Abstract
Mice have frequently been used to model human diseases involving immune dysregulation such as autoimmune and inflammatory diseases. These models help elucidate the mechanisms underlying the disease and in the development of novel therapies. However, if mice are deficient in certain cells and/or effectors associated with human diseases, how can their functions be investigated in this species? Mucosal-associated invariant T (MAIT) cells, a novel innate-like T cell family member, are a good example. MAIT cells are abundant in humans but scarce in laboratory mice. MAIT cells harbor an invariant T cell receptor and recognize nonpeptidic antigens vitamin B2 metabolites from bacteria and yeasts. Recent studies have shown that MAIT cells play a pivotal role in human diseases such as bacterial infections and autoimmune and inflammatory diseases. MAIT cells possess granulysin, a human-specific effector molecule, but granulysin and its homologue are absent in mice. Furthermore, MAIT cells show poor proliferation in vitro. To overcome these problems and further our knowledge of MAIT cells, we have established a method to expand MAIT cells via induced pluripotent stem cells (iPSCs). In this review, we describe recent advances in the field of MAIT cell research and our approach for human disease modeling with iPSC-derived MAIT cells.
Keywords: Mucosal-associated invariant T cells, Induced pluripotent stem cells, Differentiation, Adoptive transfer, Inflammatory diseases, Autoimmune diseases, Disease modeling, Infectious diseases, Immunocompromised mouse
Core tip: Mucosal-associated invariant T (MAIT) cells, a novel innate-like T cell subset abundant in humans, play a pivotal role in immune-dysregulated diseases. However, MAIT cells are quite rare in laboratory mice and show poor proliferation in vitro. This makes it difficult to delineate their physiological functions in health and disease. Therefore, we developed a method to generate human MAIT cells from induced pluripotent stem cells [redifferentiation of MAIT (reMAIT) cells]. Given that reMAIT cells harbor characteristics quasi-identical to those found in MAIT cells from human peripheral blood, they will be useful to model human diseases in animals.
INTRODUCTION
T cells are distinguished from other lymphocytes, such as B cells and natural killer cells, by the expression of T cell receptors (TCRs) on the cell surface. T cells have been well-characterized as central players in adaptive immunity, so-called conventional T cells. The TCRs in conventional T cells consist of a heterodimer of α-chain and β-chain and are highly diverse owing to gene rearrangement together with insertion and/or deletion of nucleotides at the junctions between the gene segments, enabling them to recognize a wide variety of peptide antigens presented on major histocompatibility complex (MHC) molecules, which are also highly polymorphic[1]. In recent years, however, non-conventional type T cells termed “innate-like” T cells have received keen attention in immune homeostasis and diseases[2]. In contrast to conventional T cells, innate-like T cells express a limited set (semi-invariant) of TCRs and recognize nonpeptidic antigens presented on evolutionarily conserved nonclassical MHC molecules[3,4]. Innate-like T cells develop in the thymus, similar to conventional T cells[5]. There is a time lag between the initial antigen exposure and execution of the maximum effector function in conventional T cell responses. Given that conventional T cells transit from naïve to effector/memory stage through the recognition of peptidic antigens, these T cells are ready to be activated and to expand upon receiving secondary stimuli to exert effector functions. In marked contrast, innate-like T cells have already acquired such immune competence when leave the thymus. This may be relevant to the fact that innate-like T cells, but not conventional T cells, express the transcription factor promyelocytic leukemia zinc finger (PLZF), which directs effector differentiation of these cells during thymic development[5-7]. Thus far, it has been appreciated that the raison-d’etre of innate-like T cells consists in filling a gap between innate and adaptive immunity[8].
Mucosal-associated invariant T (MAIT) cells and natural killer T (NKT) cells are representatives of innate-like T cells expressing semi-invariant αβTCR in mammals[2]. Because the discovery of NKT cell ligands has preceded that of MAIT cells, most of our knowledge on diseases has been made with NKT cells abundant in laboratory mice (but quite few in humans). NKT cells play a pivotal role in the suppression of tumor growth and/or metastasis, and in ameliorating or aggravating autoimmune diseases[9,10]. NKT cells produce a plethora of cytokines, including Th1-, Th2- and Th17-cytokines, upon stimulation, and MAIT cells also have a similar potential[11,12]. Although they are different in many aspects such as antigens, restriction molecules for development and/or differentiation, and abundance, they are common in that they play a critical role in infectious diseases and autoimmune and inflammatory diseases. Regardless of the their importance, it was not until recently that some information on MAIT cells has become available. In the last couple of years, there has been exciting progress regarding the functions of MAIT cells in the immunology field and in clinical settings. There are, however, some difficulties in studying MAIT cells, in that the frequency of MAIT cells is much lower in laboratory mice than in humans, and that MAIT cells show extremely poor proliferation in vitro with any T cell stimulants tested to date. Here, we provide an overview of recent advances in the study on MAIT cells and introduce our approach with induced pluripotent stem cell (iPSC) technology to overcome the experimental difficulties in MAIT cell study.
PHENOTYPIC FEATURES OF MAIT CELLS
MAIT cells are probably one of the most abundant T cell subsets in humans[13]. However, until quite recently, MAIT cells had been hidden behind conventional T cells because they are indistinguishable from other T cell populations by standard T cell phenotyping using cell surface markers such as CD3, CD4 and CD8. MAIT cells are distinguished from conventional T cells and other T cell subsets such as NKT cells and γδ T cells by the expression of an invariant TCR α chain, Vα7.2-Jα33 in humans and Vα19-Jα33 in mice, paired with a limited repertoire of TCR β chains; Vβ13 and Vβ2 are preferentially used in humans and homologous Vβ8 and Vβ6 in mice (Figure 1)[13,14]. Together with invariant TCRα Vα7.2, human MAIT cells express a C-type lectin CD161 and interleukin (IL)-18 receptor α chain (IL-18Rα) as specific markers[15,16]. Primarily, MAIT cells are defined as CD3+, Vα7.2+, CD161+ and IL-18Rα+. MAIT cells can further be classified into CD8+ (most abundant), CD4−CD8− [double negative (DN)] and CD4+ phenotypes (very few) in healthy human subjects[13,17]. In addition, MAIT cells display CD45RA−, CD45RO+, CD95high, and CD62Llow as their effector/memory T cell phenotype, and α4β7 integrin+, CCR9int, CCR7−, CCR5high, CXCR6high, and CCR6high, suggesting MAIT cells home to the intestines and liver[11,18,19]. High expression levels of CD161 in MAIT cells are accompanied by RORγt, IL-23R and IL-21R, markers associated with Th17/Tc17 type T cells[11,19,20]. Furthermore, MAIT cells possess PLZF, indicating the capacity to promptly produce cytokines upon stimulation without priming[7,17] and CD26+, a serine exodipeptidase, which processes chemokines in the extracellular matrix[20,21]. Accordingly, MAIT cells have the potential to release a variety of cytokines under various conditions: Interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-2, IL-4, IL-10, IL-17, IL-22, granzymes, and others, which anticipates the multifaceted roles in health and diseases[11,12,22].
Figure 1.
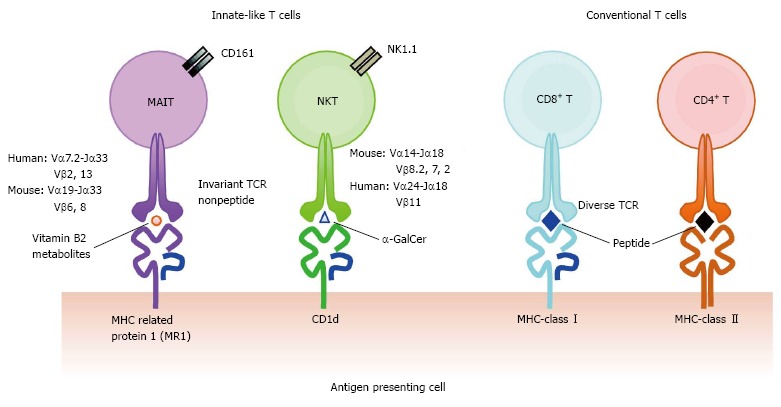
Comparison of the T cell receptors and the antigen presenting molecules among αβ T cell subsets. Invariant T cell subsets consist of mucosal-associated invariant T (MAIT) cells and natural killer T (NKT) cells expressing invariant TCRs. MAIT cells and NKT cells recognize vitamin B2 metabolites on MR1, and α-galactosylceramide (α-GalCer) on CD1d, respectively. In contrast, conventional CD8+ and CD4+ T cells possess divergent TCRs and recognize a variety of peptides on major histocompatibility complex-class I and class II, respectively. TCRs: T cell receptors; MHC: Major histocompatibility complex.
MAIT CELLS AND MR1
The TCR of MAIT cells recognizes derivatives of vitamin B2 presented on the monomorphic MHC class-related molecule 1, MR1[18,23] (Figure 1). MR1 mRNA is expressed ubiquitously in all types of cells, whereas the MR1 protein are not always on the cell surface but mainly in the endoplasmic reticulum[24,25]. Although vitamin B2 derivatives are exogenous ligands from the biosynthetic pathway that some bacteria and yeasts possess, they are indispensable for the development of MAIT cells, because MAIT cells are absent in germ-free mice[18]. TCRs for MAIT cells and MR1 are highly conserved during evolution, which suggests the functional and physiological importance of MAIT cells and MR1 in animals[26]. Indeed, mouse and human MR1 molecules crossover part of the antigen presentation and activation in MAIT cells[26].
MAIT cell development is dependent on MR1. Lymphoid progenitors derived from CD34+ hematopoietic stem cells in the bone marrow migrate to the thymus, wherein they undergo random rearrangement at the TCR loci. MAIT cell progenitors harboring the TCR Vα7.2-Jα33 are selected from CD4/CD8 double positive thymocytes that express MR1 loaded with unknown endogenous ligands[18,27]. MAIT cells then egress from the thymus as naïve cells and further differentiate into effector/memory cells by recognizing commensal microflora-derived vitamin B2 metabolites bound to MR1 at mucosal sites[18,19].
MAIT CELLS IN HEALTH AND DISEASES
MAIT cells consist of 1%-10% of T cells in the peripheral blood and of T cells in the intestinal lamina propria and 20%-50% in T cells of the liver, but they are at least 10 times less abundant in laboratory mice[11,28]. MAIT cells are already present in the tissues of second trimester fetuses. Fetal MAIT cells exhibit a naïve phenotype but have potential functions in the activation and secretion of cytokines upon antigen stimulation[29]. Although MAIT cells still showing a naïve phenotype and are low in frequency at birth, most of them have acquired a memory phenotype by 3 mo of age, and their frequency increases with age and reaches adult levels within 8-10 years after birth[11]. This corresponds to the expansion and maturation of MAIT cells by commensal microflora colonizing after birth. The highest number of MAIT cells in PBMC is observed in adults aged 30-50 years, notably in females of reproductive age[30]. MAIT cells, especially CD8+ MAIT cells as the most abundant subset, decrease drastically with age, implying an association with waning immunity in the elderly[22,30].
The diseases in which a potential implication of MAIT cells has been reported are summarized in Table 1. A well-defined function of MAIT cells in disease settings is the control of infections with bacteria and/or yeasts. MAIT cells are activated by bacteria-infected cells in a MR1-dependent manner, followed by release of proinflammatory cytokines and cytotoxic granules, and eventually killing the infected cells[16,31-33]. MAIT cells also express multidrug resistance transporter (ABCB1), which implies that MAIT cells are highly resistant to xenobiotics produced by bacteria[11]. Although MAIT cells are extremely rare in laboratory mice, Francisella tularensis-infected mice revealed a massive expansion of MAIT cells in infected tissues earlier than the migration of conventional CD4+ and CD8+ T cells[34], which suggests their unique function in host defense against bacterial infection. Vα19 iTCR (invariant TCR) transgenic mice (a MAIT-cell-enriched mouse model) and MR1 knockout mice (a MAIT-cell-deficient model), MAIT cells seemed to prevent the growth of bacterial such as Mycobacterium abscessus, M. bovis (BCG), Escherichia coli and Klebsiella pneumoniae[16,35-37]. Accordingly, patients with bacterial infections such as tuberculosis and pneumopathies showed a decrease in MAIT cells in circulating blood, which might reflect their infiltration into the diseased sites[16,37]. In HIV-infected patients, MAIT cells were also depleted from the circulating blood irrespective of the disease stage (acute or chronic infection), and even with combinatorial anti-retroviral therapy[38-43]. Although it is believed that CD4+ T cell depletion causes immunodeficiency in HIV-infected patients, innate immune cells such as MAIT cells could play a crucial role in prevention of opportunistic infections with bacteria and/or fungi, which is a manifestation of AIDS.
Table 1.
Clinical relevance of mucosal-associated invariant T cells
| Disease categories | Diseases or status | Features relevant to the diseases | Ref. |
| Infectious diseases | Pneumopathy | Decrease in frequency and absolute number of MAIT cells in peripheral blood | [16] |
| Tuberculosis (Mycobacterium tuberculosis) | Decrease in frequency and absolute number of MAIT cells in peripheral blood Enriched in the lung | [16,37,92] | |
| HIV/AIDS (opportunistic infection) | Decrease in frequency of MAIT cells in peripheral blood, guts, and lymph nodes Failure of recovery of blood MAIT cells with successful cART Long-term cART restore colonic but not blood MAIT cell levels MAIT cells are depleted but retain functionality | [38-43,93,94] | |
| Sepsis (severe bacterial infection) | Decrease in frequency and absolute number of MAIT cells in peripheral blood of patients | [95] | |
| P. aeruginosa infection with cystic fibrosis | Decrease in frequency of MAIT cells in peripheral blood of cystic fibrosis patients with P. aeruginosa infection | [96] | |
| Cholera (Vibrio cholera O1) | Activation of MAIT cells in the acute phase No change of blood MAIT cell frequency in adult patients, but persistently decreased in child patients | [97] | |
| Autoimmune diseases | Multiple sclerosis | Accumulation of MAIT cells in the central nervous system lesions Decrease in frequency of MAIT cells in peripheral blood | [44-46,48] |
| Increased CD161high CD8+ T cells in peripheral blood | [98] | ||
| Chronic inflammatory demyelinating polyneuropathy | Accumulation of MAIT cells in the peripheral nerves | [44] | |
| Psoriatic and rheumatoid arthritis Rheumatoid arthritis | Enrichment of CD161high CD8+ T cells in the joints and secretion of IL-17 from those cells | [99] | |
| Inflammatory bowel disease | Decrease in frequency and absolute number of MAIT cells (in particularly, in CD8+ and DN subsets) in peripheral blood | [53] | |
| Increased MAIT cell levels in the synovial fluid Decrease in CD8+ MAIT cells in peripheral blood of CD and UC patients Accumulation of MAIT cells in the inflamed ileon of patients with CD Reduced IFN-γ production in CD patients and increased IL-17 production in CD and UC patients | [49] | ||
| Fewer MAIT cells in in the inflamed ileon of patients with CD and UC Increased apoptosis in MAIT cells | [100] | ||
| Psoriasis | MAIT cells reside in not only the dermis of patients but also that of health donors. MAIT cells may contribute IL-17 production in the dermis of patients | [51] | |
| Celiac disease | Decrease in frequency of MAIT cells in peripheral blood and guts of adult and pediatric patients | [52] | |
| Systemic lupus erythematosus | Decrease in frequency and absolute number of MAIT cells (in particularly, in CD8+ and DN subsets) in peripheral blood Reduced IFN-γ production Elevated expression of PD-1 in MAIT cells | [53] | |
| Inflammatory diseases | Asthma | Decrease in frequency of MAIT cells in blood, sputum, and endobronchial biopsy | [101] |
| Diabetes type 2/obesity | Decrease in frequency of MAIT cells in peripheral blood Circulating MAIT cells display an activate phenotype MAIT cells are more abundant in adipose tissue | [55,56] | |
| Acute cholecystitis | Decrease in frequency and absolute number of MAIT cells in peripheral blood | [102] | |
| Fibromyalgia syndrome vs Spondyloarthritis vs Rheumatoid arthritis | Defined analysis of MAIT cell phenotype among three diseases that exhibit a similar clinical manifestation Decrease in frequency of MAIT cells in three diseases Three diseases are able to distinguish by surface marker expression | [57] | |
| Tissue transplant | Cutaneous acute graft-vs-host disease | Infiltration of CD8+ T cells, CD161+, CCR6+, RORγt+ in the epidermis and dermis of patients with GVHD | [103] |
| Hemodialyzed and kidney transplant | Decrease in frequency of MAIT cells in peripheral blood Implication for the susceptibility to infections in the patients | [104] | |
| Tumors | Kidney and brain tumors | Presence of MAIT cells in tumors | [58] |
| Physiological change | Fetus | Rare and immature in the thymus, spleen, mesenteric lymph nodes Mature and enriched in the guts, liver, and lung | [29] |
| Neonate/infant | Naïve phenotype at birth. Acquisition of effector/memory phenotype and increase in frequency and number with age | [11,30] | |
| Adult | Maximum levels in the third and fourth decenniums Higher amounts in females with reproductive age than in males | [30] | |
| Aging | Decrease in CD8+ MAIT cells and increase in CD4+ MAIT cells with age Th2 shift in cytokine profile in elderly | [22,30] |
CD: Crohn’s disease; UC: Ulcerative colitis; MAIT: Mucosal-associated invariant T; HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome; P. aeruginosa: Pseudomonas aeruginosa.
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system caused by autoreactive T cells. Although it is suggested that myelin-specific CD4+ T cells might play a central role in MS pathogenesis, recent studies have indicated that MAIT cells accumulate in brain lesions concomitantly with a decrease in peripheral blood in MS patients[44-48]. This evidence indicates that MAIT cells may play a pivotal role in MS pathology, but the underlying mechanisms are yet to be elucidated. An increase in IL-18 in the serum of MS patients could signify that MAIT cells tend to migrate into the brain[46]. In conjunction with the high levels of IL-17 and IFN-γ secretion from MAIT cells in MS patients, one study has demonstrated that MAIT cells in MS exhibited proinflammatory profiles[45], but another interpreted that these MAIT cells exhibited a regulatory function to suppress the pathogenic Th1 response[48]. Accordingly, a novel animal model will be required to examine the direct contribution of MAIT cells in MS pathogenesis, as will be described later in this review.
Inflammatory bowel diseases (IBDs), such as Crohn’s disease and ulcerative colitis (UC), are autoimmune diseases in which the potential contribution of MAIT cells is suggested owing to their anti-microbial activity, intestinal homing, and capacity to promptly induce both Th1- and Th17-cytokines. Similar to MS patients, a decrease in MAIT cells in the peripheral blood concomitant with an increase in MAIT cells in the injured ileal regions of IBD patients has been reported[49]. In addition, peripheral blood MAIT cells from IBD patients showed more activated and proliferative state compared with that in healthy controls, suggesting that such alterations impinge on their functions. In fact, MAIT cells from the IBD patients produced significantly more IL-17 than from healthy donors, whereas there was no difference in IL-2 and TNF-α production[49]. MAIT cells from UC patients produced more IL-22, a Th17-cytokine, than controls. Upon binding to its cognate receptors on respiratory and gut epithelial cells, IL-22 evoked the expression of mucin and antimicrobacterial peptides, both of which play a critical role in the protection of epithelial cells from bacteria and/or fungal invasion[50]. Expression of these proteins may in turns enhance the protection and accelerate healing of cellular damage, implying the tissue protective functions of MAIT cells[49].
Numerous studies have reported possible implications of MAIT cells in psoriasis[51], celiac disease[52], systemic lupus erythematosus[53], diabetes[54,55] and obesity[55,56]. In our recent study, MAIT cells were shown to be useful to distinguish diseases that manifest similar symptoms such as fibromyalgia syndrome, rheumatoid arthritis, and spondyloarthritis by measuring the expression of cell surface antigens, in particular, chemokine receptors associated with homing[57]. MAIT cells tend to migrate toward peripheral tissues, particularly in inflammatory conditions, because they express a variety of chemokine and cytokine receptors. Most of these studies have implied that immune-mediated tissue damage is induced by the pathogenic proinflammatory features of MAIT cells. In contrast, MAIT cells may protect against the damage caused by inflammation, as described above for UC. Furthermore, a subset of MAIT cells (CD56−) accumulated in kidney and brain tumors and may operate in tumor immune responses[58].
As detailed above, there is no doubt about the importance of understanding the functions of MAIT cells in health and disease. However, the following questions still remain to be answered: What are the underlying mechanisms in immune regulation, particularly in innate immunity? And what are the molecules that control the functions of MAIT cells other than vitamin B2 metabolites? Although laboratory mice are useful to model human diseases, the study of MAIT cells is quite limited owing to their paucity in mice[18]. Furthermore, MAIT cells hardly propagate in vitro[11]. A recent paper, however, has showed the potential for MAIT cells to proliferate in response to E. coli and to anti-CD3/CD28/CD2 antibodies[33]. The expansion most likely depends on an precise balance between proliferation and activation-induced cell death, because MAIT cells are highly sensitive to activation-induced cell death[33,38,59]. To overcome these difficulties, we attempted to produce human MAIT cells through iPSC technology.
GENERATION OF MAIT CELLS USING MAIT CELL-DERIVED iPSCS
iPSCs may be established from a variety of somatic cells[60-62] and be differentiated into T cells, as can embryonic stem cells (ESCs)[63-65]. Nonetheless, it is near-impossible to obtain a monoclonal T cell with an antigenic specificity. This is primarily due to the fact that iPSCs and ESCs carry the germline configuration of TCRα and TCRβ, which are subject to random gene rearrangement during T cell differentiation, resulting in the generation of polyclonal T cells (Figure 2)[65]. Although iPSCs have been established with terminally differentiated T cells in PBMC, the authors did not address whether or not differentiation of these iPSCs into T cells culminated in regeneration of an antigen-specific T cell clone[66-68]. Recently, however, iPSCs have been established from tumor antigen-specific or HIV-specific CD8+ T cells with intention to rejuvenate T cells harboring the original epitopes, although the efficiency of such redifferentiation into the original clone remains unclear[69,70]. Well before these reports, we have shown that the progeny of a cloned mouse from NKT cells possessed an in-frame rearranged TCRα (Vα14-Jα18) specific for NKT cells in the genome, and an increased number of NKT cells[71]. This indicated that in-frame rearranged TCRα (Vα14-Jα18) had a strong impact on the destiny of T cells in the thymus. Such a notion has been explored further in vitro. ESCs prepared through nuclear transfer with hepatic NKT cells (ntESCs), harboring in-frame rearranged TCRα (Vα14-Jα18), gave raise to T lymphocytes exclusively comprising NKT cells (> 94%) when ntESCs were subjected to the OP9/OP9-DL system, which is well-known to promote T cell lineage differentiation from pluripotent stem cells[64,65,72,73]. We have exploited a corollary that iPSCs derived from MAIT cells would efficiently redifferentiate into MAIT cells under the same conditions, because MAIT cells are innate-like T cells, and these iPSCs possess a rearranged TCRα (Vα7.2-Jα33), specific for MAIT cells[21]. This turned out to be the case.
Figure 2.
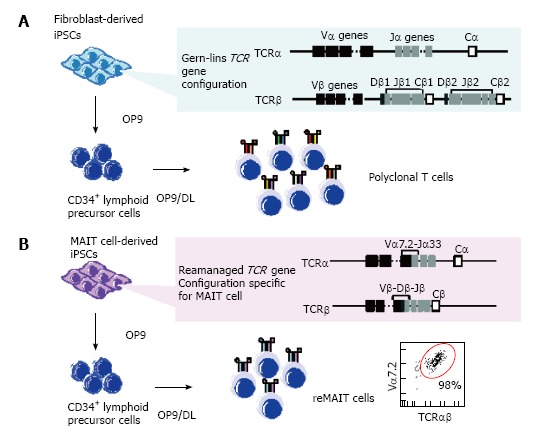
Scheme for T cell differentiation from induced pluripotent stem cells. Induced pluripotent stem cells (iPSCs) derived from normal somatic cells such as fibroblasts possess the germline configuration at T cell receptor (TCR) loci, whereas those from T cells harbor rearranged configurations (A); Upon differentiation in the T-cell-permissive conditions, the resulting T cells possess diverse sets of TCR repertoires; polyclonal T cells. In contrast, mucosal-associated invariant T (MAIT) cells-derived iPSCs exclusively confer MAIT cells in the same differentiation conditions. Note that MAIT cell-derived iPSCs possess a rearranged Vα7.2-Jα-33 specific for MAIT cells in the genome (B).
MAIT cells purified from umbilical cord blood (CB-MAIT) as TCR Vα7.2+ cells were reprogrammed with Sendai virus (SeV) vector harboring four reprogramming factors (Oct4, Sox2, Klf4 and c-Myc) (MAIT-iPSCs) without any proliferative stimulation as used in reprogramming of antigen-specific CD8+ T cells[69,70]. SeV is superior to other viruses, such as lentivirus, in that SeV does not integrate into the host genome, thus leaving the genomic DNA free from interruptions[21,67,69,74]. As expected, MAIT-iPSCs successfully redifferentiated into MAIT cell-like cells expressing Vα7.2, CD3, CD161, and IL-18Rα (reMAIT cells) with high efficiency (> 98%) in T-cell-permissive conditions (Figure 2)[21]. reMAIT cells generally display a naïve phenotype, but express a high level of CCR6 (a receptor directing mucosal tissue homing and IL-17 expression), recapitulating that CB-MAIT cells that are still in an immature stage prior to exposure to commensal flora[16,17,21,75,76]. Furthermore, reMAIT cells produce an array of cytokines, chemokines, and cytotoxic granules, such as granulysin, perforin and granzyme A, in an MR1-dependent manner. reMAIT cells also protect mice from Mycobacterial infection upon adoptive transfer, holding a promise to realize cell therapy with these cells[21]. Taken together, reMAIT cells should function as innate-like T cells, although they are still immature[16,17,21].
FUTURE PERSPECTIVES - DISEASE MODELING USING MAIT CELLS DIFFERENTIATED FROM iPSCS
reMAIT cells generated from iPSCs will be useful not only for deciphering their immunological functions in vivo but also for creating novel disease models in animals. Two types of genetically engineered mice, MR1-knockout mice and TCR transgenic mice, have been widely used to delineate the roles of MAIT cells in vivo (Tables 2 and 3). Originally, MR1-knockout mice (MR1-/-) were generated to assess the roles of MR1 in the selection and expansion of MAIT cells in vivo[18]. MR1-knockout mice possessed severely decreased TCR Vα19-Jα33 expression compared with their littermate controls[18]. Thus far, MR1-knockout mice have been used as a model devoid of MAIT cells. MR1-knockout mice may have shed light on the roles of MAIT cells in vivo. However, the findings from the mice were too complicated to interpret, maybe because in part of the insufficient number of MAIT cells in the control, and the lack of an appropriate reagent to detect mouse MAIT cells. An MR1 tetramer that has been created recently is useful to detect MAIT cells in mice and humans[77], but tetramer-positive cells may not always be functional cells. Three groups have independently reported Vα19 iTCR transgenic mice as a MAIT cell-enriched model[17,78-80]. Two reports indicated that MAIT cells in Vα19 iTCR transgenic mice harbored an effector/memory phenotype; CD44highCD69+CD25+ICOS+ and NK1.1[78-80]. With the ligand-loaded MR1 tetramer, it was found that approximately 40%-50% of MAIT cells were CD4+, and the rest being comprised of DN cells and fewer CD8+ MAIT cells[77], whereas in humans few CD4+ MAIT cells are present. Such a difference in CD4 or CD8 usage between mouse and human may reflect their physiological roles. In contrast, Martin et al[17] showed that MAIT cells from their Vα19iTCR transgenic mice were DN and CD8+ with few CD4+ . Furthermore, NK1.1, CD25, CD69, and ICOS were not present in MAIT cells. Such inconsistency demonstrated that MAIT cells are different in nature from those in transgenic mice. It is plausible that such an alteration stems from the differences in transgenes or commensal flora utilized. Should it be the case, the transgenic mouse may not be adequate to delineate the functions of MAIT cells[17]. Therefore, it is indispensable to create a novel animal model to address the physiological roles of MAIT cells in health and diseases, and harnessing the results of animals for clinical applications.
Table 2.
Mice used in study for mucosal-associated invariant T cells
| Genotype | Characteristics | Ref. | |
| Knockout mice | MR1-/- | Impaired development of MAIT cells | [79] |
| Transgenic mice | Vα19 iTCR Tg | Enriched MAIT cells | [17,78-80] |
| Vβ6 Vβ8 Tg | Increase of MAIT cells | [17] |
MAIT: Mucosal-associated invariant T; iTCR: Invariant T cell receptor.
Table 3.
Mucosal-associated invariant T cells in the diseases
| Category | Mouse strains | Disease model | Phenotype | Ref. |
| Bacterial infection | MR1-/- Vα19 iTCR Tg Vβ6 Vβ8 Tg | Escherichia coli Micobacterium abcessus | Increase in the bacterial burden Repression of the bacterial burden | [16] |
| MR1-/- | Klebsiella pneumoniae | Increased susceptibility to K. pneumoniae infection | [36] | |
| MR1-/- | Mycobacterium bovis BCG | Enhanced bacterial growth at the early stage of infection | [35] | |
| Francisella tularensis | Delayed adaptive immune reaction | [34] | ||
| Autoimmune diseases | Vα19 iTCR Tg | Experimental autoimmune encephalomyelitis (model of MS) | Suppressed disease induction and progression | [78] |
| MR1-/- | Collagen-induced arthritis (model of rheumatoid arthritis) | Improved CIA score | [86] | |
| Adoptive transfer Jα33+ MAIT cells into BALB/c | TNBS induced colitis | Improved disease index | [105] | |
| B10.RIII | Spondyloarthropathy by IL-23 | Enthesitis induced by IL-22 produced from IL-23R+RORγt+CD4-CD8- T cells (MAIT cells?) in the entheses | [91] | |
| Others | Vα19 iTCR Tg NOD | Non-obese diabetes | Delayed disease onset | [106] |
| Vα19 iTCR Tg | Delayed-type hypersensitivity to sheep erythrocytes (type IV allergy) | Suppression of the disease | [106] |
MAIT: Mucosal-associated invariant T; IL: Interleukin; CIA: Collagen-induced arthritis; iTCR: Invariant T cell receptor.
In this context, use of humanized mice can be envisaged, because the human cells in question can be engrafted and their functions and development may be examined in vivo[81]. To study the physiological roles in vivo, reMAIT cells were adoptively transferred to NOD/SCID or NOG (NOD/Shi-scid IL2Rγnull) mice, both of which are devoid of mature B, T cells, and the later deficient in NK cells, functional macrophages, and dendritic cells[21]. reMAIT cells migrated and engrafted in tissues such as the intestines, bone marrow, liver, and spleen, which probably mirrors the distribution of MAIT cells in humans[18,71]. In addition, reMAIT cells dramatically changed the phenotype from naïve to mature concomitant with the expression of the chemokine receptors required for the tissue-specific homing. Moreover, reMAIT cells appeared to proliferate in mice, whereas they did not in vitro. These results indicated that reMAIT cells from iPSCs responded to external cues, migrated to different tissues, and proliferated in mice. Such interactions most likely occur via chemokine receptors on reMAIT cells and via mouse MR1 bound with ligands from commensal flora or with an endogenous one. The data suggest that the function of reMAIT cells could be assessed in vivo, which opens up new horizons for modeling human diseases in mice.
Accordingly, the protective mechanisms of MAIT cells against bacterial infection have been examined using reMAIT cells[71]. Upon adoptive transfer, reMAIT cells protected mice from M. abscessus, as demonstrated by a decrease in bacterial burden. Such an protective activity mirrors that observed with MAIT cells from PBMCs[16]. Granulysin has been identified as an effector molecule in the control of mycobacterial infection. Granulysin is present together with granzymes and perforin in the cytolytic granules of cytotoxic cells such as CD8+ T and NK cells as well as MAIT cells[32,82]. Granulysin plays a crucial role not only in the destruction of infected cells but also in killing pathogens[83,84]. Given that mice are devoid of granulysin and its homologue[85], mice harboring reMAIT cells could serve as a novel model to decipher the roles of human-specific factors.
There is accumulating evidence that MAIT cells play a pivotal role in inflammatory and autoimmune diseases. Nonetheless, delineating how MAIT cells are implicated in these diseases has to await the advent of appropriate animal models. Experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) are animal models for MS and RA, respectively. By using Vα19 iTCR transgenic mice and/or MR1-knockout mice, the implication of MAIT cells in autoimmune diseases has been investigated[78,86]. In Vα19 iTCR mice, the severity of EAE was ameliorated in both induction and progression of demyelination compared with control littermates[78,86]. In marked contrast, the severity of CIA was improved in MR1-knockout mice, whereas adoptive transfer of MAIT cells from Vα19 iTCR transgenic mice resulted in aggravation of the disease[78,86]. EAE and CIA are intended to induce autoreactive T cells, especially focused on Th17 or Th1 responses, through hyperimmunization of putative target antigens (myelin basic proteins or type II collagen) with Freund’s adjuvant. Induced T cells could migrate to target tissues and secrete proinflammatory or anti-inflammatory cytokines, which may further worsen tissue damage or help resolve the damage. It has been believed that such mechanisms recapitulate the etiology and pathology of human diseases. Nonetheless, it is not appropriate to use such mice for disease modeling because MAIT cells do not react with peptide antigens, although they may respond to the components of adjuvant such as those from M. tuberculosis. Furthermore, the paucity of murine MAIT cells is another issue. Even though Vα19 iTCR transgenic mice can be used in a disease model, the nature of transgenic MAIT cells may be different from that present in the control. Given that MAIT cells are competent to produce a plethora of cytokines, a nature prerequisite for immunoregulatory functions, the above disease models may not be suitable for deciphering the etiology and pathology, in that such a crucial feature of MAIT cells is largely overlooked or distorted.
Exploring a disease model with reMAIT cells could further our knowledge of the etiology and pathology of MS. It has been reported that inflammatory demyelinating lesions are infiltrated by IL-17-expressing T cells in the mouse brain when they received cerebrospinal fluid from a progressive MS patients[87]. A longitudinal study in MS patients indicated massive expansion of MAIT cells or MAIT cell-like cells, harboring canonical or atypical TCR Vα and β chains but do not react with bacterial antigens, could play an important role in the onset and the formation of early active MS lesions[47]. The above data implied the presence of yet-to-be-identified ligands responsible for the negative effects of MAIT cells in disease. Use of reMAIT cells could make it possible to examine whether or not sole ligands for MR1 or any epigenetic modifications of MAIT cells are responsible for disease. In either case, mice with reMAIT cells are useful to identify such ligands and to create a novel autoimmune disease model.
Should MAIT cells play a pivotal role in autoimmune diseases, it is tempting to anticipate that granulysin per se or in combination with granzymes and perforin exerts a cytolytic activity against the target tissue. In line with this hypothesis, granulysin may play crucial roles in transplant rejection and epidermal necrosis in toxic epidermal necrolysis and Stevens-Johnson syndrome[85,88,89]. Furthermore, combined with the ectopic expression of human cytokines and/or chemokines, mice with reMAIT cells could be further fine-tuned to mimic human diseases by controlling tissue migration[90,91]. Provided such an exquisite model is available, we can go to the next step of drug discovery and/or screening. Compounds that interfere either with the development of MAIT cells or the function of MAIT cells can be screened in such a mouse model (Figure 3).
Figure 3.
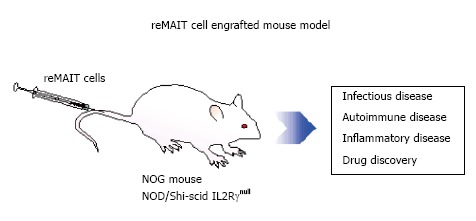
Utility of mucosal-associated invariant T cells from induced pluripotent stem cells (redifferentiation of mucosal-associated invariant T cells) for modeling human diseases. Severely immunocompromised mice received MAIT cells from induced pluripotent stem cells. reMAIT cells are useful for deciphering the physiological functions of MAIT cells in health and disease. MAIT: Mucosal-associated invariant T; reMAIT: Redifferentiation of MAIT.
CONCLUSION
Recent studies have shed light on the unique properties of MAIT cells and on their possible involvement in a variety of human diseases, although MAIT cells have been overlooked behind conventional T cells and other innate immune cells for a long time. The paucity of MAIT cells in laboratory mice and their extremely poor proliferative capacity are the biggest obstacles to fully understand the function of MAIT cells in health and diseases. Reprograming and redifferentiation of MAIT cells from iPSCs have overcome these difficulties. Furthermore, mice with reMAIT cells will pave the way for unveiling the mechanisms underlying the diseases and open up new horizons in medical research.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 20, 2015
First decision: August 14, 2015
Article in press: February 16, 2016
P- Reviewer: Politi LE S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
References
- 1.Gulati P. Janeway’s Immunobiology. In: Murphy K, Travers P, Walport M, editors. USA: Wiley Online Library; 2009. [Google Scholar]
- 2.Treiner E, Lantz O. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr Opin Immunol. 2006;18:519–526. doi: 10.1016/j.coi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 4.Treiner E, Duban L, Moura IC, Hansen T, Gilfillan S, Lantz O. Mucosal-associated invariant T (MAIT) cells: an evolutionarily conserved T cell subset. Microbes Infect. 2005;7:552–559. doi: 10.1016/j.micinf.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–366. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 6.Alonzo ES, Sant’Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011;23:220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res. 2011;343:43–55. doi: 10.1007/s00441-010-1023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 11.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 12.Lepore M, Kalinichenko A, Colone A, Paleja B, Singhal A, Tschumi A, Lee B, Poidinger M, Zolezzi F, Quagliata L, et al. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nat Commun. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 13.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 17.Martin E, Treiner E, Duban L, Guerri L, Laude H, Toly C, Premel V, Devys A, Moura IC, Tilloy F, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 19.Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, Sahgal N, Leslie A, Oo Y, Geremia A, et al. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119:422–433. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma PK, Wong EB, Napier RJ, Bishai WR, Ndung’u T, Kasprowicz VO, Lewinsohn DA, Lewinsohn DM, Gold MC. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015;145:443–453. doi: 10.1111/imm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakao H, Yoshikiyo K, Koshimizu U, Furukawa T, Enomoto K, Matsunaga T, Tanaka T, Yasutomi Y, Yamada T, Minakami H, et al. Expansion of functional human mucosal-associated invariant T cells via reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:546–558. doi: 10.1016/j.stem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Lee OJ, Cho YN, Kee SJ, Kim MJ, Jin HM, Lee SJ, Park KJ, Kim TJ, Lee SS, Kwon YS, et al. Circulating mucosal-associated invariant T cell levels and their cytokine levels in healthy adults. Exp Gerontol. 2014;49:47–54. doi: 10.1016/j.exger.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, Bhati M, Chen Z, Kostenko L, Reantragoon R, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 24.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998;161:4066–4077. [PubMed] [Google Scholar]
- 25.Yamaguchi H, Hashimoto K. Association of MR1 protein, an MHC class I-related molecule, with beta(2)-microglobulin. Biochem Biophys Res Commun. 2002;290:722–729. doi: 10.1006/bbrc.2001.6277. [DOI] [PubMed] [Google Scholar]
- 26.Huang S, Martin E, Kim S, Yu L, Soudais C, Fremont DH, Lantz O, Hansen TH. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc Natl Acad Sci USA. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seach N, Guerri L, Le Bourhis L, Mburu Y, Cui Y, Bessoles S, Soudais C, Lantz O. Double-positive thymocytes select mucosal-associated invariant T cells. J Immunol. 2013;191:6002–6009. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 28.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun. 2014;5:3143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol. 2014;80:271–275. doi: 10.1111/sji.12193. [DOI] [PubMed] [Google Scholar]
- 31.Gold MC, Lewinsohn DM. Mucosal associated invariant T cells and the immune response to infection. Microbes Infect. 2011;13:742–748. doi: 10.1016/j.micinf.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Bourhis L, Dusseaux M, Bohineust A, Bessoles S, Martin E, Premel V, Coré M, Sleurs D, Serriari NE, Treiner E, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci USA. 2013;110:E3119–E3128. doi: 10.1073/pnas.1302799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80:3256–3267. doi: 10.1128/IAI.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48:769–775. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosgrove C, Ussher JE, Rauch A, Gärtner K, Kurioka A, Hühn MH, Adelmann K, Kang YH, Fergusson JR, Simmonds P, et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood. 2013;121:951–961. doi: 10.1182/blood-2012-06-436436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eberhard JM, Hartjen P, Kummer S, Schmidt RE, Bockhorn M, Lehmann C, Balagopal A, Hauber J, van Lunzen J, Schulze zur Wiesch J. CD161+ MAIT cells are severely reduced in peripheral blood and lymph nodes of HIV-infected individuals independently of disease progression. PLoS One. 2014;9:e111323. doi: 10.1371/journal.pone.0111323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez CS, Amarasena T, Kelleher AD, Rossjohn J, McCluskey J, Godfrey DI, Kent SJ. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol. 2015;93:177–188. doi: 10.1038/icb.2014.91. [DOI] [PubMed] [Google Scholar]
- 41.Greathead L, Metcalf R, Gazzard B, Gotch F, Steel A, Kelleher P. CD8+/CD161++ mucosal-associated invariant T-cell levels in the colon are restored on long-term antiretroviral therapy and correlate with CD8+ T-cell immune activation. AIDS. 2014;28:1690–1692. doi: 10.1097/QAD.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 42.Leeansyah E, Ganesh A, Quigley MF, Sönnerborg A, Andersson J, Hunt PW, Somsouk M, Deeks SG, Martin JN, Moll M, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121:1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong EB, Akilimali NA, Govender P, Sullivan ZA, Cosgrove C, Pillay M, Lewinsohn DM, Bishai WR, Walker BD, Ndung’u T, et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS One. 2013;8:e83474. doi: 10.1371/journal.pone.0083474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Illés Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of Valpha7.2-Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol. 2004;16:223–230. doi: 10.1093/intimm/dxh018. [DOI] [PubMed] [Google Scholar]
- 45.Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL, Carassiti D, Reynolds R, Salvetti M, Calabresi PA, et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain. 2013;136:2888–2903. doi: 10.1093/brain/awt182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willing A, Leach OA, Ufer F, Attfield KE, Steinbach K, Kursawe N, Piedavent M, Friese MA. CD8⁺ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur J Immunol. 2014;44:3119–3128. doi: 10.1002/eji.201344160. [DOI] [PubMed] [Google Scholar]
- 47.Held K, Bhonsle-Deeng L, Siewert K, Sato W, Beltrán E, Schmidt S, Rühl G, Ng JK, Engerer P, Moser M, et al. αβ T-cell receptors from multiple sclerosis brain lesions show MAIT cell-related features. Neurol Neuroimmunol Neuroinflamm. 2015;2:e107. doi: 10.1212/NXI.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyazaki Y, Miyake S, Chiba A, Lantz O, Yamamura T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int Immunol. 2011;23:529–535. doi: 10.1093/intimm/dxr047. [DOI] [PubMed] [Google Scholar]
- 49.Serriari NE, Eoche M, Lamotte L, Lion J, Fumery M, Marcelo P, Chatelain D, Barre A, Nguyen-Khac E, Lantz O, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 51.Teunissen MB, Yeremenko NG, Baeten DL, Chielie S, Spuls PI, de Rie MA, Lantz O, Res PC. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J Invest Dermatol. 2014;134:2898–2907. doi: 10.1038/jid.2014.261. [DOI] [PubMed] [Google Scholar]
- 52.Dunne MR, Elliott L, Hussey S, Mahmud N, Kelly J, Doherty DG, Feighery CF. Persistent changes in circulating and intestinal γδ T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLoS One. 2013;8:e76008. doi: 10.1371/journal.pone.0076008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho YN, Kee SJ, Kim TJ, Jin HM, Kim MJ, Jung HJ, Park KJ, Lee SJ, Lee SS, Kwon YS, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol. 2014;193:3891–3901. doi: 10.4049/jimmunol.1302701. [DOI] [PubMed] [Google Scholar]
- 54.Harms RZ, Lorenzo KM, Corley KP, Cabrera MS, Sarvetnick NE. Altered CD161 bright CD8+ mucosal associated invariant T (MAIT)-like cell dynamics and increased differentiation states among juvenile type 1 diabetics. PLoS One. 2015;10:e0117335. doi: 10.1371/journal.pone.0117335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magalhaes I, Pingris K, Poitou C, Bessoles S, Venteclef N, Kiaf B, Beaudoin L, Da Silva J, Allatif O, Rossjohn J, et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest. 2015;125:1752–1762. doi: 10.1172/JCI78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, Geoghegan J, Cody D, O’Connell J, Winter DC, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194:5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 57.Sugimoto C, Konno T, Wakao R, Fujita H, Fujita H, Wakao H. Mucosal-associated invariant T cell is a potential marker to distinguish fibromyalgia syndrome from arthritis. PLoS One. 2015;10:e0121124. doi: 10.1371/journal.pone.0121124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterfalvi A, Gomori E, Magyarlaki T, Pal J, Banati M, Javorhazy A, Szekeres-Bartho J, Szereday L, Illes Z. Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. 2008;20:1517–1525. doi: 10.1093/intimm/dxn111. [DOI] [PubMed] [Google Scholar]
- 59.Gérart S, Sibéril S, Martin E, Lenoir C, Aguilar C, Picard C, Lantz O, Fischer A, Latour S. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood. 2013;121:614–623. doi: 10.1182/blood-2012-09-456095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue H, Nagata N, Kurokawa H, Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, Dang CV, Spivak JL, Moliterno AR, Cheng L. Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009;114:5473–5480. doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galic Z, Kitchen SG, Kacena A, Subramanian A, Burke B, Cortado R, Zack JA. T lineage differentiation from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:11742–11747. doi: 10.1073/pnas.0604244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lei F, Haque R, Weiler L, Vrana KE, Song J. T lineage differentiation from induced pluripotent stem cells. Cell Immunol. 2009;260:1–5. doi: 10.1016/j.cellimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zúñiga-Pflücker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 66.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, Urbach A, Heffner GC, Grskovic M, Vigneault F, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7:15–19. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, Nakata H, Tohyama S, Hashimoto H, Kodaira M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7:11–14. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 68.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, Mostoslavsky G, Jaenisch R. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7:20–24. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, Nakayama-Hosoya K, Iriguchi S, Uemura Y, Shimizu T, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Wakao H, Kawamoto H, Sakata S, Inoue K, Ogura A, Wakao R, Oda A, Fujita H. A novel mouse model for invariant NKT cell study. J Immunol. 2007;179:3888–3895. doi: 10.4049/jimmunol.179.6.3888. [DOI] [PubMed] [Google Scholar]
- 72.Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 73.Wakao H, Wakao R, Sakata S, Iwabuchi K, Oda A, Fujita H. In vitro induction of natural killer T cells from embryonic stem cells prepared using somatic cell nuclear transfer. FASEB J. 2008;22:2223–2231. doi: 10.1096/fj.07-104687. [DOI] [PubMed] [Google Scholar]
- 74.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito T, Carson WF, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317:613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, Eckle SB, Uldrich AP, Birkinshaw RW, Patel O, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Croxford JL, Miyake S, Huang YY, Shimamura M, Yamamura T. Invariant V(alpha)19i T cells regulate autoimmune inflammation. Nat Immunol. 2006;7:987–994. doi: 10.1038/ni1370. [DOI] [PubMed] [Google Scholar]
- 79.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-restricted V alpha 19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol. 2006;176:1618–1627. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 80.Okamoto N, Kanie O, Huang YY, Fujii R, Watanabe H, Shimamura M. Synthetic alpha-mannosyl ceramide as a potent stimulant for an NKT cell repertoire bearing the invariant Valpha19-Jalpha26 TCR alpha chain. Chem Biol. 2005;12:677–683. doi: 10.1016/j.chembiol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 81.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krensky AM, Clayberger C. Granulysin: a novel host defense molecule. Am J Transplant. 2005;5:1789–1792. doi: 10.1111/j.1600-6143.2005.00970.x. [DOI] [PubMed] [Google Scholar]
- 83.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 84.Walch M, Dotiwala F, Mulik S, Thiery J, Kirchhausen T, Clayberger C, Krensky AM, Martinvalet D, Lieberman J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell. 2014;157:1309–1323. doi: 10.1016/j.cell.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krensky AM, Clayberger C. Biology and clinical relevance of granulysin. Tissue Antigens. 2009;73:193–198. doi: 10.1111/j.1399-0039.2008.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiba A, Tajima R, Tomi C, Miyazaki Y, Yamamura T, Miyake S. Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum. 2012;64:153–161. doi: 10.1002/art.33314. [DOI] [PubMed] [Google Scholar]
- 87.Cristofanilli M, Rosenthal H, Cymring B, Gratch D, Pagano B, Xie B, Sadiq SA. Progressive multiple sclerosis cerebrospinal fluid induces inflammatory demyelination, axonal loss, and astrogliosis in mice. Exp Neurol. 2014;261:620–632. doi: 10.1016/j.expneurol.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 88.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 89.Hidalgo LG, Einecke G, Allanach K, Mengel M, Sis B, Mueller TF, Halloran PF. The transcriptome of human cytotoxic T cells: measuring the burden of CTL-associated transcripts in human kidney transplants. Am J Transplant. 2008;8:637–646. doi: 10.1111/j.1600-6143.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- 90.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, et al. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 92.Kwon YS, Cho YN, Kim MJ, Jin HM, Jung HJ, Kang JH, Park KJ, Kim TJ, Kee HJ, Kim N, et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb) 2015;95:267–274. doi: 10.1016/j.tube.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Saeidi A, Tien Tien VL, Al-Batran R, Al-Darraji HA, Tan HY, Yong YK, Ponnampalavanar S, Barathan M, Rukumani DV, Ansari AW, et al. Attrition of TCR Vα7.2+ CD161++ MAIT cells in HIV-tuberculosis co-infection is associated with elevated levels of PD-1 expression. PLoS One. 2015;10:e0124659. doi: 10.1371/journal.pone.0124659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sandberg JK, Dias J, Shacklett BL, Leeansyah E. Will loss of your MAITs weaken your HAART [corrected]? AIDS. 2013;27:2501–2504. doi: 10.1097/QAD.0b013e3283620726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grimaldi D, Le Bourhis L, Sauneuf B, Dechartres A, Rousseau C, Ouaaz F, Milder M, Louis D, Chiche JD, Mira JP, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 2014;40:192–201. doi: 10.1007/s00134-013-3163-x. [DOI] [PubMed] [Google Scholar]
- 96.Smith DJ, Hill GR, Bell SC, Reid DW. Reduced mucosal associated invariant T-cells are associated with increased disease severity and Pseudomonas aeruginosa infection in cystic fibrosis. PLoS One. 2014;9:e109891. doi: 10.1371/journal.pone.0109891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leung DT, Bhuiyan TR, Nishat NS, Hoq MR, Aktar A, Rahman MA, Uddin T, Khan AI, Chowdhury F, Charles RC, et al. Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl Trop Dis. 2014;8:e3076. doi: 10.1371/journal.pntd.0003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Annibali V, Ristori G, Angelini DF, Serafini B, Mechelli R, Cannoni S, Romano S, Paolillo A, Abderrahim H, Diamantini A, et al. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–554. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- 99.Billerbeck E, Kang YH, Walker L, Lockstone H, Grafmueller S, Fleming V, Flint J, Willberg CB, Bengsch B, Seigel B, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010;107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hiejima E, Kawai T, Nakase H, Tsuruyama T, Morimoto T, Yasumi T, Taga T, Kanegane H, Hori M, Ohmori K, et al. Reduced Numbers and Proapoptotic Features of Mucosal-associated Invariant T Cells as a Characteristic Finding in Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1529–1540. doi: 10.1097/MIB.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 101.Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136:323–333. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim JC, Jin HM, Cho YN, Kwon YS, Kee SJ, Park YW. Deficiencies of Circulating Mucosal-associated Invariant T Cells and Natural Killer T Cells in Patients with Acute Cholecystitis. J Korean Med Sci. 2015;30:606–611. doi: 10.3346/jkms.2015.30.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malard F, Bossard C, Brissot E, Chevallier P, Guillaume T, Delaunay J, Mosnier JF, Moreau P, Grégoire M, Gaugler B, et al. Increased plasmacytoid dendritic cells and RORγt-expressing immune effectors in cutaneous acute graft-versus-host disease. J Leukoc Biol. 2013;94:1337–1343. doi: 10.1189/jlb.0513295. [DOI] [PubMed] [Google Scholar]
- 104.Baron M, Belo R, Cathelin D, Moreira-Teixeira L, Cartery C, Rondeau E, Mesnard L, Leite-de-Moraes M. Innate-like and conventional T cell populations from hemodialyzed and kidney transplanted patients are equally compromised. PLoS One. 2014;9:e105422. doi: 10.1371/journal.pone.0105422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruijing X, Mengjun W, Xiaoling Z, Shu P, Mei W, Yingcheng Z, Yuling H, Jinquan T. Jα33+ MAIT cells play a protective role in TNBS induced intestinal inflammation. Hepatogastroenterology. 2012;59:762–767. doi: 10.5754/hge11432. [DOI] [PubMed] [Google Scholar]
- 106.Shimamura M, Huang YY, Goji H, Endo S, Migishima R, Yokoyama M. Regulation of immunological disorders by invariant Vα19-Jα33 TCR-bearing cells. Immunobiology. 2011;216:374–378. doi: 10.1016/j.imbio.2010.08.003. [DOI] [PubMed] [Google Scholar]


