Abstract
Stream metacommunities are structured by a combination of local (environmental filtering) and regional (dispersal) processes. The unique characters of high mountain streams could potentially determine metacommunity structuring, which is currently poorly understood. Aiming at understanding how these characters influenced metacommunity structuring, we explored the relative importance of local environmental conditions and various dispersal processes, including through geographical (overland), topographical (across mountain barriers) and network (along flow direction) pathways in shaping benthic diatom communities. From a trait perspective, diatoms were categorized into high-profile, low-profile and motile guild to examine the roles of functional traits. Our results indicated that both environmental filtering and dispersal processes influenced metacommunity structuring, with dispersal contributing more than environmental processes. Among the three pathways, stream corridors were primary pathway. Deconstructive analysis suggested different responses to environmental and spatial factors for each of three ecological guilds. However, regardless of traits, dispersal among streams was limited by mountain barriers, while dispersal along stream was promoted by rushing flow in high mountain stream. Our results highlighted that directional processes had prevailing effects on metacommunity structuring in high mountain streams. Flow directionality, mountain barriers and ecological guilds contributed to a better understanding of the roles that mountains played in structuring metacommunity.
Dispersal is one of the most important processes in biogeography, evolutionary biology and ecology. Dispersal affects the distribution of individuals in space, which in turn gives rise to genetic differentiation and changes the potential for local adaptation1. Traditionally, dispersal has been regarded as a population level character2; however, it also plays a significant role in community dynamics3,4. At the regional scale, dispersal shapes species diversity patterns5, and determines how much a regional pool contributes to species composition of local communities6. At the local scale, dispersal controls the loss (extinction, emigration) and gain (immigration, speciation) of individuals, regulates species coexistence through competition-colonization trade-off 7 and determines structure and function of local communities8. The theory of island biogeography and the neutral community models (NCM) are two examples which exemplify the effects of dispersal at the regional and local scales9,10.
Understanding the degree to which assemblages are dispersal limited could provide insights into the mechanism of community assembly and the maintenance of biodiversity3,5. On one hand, any factors which prevent organisms from moving between local habitats can be considered as barriers to dispersal11. The most common dispersal barriers are geographical barriers: dispersal limitation typically increased with increasing spatial distance between sites. Distance decay relationships (DDRs) describe this biogeographical phenomenon12. In addition, topographical and geomorphological factors (e.g. high mountains), hydrological factors, unsuited environmental conditions (e.g. deserts between sites), low connectivity of habitats, and artificial obstacles (e.g. dams for fish) also impede the dispersal of organisms13,14,15. On the other hand, ecological traits of species, such as dispersal ability or dispersal mode also determine the dispersal limitation16.
Community assembly is not only affected by dispersal occurring at regional scale, but also structured by local processes, such as environmental filtering and species interactions17,18. Metacommunity theory has provided an insightful view and a mechanism approach to study the dynamics of community patterns, species diversity and distribution at both local and regional scales18, which addresses ‘a set of local communities that are linked by dispersal of multiple potentially interacting species’19. Four conceptual paradigms have been put forward by Leibold et al.17 to describe metacommunities, depending on the relative importance of local and regional processes, termed as patch-dynamic (PD), species-sorting (SS), mass-effect (ME) and neutral-model (NM) paradigms. In the PD perspective, patches are identical and competition-colonization trade-off regulates metacommunity20. NM also assumes that patches are identical, but there is no competition–colonization trade-off (species are ecologically identical) and that stochastic speciation, extinction, immigration and migration determine species composition21. In contrast, SS and ME both assume patches and species are not identical. In SS, dispersal is not limited (moderate), so that species can track environmental gradients22, while in ME, the rate of dispersal is high enough to allow species survive in suboptimal patches via source-sink dynamics18,23. Another situation may arise if limiting dispersal (LD) occurs among patches, in that situation, the low-rate dispersal prevents species from being sorted to their optimal patches, and metacommunity presents a patch-dynamic paradigm23,24,25. Recently, many studies in this field have been published across a wide range of organisms and covering the terrestrial, marine and freshwater realms26,27. However, to our knowledge, few studies have been carried out in high mountain streams (but see28). Furthermore, most previous metacommunity studies were more concerned with geographic distance and many other dispersal limitation factors were largely ignored (but see14,15,25,29).
High mountain streams are often characterized by steep valleys, high elevational gradients and rushing flow. Geographical barriers occur frequently and across large areas11,30. For the organisms inhabiting high mountain streams, the steep slopes on both sides of the streams may constitute significant physical barriers to dispersal, even across small spatial extents31. For example, the Southern Rocky Mountains (mean altitude of ca. 3500 m) were proven to be a topographical barrier to the dispersal of a species of black fly (Simuliidae) (<800 km2)32. Similar results were shown by Šlechtová et al. for the fish in the Alps33. However, both studies mainly focused on the barrier effects at metapopulation level through molecular approaches, rather than from the metacommunity perspective. Furthermore, steep elevational gradients and high discharge can impose a significant directional dispersal along stream channels34,35. As a result, alternative dispersal pathways would be restricted compared with other systems, such as lakes or streams in flat areas13. Heino et al. who worked in River Oulankajoki basin which was characterized by considerable altitudinal differences suggested that high-rate dispersal would be important within streams36. Also a conceptual model focusing on the scale dependency of the mechanisms affecting metacommunities has supported the high-rate dispersal within streams and weaker dispersal among streams37. Steep elevational gradients also create more intense environmental gradients, such as temperature and conductivity38, which can influence the local processes more strongly than occurring in gentle slope areas. Accordingly, attempts to fully understand the mechanisms behind metacommunity structuring in high mountain streams must account for the intense environmental gradients and all kinds of dispersal limitation.
In this study, we investigated 63 sites in high mountain streams (elevation ranges from 1600 to 2900 m a.s.l.), across a small spatial extent in Southwestern China (<500 km2, Fig. 1). Benthic diatoms were selected to study metacommunity structuring in high mountain streams for the following reasons: (1) benthic diatoms are dominant primary producers, and one of the most important and ubiquitous assemblages in streams, however, to our knowledge, metacommunity study on benthic diatoms is still scarce up to now, especially those considering the dispersal ability or some other ecological traits when comparing with macroinvertebrates11,39 (but see40,41); (2) diatoms are widely regarded as ubiquitously distributed organisms42, but recent studies suggested that dispersal limitation also played an important role in structuring benthic diatom metacommunities in streams at a large scale30,39,43; (3) whether dispersal limitation occurs in high mountains at a small scale remains unknown; and (4) it is relatively easy to study the traits related to dispersal and habitat affinities of benthic diatoms44.
Figure 1. Map of the study area and the distribution of sampling sites in the Cangshan Erhai National Nature Reserve, Yunnan Province, Southwestern China.
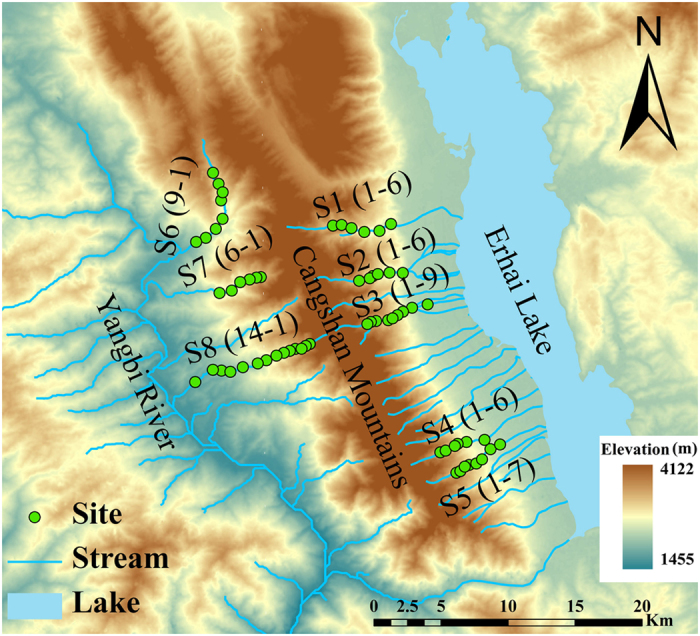
Green dots represent sampling sites (S1–1 represents the site most upstream in stream 1, and S1–6 represents the site most downstream in stream 1); blue lines and polygon depict the streams and the Erhai Lake. The map is based on a digital elevation model at 30 m resolution and created using ArcGIS 10.0 software (http://www.esri.com/software/arcgis).
The aim of our study was to understand diatom metacommunity structuring in high mountain streams. To cover the unique characters of high mountain streams, geographical distance, topographic distance and network distance along flow direction were applied to model all pathways of dispersal (Fig. 2). Relative contributions of these three dispersal processes were then assessed to understand how high mountains restricted dispersal processes. Afterwards, we analyzed the relative influences of environmental vs. dispersal processes to explore the mechanism of community assembly. For a better understanding, benthic diatoms were categorized into three ecological guilds (namely high-profile, low-profile and motile guild44) to further examine the roles of functional traits. We proposed three hypotheses based on the unique characters of high mountain streams and species traits of benthic diatoms:
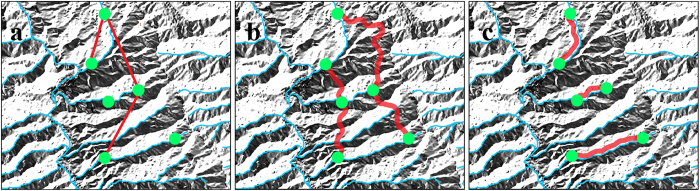
Figure 2. Hypothetical pathways of organisms’ dispersal among sites along or among streams in high mountains.
(a) Geographical pathway (overland dispersal); (b) topographical pathway (across mountain barriers); (c) network pathway (along flow direction). The locations of communities are shown as green dots, streams are depicted by blue lines and red lines represent dispersal pathways between locations. The schematic diagrams were created with ArcGIS 10.0 (http://www.esri.com/software/arcgis) and modified with Adobe Photoshop CS6.
H1: Owing to steep slopes and high discharge, dispersal among streams will be limited, downstream along streams will be promoted.
H2: Steep elevational gradients will create intense environmental gradients in high mountain streams, giving rise to strong environmental effects.
H3: Ecological guilds are expected to respond to environmental and spatial factors differently: all guilds are expected to have significant relationships with environmental factors. While weak dispersers (the low-profile guild) will show strong spatial structure due to dispersal limitation, while strong dispersers (the high-profile and motile guild) will not.
Results
Community composition patterns
Across all 63 sites in our study area, a total of 149 diatom species belonging to 34 genera were identified. The number of species assigned to the high-profile, low-profile and motile guild were 49, 68 and 32 respectively. There were three dominant species in our study: Achnanthidium rivulare Potapova & Ponader, Achnanthidium minutissimum (Kützing) Czarnecki, and Achnanthidium minutissimum f. inconspicuum (Østrup) Compère & Riaux-Gobin, with relative abundance of 29.7%, 21.4%, and 13.2%, all belonging to the low-profile guild. The low-profile guild outweighed the other two guilds, contributing 45.6% of total richness and 95.3% of total abundance. Details on the species of each ecological guild were described in Supplementary Tables S1, S4 and S5.
Benthic diatom communities among the eight streams showed significant differences (adonis statistic R2 = 0.319, P = 0.001; ANOSIM statistic R = 0.24, P = 0.001; A = 0.157, P = 0.001 based on MRPP). Communities on the eastern slope were dissimilar from those on the western slope (adonis statistic R2 = 0.041, P = 0.022; ANOSIM statistic R = 0.062, P = 0.022; A = 0.018, P = 0.008 based on MRPP). Communities in the five streams on the eastern slope (adonis statistic R2 = 0.386, P = 0.001; ANOSIM statistic R = 0.443, P = 0.001; A = 0.197, P = 0.001 based on MRPP) and in the three streams on the western slope (adonis statistic R2 = 0.184, P = 0.003; ANOSIM statistic R = 0.111, P = 0.05; A = 0.079, P = 0.003 based on MRPP) were all distinctive in their composition.
Relative importance of environmental and spatial factors
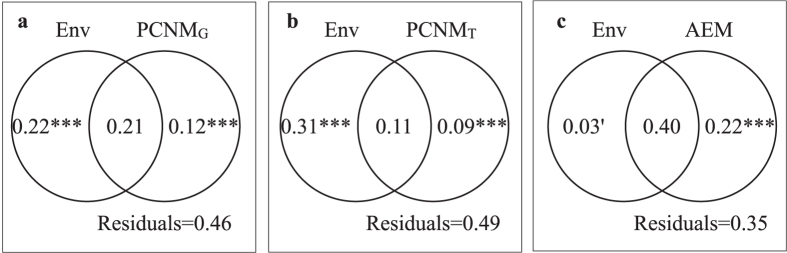
Variation partitioning was firstly performed on environmental variables (Env) and geographical spatial variables (PCNMG) for diatom communities. Both sets of variables were significant and could explain 54% variation of diatom communities (Fig. 3a). The variation purely explained by Env and PCNMG was 22% and 12% respectively, while the shared fraction was 21% (Fig. 3a). Env and topographic spatial variables (PCNMT) explained 51% of the community variation. Env alone explained much more than PCNMT (31% and 9%), while both explained 11% community variation (Fig. 3b). Directional spatial variables (AEM) had a unique contribution of 22% to metacommunity structuring, and the total explained variation increased to 65%. Furthermore, the unique faction of Env (3%) turned to be marginally significant (Fig. 3c).
Figure 3.
Venn-diagrams showing the results of variation partitioning performed on (a) Environmental model (Env) and Geographical model (PCNMG), (b) Environmental model (Env) and Topographic model (PCNMT), and (c) Environmental model (Env) and Directional spatial model (AEM) for benthic diatom metacommuntiy in the study area. Variation explained uniquely and jointly, and the unexplained fractions were shown as the number in each part of the figures (total variation = 1). The significance of each testable fraction was expressed as ■P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001.
Comparison of dispersal modes
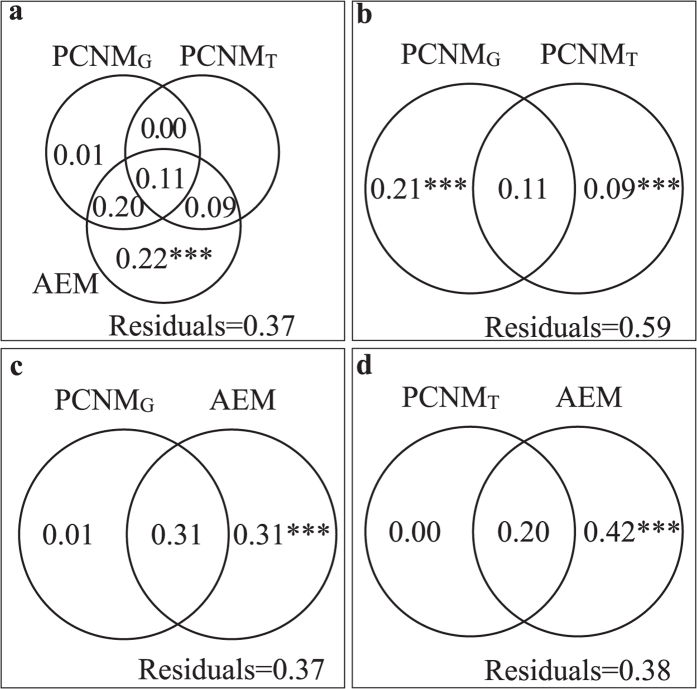
All of the three sets of spatial variables (i.e. PCNMG, PCNMT and AEM) could significantly explain variation of diatom communities (P = 0.001 for PCNMG, P = 0.019 for PCNMT, and P = 0.016 for AEM, Table 1). Among them, AEM contained the most important spatial variables, as it accounted for 22% of the unique fraction and 40% of the shared variance (Fig. 4a). When variation partitioning was performed on PCNMG and PCNMT only, both of them had unique contributions (Fig. 4b), while AEM shared almost all explained variation with PCNMG and PCNMT (Fig. 4c,d).
Table 1. Results of global test and forward selection for all taxa and each guild.
| Group | Global test significance | Variables retained for variation partitioning from forward selection (AdjR2Cum) | ||||||
|---|---|---|---|---|---|---|---|---|
| P(Env) | P (PCNMG) | P (PCNMT) | P (AEM) | Env | PCNMG | PCNMT | AEM | |
| All | 0.001*** | 0.001*** | 0.019* | 0.016* | Conductivity, Built-up%, Agriculture%, TOC, DO, Width, Grass%, PO4-P, Altitude, Barren land%, T-N, NO3-N (0.42) | PCNMG 2, 6, 1, 25, 3, 13, 7 (0.32) | PCNMT 5, 1, 3 (0.20) | AEM 4, 2, 8, 5, 1, 14, 10, 9, 3, 16, 15, 18, 6, 23, 11, 17, 26, 54, 19, 20, 13, 12, 60, 7, 27, 34, (0.61) |
| High | 0.001*** | 0.146 | 0.026* | 0.878 | Altitude, Depth, pH, Built-up%, PO4-P, T-N (0.14) | PCNMT 7, 5, 3, 43, 41, 1 (0.13) | ||
| Low | 0.001*** | 0.001*** | 0.013* | 0.031* | Conductivity, Built-up%, TOC, DO, Agriculture%, Width, T-N, NO3-N (0.41) | PCNMG 2, 6, 1, 25, 3, 13, 7, 21 (0.38) | PCNMT 5, 1, 3 (0.23) | AEM 4, 2, 8, 5, 1, 14, 10, 3, 9, 15, 16, 18, 6, 23, 26, 54, 17, 11, 19, 20, 12, 13, 27, 28, 7, 60, 34 (0.68) |
| Motile | 0.031* | 0.184 | 0.832 | 0.464 | Built-up%, NO3-N (0.05) | |||
Environmental model, Geographical model, Topographic model and Directional spatial model were expressed as Env, PCNMG, PCNMT and AEM. P (Env), P (PCNMG), P (PCNMT) and P (AEM) give the significance of global tests (i.e. using all variables in each model). Only when global tests were significant, forward selections could be proceeded to get parsimonious models. The final retained variables are shown in the order in which they were selected in the forward selection procedure, with the AdjR2Cum of all retained variables in the following parentheses. The variables of spatial model were indicated as numbers, where small numbers represented broad-scales patterns and large numbers represented fine-scales. High, low and motile represented the high-profile, low-profile and motile guild, respectively. Significance was expressed as *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
Venn-diagrams showing the results of variation partitioning performed on (a) Geographical model (PCNMG), Topographic model (PCNMT) and directional spatial model (AEM), (b) PCNMG and PCNMT, (c) PCNMG and AEM, (d) PCNMT and AEM for benthic diatom metacommuntiy in the study area. Variation explained uniquely and jointly, and the unexplained fractions were shown as the number in each part of the figures (total variation = 1). The significance of each testable fraction was expressed as *P < 0.05, **P < 0.01, ***P < 0.001.
Analysis of deconstructed matrices
Among the three ecological guilds, different responses to environmental and spatial variables were revealed. Similar to the whole community, the low-profile guild had significant relationships with the environmental and the spatial variables (Table 1). The directional variables (AEM) also influenced the low-profile guild, with 13% of unique and 53% of shared fraction of variation explained (Table 2). In contrast, the high-profile guild could only be explained by environmental and topographic variables, with a total explained variation of 20%. Local effects were different between the high-profile and low-profile guild: conductivity, built-up% and TOC were the most important environmental variables for the low-profile guild, while altitude, depth, and pH were for the high-profile guild. Environmental variables contributed only a little to variation in the motile guild (5%), with spatial variables showing non-significant effects (P = 0.184, 0.832 and 0.464 for PCNMG, PCNMT and AEM, Table 1).
Table 2. Fractions of variation partitioning for all taxa and each guild.
| a | b | c | d | e | f | g | h | i | j | k | l | m | n | o | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 0.02 | 0.01 | — | 0.09** | — | 0.003 | 0.01 | 0.12 | 0.03 | 0.03 | 0.17 | — | 0.06 | 0.1 | 0.04 | 0.35 |
| High | 0.05** | — | 0.06*** | — | — | — | 0.09 | — | — | — | — | — | — | — | — | 0.80 |
| Low | 0.02■ | 0.07** | — | 0.13*** | — | — | 0.004 | 0.11 | 0.07 | 0.03 | 0.14 | 0.005 | 0.04 | 0.07 | 0.08 | 0.25 |
| Motile | 0.05* | 0.95 |
Environmental model, Geographical model, Topographic model and Directional spatial model were expressed as Env, PCNMG, PCNMT and AEM. [a] unique environmental fraction; [b] unique PCNMG fraction; [c] unique PCNMT fraction; [d] unique AEM fraction; [e] shared fraction of Env and PCNMG; [f] shared fraction of PCNMG and PCNMT; [g] shared fraction of Env and PCNMT; [h] shared fraction of Env and AEM; [i] shared fraction of PCNMG and AEM; [j] shared fraction of PCNMT and AEM; [k] shared fraction of Env, PCNMG and AEM; [l] shared fraction of Env, PCNMG and PCNMT; [m] shared fraction of PCNMG, PCNMT and AEM; [n] shared fraction of Env, PCNMT and AEM; [o] shared fraction of the four models; [p] unexplained fraction. High, low and motile represented the high-profile, low-profile and motile guild, respectively. Values <0 and no data were shown as —. Significance of each testable fraction (a–d) was expressed as ■P < 0.1, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
From the four conceptual metacommunity paradigms17, species-sorting (SS) and mass-effect (ME) are the most commonly tested and supported paradigms in natural communities, both of which assumed that environmentally heterogeneous habitats were linked via sufficient dispersal18,45. The SS paradigm is highly related to niche theory, in which dispersal is unlimited and the environmental gradients along which species sort is the determinant of species distribution. As a comparison, in the ME paradigm, the local environment condition still plays an important role in structuring communities, but due to high rate of dispersal, the regional processes also show significant effects12,18. In ME (equivalent to SS+ high dispersal or SS+ HD in Ng et al. and SS+ ME in Cottenie)23,45, dispersal is so high that individuals can inhabit in less suitable habitats through source-sink dynamics17, and sink communities will be spatially structured. Nevertheless, spatial significance may result from not only ME, but also limiting dispersal (LD, synonymous with SS+ LD in Ng et al.)23, which can result in spatial significance by clustering individuals with similar dispersal ability across the landscape.
In our study, environmental factors contributed 42% of community variations (Table 1), supporting hypothesis H2, i.e. that steep elevational gradients create intense environmental gradients in high mountain streams, giving rise to strong environmental effects. In order to test metacommunity paradigms and to compare with previous studies, we firstly performed variation partitioning on environmental and geographical factors (the spatial factors which were most commonly used in testing metacommunity paradigms in previous studies), and both environmental and geographical effects controlled benthic diatom metacommunity (Fig. 3a). We summarized most previous researches on benthic diatom metacommunities in lotic systems (see Supplementary Table S2), and found that the relative importance of environmental and spatial factors varied among them. Besides differences in characters of the study areas, the importance of these factors seemed to depend on the spatial extent of the study11,39,46. Generally, diatoms were always species-sorting (only environment variables had significant effects), and the importance of spatial effects increased with geographical distance as dispersal limitation45,47, which was in line with the conceptual model from Soininen39. At large scales, spatial effects might even overcome environmental effects30,43. In general, the paradigms of benthic diatom metacommunities at large scales were always marked by SS+ LD. Also, Heino et al.11 suggested that at small extents, mass effects would increase in importance with shorter distances. Nevertheless, besides geographical distances, the influences of topographical and hydrological barriers for dispersal haven’t been considered in previous studies on benthic diatoms. At our study extent (spatial extent: <500 km2, max. distance between sites ca. 30 km), spatial effects (geographical factor, 12%) also played a significant role in structuring the metacommunity. However, given the characters of high mountain areas, whether the spatial effects attributed to mass effects arising from fast flow along streams or limiting dispersal (LD) caused by mountain barriers even at a small scale should be further analyzed. As a result, we investigated dispersal mechanism by comparison of dispersal modes, i.e. across topographic distance or along with flow.
Kristiansen48 has reviewed the four main modes of algae dispersal, among which dispersal by flow was the most natural way. Overland dispersal could be airborne dispersal by wind, or occur by virtue of organisms from aquatic insects, water birds to water-living mammals, even by humans. Previous studies on benthic diatoms considered mostly geographical distances as spatial factors in metacommunity analyses (Table S2), but ignored other barriers to dispersal. In our study, we modeled the possible ways of diatom dispersal, and transformed them into different sets of spatial factors (geographical, topographical and flow directionality) to explain metacommunity structure. Geographical distance was one of spatial factors controlling benthic diatom communities at a small spatial extent (P = 0.001, Table 1), which was in agreement with the study in a stream in Laojun Mountain49. Topographic factor also had a significant relationship with communities (P = 0.019, Table 1), indicating that the mountains acted as barriers for dispersal, similar results were also shown by Kärnä et al.15. Through nonparametric tests, significant distinctness of community composition was shown between streams, supporting hypothesis H1 that mountains could limit the dispersal among streams. Furthermore, directionality played a key role in shaping the metacommunity, confirming H1 that downstream along streams would be promoted by steep slopes and high discharge. The directional factor (AEM) not only uniquely explained the largest fraction of variation, but also substantially shared all of the other explained fractions with environmental and other spatial factors (Table 2; Figs 3c and 4a,c,d). This suggested that ecological processes were almost constrained along the stream corridors with directional flow in our study area. In conclusion, the strong spatial patterns of the metacommunity structure could be mainly ascribed to the strong directionality of flow, which could be regarded as a manifestation of mountain barriers. Flow promoted high rate of asymmetric dispersal of benthic diatoms, and generated a ME paradigm. Similar ideas have been suggested by Heino et al.36 and Heino, Melo & Bini37. Moreover, in the full model, the environmental factors (Env) could not singly contribute a faction, indicating that not only the dispersal processes constrained within streams, but also environmental gradients shaped by directional processes. The unexplained fraction (35%) in the full model might be explained by biotic interaction and some other factors.
Compared to the study in the Dong River41, one of the few available studies on effects of directionality on diatoms in lotic systems, our result demonstrated much stronger directional effects (unique contribution of 22% in the Cangshan versus <10% for all seasons or function groups in the Dong River). The specific characteristics of the high mountains, such as steep slope, gave rise to stronger and more powerful directional spatial processes, consistent with the viewpoint of Adams et al., Lowe et al. and Altermatt et al.34,35,50. A study on headwater macroinvertebrates of Sweden demonstrated a significant but less marked directional spatial process compared with our study25. Similar results were also illustrated by studies on fish larvae in a fluvial lake system51 and coastal Mediterranean polychaetes26. Our results and those from other publications mentioned here suggest that directional processes are crucial in structuring diatom metacommunities, especially in high mountain streams, and cannot be neglected in future researches.
The deconstructive analysis based on ecological traits allowed a better understanding of processes underlying community structure patterns16,40. The ecological guilds of diatoms could reflect not only the difference of dispersal ability, but also the environmental adaptability44. Our results based on species traits suggested that ecological guilds were structured by different dynamic paradigms, which partly supported our hypothesis H3, i.e. that ecological guilds would respond to environmental and spatial factors differently. The low-profile guild could resist high disturbance but not resource stress44, which explained why the low-profile guild prevailed in high mountain streams, which were often characterized by oligotrophy and high discharge. Considering the steep slopes and the high water velocities, the low-profile species were still strongly controlled by directional factor (AEM), leading to a mass-effect paradigm (i.e. species sorting + high dispersal, SS+ HD). When compared to the low-profile guild, the dispersal of the high-profile guild seemed easier either along stream or among streams with flying grazers, such as water birds or odonata. However, limited by hydrological conditions, the total abundance of the high-profile species (only accounted for 4%) was too low to generate mass effects along with stream flow (i.e. species sorting + moderate dispersal, SS). In addition, it showed dispersal limitation for topographical barriers. The environmental and topographical effects on the high-profile guild indicated a combination of species sorting within streams and limiting dispersal among streams (SS+ LD, Table 2). The motile guild had a significant relationship with environmental variables, but none with spatial variables. This was not surprising, the active moving processes of the motile guild were too weak to resist strong flow and grazers to select suitable microhabitats, and consequently, the guild was still imposed by flow to track environmental gradients. Similar to the high-profile guild, low abundance gave rise to a non-significant spatial structure (species sorting + moderate dispersal, SS). High mountains have shaped the benthic diatom assemblages dominated by the low-profile guild with overwhelming abundance, so that the traits of the low-profile guild played decisive roles in structuring metacommunity. In conclusion, metacommunity structuring differed among the three ecological guilds, and the relative abundance of each guild finally determined the paradigm of the whole metacommunity. However, the species composition varies depending on the study area. In Sweden, for example, headwater streams, lower altitude (mean altitude 321 m a.s.l.) and gentle slopes assembled communities dominated by the high-profile guild40, and thus the metacommunity were structuring as a SS paradigm, which supported our conclusion. The deconstructive analysis allowed to better understand how flow directionality and mountain barriers acted as determining factors in structuring metacommunity of streams: regardless of how strong the dispersal ability was, the dispersal within streams was unconstrained, while among streams was limited by mountains.
Conclusions
The unique characters of high mountain streams determined metacommunity structuring through: (1) considerable environmental gradients; (2) dispersal limitation among streams by mountain barriers and dispersal facilitation along stream channels by rushing flow, regardless of dispersal ability; (3) directional processes overriding any other effects and generating a mass-effect (ME) paradigm; (4) finally, the domination of the low-profile guild, so that the way by which the low-profile guild structured profoundly shaped the paradigm of the whole metacommunity.
Our results highlighted that in diatoms, stream corridors are the primary dispersal pathway in high mountains, where rushing flow and steep slope facilitate dispersal of benthic organisms. For biodiversity conservation, maintaining the instream environmental flow and keeping various habitats among streams are of vital importance in high mountain streams. The unique characters of high mountain streams are needed to be better understood and considered in further research.
Methods
Study areas
The study streams are located in the Cangshan Erhai National Nature Reserve, Yunnan Province, Southwestern China (25.64–25.85°N, 99.95–100.20°E, Fig. 1). The Cangshan Mountain, belonging to the Hengduan Mountains, is a part of the biodiversity hotspot “The Mountains of Southwest China” (Conservation International, http://www.conservation.org/Pages/default.aspx). The Cangshan Mountain summit reaches 4122 m a.s.l., and has 18 additional peaks all over 3000 m a.s.l., covering an area of about 950 km2. For these streams, the slope gradients range from 2.3 to 8.5%, and the length from 10 to 15 km. Land use is dominated by secondary forest (Table 3). A subtropical plateau monsoon climate prevails, characterized by two distinct seasons: wet (from May to October) and dry (from November to April of next year), where the rainfall in the wet season can account for up to 84% of the annual precipitation52. During November 2012, we investigated a total of 63 riffle sites from eight streams, five streams on the eastern slope and three on the western slope. Most sites were of difficult access to humans and in an almost pristine state.
Table 3. Summary of environmental variables across 63 sites in the study area.
| Environmental vaiables | Mean ± SD | Min-Max | Transformation |
|---|---|---|---|
| Altitude (m) | 2309 ± 282.59 | 1623 ~ 2905 | None |
| Conductivity (μs cm−1) | 65.52 ± 30.82 | 16.80 ~ 151 | None |
| DO (mg L−1) | 7.88 ± 0.69 | 6.52 ~ 10.03 | None |
| pH | 7.96 ± 0.24 | 7.44 ~ 8.55 | None |
| Stream width (m) | 2.86 ± 1.69 | 0.36 ~ 10.70 | Log |
| Depth (m) | 0.20 ± 0.08 | 0.05 ~ 0.45 | Log |
| Mean velocity (m s−1) | 0.38 ± 0.22 | 0.06 ~ 1.27 | Squ.root |
| T-N (mg L−1) | 0.27 ± 0.27 | 0.02 ~ 1.29 | Log |
| NO3-N (mg L−1) | 0.22 ± 0.25 | 0 ~ 1.12 | None |
| NH4-N (mg L−1) | 0.02 ± 0.01 | 0.01 ~ 0.07 | Log |
| T-P (mg L−1) | 0.01 ± 0.004 | 0 ~ 0.03 | None |
| PO4-P (mg L−1) | 0.01 ± 0.004 | 0 ~ 0.02 | Log |
| SiO2-Si (mg L−1) | 4.31 ± 1.41 | 2 ~ 8.21 | Squ.root |
| TOC (mg L−1) | 0.38 ± 0.14 | 0.15 ~ 0.90 | None |
| Forest % | 81.93 ± 13.15 | 48.66 ~ 96.44 | Centred log ratio |
| Grass % | 9.05 ± 7.15 | 1.32 ~ 32.93 | Centred log ratio |
| Agriculture % | 0.52 ± 1.19 | 0 ~ 6.3 | Centred log ratio |
| Built-up% | 3.31 ± 2.40 | 0.13 ~ 9.32 | Centred log ratio |
| Barren land % | 5.19 ± 6.51 | 0.04 ~ 28.13 | Centred log ratio |
Transformations applied to meet normality assumptions are listed. None: none transformation; Log: logarithmic transformation; Squ.root: square root transformation; Centred log ratio: a transformation specially for compositional data.
Diatom sampling and identification
At each site, 12 pebble-to-cobble-sized stones were collected randomly from riffle-run habitats in three typical sections within a 30 m stretch. Benthic diatoms attached on stone surface covered within a lid (with radius of 2.7 cm) were vigorously scrubbed by a nylon brush and flushed 3–4 times with distilled water. Three subsamples were subsequently pooled into one composite sample, and collected in a pre-defined volume container (350 ml) with 4% formalin.
Permanent diatom slides were prepared for diatoms identification after acid digestion. A minimum of 500 valves were counted at 1000 magnification under oil immersion (Olympus CX21, Japan). Diatoms were identified to the species level following the identification references of Krammer and Lange-Bertalot53 and Qi et al.54. The total number of diatoms were calculated and converted to abundance in unit area expressed as cells m−2.
Species traits of diatom
Diatom species were assigned to three ecological guilds species-specifically based on their growth morphologies referring to Passy44, i.e. high-profile, low-profile and motile guild. The high-profile guild is composed of the species of tall stature beyond biofilm boundary, which are comparatively vulnerable to physical disturbances by flow and grazers, but relatively tolerant to resource limitation by extending beyond the boundary layer to exploit light and nutrients. The low-profile guild consists of species within boundary layers of biofilm, which are always resource-limited and much less prone to physical disturbance. The motile guild encompasses the relatively fast moving species, and therefore can actively select suitable microhabitats in some cases, comparatively free of both resource limitation and disturbance stress44. Based on above and considering environmental affinities, the low-profile guild should be subject to niche filtering because of limited resources; the high-profile guild is expected to be stressed by hydrological condition and grazers; while the motile guild is less sensitive to environmental filtering relatively. Concerning dispersal ability, the high-profile guild may be regarded as strong dispersers along stream flow or with flying grazers; as well as the motile species because of their active moving, while the low-profile guild is considered as weak dispersers. We also added some taxa recorded in this study but not mentioned by Passy44 to corresponding guilds based on their growth morphologies. Since the heterogeneous genera (i.e. Navicula and Gomphonema) just had one type of growth morphology in our study, the assignments of all species into three ecological guilds were based on their genera. Each genus assigned to three ecological guilds was shown on Supplementary Table S3.
Environmental variables
At each site, conductivity (Cond), dissolved oxygen (DO), and pH were measured in situ with a multiparameter water quality meters (YSI Professional plus, US). Stream width was measured along 3 representative cross-transects. Depth and velocity were measured at 50 cm intervals across a transect using a digital water velocity meter (Global Water FP201, US). The mean values of stream width, depth and velocity were calculated for further analyses. Geographic coordinates and altitude were recorded using a hand-held GPS (Magellan 500E, US). A 350 ml water sample was collected simultaneously with diatom sampling and preserved by adding sulfuric acid to regulate pH < 2 in the field. Concentration of total phosphorus (T-P), Orthophosphate (PO4-P), total nitrogen (T-N), Nitrate nitrogen (NO3-N), Ammonium nitrogen (NH4-N), and dissolved silicon (SiO2-Si) were measured in the lab using a segmented flow analyzer (Skalar San++, Netherlands). Total organic carbon (TOC) was determined by using a TOC analyzer (Shimadzu TOC-V CPH, Japan).
Landsat data were downloaded from International Scientific & Technical Data Mirror Site, Computer Network Information Center, Chinese Academy of Sciences (http://www.gscloud.cn), and interpreted using ENVI (Version 4.1, ITT Visual Information Solutions Inc., US) to determine land cover/land use (LCLU) of our study area. The LCLU classification we used was a modification of Chinese National Standard “GB/T 21010-2007”. The LCLU categories included forest, grass, agriculture, built-up, water, and other (mainly barren land). ArcGIS software (Version 10.0, ESRI, US) was used to calculate the area of each land use category in the upstream watershed of each site47. The land-use areas were converted to proportions for use in future data analyses. A general description of the environmental variables used in our study is given in Table 3.
Spatial variables and statistical analyses
Distance matrices
Three distance matrices were calculated: (a) a geographical distance matrix as Euclidean distance between each pair of sampling sites calculated using the earth.dist function in the package fossil in R (Version 3.0.0, R Development Core Team; package fossil Version 0.3.7); (b) a topographic distance matrix with pairwise landscape resistance distances to dispersal based on circuit theory55,56 generated with the program CIRCUITSCAPE (Version 4.0)55 using a digital elevation model (DEM, 30 m resolution, International Scientific & Technical Data Mirror Site, Computer Network Information Center, Chinese Academy of Sciences; http://www.gscloud.cn) and transformed into a curvature map from which resistance distances were calculated as the sum of the resistance of individual pixels in relation to topography (i.e. mountain barriers)14,56; and (c) a network distance matrix, calculated to model the least-cost dispersal route between two sites along the stream network, using the Network Analyst extension/OD Cost Matrix tool in ArcGIS 10.0.
Spatial variables
Three sets of spatial variables were extracted from the three distance matrices by eigenfunction-based spatial models in R. Principal Coordinates of Neighborhood Matrix (PCNM) analysis based on both geographical distance and topographic distance were used to model spatial variables representing geographical positions and dispersal across the mountains respectively57, through the pcnm function in package vegan (Version 2.0-10). We applied asymmetric eigenvector map (AEM) analysis, which was specifically designed to model directional patterns58, to generate spatial variables along directional flow. A site-by-edge binary matrix was constructed based on coordinates of the sites and the directional links (edges) among sites using the build.binary function in R package AEM (version 0.5-1/r188). Thereafter, the following weighting function was applied: weight = 1 − (d/dmax)2, where d is the network distance between linked sites and dmax was the maximum distances among value d59, were assigned to each edge. Finally, the aem function in R package AEM was used to create eigenvectors. Details of AEM were described in Blanchet et al.58. The generated eigenvectors were used as spatial variables (i.e., PCNMG, PCNMT and AEM components, PCNMGs, PCNMTs and AEMs) and hereafter referred to as PCNMG, PCNMT and AEM.
Statistical analyses
All statistical analyses were conducted in R. Three nonparametric tests: permutational multivariate analysis of variance using distance matrices (adonis), analysis of similarity (ANOSIM) and multi-response permutation procedure (MRPP) were performed to test the differences of community composition and structure among the 8 streams in the study area, and between the eastern and western slope using the functions adonis, anosim and mrpp respectively in the package vegan.
To calculate the unique and shared effects of environmental and spatial variables, as well as how much community variation was explained by each set of the spatial variables, a variation partitioning analysis (partial RDA) was performed. First, a diatom abundance matrix (site-by-species) was Hellinger-transformed for further analyses59. Second, the environmental and spatial variables were prepared as site-by-variables matrices. Water chemistry and hydromorphological data were normalized by logarithmic or square root if necessary, and land-use data was transformed by a centred log ratio transformation (Table 3)25. Only the PCNM eigenvectors with positive eigenvalues were selected into spatial matrices. Third, the diatom matrix was analyzed by four explanatory matrices (one environmental and three spatial matrices). We performed a global test with redundancy analysis (RDA) using the rda function for each explanatory matrix and tested the significance using the anova function. Only if it was significant, a forward selection could be proceeded to get a parsimonious model with two stopping criteria: significance level and the adjusted coefficient of determination (R2adj) of the global model60. Forward selection was performed by the forward.sel function in the packfor package (version 0.0–8/r109). The selected variables were then used as explanatory variables for the following variation partitioning analysis by using varpart function. The significance of each testable fraction in variation partitioning analysis was obtained from the functions rda and anova. Similarly, the analyses mentioned above were implemented separately for each diatom ecological guild to test their different responses. The functions varpart, rda and anova are all found in the package vegan.
Additional Information
How to cite this article: Dong, X. et al. Flow directionality, mountain barriers and functional traits determine diatom metacommunity structuring of high mountain streams. Sci. Rep. 6, 24711; doi: 10.1038/srep24711 (2016).
Supplementary Material
Acknowledgments
This study was financially supported by Major Science and Technology Program for Water Pollution Control and Treatment (2012ZX07501002-007). We thank Yihao Fang and Yun Zhou who assisted in field sampling; Guillaume Blanchet for help with the R package AEM; Brad McRae for help with the program CIRCUITSCAPE. Special thanks to Mathias Kuemmerlen and Naicheng Wu for language improvement and thoughtful comments.
Footnotes
Author Contributions Conceiving and designation of the experiments: Q.C., X.D. and B.L. Field sampling and collecting data: X.D., B.L., F.H., M.S., H.Z., W.X., S.L. and Q.C. Interpreting land cover/land use (LCLU) data: M.S. Diatoms identification: X.D. Chemical experiments: B.L., Y.G. and L.T. Data analyses: X.D. and B.L. Writing the main manuscript text: X.D. Preparing figures: B.L. and X.D.
References
- Clobert J., Baguette M., Benton T. G. & Bullock J. M. Dispersal Ecology and Evolution. 1–496 (Oxford University Press, Oxford, 2012). [Google Scholar]
- Kendrick G. A. et al. The central role of dispersal in the maintenance and persistence of seagrass populations. Bio Science 62, 56–65 (2012). [Google Scholar]
- Shurin J. B., Cottenie K. & Hillebrand H. Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159, 151–159 (2009). [DOI] [PubMed] [Google Scholar]
- Lowe W. H. & McPeek M. A. Is dispersal neutral? Trends Ecol. Evol. 29, 444–450 (2014). [DOI] [PubMed] [Google Scholar]
- Thompson R. & Townsend C. A truce with neutral theory: local deterministic factors, species traits and dispersal limitation together determine patterns of diversity in stream invertebrates. J. Anim. Ecol. 75, 476–484 (2006). [DOI] [PubMed] [Google Scholar]
- Flinn K. M., Gouhier T. C., Lechowicz M. J. & Waterway M. J. The role of dispersal in shaping plant community composition of wetlands within an old-growth forest. J. Ecol. 98, 1292–1299 (2010). [Google Scholar]
- Cadotte M. W. Competition-colonization trade-offs and disturbance effects at multiple scales. Ecology 88, 823–829 (2007). [DOI] [PubMed] [Google Scholar]
- Lindström E. S. & Östman Ö. The importance of dispersal for bacterial community composition and functioning. Plos One 6, e25883 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoak M. & Loreau M. Reconciling empirical ecology with neutral community models. Ecology 87, 1370–1377 (2006). [DOI] [PubMed] [Google Scholar]
- Warren B. H. et al. Islands as model systems in ecology and evolution: prospects fifty years after MacArthur-Wilson. Ecol. Lett. 18, 200–217 (2015). [DOI] [PubMed] [Google Scholar]
- Heino J. et al. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biol. 60, 845–869 (2015). [Google Scholar]
- Brown B. & Swan C. Dendritic network structure constrains metacommunity properties in riverine ecosystems. J. Anim. Ecol. 79, 571–580 (2010). [DOI] [PubMed] [Google Scholar]
- Kerby J. L., Riley S. P., Kats L. B. & Wilson P. Barriers and flow as limiting factors in the spread of an invasive crayfish (Procambarus clarkii) in southern California streams. Biol. Conserv. 126, 402–409 (2005). [Google Scholar]
- Cañedo-Argüelles M. et al. Dispersal strength determines meta-community structure in a dendritic riverine network. J. Biogeogr. 42, 778–790 (2015). [Google Scholar]
- Kärnä O. M. et al. Inferring the effects of potential dispersal routes on the metacommunity structure of stream insects: as the crow flies, as the fish swims or as the fox runs? J. Anim. Ecol. 84, 1342–1353 (2015). [DOI] [PubMed] [Google Scholar]
- Algarte V. M., Rodrigues L., Landeiro V. L., Siqueira T. & Bini L. M. Variance partitioning of deconstructed periphyton communities: does the use of biological traits matter? Hydrobiologia 722, 279–290 (2014). [Google Scholar]
- Leibold M. A. et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613 (2004). [Google Scholar]
- Logue J. B., Mouquet N., Peter H., Hillebrand H. & Group M. W. Empirical approaches to metacommunities: a review and comparison with theory. Trends Ecol. Evol. 26, 482–491 (2011). [DOI] [PubMed] [Google Scholar]
- Wilson D. S. Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology 73, 1984–2000 (1992). [Google Scholar]
- Holyoak M., Leibold M. A., Mouquet N., Holt R. D. & Hoopes M. F. In Metacommunities: Spatial Dynamics And Ecological Communities (eds Holyoak M., Leibold M. A. & Holt R. D.) Ch. 1, 1–31 (University of Chicago Press, Chicago, 2005). [Google Scholar]
- Hubbell, S. P. In The Unified Neutral Theory Of Biodiversity And Biogeography (eds Hubbell S. P.) Ch. 5, 113–151 (Princeton University Press, Princeton, 2001). [Google Scholar]
- Soininen J. A quantitative analysis of species sorting across organisms and ecosystems. Ecology 95, 3284–3292 (2014). [Google Scholar]
- Ng I. S., Carr C. M. & Cottenie K. Hierarchical zooplankton metacommunities: distinguishing between high and limiting dispersal mechanisms. Hydrobiologia 619, 133–143 (2009). [Google Scholar]
- Winegardner A. K., Jones B. K., Ng I. S., Siqueira T. & Cottenie K. The terminology of metacommunity ecology. Trends Ecol. Evol. 27, 253–254 (2012). [DOI] [PubMed] [Google Scholar]
- Göthe E., Angeler D. G. & Sandin L. Metacommunity structure in a small boreal stream network. J. Anim. Ecol. 82, 449–458 (2013). [DOI] [PubMed] [Google Scholar]
- Moritz C. et al. Disentangling the role of connectivity, environmental filtering, and spatial structure on metacommunity dynamics. Oikos 122, 1401–1410 (2013). [Google Scholar]
- Sancha N. U., Higgins C., Presley S. J. & Strauss R. E. Metacommunity structure in a highly fragmented forest: has deforestation in the Atlantic Forest altered historic biogeographic patterns? Divers. Distrib. 20, 1058–1070 (2014). [Google Scholar]
- Finn D. S. & Poff N. L. Examining spatial concordance of genetic and species diversity patterns to evaluate the role of dispersal limitation in structuring headwater metacommunities. J. N. Am. Benthol. Soc. 30, 273–283 (2011). [Google Scholar]
- Landeiro V. L., Magnusson W. E., Melo A. S., Espírito-Santo H. M. V. & Bini L. M. Spatial eigenfunction analyses in stream networks: do watercourse and overland distances produce different results? Freshwater Biol. 56, 1184–1192 (2011). [Google Scholar]
- Heino J. et al. Geographical patterns of micro-organismal community structure: are diatoms ubiquitously distributed across boreal streams? Oikos 119, 129–137 (2010). [Google Scholar]
- Sabando M., Vila I., Penaloza R. & Veliz D. Contrasting population genetic structure of two widespread aquatic insects in the Chilean high-slope rivers. Mar. Freshwater Res. 62, 1–10 (2011). [Google Scholar]
- Finn D. S., Theobald D. M., Black W. C. & Poff N. L. Spatial population genetic structure and limited dispersal in a Rocky Mountain alpine stream insect. Mol. Ecol. 15, 3553–3566 (2006). [DOI] [PubMed] [Google Scholar]
- Šlechtová V., Bohlen J., Freyhof J., Persat H. & Delmastro G. B. The Alps as barrier to dispersal in cold-adapted freshwater fishes? Phylogeographic history and taxonomic status of the bullhead in the Adriatic freshwater drainage. Mol. Phylogenet. Evol. 33, 225–239 (2004). [DOI] [PubMed] [Google Scholar]
- Adams S. B., Frissell C. A. & Rieman B. E. Movements of nonnative brook trout in relation to stream channel slope. T. Am. Fish. Soc. 129, 623–638 (2000). [Google Scholar]
- Lowe W. H., Likens G. E., McPeek M. A. & Buso D. C. Linking direct and indirect data on dispersal: isolation by slope in a headwater stream salamander. Ecology 87, 334–339 (2006). [DOI] [PubMed] [Google Scholar]
- Heino J. et al. Environmental heterogeneity and β diversity of stream macroinvertebrate communities at intermediate spatial scales. Freshw. Sci. 32, 142–154 (2013). [Google Scholar]
- Heino J., Melo A. S. & Bini L. M. Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshwater Biol. 60, 223–235 (2015). [Google Scholar]
- Sundqvist M. K., Sanders N. J. & Wardle D. A. Community and ecosystem responses to elevational gradients: processes, mechanisms, and insights for global change. Annu. Rev. Ecol. Evol. S. 44, 261–280 (2013). [Google Scholar]
- Soininen J. Environmental and spatial control of freshwater diatoms—a review. Diatom Res. 22, 473–490 (2007). [Google Scholar]
- Göthe E., Angeler D. G., Gottschalk S., Löfgren S. & Sandin L. The influence of environmental, biotic and spatial factors on diatom metacommunity structure in Swedish headwater streams. Plos One 8, e72237 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Soininen J., Han B. P. & Declerck S. A. Effects of connectivity, dispersal directionality and functional traits on the metacommunity structure of river benthic diatoms. J. Biogeogr. 40, 2238–2248 (2013). [Google Scholar]
- Finlay B. J. Global dispersal of free-living microbial eukaryote species. Science 296, 1061–1063 (2002). [DOI] [PubMed] [Google Scholar]
- Wu N., Cai Q. & Fohrer N. Contribution of microspatial factors to benthic diatom communities. Hydrobiologia 732, 49–60 (2014). [Google Scholar]
- Passy S. I. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 86, 171–178 (2007). [Google Scholar]
- Cottenie K. Integrating environmental and spatial processes in ecological community dynamics. Ecol. Lett. 8, 1175–1182 (2005). [DOI] [PubMed] [Google Scholar]
- Meynard C. N. et al. Disentangling the drivers of metacommunity structure across spatial scales. J. Biogeogr. 40, 1560–1571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potapova M. G. & Charles D. F. Benthic diatoms in USA rivers: distributions along spatial and environmental gradients. J. Biogeogr. 29, 167–187 (2002). [Google Scholar]
- Kristiansen J. 16. Dispersal of freshwater algae—a review. Hydrobiologia 336, 151–157 (1996). [Google Scholar]
- Wang J. et al. Patterns of elevational beta diversity in micro-and macroorganisms. Global Ecol. Biogeogr. 21, 743–750 (2012). [Google Scholar]
- Altermatt F., Schreiber S. & Holyoak M. Interactive effects of disturbance and dispersal directionality on species richness and composition in metacommunities. Ecology 92, 859–870 (2011). [DOI] [PubMed] [Google Scholar]
- Bertolo A. et al. Inferring processes from spatial patterns: the role of directional and non–directional forces in shaping fish larvae distribution in a freshwater lake system. Plos One 7, e50239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. In Scientific Investigation Of The Plant On Cangshan Mountain (eds Duan C.) 28–137 (Yunnan Science and Technology Press, Kunming, 1995). [Google Scholar]
- Krammer K. & Lange-Bertalot H. Bacillariophyceae. Süßwasserflora von Mitteleuropa, Vol. 2 (1–4). (Gustav Fischer Verlag, Stuttgart, 1986–1991). [Google Scholar]
- Qi Y., Li J. & Shi Z. Flora Algarum Sinicarum Aquae Dulcis (Tomus IV, X, XII, XIV, XVI). (Science Press, Beijing, 1995–2013). [Google Scholar]
- McRae B. H., Dickson B. G., Keitt T. H. & Shah V. B. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 89, 2712–2724 (2008). [DOI] [PubMed] [Google Scholar]
- Phillipsen I. C. & Lytle D. A. Aquatic insects in a sea of desert: population genetic structure is shaped by limited dispersal in a naturally fragmented landscape. Ecography 36, 731–743 (2013). [Google Scholar]
- Dray S., Legendre P. & Peres-Neto P. R. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol. Model. 196, 483–493 (2006). [Google Scholar]
- Blanchet F. G., Legendre P., Maranger R., Monti D. & Pepin P. Modelling the effect of directional spatial ecological processes at different scales. Oecologia 166, 357–368 (2011). [DOI] [PubMed] [Google Scholar]
- Borcard D., Gillet F. & Legendre P. In Numerical Ecology With R (eds Borcard D., Gillet F. & Legendre P.) Ch. 7, 227–292 (Springer, New York, 2011). [Google Scholar]
- Blanchet F. G., Legendre P. & Borcard D. Forward selection of explanatory variables. Ecology 89, 2623–2632 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.