independently associated with frequent MPM prescription was age over 55 when compared with the group of age under 40. This might be a consequence of increased concern about health issue, higher incidence of co-morbidity, and more financial capability for MPM in older population.
Our study harbors several limitations. First, this is a single center experience and travel destinations were skewed to areas where yellow fever is prevailing because our institution was the only place offering YFV in the area with ten million residents. Second, several large groups providing voluntary services visited our clinic, and they influenced the distribution of visiting country frequencies. In the case of Peru and Sri Lanka, a single group comprised more than half of all visitors to each country among those in the non-YFV group. Third, we included only MQ and AT/PGU for MPM analysis. However, doxycycline, primaquine, and hydroxychlorquine were prescribed in just about 1% among the visitors who received MPM prescription during the study period and we suppose that they might have made little influence on our study result.
Despite these limitations, we believe that our study may provide valuable information about the demographics and travel patterns of travel clinic visitors in South Korea. YFV is one of the key subjects during travel clinic consultation and travelers to the areas of yellow fever epidemic are also vulnerable to other major travel related morbidities such as malaria, typhoid fever, and hepatitis A [2]. Therefore, our study population represents one of the main target groups for pre-travel medical assistance. Besides YFV, demographic and epidemiologic features should be considered for hepatitis A vaccination and preventive measures for malaria should be emphasized to the groups of younger age, travel duration >10 days, and purpose of providing service during the pre-travel consultation in South Korea.
Acknowledgement
Ms. Yoo Jeongsuk (Department of Medical Record Service, National Medical Center) sophisticatedly constructed travel clinic visitor cohort from the electrical medical database. This study was supported by University of California San Diego, Center for AIDS Research (UCSD CFAR) TC AI036214, UCSD CFAR 5 P30 AI036214, University of California San Francisco-Gladstone Institute of Virology and Immunology, Center for AIDS Research (UCSF-GIVI CFAR) 5 P30 AI027763, San Diego Primary Infections Resource Consortium (PIRC) 5 R24 AI106039.
Supplementary material
Guideline Korean version.
Supplementary material can be found with this article on-line http://www.icjournal.org/src/sm/ic-48-20-s001.pdf.
Countries with high or moderate relative risk of malaria infection nationwide during travellinga
References
- 1.LaRocque RC, Rao SR, Lee J, Ansdell V, Yates JA, Schwartz BS, Knouse M, Cahill J, Hagmann S, Vinetz J, Connor BA, Goad JA, Oladele A, Alvarez S, Stauffer W, Walker P, Kozarsky P, Franco-Paredes C, Dismukes R, Rosen J, Hynes NA, Jacquerioz F, McLellan S, Hale D, Sofarelli T, Schoenfeld D, Marano N, Brunette G, Jentes ES, Yanni E, Sotir MJ, Ryan ET Global TravEpiNet Consortium. Global TravEpiNet: a national consortium of clinics providing care to international travelers--analysis of demographic characteristics, travel destinations, and pretravel healthcare of high-risk US international travelers, 2009-2011. Clin Infect Dis. 2012;54:455–462. doi: 10.1093/cid/cir839. [DOI] [PubMed] [Google Scholar]
- 2.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS GeoSentinel Surveillance Network. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 3.van Genderen PJ, van Thiel PP, Mulder PG, Overbosch D Dutch Schiphol Airport Study Group. Trends in knowledge, attitudes, and practices of travel risk groups toward prevention of hepatitis A: results from the Dutch Schiphol Airport survey 2002 to 2009. J Travel Med. 2012;19:35–43. doi: 10.1111/j.1708-8305.2011.00578.x. [DOI] [PubMed] [Google Scholar]
- 4.Lobel HO, Baker MA, Gras FA, Stennies GM, Meerburg P, Hiemstra E, Parise M, Odero M, Waiyaki P. Use of malaria prevention measures by North American and European travelers to East Africa. J Travel Med. 2001;8:167–172. doi: 10.2310/7060.2001.22206. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M, Liu Z, He H, Luo L, Wang S, Bu H, Zhou X. Knowledge, Attitudes, and Practices on Malaria Prevention Among Chinese International Travelers. J Travel Med. 2011;18:173–177. doi: 10.1111/j.1708-8305.2011.00512.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilder-Smith A, Khairullah NS, Song JH, Chen CY, Torresi J. Travel health knowledge, attitudes and practices among Australasian travelers. J Travel Med. 2004;11:9–15. doi: 10.2310/7060.2004.13600. [DOI] [PubMed] [Google Scholar]
- 7.Heywood A, Watkins RE, Iamsirithaworn S, Nilvarangkul K, MacIntyre CR. A cross-sectional study of pre-travel health-seeking practices among travelers departing Sydney and Bangkok airports. BMC Public Health. 2012;12:321. doi: 10.1186/1471-2458-12-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo YJ, Bae GO, Choi JH, Shin HC, Ga H, Shin SR, Kim MS, Joo KJ, Park CH, Yoon HJ, Baek HJ. Korean travelers' knowledge, attitudes, and practices regarding the prevention of malaria: measures taken by travelers departing for India from Incheon International Airport. J Travel Med. 2007;14:381–385. doi: 10.1111/j.1708-8305.2007.00157.x. [DOI] [PubMed] [Google Scholar]
- 9.Choi SH, Kim YJ, Shin JH, Yoo KH, Sung KW, Koo HH. International travel of Korean children and Dengue fever: A single institutional analysis. Korean J Pediatr. 2010;53:701–704. doi: 10.3345/kjp.2010.53.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, Gwack J, Yoon SK. Epidemiologic characteristics of imported malaria in Korea, 2003-2012. Public Health Wkly Rep. 2014;7:1–7. [Google Scholar]
- 11.Boggild AK, Castelli F, Gautret P, Torresi J, von Sonnenburg F, Barnett ED, Greenaway CA, Lim PL, Schwartz E, Wilder-Smith A, Wilson ME GeoSentinel Surveillance Network. Vaccine preventable diseases in returned international travelers: results from the GeoSentinel Surveillance Network. Vaccine. 2010;28:7389–7395. doi: 10.1016/j.vaccine.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Sicard S, Simon F, Soula G, Gazin P. Efficacy of antimalarial chemoprophylaxis for travelers. J Travel Med. 2009;16:66–67. doi: 10.1111/j.1708-8305.2008.00265.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang JH, Kim HB, Sohn JW, Lee SO, Chung MH, Cheong HJ, Choi YH, Choi JH, Choi JY, Choe HJ. Adult immunization schedule recommended by the Korean Society of Infectious Diseases, 2007. Infect Chemother. 2008;40:1–13. [Google Scholar]
- 14.Chung SJ, Kim TY, Kim SM, Roh M, Yu MY, Lee JH, Oh C, Lee EY, Lee S, Jeon YC, Yoo KS, Sohn JH. Changes in the seroprevalence of IgG anti-hepatitis A virus between 2001 and 2013: experience at a single center in Korea. Clin Mol Hepatol. 2014;20:162–167. doi: 10.3350/cmh.2014.20.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamada A. Past, present and future of travel medicine in Japan. Travel Med Infect Dis. 2011;9:187–191. doi: 10.1016/j.tmaid.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno Y, Kudo K. Travel-related health problems in Japanese travelers. Travel Med Infect Dis. 2009;7:296–300. doi: 10.1016/j.tmaid.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Ahn JY, Chung JW, Chang KJ, You MH, Chai JS, Kang YA, Kim SH, Jeoung H, Cheon D, Jeoung A, Choi ES. Clinical characteristics and etiology of travelers' diarrhea among Korean travelers visiting South-East Asia. J Korean Med Sci. 2011;26:196–200. doi: 10.3346/jkms.2011.26.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung KK, Lin AK, Cheng CK, Chan EY, Graham CA. Travel health risk perceptions and preparations among travelers at Hong Kong international airport. J Travel Med. 2014;21:288–291. doi: 10.1111/jtm.12112. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Velez R, Bayas JM. Spanish travelers to high-risk areas in the tropics: airport survey of travel health knowledge, attitudes, and practices in vaccination and malaria prevention. J Travel Med. 2007;14:297–305. doi: 10.1111/j.1708-8305.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 20.Angelin M, Evengård B, Palmgren H. Travel and vaccination patterns: a report from a travel medicine clinic in northern Sweden. Scand J Infect Dis. 2011;43:714–720. doi: 10.3109/00365548.2011.581306. [DOI] [PubMed] [Google Scholar]
- 21.Pavli A, Smeti P, Spilioti A, Silvestros C, Katerelos P, Maltezou HC. Vaccinations and malaria prophylaxis for long-term travellers travelling from Greece: A prospective, questionnaire-based analysis. Travel Med Infect Dis. 2014;12:764–770. doi: 10.1016/j.tmaid.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Durham MJ, Goad JA, Neinstein LS, Lou M. A comparison of pharmacist travel-health specialists' versus primary care providers' recommendations for travel-related medications, vaccinations, and patient compliance in a college health setting. J Travel Med. 2011;18:20–25. doi: 10.1111/j.1708-8305.2010.00470.x. [DOI] [PubMed] [Google Scholar]
- 23.Askling HH, Rombo L, Andersson Y, Martin S, Ekdahl K. Hepatitis A risk in travelers. J Travel Med. 2009;16:233–238. doi: 10.1111/j.1708-8305.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- 24.Schilthuis HJ, Goossens I, Ligthelm RJ, de Vlas SJ, Varkevisser C, Richardus JH. Factors determining use of pre-travel preventive health services by West African immigrants in The Netherlands. Trop Med Int Health. 2007;12:990–998. doi: 10.1111/j.1365-3156.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 25.Wieten RW, Harting J, Biemond PM, Grobusch MP, van Vugt M. Towards improved uptake of malaria chemoprophylaxis among West African travellers: identification of behavioural determinants. Malar J. 2013;12:360. doi: 10.1186/1475-2875-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leder K, Tong S, Weld L, Kain KC, Wilder-Smith A, von Sonnenburg F, Black J, Brown GV, Torresi J GeoSentinel Surveillance Network. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–1193. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee S. Compliance of malaria chemoprophylaxis among travelers to India. J Travel Med. 1999;6:7–11. doi: 10.2310/7060.1999.00003. [DOI] [PubMed] [Google Scholar]
- 28.Seringe E, Thellier M, Fontanet A, Legros F, Bouchaud O, Ancelle T, Kendjo E, Houze S, Le Bras J, Danis M, Durand R French National Reference Center for Imported Malaria Study Group. Severe imported Plasmodium falciparum malaria, France, 1996-2003. Emerg Infect Dis. 2011;17:807–813. doi: 10.3201/eid1705.101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senn N, D'Acremont V, Landry P, Genton B. Malaria chemoprophylaxis: what do the travelers choose, and how does pretravel consultation influence their final decision. Am J Trop Med Hyg. 2007;77:1010–1014. [PubMed] [Google Scholar]
- 30.Goodyer L, Rice L, Martin A. Choice of and adherence to prophylactic antimalarials. J Travel Med. 2011;18:245–249. doi: 10.1111/j.1708-8305.2011.00534.x. [DOI] [PubMed] [Google Scholar]
- 31.Schlagenhauf P, Tschopp A, Johnson R, Nothdurft HD, Beck B, Schwartz E, Herold M, Krebs B, Veit O, Allwinn R, Steffen R. Tolerability of malaria chemoprophylaxis in non-immune travellers to sub-Saharan Africa: multicentre, randomised, double blind, four arm study. BMJ. 2003;327:1078. doi: 10.1136/bmj.327.7423.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Countries with high or moderate relative risk of malaria infection nationwide during travellinga