Abstract
The purpose of this study was to investigate spatial variations in measured wave speed in the relaxed and stretched Achilles tendons of young and middle-aged adults. Wave speed was measured from the distal Achilles tendon, soleus aponeurosis, medial gastrocnemius aponeurosis and medial gastrocnemius muscle in healthy young (n = 15, aged 25 ± 4 years) and middle-aged (n = 10, aged 49 ± 4 years) adults in resting, dorsiflexed and plantarflexed postures. In both age groups, Achilles tendon wave speed decreased proximally, with the lowest wave speed measured in the gastrocnemius aponeurosis. Measured wave speed increased with passive dorsiflexion, reflecting the strain-stiffening behavior of tendons. There were no significant aging effects on wave speed in the free tendon or soleus aponeurosis. However, a significant, inverse relationship between gastrocnemius aponeurosis wave speed and age was observed in the dorsiflexed posture. We also observed significantly lower wave speeds in the gastrocnemius muscles of middle-aged adults when compared with young adults. These results suggest that Achilles tendon compliance increases in a distal-to-proximal pattern, with middle-aged adults exhibiting greater compliance in the distal gastrocnemius muscle and tendinous structures. An age-related change in the spatial variation in Achilles tendon compliance could affect localised tissue deformation patterns and injury potential within the triceps surae muscle-tendon units.
Keywords: Tendon Compliance, Aponeurosis, Supersonic Shear Imaging, Aging, Triceps Surae
1. Introduction
The incidence of gastrocnemius muscle strain and Achilles tendon injury increases in middle-age. For example, a recent cross-sectional study (n = 2886) found that middle-aged runners (aged 40+ years) reported nearly twice as many plantarflexor muscle and Achilles tendon injuries as their younger (aged <40 years) counterparts (McKean et al, 2006). These soft tissue injuries can be challenging to treat and often have long-term consequences. For example, prior muscle strains are linked to impaired performance and contribute to future injury risk (Kannus et al, 1989; Garrett, 1990; Orchard, 2001). Thus, evaluating the age-related changes to muscle and tendon tissue properties may provide relevant diagnostic information to guide treatment and monitor recovery.
Prior studies have sought to understand the effects of aging on both the structural and mechanical properties of tendon. In comparative animal models, aging has been observed to lead to decreased collagen fibril diameters (Nakagawa et al, 1994), decreased collagen crimp angle (Patterson-Kane et al, 1997; Tuite et al, 1997), and changes in collagen type III content (Birch et al, 1999), though specific age-effects may vary based on the tendon’s primary function (Thorpe et al, 2013; Birch et al, 1999). It is generally believed that microstructural changes lead to an increase in tendon compliance with aging. Biomechanical studies on isolated tendon specimens have supported this supposition with a variety of studies reporting an age-related increase in compliance following maturation (Vogel, 1980, 1983; Blevins et al, 1994). However, in vivo studies have produced conflicting results, with some studies finding a positive (Onambele et al, 2006; Stenroth et al, 2012; Narici et al, 2005; Csapo et al, 2014), negative (Kubo et al, 2007), or lack of (Karamanidis and Arampatzis, 2005) correlation between Achilles tendon compliance and aging. This discrepancy could, in part, reflect limitations of prior measurement methodologies. In the most common approach for measuring in vivo Achilles tendon compliance, ultrasound images are collected while the subject performs a specific task, such as isometric plantarflexion. The motion of the muscle-tendon junction is then tracked to estimate average tendon stretch along the Achilles tendon, which can include the distal free tendon, the soleus aponeurosis and, in some studies, the gastrocnemius aponeurosis (Kubo et al, 2003; Child et al, 2010; Karamanidis and Arampatzis, 2005; Kubo et al, 2007; Rosager et al, 2002). However, this averaging approach may mask non-uniform tendon deformations that can arise from variations in loading, material properties and architecture along the tendon (Finni et al, 2003; Magnusson et al, 2003; Arampatzis et al, 2005; Maganaris and Paul, 2000; Magnusson et al, 2001; Slane and Thelen, 2014; DeWall et al, 2014). Thus, regional characterization of tendon tissue compliance may be important for elucidating aging effects.
Supersonic Shear Imaging (SSI) is a quantitative ultrasound imaging technique for noninvasively inducing and tracking the speed of transient shear waves in tissues (Bercoff et al, 2004). SSI was first used to assess pathological compliance changes in a variety of soft tissues, including the breast (Athanasiou et al, 2010), liver (Bavu et al, 2011), and thyroid (Sebag et al, 2010). SSI has more recently been applied to assess muscle and tendon tissue properties (Brum et al, 2014; Arda et al, 2011; Aubry et al, 2013; Hug et al, 2013; Chen et al, 2013; Chernak et al, 2013; DeWall et al, 2014). Tendon tissue has a transversely isotropic structure, relatively high stiffness and a thickness that is less than the induced shear wave wavelengths, which likely leads to guided wave propagation (Brum et al, 2014). Further, tendon exhibits strain-stiffening behavior at lower loads, which gives rise to an increase in wave speed with passive stretch (Aubry et al, 2013; Hug et al, 2013; DeWall et al, 2014).
The influence of age-related changes in tendon on tissue wave speeds are not well understood. A recent study involving 80 subjects from a wide range of ages (aged 20–83 years, mean: 45.4) found a significant decrease in Achilles free tendon shear wave speed with age when the ankle was plantarflexed, but found no significant age effect when the ankle was in a dorsiflexed or resting posture (Aubry et al, 2013). However, a second study (subjects aged 17–63 years, mean: 37.7) found that shear wave speeds in the free tendon in a relaxed (i.e. slightly plantarflexed) posture did not vary with age (Arda et al, 2011). These disparate results may partly reflect the posture and location dependent nature of Achilles tendon shear wave speeds (DeWall et al, 2014). In a recent study on young adults (DeWall et al, 2014), we observed significant variation in shear wave speed along the Achilles tendon length, with much lower speeds in the soleus and gastrocnemius aponeurosis than in the distal free tendon.
The purpose of this study was to investigate longitudinal spatial variations in measured wave speed in the relaxed and stretched Achilles tendons of middle-aged adults, and to compare these results to data collected from young adults. We hypothesised that, relative to young adults, middle-aged adults would exhibit smaller stretch-induced increases in measured Achilles tendon wave speed, which would reflect a more compliant tendon. Further, we hypothesised that aging would have the greatest effect on measured wave speed in the gastrocnemius aponeurosis, which is adjacent to the location where muscle strain injuries are most prevalent (Garrett, 1990; Kirkendall and Garrett, 2002; Speer et al, 1993).
2. Materials and Methods
Fifteen healthy young adults, and ten healthy middle-aged adults, with no history of Achilles tendon injury, were recruited for this study (Table 1). All subjects gave written consent to participate as per the requirements of the University of Wisconsin-Madison Institutional Review Board. Subjects completed a survey recording average weekly number of hours engaged in mild to strenuous exercise. Prior to testing, subjects walked for six minutes to precondition their muscle-tendon structures (Hawkins et al, 2009). Subjects were then positioned prone on an examination table, fully relaxed, with their knees extended and feet extended over the end of the table. We tested the right leg of each subject, and the resting ankle angle was measured with a goniometer.
Table 1.
Comparison of subject characteristics. There were no significant group differences in resting ankle angle (expressed in degrees of plantarflexion) or tendon thickness, but middle-aged adults exhibited slightly smaller pennation angles (p < 0.05) in a resting ankle posture.
| Young adults | Middle-aged adults | |
|---|---|---|
| Age: | 25 ± 4 years | 49 ± 4 years |
| Gender: | 8 M, 7 F | 5 M, 5 F |
| Height: | 69 ± 3 inches | 70 ± 5 inches |
| Weekly exercise: | 11 ± 5 hours | 10 ± 5 hours |
| Resting ankle angle: | 25 ± 5 deg. | 25 ± 6 deg. |
| Tendon thickness: | 4.2 ± 0.6 mm | 4.7 ± 0.9 mm |
| Resting pennation angle: | 22 ± 4 deg. | 19 ± 3 deg. |
Subjects were positioned in three ankle postures in a random order: resting ankle angle (R; Table 1), a dorsiflexed angle (R − 15 deg) and a plantarflexed angle (R + 15 deg). After positioning, sufficient time (>1 min) was allowed for the subject to relax and thereby mitigate any stretch-induced reflexes (Hirata et al, 2015). Ultrasound B-mode and shear wave data were collected from the Achilles tendon using a 50 mm linear array transducer (L15-4, Aixplorer, Supersonic Imagine, Aix-en-Provence, France; software version: 5; preset: superficial MSK persist: high; smoothing: 7). The transducer was first placed over the free Achilles tendon with the distal edge of the image approximately 10 mm distal to the proximal edge of the calcaneus. Shear wave data were collected from manually positioned regions (10 mm in width) centered on the tendon. This shear wave region was initially placed over the most distal aspect of the tendon, and was sequentially moved in ~10 mm increments proximally along the Achilles tendon into the medial gastrocnemius aponeurosis, such that the full tendon width was captured in five sequential region locations. For each region location, five repeat images were acquired. The transducer was translated proximally every 50 mm, with external markers placed on the skin to mark transducer locations. A custom standoff pad (178 × 127 mm, 16 mm thick), remolded from commercial pads (Aquaflex, Parker Laboratories, Fairfield, NJ), was used in the two most distal transducer positions. The most proximal shear wave region was defined as the most proximal location where the gastrocnemius aponeurosis was still clearly visible within the B-mode image and there were no notable artifacts or missing shear wave data. This position was, on average, 112 mm proximal to the gastrocnemius muscle-tendon junction.
Shear wave data were evaluated post-hoc from exported DICOM images using a custom MATLAB (2011a, Mathworks, Inc., Natick, MA) graphical user interface. Regions of interest (ROIs) were manually defined within the tendon boundaries from the B-mode images, and the average measured wave speed for the repeat images was computed. Beyond the gastrocnemius muscle-tendon junction, ROIs were also defined within the gastrocnemius muscle, from which wave speeds were obtained.
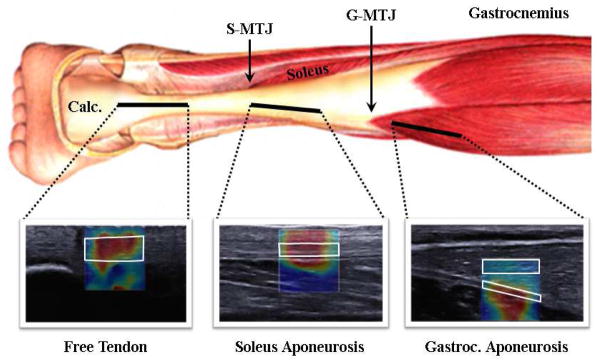
For each image set, the soleus muscle-tendon junction (S-MTJ) and medial gastrocnemius muscle-tendon junction (G-MTJ) were identified. The edge of the calcaneus (C) was defined as the most distal point for image analysis. To enable inter-subject comparisons, shear wave measures were extracted from all ROIs and manually defined to fall within three anatomical regions: the free tendon (FT; from C to S-MTJ), the soleus aponeurosis (SA; S-MTJ to G-MTJ) and the gastrocnemius aponeurosis (GA; G-MTJ to most proximal imaging location; Fig. 1). Data were then interpolated for all subjects to create a dataset with ten uniformly distributed points in the free tendon, thirty within the soleus aponeurosis and ten within the gastrocnemius aponeurosis, mimicking the approach used previously (DeWall et al, 2014). Gastrocnemius muscle (GM) wave speeds were also obtained from the same positions used to assess gastrocnemius aponeurosis wave speeds. Regional wave speeds were then computed by averaging the data points within each region for each subject for use in statistical analyses.
Figure 1.
B-mode ultrasound images and wave speed data were collected along a path that extended from the Achilles tendon to the medial gastrocnemius aponeurosis. Distal images from the free tendon were collected with the calcaneus (Calc.) in view. The soleus aponeurosis was defined as the region from the soleus muscle-tendon junction (S-MTJ) to the gastrocnemius muscle-tendon junction (G-MTJ). In post-hoc analysis, regions of interest (examples outlined in white) were defined within the tendon boundaries. In the portion of the tendon proximal to the G-MTJ, regions of interest were also defined in the gastrocnemius muscle tissue to compare with tendon wave speeds. Note that the 50 mm wide images have been cropped to ~30 mm sections for illustrative purposes.
Tendon thickness was measured by manually delineating the edges of the free tendon in a resting posture. Thickness was defined as the distance between the superficial and deep edges of the tendon in the distal 20% of the free tendon region. Gastrocnemius resting pennation angle was defined at the fascicle insertion onto the deep aponeurosis (Legerlotz et al, 2010), manually delineated in MATLAB. The pennation angle was measured three times for each subject from the most proximal image collected in the resting posture. The maximum measureable wave speed of the system is 16.3 m/s, a value that has been observed previously in stretched tendon (Aubry et al, 2013; DeWall et al, 2014). Thus, we also computed the percentage of pixels within each ROI that exhibited wave speed magnitudes equal to 16.3 m/s to assess potential saturation effects.
A two-way ANOVA was used to evaluate the effects of ankle posture (D, R, P) and region (FT, SA, GA, GM) on average measured wave speed in middle-aged adults. For each image location (FT, SA, GA, GM), a two way ANOVA was used to evaluate the effects of age (young, middle-aged) and ankle posture (D, R, P) on wave speed. Post-hoc Tukey comparisons were used as follow-up to the significant ANOVA results. In the tendon region(s) with significant aging effects, linear regressions of ankle posture and wave speed were then evaluated. Significance was set at p < 0.05 for all statistical comparisons.
3. Results
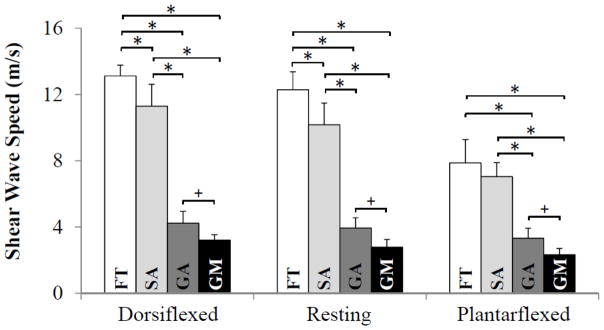
There were no significant differences between young and middle-aged adult subjects in terms of height, average weekly hours of exercise, resting ankle angle or distal tendon thickness (Table 1). We did measure a slightly larger medial gastrocnemius pennation angle in young adults than middle-aged adults (22 vs 19 deg, p = 0.03). For both age groups, wave speed was observed to vary significantly with imaging location and ankle posture (Fig. 2). The highest wave speeds were measured in the distal free tendon, with lower speeds measured proximally (Fig. 3). For example, in middle-aged adults in the resting posture, wave speed in the free tendon (12.3 ± 1.1 m/s) was significantly higher (p < 0.001) than the soleus aponeurosis (10.2 ± 1.3 m/s), the gastrocnemius aponeurosis (3.9 ± 0.6 m/s), and the gastrocnemius muscle (2.8 ± 0.5 m/s). Measured wave speed in all three regions of the tendon significantly increased from a plantarflexed to resting posture, and from a resting to dorsiflexed posture. In the gastrocnemius muscle, there was a significant difference in measured wave speed between the dorsiflexed and plantarflexed postures. Portions of the Achilles free tendon reached the maximum measureable wave speed of 16.3 m/s in both resting (12% saturation) and dorsiflexed (16%) postures.
Figure 2.
The average (± standard deviation) measured wave speed for fifteen young and ten middle-aged adults is plotted along the length of the tendon for the (a) plantarflexed, (b) resting and (c) dorsiflexed postures. Measured wave speeds decreased proximally, with the highest wave speeds observed in the distal free tendon (FT), lower values observed in the soleus aponeurosis (SA) and the lowest values in the proximal gastrocnemius aponeurosis (GA). Wave speed was significantly lower in the GA of middle-aged adults in the dorsiflexed posture. +p < 0.05
Figure 3.
The average (+ standard deviation) measured wave speed from ten middle-aged subjects for each ankle posture and each region: the Achilles free tendon (FT), soleus aponeurosis (SA), gastrocnemius aponeurosis (GA) and the gastrocnemius muscle (GM). For each ankle posture, measured wave speed varied significantly between muscle and tendon regions. *p<0.001, +p<0.05.
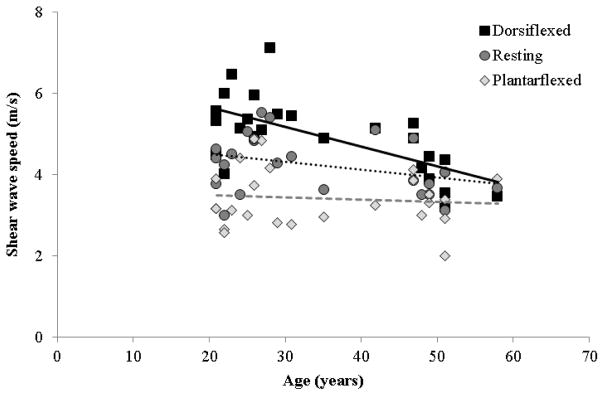
Aging effects on measured tendon wave speed were location dependent. In the free tendon and soleus aponeurosis, measured wave speed was similar between age groups in all postures (Fig. 2). In the gastrocnemius aponeurosis, however, significantly lower wave speeds were measured in middle-aged adults when the ankle was in a dorsiflexed posture (p < 0.05). There was a significant inverse relationship between measured gastrocnemius aponeurosis wave speed and age in the dorsiflexed posture (R2 = 0.42, p = 0.0004; Fig. 4). We also observed significantly lower wave speeds in the gastrocnemius muscles of middle-aged adults compared with young adults (p = 0.005), with no interactions between age group and ankle angle.
Figure 4.
Wave speed in the gastrocnemius aponeurosis decreased with age in the dorsiflexed (R2 = 0.42, p = 0.0004) posture, but not in the resting (p = 0.083) or plantarflexed postures (p = 0.62). The equation of the line fit for the dorsiflexed (DF) postures is: SWSDF = Age · −0.048(m/s)/years + 6.64m/s
4. Discussion
In this study, we compared postural and spatial variations in Achilles tendon wave speed between young and middle-aged adults. We had hypothesised that middle-aged adults would exhibit lower wave speeds than young adults, reflecting more compliant tendinous tissue. This hypothesis was only supported for the gastrocnemius aponeurosis, in which middle-aged adults exhibited smaller increases in aponeurosis wave speed with passive stretch. Thus, our results suggest that age-related variations in tendon tissue compliance may be location dependent, an effect which could alter localised tissue strain patterns.
Achilles tendon wave speeds exhibited postural and spatial variations that were generally similar to those observed previously in young adults (DeWall et al, 2014). Specifically, measured wave speeds increased progressively with passive tendon stretch, and were lower in the proximal tendon (i.e. the gastrocnemius aponeurosis) than in the distal tendon (i.e. the free tendon). However, there were distinct age-related differences in the rate of increase in measured gastrocnemius aponeurosis wave speed with passive stretch (Fig. 4). This result suggests that the gastrocnemius aponeurosis compliance is altered with aging, though the complex mechanical interaction of muscle and aponeurosis tissue (Epstein et al, 2006) makes it challenging to infer the underlying mechanics that give rise to this observation.
The increase in medial gastrocnemius aponeurosis tissue compliance could reflect either an age-related change in the tissue material properties or, alternatively, a shift in load distribution between the aponeurosis and muscle tissue. In support of the former hypothesis, there is substantial evidence of age-related changes in the microstructural composition of tendon that are generally believed to give rise to an increase in tissue compliance (Nakagawa et al, 1994; Patterson-Kane et al, 1997; Tuite et al, 1997; Birch et al, 1999). Although it is not clear why changes in tendon tissue material properties with middle-age would be isolated to the aponeurosis, there is increasing evidence that tendinous tissue is highly specialised to function and loading (Thorpe et al, 2012), with significant variations in cellularity, matrix composition and crimp angle observed even within regions of the same tendon (Birch et al, 1999; Patterson-Kane et al, 1997). Further, in equine tendons, aging has been observed to progress differently in functionally dissimilar tendons (Thorpe et al, 2013) and even within tendon regions (Birch et al, 1999; Patterson-Kane et al, 1997). Thus, it is conceivable that the aging process could have region-dependent effects on the Achilles free tendon and gastrocnemius aponeurosis.
An alternate hypothesis is that the relative load taken up by the middle-aged gastrocnemius aponeuroses during passive dorsiflexion is reduced, which could be due to greater load transmission across muscle tissues that are in parallel with the aponeurosis (i.e. the soleus and portions of the gastrocnemius muscle). A lower medial gastrocnemius aponeurosis load would result in a lower regional strain, which may manifest as a smaller increase in wave speed with dorsiflexion. We did observe significantly lower measured wave speeds in the gastrocnemius muscle in middle-aged adults. This result suggests that there may have been less passive loading of the middle-aged gastrocnemius with ankle dorsiflexion, such that the gastrocnemius aponeurosis underwent less strain-stiffening with dorsiflexion. It is also interesting to note that the age-related decrease in muscle wave speed was observed in all three ankle postures, which could reflect a different slack muscle-tendon length for the middle-aged subjects. One possibility is that the lateral gastrocnemius and soleus are undergoing relatively greater load with passive stretch with middle-age, which could reflect differing slack lengths of the muscle-tendon units. A prior study showed that shear wave imaging can be used to estimate slack lengths by detecting the posture-dependent onset of increased wave speed in muscle-tendon structures (Hug et al, 2013), such that SSI could be used to test potential variations of inter-muscle load distribution. Architectural variations within the plantarflexors (O’Brien, 1984) are also relevant to consider. In this regard, we did measure slightly smaller gastrocnemius pennation angles (−3 deg on average) in middle-aged adults. However, a simple Hill-type model of the muscle-tendon unit (Zajac, 1989) would suggest that smaller pennation angles would actually lead to less rotation and hence increased muscle fibre stretch with passive dorsiflexion, which should increase load on the aponeurosis. Hence, pennation angle differences between groups seem unlikely to explain the lower aponeurosis wave speeds in middle-aged subjects, and further investigation is needed to understand the underlying tissue properties and muscle-tendon architectural factors more fully.
An age-related change in aponeurosis compliance may be relevant to consider in the context of muscle strain injuries. Muscle strain injuries commonly occur in the gastrocnemius muscle (Orchard, 2001; Speer et al, 1993; Garrett, 1990) and arise more frequently in middle-age (McKean et al, 2006). Imaging studies have found that the location of gastrocnemius muscle strain injuries is often near the distal aponeurosis (Garrett, 1990; Kirkendall and Garrett, 2002; Speer et al, 1993), which is the region where we observed decreases in measured tissue wave speed in the middle-aged subject population. Thus, it is possible that this reflects a change in localised tendon compliance, which could alter nearby tissue deformation patterns and thereby affect risk for localised tissue injury. Given the complexity of the plantarflexor muscle-tendon architecture and mechanics (Cummins et al, 1946), there is a clear need for further modeling and imaging investigations to clarify muscle-tendon load transmission and its effects on localised tissue strain patterns.
We did not observe a significant difference in Achilles tendon wave speeds between young and middle-aged adults in the free tendon and soleus aponeurosis. We had hypothesised that middle-aged adults would exhibit a decrease in tendon compliance based on known structural changes to tendinous tissue with age (Birch et al, 1999; Patterson-Kane et al, 1997; Nakagawa et al, 1994; Tuite et al, 1997). To our knowledge, there are no prior studies that have used SSI to evaluate age-related changes in wave speed in the soleus or gastrocnemius aponeuroses, and only two that have considered the Achilles free tendon. Of these, Arda et al. (2011) showed results in agreement with ours, with no observed relationship between age and Achilles free tendon wave speed from a large sample (n = 127, aged 17–63 years, mean: 37.7). In contrast, Aubry et al. (2013) did measure an age-related decrease in free tendon wave speed (n = 80, aged 20–83 years, mean: 45.4), but only when the ankle was in plantarflexed postures. Methodologically, these prior studies differ substantially from what is described here, in that both evaluated wave speed within a single location of the Achilles tendon, rather than along the length of the free tendon. Additionally, the subject populations in these prior studies are of somewhat different age ranges than in our study (n = 25, aged 21–58 years, mean: 35.0). Future studies including older adult populations (aged 61+) will be necessary to fully elucidate the relationship between aging and measured wave speeds in the Achilles free tendon, soleus aponeurosis and gastrocnemius muscle.
There are a few limitations in this study that should be considered. First, wave speed saturation is a challenge when using SSI technology on loaded tendinous tissue. SSI uses high frame-rate planar imaging (>5000 frames/sec (Bercoff et al, 2004)) to track ultrasonically induced shear waves. However, the induced shear waves only propagate a few millimeters before dispersing such that the current range of measurable wave speeds is <16.3 m/s. Maximum wave speeds were reached in the stretched free tendon, with 16% and 12% of the ROI exhibiting the fastest measurable speed in dorsiflexed and resting postures, respectively. Partially saturated wave speeds were previously observed in the same postures in young adults (DeWall et al, 2014), such that this limitation could potentially mask the effects of age on load-dependent changes in free tendon wave speed. Spatial smoothing, ROI size and border effects may also influence wave speed measurements, particularly in regions where there is a large differential of wave speed between neighboring tissues (e.g. between gastrocnemius aponeurosis and muscle). For example, in a pilot study, we tested the effect of reducing the ROI width to minimize border effects and found that this led to a consistent increase in measured wave speed, with no difference between age groups. In order to maintain consistency with our prior study [1], we chose to report here the measured wave speeds when the same ROI sizes and smoothing parameters were used as in the prior study, so as not to bias the measures from either group. However, it is important to note that the results here likely underestimate the true wave speeds due to these border effects as well as the aforementioned data saturation. It is also important to note that in this study we controlled ankle position, which is not a direct surrogate for tissue loading. Finally, prior studies have used shear wave speeds to estimate Young’s modulus (Aubry et al, 2013; Arda et al, 2011), however the underlying assumption of tissue isotropy necessary for the Young’s modulus computation is not valid in tendon, which is better represented as a transversely isotropic material (Royer et al, 2011). We are currently pursuing a variety of research studies so that we may better understand the implications of these results. Our current and future work is focused around two specific areas. First, we are working with a variety of ex vivo tendon setups to better understand the relationships between tendon shear wave speeds, loading conditions and mechanical behaviour. Second, we are working with collaborators to develop three dimensional computational models of the triceps surae muscle-tendon units, that will enable us to investigate the inter-relationship of material properties, muscle-tendon architecture and localised tissue mechanics.
In summary, we observed aging effects on longitudinal muscle and tendon wave speed that were location dependent. Wave speeds decreased proximally, with the gastrocnemius muscle and aponeurosis of middle-aged adults exhibiting much lower wave speeds in response to passive stretch than young adults. We did not observe any effects of aging on wave speed in the free tendon or soleus aponeurosis. Our results suggest that spatial variations in the aponeurosis compliance may be altered in middle-aged adults, which may have implications for localised tissue strain patterns and injury potential.
Acknowledgments
This project was supported by NIH F31AG043216, the Radiological Society of North American Scholar Grant Award (#RSCH1317), the University of Wisconsin-Madison Radiology Department Research and Development Fund (#1204-001), and the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR #1UL1RR025011) and now by the National Center for Advancing Translational Sciences (NCATS # 9U54TR000021).
References
- Arampatzis A, Stafilidis S, DeMonte G, Karamanidis K, Morey-Klapsing G, Bruggenmann GP. Strain and elongation of the human gastrocnemius tendon and aponeurosis during maximal plantarflexion effort. J Biomech. 2005;38:833–41. doi: 10.1016/j.jbiomech.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol. 2011;197:532–6. doi: 10.2214/AJR.10.5449. [DOI] [PubMed] [Google Scholar]
- Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, Gennisson JL, Fink M, Neuenschwander S. Breast lesions: quantitative elastography with supersonic shear imaging--preliminary results. Radiol. 2010;256:297–303. doi: 10.1148/radiol.10090385. [DOI] [PubMed] [Google Scholar]
- Aubry S, Risson JR, Kastler A, Barbier-Brion B, Siliman G, Runge M, Kastler B. Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skeletal Radiol. 2013;42:1143–50. doi: 10.1007/s00256-013-1649-9. [DOI] [PubMed] [Google Scholar]
- Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, Badel A, Vallet-Pichard A, Nalpas B, Tanter M, Pol S. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37:1361–73. doi: 10.1016/j.ultrasmedbio.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:396–409. doi: 10.1109/tuffc.2004.1295425. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey JV, Bailey AJ, Goodship AE. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet J. 1999;31:391–6. doi: 10.1111/j.2042-3306.1999.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Blevins FT, Hecker AT, Bigler GT, Boland AL, Hayes WC. The effects of donor age and strain rate on the biomechanical properties of bone-patellar tendon-bone allografts. Am J Sports Med. 1994;22:328–33. doi: 10.1177/036354659402200306. [DOI] [PubMed] [Google Scholar]
- Brum J, Bernal M, Gennisson JL, Tanter M. In vivo evaluation of the elastic anisotropy of the human Achilles tendon using shear wave dispersion analysis. Phys Med Biol. 2014;59:505–23. doi: 10.1088/0031-9155/59/3/505. [DOI] [PubMed] [Google Scholar]
- Chen XM, Cui LG, He P, Shen WW, Qian YJ, Wang JR. Shear wave elastographic characterization of normal and torn achilles tendons: a pilot study. J Ultrasound Med. 2013;32:449–55. doi: 10.7863/jum.2013.32.3.449. [DOI] [PubMed] [Google Scholar]
- Chernak LA, DeWall RJ, Lee KS, Thelen DG. Length and activation dependent variations in muscle shear wave speed. Physiol Meas. 2013;34:713–21. doi: 10.1088/0967-3334/34/6/713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child S, Bryant AL, Clark RA, Crossley KM. Mechanical Properties of the Achilles Tendon Aponeurosis Are Altered in Athletes With Achilles Tendinopathy. Am J Sports Med. 2010;38:1885–93. doi: 10.1177/0363546510366234. [DOI] [PubMed] [Google Scholar]
- Csapo R, Malis V, Hodgson J, Sinha S. Age-related greater Achilles tendon compliance is not associated with larger plantar flexor muscle fascicle strains in senior women. J Appl Physiol. 2014;116:961–9. doi: 10.1152/japplphysiol.01337.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins EJ, Anson BJ, et al. The structure of the calcaneal tendon (of Achilles) in relation to orthopedic surgery, with additional observations on the plantaris muscle. Surg Gynecol Obstet. 1946;83:107–16. [PubMed] [Google Scholar]
- DeWall RJ, Slane LC, Lee KS, Thelen DG. Spatial variations in Achilles tendon shear wave speed. J Biomech. 2014;47:2685–92. doi: 10.1016/j.jbiomech.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An KN. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–7. doi: 10.1016/j.jbiomech.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M, Wong M, Herzog W. Should tendon and aponeurosis be considered in series? J Biomech. 2006;39:2020–5. doi: 10.1016/j.jbiomech.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol. 2003;95:829–37. doi: 10.1152/japplphysiol.00775.2002. [DOI] [PubMed] [Google Scholar]
- Garrett WE. Muscle Strain Injuries - Clinical and Basic Aspects. Med Sci Sport Exer. 1990;22:436–43. [PubMed] [Google Scholar]
- Hawkins D, Lum C, Gaydos D, Dunning R. Dynamic creep and pre-conditioning of the Achilles tendon in-vivo. J Biomech. 2009;42:2813–7. doi: 10.1016/j.jbiomech.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Hirata K, Kanehisa H, Miyamoto-Mikami E, Miyamoto N. Evidence for intermuscule difference in slack angle in human triceps surae. J Biomech. 2015 doi: 10.1016/j.jbiomech.2015.01.039. in press. [DOI] [PubMed] [Google Scholar]
- Hug F, Lacourpaille L, Maisetti O, Nordez A. Slack length of gastrocnemius medialis and Achilles tendon occurs at different ankle angles. J Biomech. 2013;46:2534–8. doi: 10.1016/j.jbiomech.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Kannus P, Niittymaki S, Jarvinen M, Lehto M. Sports injuries in elderly athletes: a three-year prospective, controlled study. Age Ageing. 1989;18:263–70. doi: 10.1093/ageing/18.4.263. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A. Mechanical and morphological properties of different muscle-tendon units in the lower extremity and running mechanics: effect of aging and physical activity. J Exp Biol. 2005;208:3907–23. doi: 10.1242/jeb.01830. [DOI] [PubMed] [Google Scholar]
- Kirkendall DT, Garrett WE. Clinical perspectives regarding eccentric muscle injury. Clin Orthop Relat R. 2002;403(Suppl):S81–S9. doi: 10.1097/00003086-200210001-00010. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Euro J Appl Physiol. 2003;88:520–6. doi: 10.1007/s00421-002-0744-8. [DOI] [PubMed] [Google Scholar]
- Kubo K, Morimoto M, Komuro T, Tsunoda N, Kanehisa H, Fukunaga T. Age-related differences in the properties of the plantar flexor muscles and tendons. Med Sci Sports Exerc. 2007;39:541–7. doi: 10.1249/01.mss.0000247006.24965.74. [DOI] [PubMed] [Google Scholar]
- Legerlotz K, Smith HK, Hing WA. Variation and reliability of ultrasonographic quantification of the architecture of the medial gastrocnemius muscle in young children. Clin Physiol Funct Imaging. 2010;30:198–205. doi: 10.1111/j.1475-097X.2010.00925.x. [DOI] [PubMed] [Google Scholar]
- Maganaris CN, Paul JP. Load-elongation characteristics of in vivo human tendon and aponeurosis. J Exp Biol. 2000;203:751–6. doi: 10.1242/jeb.203.4.751. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol. 2001;531:277–88. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand. 2003;177:185–95. doi: 10.1046/j.1365-201X.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- McKean KA, Manson NA, Stanish WD. Musculoskeletal injury in the masters runners. Clin J Sport Med. 2006;16:149–54. doi: 10.1097/00042752-200603000-00011. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Majima T, Nagashima K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand. 1994;152:307–13. doi: 10.1111/j.1748-1716.1994.tb09810.x. [DOI] [PubMed] [Google Scholar]
- Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005;15:392–401. doi: 10.1111/j.1600-0838.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- O’Brien T. The needle test for complete rupture of the Achilles tendon. J Bone Joint Surg Am. 1984;66:1099–101. [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100:2048–56. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29:300–3. doi: 10.1177/03635465010290030801. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane JC, Firth EC, Goodship AE, Parry DA. Age-related differences in collagen crimp patterns in the superficial digital flexor tendon core region of untrained horses. Aust Vet J. 1997;75:39–44. doi: 10.1111/j.1751-0813.1997.tb13829.x. [DOI] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12:90–8. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Royer D, Gennisson JL, Deffieux T, Tanter M. On the elasticity of transverse isotropic soft tissues. J Acoust Soc Am. 2011;129:2757–60. doi: 10.1121/1.3559681. [DOI] [PubMed] [Google Scholar]
- Sebag F, Vaillant-Lombard J, Berbis J, Griset V, Henry JF, Petit P, Oliver C. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J Clin Endocrinol Metab. 2010;95:5281–8. doi: 10.1210/jc.2010-0766. [DOI] [PubMed] [Google Scholar]
- Slane LC, Thelen D. Non-Uniform Displacements within the Achilles Tendon observed during Passive and Eccentric Loading. J Biomech. 2014;47:2831–5. doi: 10.1016/j.jbiomech.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer KP, Lohnes J, Garrett WE., Jr Radiographic imaging of muscle strain injury. Am J Sports Med. 1993;21:89–95. doi: 10.1177/036354659302100116. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;113:1537–44. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HR. Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface. 2012;9:3108–17. doi: 10.1098/rsif.2012.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, Clegg PD, Screen HRC. Capacity for Sliding between Tendon Fascicles Decreases with Ageing in Injury Prone Equine Tendons: A Possible Mechanism for Age-Related Tendinopathy? Eur Cells Mater. 2013;25:48–60. doi: 10.22203/ecm.v025a04. [DOI] [PubMed] [Google Scholar]
- Tuite DJ, Renstrom PA, O’Brien M. The aging tendon. Scand J Med Sci Sports. 1997;7:72–7. doi: 10.1111/j.1600-0838.1997.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Influence of maturation and aging on mechanical and biochemical properties of connective tissue in rats. Mech Ageing Dev. 1980;14:283–92. doi: 10.1016/0047-6374(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Age dependence of mechanical properties of rat tail tendons (hysteresis experiments) Aktuelle Gerontol. 1983;13:22–7. [PubMed] [Google Scholar]
- Zajac F. Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]