Abstract
Phosphorylation of the nicotinic acetyl-choline receptor (nAChR) is believed to play a critical role in its nicotine-induced desensitization and up-regulation. We examined the contribution of a consensus PKC site in the α4 M3/M4 intracellular loop (α4S336) on the desensitization and up-regulation of α4β2 nAChRs expressed in oocytes. Position α4S336 was replaced with either alanine to abolish potential phosphorylation at this site or with aspartic acid to mimic phosphorylation at this same site. Mutations α4S336A and α4S336D displayed a threefold increase in the ACh-induced response and an increase in ACh EC50. Epibatidine binding revealed a three and sevenfold increase in surface expression for the α4S336A and α4S336D mutations, respectively, relative to wild-type, therefore, both mutations enhanced expression of the α4β2 nAChR. Interestingly, the EC50’s and peak currents for nicotine activation remained unaffected in both mutants. Both mutations abolished the nicotine-induced up-regulation that is normally observed in the wild-type. The present data suggest that adding or removing a negative charge at this phosphorylation site cannot be explained by a simple straightforward on-and-off mechanism; rather a more complex mechanism(s) may govern the functional expression of the α4β2 nAChR. Along the same line, our data support the idea that phosphorylation at multiple consensus sites in the α4 subunit could play a remarkable role on the regulation of the functional expression of the α4β2 nAChR.
Keywords: Whole-mount immunofluorescence assay, Epibatidine binding, Nicotine, Voltage-clamp, Confocal imaging
Introduction
Chronic nicotine exposure induces an up-regulation of the α4β2 nicotinic acetylcholine receptors (nAChRs) in the CNS (Benwell et al. 1988; Breese et al. 1997; Flores et al. 1992; Marks et al. 1983; Schwartz and Kellar 1985; Whiteaker et al. 1998). Despite this increase in receptor number, chronic nicotine treatment produces loss in function as a result of their rapid and persistent desensitization (Eilers et al. 1997; Ke et al. 1998; Lapchack et al. 1989; Lukas 1991). It is proposed that the property of desensitization of nAChRs is the driving force for the up-regulation (Fenster et al. 1999b; Marks et al. 1983; Schwartz and Kellar 1985).
Factors that regulate α4β2 nAChR desensitization may contribute to the long-term effects of nicotine on receptor number and function (Fenster et al. 1999a). One form of physiological regulation of nicotinic receptor desensitization is phosphorylation of nAChR subunits. Evidence for the role of phosphorylation on the desensitization process initially came from the study of muscle nAChRs; direct phosphorylation of γ and δ subunits at serine residues by protein kinase A (PKA) increased the rate of desensitization (Huganir et al. 1986). Subsequently, studies of neuronal nicotinic receptors in chick sympathetic ganglia (containing α3, α4, α5, α7, β2, β3, and β4 subunits) have indicated that cyclic AMP-dependent PKA and PKC can phosphorylate these receptors, and that phosphorylation may regulate agonist-induced desensitization (Downing and Role 1987; Vijayaraghavan et al. 1990).
The majority of the consensus phosphorylation sites are located in the cytoplasmic domain between M3 and M4 transmembrane regions (Swope et al. 1992). The α4 subunit contains at least 13 putative phosphorylation consensus sites. Among them are nine serine residues (S336, S364, S438, S469, S471, S490, S504, S516, and S589) that could be phosphorylated by PKC, PKA, and Casein kinase II. Two of the serine residues, S336 and S516, in the α4 M3/ M4 intracellular domain, are part of PKC phosphorylation consensus sites. Viseshakul et al. (1998) showed that the α4 subunit of α4β2 nAChRs was phosphorylated in vivo. Furthermore, Wecker et al. (2001) demonstrated that the α4 M3/M4 intracellular domain was phosphorylated by PKA and PKC.
Fenster et al. (1999a) provided compelling evidence suggesting that phosphorylation/dephosphorylation of S336 plays an important role in the modulation of nAChR desensitization as a result of short-term nicotine incubation. It is important to mention that Wecker et al. (2001)later referred this residue as S368 (numbered to include the signal peptide) and will be equivalent to S364 in our numbering. This residue is contained within the XRRXSX sequence that is phosphorylated by PKA (Wecker et al. 2001) and is different from the residue mutated in this study, which is part of a putative consensus sequence phosphorylated by PKC (HHRSPRTHT) (Viseshakul et al. 1998).
In this study, we introduced alanine and aspartic acid residues at the α4S336 position. The replacement of serine by alanine was used to eliminate the PKC consensus site, whereas the introduction of aspartic acid should mimic a permanent phosphorylated state. We found that for both mutations the activation induced by ACh differ from the one induced by nicotine. Both α4S336A and α4S336D exhibited changes in ACh-induced macroscopic current and in the EC50 value for ACh, whereas there were no changes in these two properties when using nicotine as agonist. Given that α4S336 position appears to be critical for the functional regulation of α4β2 nAChRs, it is reasonable to suggest that phosphorylation of several serine, threonine, or tyrosine residues on the α4 and β2 M3/M4 cytoplasmic domains could play a major role in nAChR function during chronic nicotine exposure.
Materials and Methods
Site-directed Mutagenesis for the Generation of Mutant Neuronal α4 Subunits
The mutant α4 subunits were created using Quik-Change site-directed mutagenesis kit (Stratagene, La Jolla, CA). pGEMHE vector with Rattus norvegicus cDNA coding for α4 neuronal nAChR subunit was used as DNA template in the PCR reaction. The oligonucleotide primers containing the desired mutation (Life Technologies-Gibco BRL, Gaithersburg, MD), each complementary to opposite strands of the vector, were extended during temperature cycling by the polymerase. The reaction mixture contains 5 µl of 10× reaction buffer, 50 ng of DNA template, 125 ng of each oligonucleotide primers, 1 µl of dNTP mix, 1 µl (2.5 units) of Pfu Turbo polymerase and water to a final volume of 50 µl. The cycling parameters consisted of 16 cycles of plasmid denaturation for 30 s at 95°C, primers annealing for 1 min at 55°C, and extension for 10.20 min at 68°C. Incorporation of the oligonucleotide primers generates a mutated plasmid containing staggered nicks. After DNA amplification the samples were digested with Dpn I endonuclease in order to digest the parental DNA template and to select for mutation-containing synthesized DNA. The nicked vector DNA containing the desired mutation was transformed into Epicurian coli XL1-Blue supercompetent cells. The DNA was purified using QIAprep spin miniprep kit (Quiagen, Germantown, MD) and then sequenced.
In vitro Synthesis of mRNA and Oocyte Microinjection
Subunit mRNA was synthesized in vitro from linearized pGEMHE plasmid templates of R. norvegicus cDNA coding for α4 and β2 nAChR subunits using the mMessage mMachine RNA transcription kit (Ambion, Austin, TX). mRNA mixtures of α4 and β2 subunits were prepared at 2 µg:3 µg ratio. The mRNA mixture was microinjected, using a displacement injector (Drummond Instruments, Broomhall, PA), into stages V and VI oocytes that had been extracted, incubated in collagenase Type 1A (Sigma, St. Louis, MO) and defolliculated by manual dissection. The injected oocytes were incubated at 19°C for 3 days in 0.5 × Leibovitz’s L-15 medium (Invitrogen) supplemented with 400 µg/ml bovine serum albumin, 119 mg/ml penicillin, 200 mg/ml streptomycin, and 110 mg/ml pyruvic acid. Electrophysiological experiments were performed after the third day of mRNA injection.
Electrophysiological Characterization of α4β2 nAChRs
Oocytes injected with the mRNA transcripts of α4 and β2 subunits were characterized using two-electrode voltage clamp. ACh- and nicotine-induced currents were recorded at 20°C, 3 days after mRNA injection, with a GeneClamp 500B Amplifier (Axon Instruments, Foster City, CA). Electrodes were filled with 3 M KCl and had a resistance of less than 2 MX. Impaled oocytes in the recording chamber were continuously perfused at a rate of 0.75 ml/s with MOR2 buffer (115 mM NaCl, 2.5 mM KCl, 5 mM HEPES, 1 mM Na2HPO4, 0.2 mM CaCl2, 5 mM MgCl2, and 0.5 mM EGTA, pH 7.4). All the reagents used were purchased from Sigma–Aldrich, Co. (St. Louis, MO). For dose–response curves, each oocyte was held at a membrane potential of −70 mV. Membrane currents were digitized using the DigiData 1200 interface (Axon Instruments, CA), filtered at 2 kHz during recording. The Whole Cell Program 2.3 (provided by Dr. J. Dempster, University of Strathclyde, UK) running on a Pentium III-based computer was used for data acquisition. Data analysis was performed using Prism 3.0 (Graphpad Software, San Diego, CA). Dose–response data for the α4β2 combination were collected using nine ACh doses (0.1, 0.3, 1, 3, 10, 30, 100, 300, and 3,000 µM) or seven nicotine concentrations (0.1, 1, 3, 10, 30, 100, and 300 µM). The data were fit using one- and two-component sigmoidal dose–response equations, Y = I/ImaxBottom + (I/ImaxTop – I/ImaxBottom)/ (1 + 10^((LogEC50 – X) × HillSlope)) and Y = I/ImaxBottom + [(I/ImaxTop – I/ImaxBottom) × Fraction/ (1 + 10^((LogEC50_1 – X) × HillSlope_1))] + [(I/Imax Top – I/ImaxBottom) × (1 – Fraction)/(1 + 10^ ((Log EC50_2 – X) × HillSlope_2))], respectively, where X is the logarithm of concentration and Y is the response. Additionally, four consecutive pulses of 300 µM ACh or 30 µM nicotine were applied to each oocyte and the nAChR macroscopic responses were recorded. Between each agonist application the oocyte was washed with MOR2 buffer for 5 min. In order to evaluate recovery, after the consecutive ACh or nicotine pulses, oocytes were washed with MOR2 buffer for 20 min and the agonist-induced macroscopic currents were measured. The protocol followed in the experiments involving acute nicotine exposure consisted of measuring the current induced by four consecutive applications of 300 µM ACh. Between each ACh application, the oocyte was washed with 1.0 µM nicotine-containing MOR2 for 5 min. That is, a total of three washes for the four ACh applications. We chose 300 µM ACh based on the maximal response observed for α4β2 nAChRs using this ACh concentration. In order to calculate percent recovery, oocytes were washed for 20 min with nicotine-free MOR2 buffer after the last nicotine wash. We also conducted experiments to assess the effect of chronic nicotine exposure on the ACh-induced responses for wild-type and mutant α4β2 nAChRs. To that end, we obtained ACh dose–response curves (0.1, 0.3, 1, 3, 10, 30, 100, 300, and 3,000 µM) before and after prolonged nicotine incubation. For this nicotine incubation, oocytes were placed in L-15 medium supplemented with nicotine at a final concentration of 1 µM for 24 h. Subsequently, oocytes were washed for 6 min by perfusing with MOR2 buffer. Finally, ACh dose–responses were determined as were done prior to the chronic nicotine incubation. The peak currents at 300 µM ACh were measured before and after 1 µM nicotine exposure. Data are expressed as mean ± SEM. One-way ANOVA was performed using Prism 3.0 (Graphpad Software, San Diego, CA). Results were compared with two-tailed Student’s t-test using Graphpad Prism 3.0.
Immunofluorescence Assay for α4β2 Neuronal nAChR
Xenopus oocytes were injected with the mRNA transcripts of α4 and β2 in subunit ratio of 2α:3β. Oocytes were incubated 72 h (3 days) in L-15 medium supplemented with antibiotics and antimycotics. On the third day of injection, 300 µM ACh-induced currents were measured using the two-electrode voltage clamp technique. Oocytes to be compared were paired according to their similar current profile, i.e., with not more than 400 nA difference. Nicotine pre-incubation consisted of 24 h in L-15 medium supplemented with 1.0 µM nicotine. Non-incubated oocytes were placed on L-15 nicotine-free solution. Following chronic exposure to nicotine, incubated and non-incubated oocytes were dehydrated with 1:1 Me2SO:methanol solution mixture, rehydrated with MOR2 buffer without EGTA (115 mM NaCl, 2.5 mM KCl, 5 mM HEPES, 1 mM Na2HPO4, 0.2 mM CaCl2, and 5 mM MgCl2, pH 7.4), and then fixed in 4% paraformaldehyde. Oocytes were then incubated in goat serum blocking solution prior to antibody treatment. They were then incubated overnight with a monoclonal antibody against the extracellular domain of the rat α4 nAChR subunit (mAb299, purchased from Covance Co.), and then washed with MOR2 solution. Finally, oocytes were incubated overnight with a secondary antibody conjugated to fluorescein isothiocyanate (Kirkegaard and Perry Laboratories, Inc., Gaithersburg, MD). Goat serum and antibodies were diluted in MOR2 buffer supplemented with 0.1% Triton X-100.
All images were acquired using a Zeiss LSM 510 confocal microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a Plan-Neofluar 10×/0.3 objective. Multiple images (optical slices; (5-µm thick) were acquired to construct Z stacks from each oocyte. Pinhole size, photomultiplier gain and offset, pixel size, and data depth settings were kept the same for all the images acquired in each experiment. Autofluorescence and background noise intensity values were obtained from non-injected oocytes in each experiment. We used MetaMorph® (Universal Imaging Corporation™, Downingtown, PA) to process each image. The threshold function was used to eliminate the fluorescence intensity values from pixels not contributing to the image (e.g., background and autofluorescence). In addition, the same area (µm2) was selected for each control–experimental pair analyzed in order to make comparisons between optical slices. The total fluorescence intensity value was then calculated from each optical slice from the control and nicotine-incubated oocyte.
Epibatidine Binding Assay
[125I]-epibatidine (PerkinElmer Life Sciences, Boston, MA) binding assays were performed to determine membrane expression of nAChR in oocytes. The oocytes were incubated in 50 pM [125I]-epibatidine with 5 mg/ml albumin serum bovine in MOR2 without EGTA at room temperature for 2 h. Non-injected oocytes were also incubated in [125I]-epibatidine to measure non-specific binding (Beckman Gamma 5500). Excess epibatidine was removed by washing each oocyte with 60 ml of MOR2 without EGTA. A standard linear regression was obtained by plotting the counts per minute against [125I]-epibatidine concentration (0.5–20 fmol).
Results
Functional Properties and Expression of α4β2 Mutant nAChRs
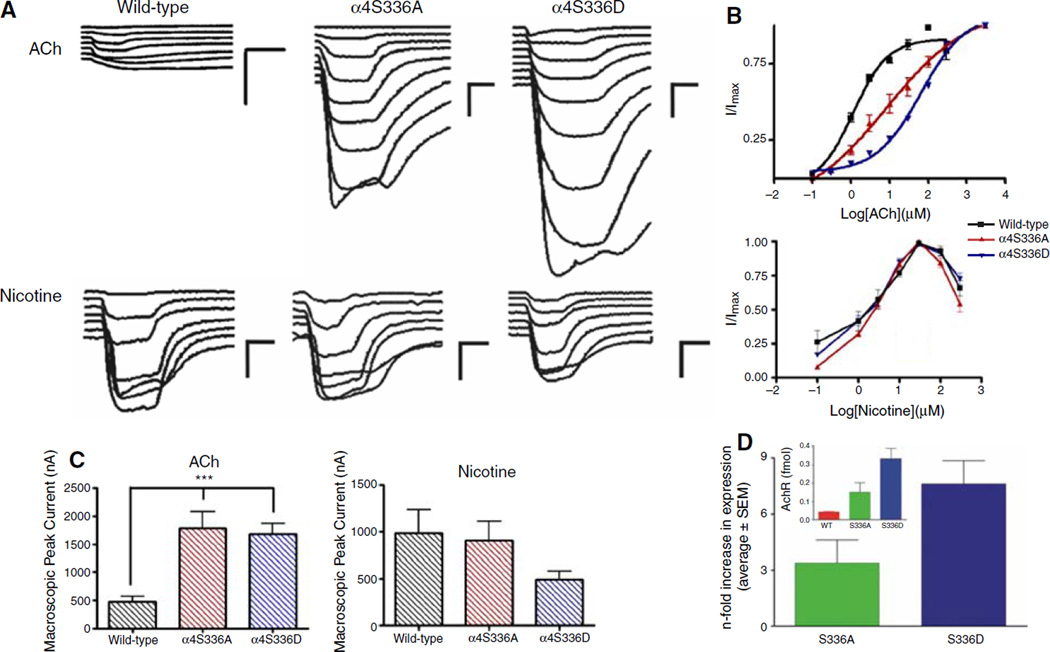
The functional effects of substitution of a serine residue at position 336 of the α4 subunit on the activation properties of α4β2 nAChR were evaluated using two-electrode voltage clamp recording. We measured the current response to several concentrations of ACh and nicotine. The average ACh- and nicotine-induced macroscopic peak currents are shown in Fig. 1c. The α4S336 mutants displayed a significant threefold increase in the ACh-induced macroscopic peak current (Fig. 1c) compared to the wild-type receptor. There was no significant difference in the nicotine-induced macroscopic peak current among the α4β2 nAChRs studied (Fig. 1c). The corresponding dose–response curves for ACh and nicotine are shown in Fig. 1b. ACh dose– response curves were fitted using a single Hill equation and a two-component Hill equation. A better fit (R2 = 0.995) was obtained using the two-component equation for the α4S336D mutant (Fig. 1b and Fig. 3b). The estimated ACh EC50 values for the α4S336D mutant receptor were 17.4 ± 1.4 and 137.7 ± 0.1 µM (Table 1). The EC50 values for ACh activation of wild-type and α4S336A mutant receptors were 1.1 ± 0.1 and 8.6 ± 0.2 µM, respectively (Table 1). Statistical analysis using unpaired t-test showed significant difference in the ACh EC50 values between wild-type and α4S336 mutants (P < 0.05). The dose–response curves for nicotine showed a bell-shaped profile for the three α4β2 nAChR subtypes studied (Fig. 1b). No significant difference was found among the mean nicotine EC50 and Hill slope values for the mutant and wild-type receptors (Table 1).
Fig. 1.
Effects of mutations at residue S336 of the α4 subunit in the functional properties of α4β2 nAChRs. Voltage clamp recording was used to determine the macroscopic response of mutant and wild-type α4β2 nAChRs to several ACh or nicotine concentrations. (a) Family of ACh- and nicotine-induced macroscopic currents recorded from Xenopus oocytes expressing wild-type, α4S336A, and α4S336D α4β2 nAChRs. Calibration bars are shown at the right of each current family, 500 nA (vertical bars) and 8 s (horizontal bars). (b) Dose– response relationships using ACh and nicotine as agonists for wild-type (black), α4S336A (red), and α4S336D (blue) α4β2 receptors. ACh dose–response curves for mutants were determined using nine concentrations (0.1, 0.3, 1, 3, 10, 30, 100, 300, and 3000 µM), whereas seven ACh concentrations (0.1, 1, 3, 10, 30, 100, and 300 µM) were tested to characterize wild-type receptor. Nicotine dose–response relationships were obtained from seven agonist concentrations (0.1, 1, 3, 10, 30, 100, and 300 µM). Agonist-induced responses were normalized to the maximum response (I/Imax). ACh dose–response curves were fitted using a single Hill equation and a two-component Hill equation. A better fit was obtained using a two-component Hill equation for the α4S336D mutant. Dose–response relationships for both agonists represent the average and standard error of the mean for 5–13 oocytes. (c) Bar graphs illustrating ACh- or nicotine-induced peak current for wild-type, α4S336A, and α4S336D α4β2 nAChRs. The peak currents were obtained at 300 µM ACh and 30 µM nicotine. Statistical analysis was performed using one-way ANOVA with Dunnett’s multiple comparison test (***P < 0.01). (d) Bar graphs illustrating a summary of the results of the [125I]-epibatidine binding assays. Insert illustrates the expression values in fmols for wild-type (n = 3; red), α4S336A (n = 6; green), and α4S336D (n = 7; blue). The main bar graph illustrates the n-fold increase (mutant expression divided by wild-type expression) in expression for the α4S336A (green) and α4S336D (blue) mutants. Values are presented as mean ± SEM
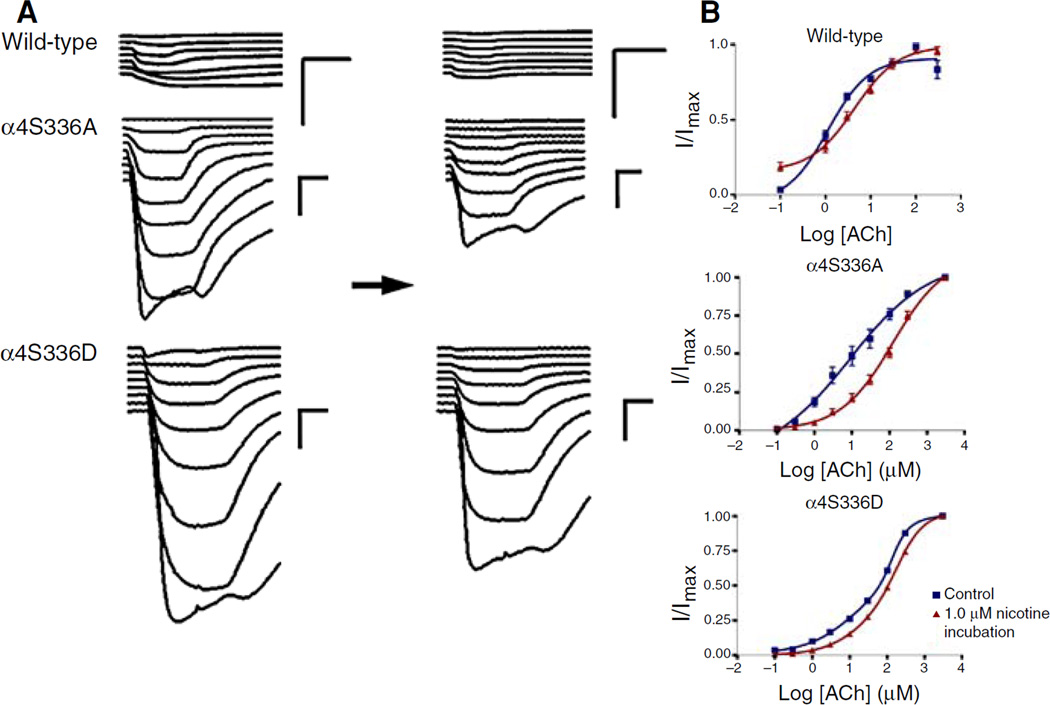
Fig. 3.
Effect of chronic nicotine exposure on the ACh-induced response of α4S336 mutants expressed in Xenopus oocytes. Voltage clamp recording was used to determine the ACh-induced response of Xenopus oocytes expressing wild-type, α4S336A, and α4S336D α4β2 nAChRs before and after chronic nicotine exposure. (a) Left side shows the family of currents for the control experiments (without chronic nicotine incubation), whereas right side illustrates the currents after chronic nicotine exposure. Horizontal black arrow represents 24 h of 1.0 µM nicotine incubation. After chronic nicotine treatment and prior to voltage clamp recording, oocytes were washed for 6 min perfusing with MOR2 buffer. Calibration bars are shown at the right of each current family, 500 nA (vertical bars) and 8 s (horizontal bars). (b) ACh dose–response curves for wild-type, α4S336A, and α4S336D α4β2 receptors, before and after chronic nicotine treatment. Dose–response relationships were determined using nine ACh concentrations for α4S336A and α4S336D mutants (0.1, 0.3, 1, 3, 10, 30, 100, 300, and 3,000 µM) and seven doses for wild-type α4β2 nAChR (0.1, 1, 3, 10, 30, 100, and 300 µM). ACh-induced currents were normalized to the maximum response (I/Imax). A better curve fit was obtained using the two-component Hill equation for α4S336D mutant. Data from control oocytes are shown in blue, whereas the results after 24 h of 1.0 µM nicotine incubation are shown in red. Dose– response curves represent the average and standard error of the mean for 5–9 oocytes
Table 1.
Effects of mutations at α4S336 in the functional properties of α4β2 nAChRs expressed in Xenopus oocytes
| AChR type (α4β2) | ACh |
Nicotine |
||||
|---|---|---|---|---|---|---|
| EC50 ± SEM (µM) | Hill coefficient | n | EC50 ± SEM (µM) | Hill coefficient | n | |
| Wild-type | 1.1 ± 0.1 | 0.97 ± 0.1 | 9 | 2.5 ± 0.1 | 1.6 ± 0.7 | 7 |
| α4S336A | 8.6 ± 0.2* | 0.4 ± 0.1* | 9 | 1.8 ± 0.1 | 1.7 ± 0.6 | 5 |
| α4S336D | 17.4 ± 1.4* | 0.6 ± 0.4* | 5 | 2.0 ± 0.1 | 1.4 ± 0.3 | 13 |
| 137.7 ± 0.1* | 2.2 ± 2.7* | |||||
Values are given as the mean ± SEM.
Indicates P < 0.05, n indicates the number of oocytes tested
The increase in macroscopic peak current induced by the α4S336 mutants (Fig. 1c) suggests that these mutations result in an increase in nAChR expression. [125I]-epibatidine assays were thus performed to determine membrane nAChR expression for wild-type and the α4S336 mutants (Fig. 1d). The calculated expressions (fmol) for wild-type, α4S336A and α4S336D, were 0.044 ± 0.003, 0.15 ± 0.05, and 0.33 ± 0.05 (Fig. 1d, insert). These numbers correspond to an n-fold increase in expression, relative to that of wild-type, of 3.40 ± 1.21 and 7.61 ± 1.25 for the α4S336A and α4S336D mutants, respectively (Fig. 1d).
Desensitization of α4β2 Mutant Receptors
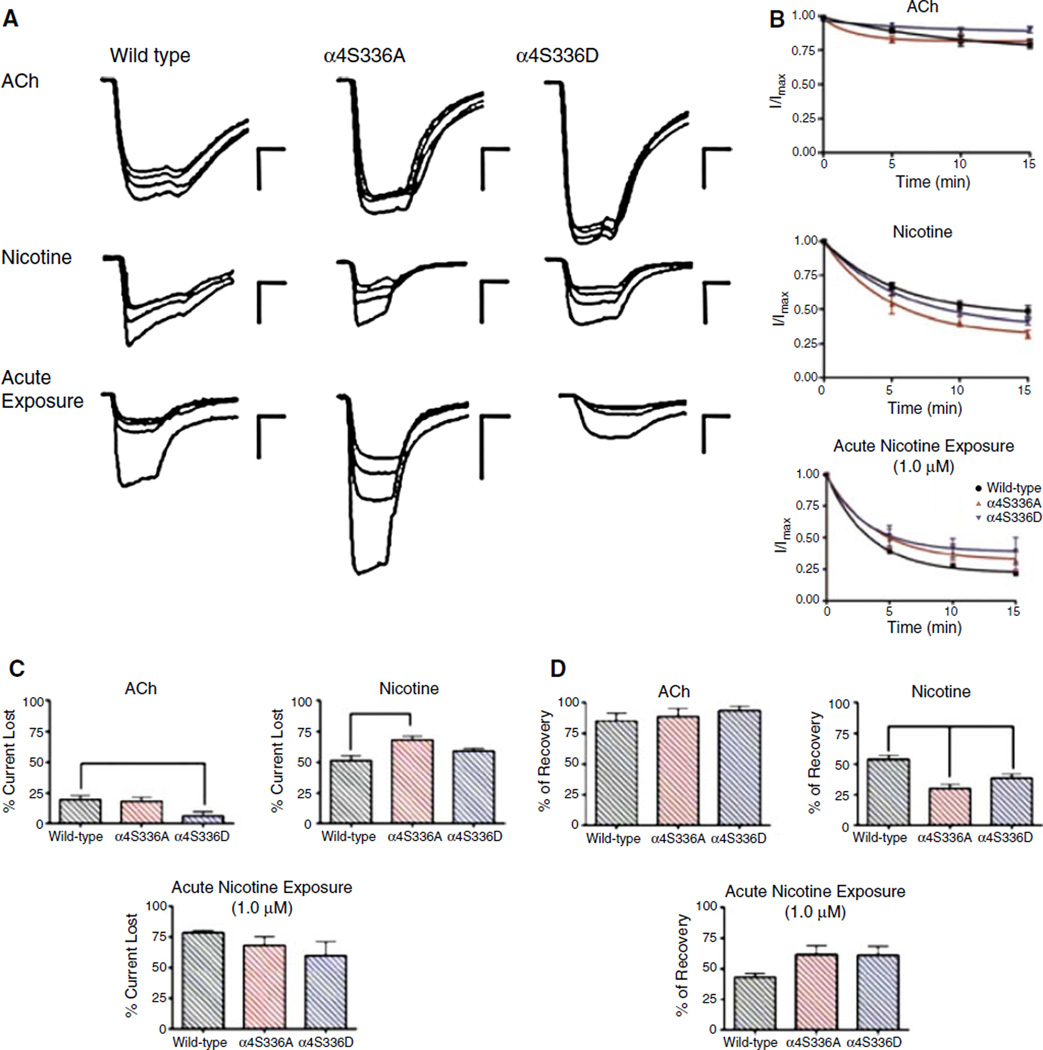
Figure 2 illustrates the effect of consecutive applications of 300 µM ACh and 30 µM nicotine in the macroscopic response of mutant receptors. Consecutive ACh applications produced only a mild current loss in the mutant and wild-type α4β2 receptors (Fig. 2c). The percentages of current lost from their original macroscopic current after repetitive pulses of 300 µM ACh were 19 ± 4, 18 ± 3, and 6 ± 3 for wild-type, α4S336A, and α4S336D, respectively. The α4S336D mutant exhibited the lowest loss of macroscopic current as result of consecutive applications of ACh (Fig. 2b, c). This lower percent of current lost for α4S336D was significantly different from the wild-type receptor (Fig. 2c). The mutants and wild-type receptor displayed an increase in the progressive loss of macroscopic current after consecutive applications of 30 µM nicotine. The percentages of current loss were 51 ± 4 for wild-type, 68 ± 3 for α4S336A, and 59 ± 2 for α4S336D (Fig. 2c). The mean current loss after consecutive applications of nicotine was significantly different between α4S336A mutant and wild-type (P < 0.01). Also acute nicotine exposure experiments were performed in which 1.0 µM nicotine was acutely applied between ACh applications as described under section “Materials and methods” (Fig. 2). The percentages of current lost from their original macroscopic current after acute nicotine exposure were 78 ± 2, 68 ± 7, and 60 ± 11 for wild-type, α4S336A, and α4S336D, respectively. The mean current loss after acute nicotine exposure was not significantly different.
Fig. 2.
ACh- and nicotine-induced desensitization of mutant α4β2 nAChRs. (a) Family of current traces for wild-type, α4S336A, and α4S336D α4β2 nAChRs representing the response to consecutive applications of agonist. The macroscopic responses to four consecutive applications of 300 µM ACh or 30 µM nicotine were recorded for each α4β2 subtype tested. Oocytes were washed with MOR2 buffer between each agonist pulse for 5 min (a total of three washes for four agonist applications). The protocol of acute nicotine exposure experiment consisted of measuring the current induced by four consecutive applications of 300 µM ACh. Between each ACh application, the oocyte was washed with 1.0 µM nicotine-containing MOR2 for 5 min. Calibration bars are shown at the right of each current family, 500 nA (vertical bars) and 8 s (horizontal bars). (b) Graphs illustrating the macroscopic response decay with respect to time of agonist exposure; wild-type in black, α4S336A in red, and α4S336D in blue. (c) Bar graphs showing the average percentage of current lost after repetitive applications of agonist. (d) Percent recovery of the original macroscopic response after 20 min of agonist withdrawal for wild-type, α4S336A, and α4S336D α4β2 nAChRs. Statistical analyses were performed using one-way ANOVA with Dunnett’s multiple comparison test (*P < 0.05; **P < 0.01; ***P < 0.001). Data were obtained from 7 to 29 oocytes
The amount of recovery was measured (see section “Materials and methods”) for each experimental condition tested (Fig. 2d). After consecutive applications of 300 µM ACh all three subtypes recovered a considerable amount of their initial current, 85 ± 6% for wild-type, 89 ± 6% for α4S336A, and 94 ± 4% for α4S336D. The means of the amount of current recovered after consecutive applications of ACh were not significantly different. In contrast, after repetitive pulses of 30 µM nicotine the mutant receptors recovered <50% of their initial current. The percentages of current recovered were 54 ± 4, 30 ± 3, and 38 ± 3 for wild-type, α4S336A, and α4S336D, respectively. The mean values of the recovered current after consecutive application of 30 µM nicotine of both mutants were significantly different from wild-type α4β2 nAChR (P < 0.01). After acute exposure to 1.0 µM nicotine, the percentages of current recovered were 43 ± 3, 61 ± 7, and 61 ± 8 for wild-type, α4S336A, and α4S336D, respectively.
Chronic Nicotine Exposure and Activation of Mutant α4β2 nAChRs
In order to evaluate the effect of chronic nicotine exposure on the functional activation of oocytes expressing mutant or wild-type α4β2 nAChRs, their responses to several concentrations of ACh were measured after 24 h incubation in 1.0 µM nicotine (Fig. 3a, right traces). After chronic nicotine treatment the mutants and wild-type α4β2 nAChRs exhibited a reduced state of activation as evidenced by a significant decrease in their respective peak currents (Table 2). Furthermore, after chronic nicotine exposure all three α4β2 nAChR subtypes displayed a significant increase in the EC50 values for ACh (Fig. 3 and Table 2), whereas there was no significant difference in the Hill coefficient values. The α4S336A mutant exhibited a dramatic inactivation evidenced by a 15-fold shift in the EC50 value for ACh. In the case of the α4S336D mutant only the component of lower ACh sensitivity showed a significant increase in the EC50 value for ACh after chronic nicotine exposure (Table 2).
Table 2.
Effects of chronic nicotine exposure in the functional properties of α4S336 mutants
| Wild-type | α4S336A | α4S336D | ||
|---|---|---|---|---|
| Peak current at 300 µM ACh (nA ± SE) | ||||
| Control | 479 ± 86 | 1,790 ± 280 | 1,684 ± 176 | |
| 1 µM nicotine incubation | 246 ± 60* | 1,128 ± 251* | 1,257 ± 163* | |
| (n = 19) | (n = 22) | (n = 21) | ||
| EC50 ACh (µM ± SE) | ||||
| Control | 1.1 ± 0.1 | 8.6 ± 0.2 | 17.4 ± 1.4 | 137.7 ± 0.1 |
| 1 µM nicotine incubation | 4.38 ± 0.1* | 124.7 ± 0.1* | 18.2 ± 1.4 | 194.1 ± 0.2* |
| (n = 9) | (n = 9) | (n = 5) | ||
| Hill slope ACh | ||||
| Control | 0.97 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.4 | 2.2 ± 2.7 |
| 1 µM nicotine incubation | 0.9 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.4 | 1.2 ± 0.4 |
| (n = 9) | (n = 9) | (n = 5) | ||
Values are given as the mean ± SEM.
Indicates P < 0.05, n indicates the number of ooctyes tested, 300 µM ACh and 30 µM nicotine were used to estimate the peak currents
Up-regulation of α4β2 nAChRs
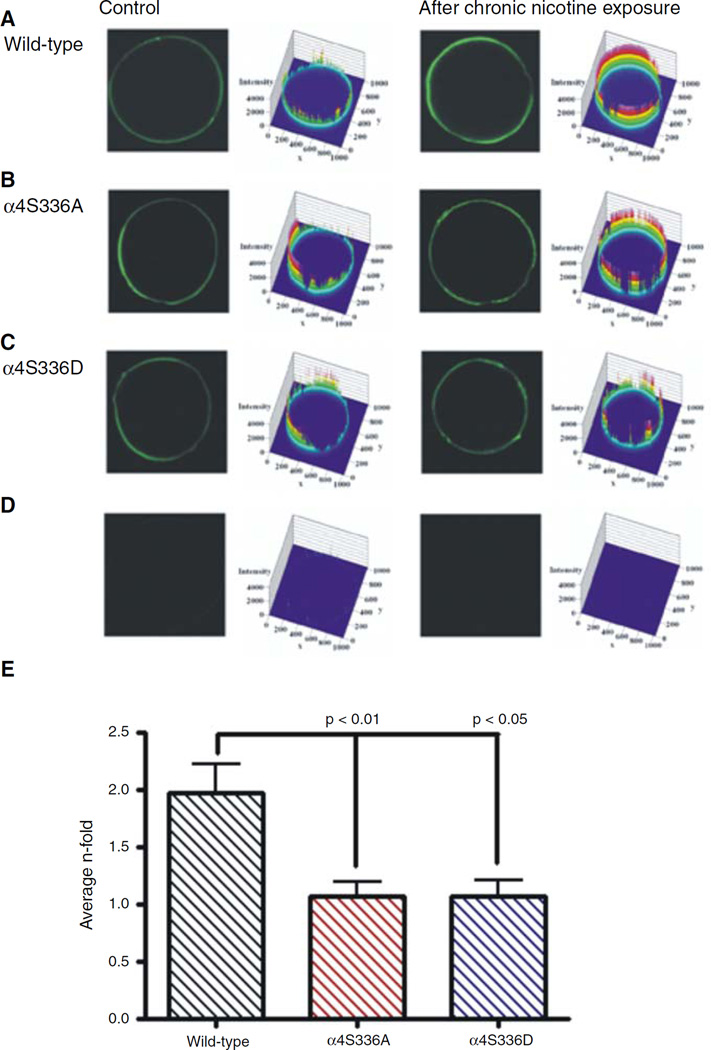
Whole-mount immunofluorescence assays for the detection of surface α4 subunit and confocal imaging were performed to determine the effects of chronic nicotine exposure on the up-regulation of mutant α4β2 nAChRs. Oocytes were paired according to their similar current profile (i.e., not more than 400 nA difference); oocytes incubated for 24 h with 1.0 µM nicotine were compared with oocytes without nicotine exposure. Figure 4 illustrates representative confocal sections of immunolabeled α4 subunit on the surface membranes of Xenopus oocytes and their corresponding fluorescence intensity profiles for each α4β2 subtype studied. The total fluorescence intensity for each confocal section was calculated as explained under section “Materials and methods.” Figure 4b shows a plot of the average n-fold values (intensity after chronic nicotine exposure divided by the intensity before chronic nicotine exposure) for each α4β2 subtype. The average n-fold values were 1.1 ± 0.1 and 1.1 ± 0.1 for the α4S336A and α4S336D mutants, respectively. In contrast, the average n-fold value for the wild-type α4β2 receptor was 2.0 ± 0.2 evidenced by the increment in the fluorescence intensity observed in oocytes expressing wild-type α4β2 nAChRs that were exposed to chronic nicotine incubation (Fig. 4a). The average n-fold values were significantly different between both mutants and wild-type α4β2 nAChRs (P = 0.0069).
Fig. 4.
Confocal images of the immunofluorescence surface detection of the α4 subunit after chronic nicotine exposure. (a) Fluorescence intensity of oocytes injected with wild-type α4β2. The initial response at 300 µM ACh for the incubated oocyte was 3,333 and 2,834 nA for the control oocyte. (b) Fluorescence intensity of oocytes injected with α4S336A mutant. The initial responses at 300 µM ACh for the incubated and control ocytes were 1,968 and 1,704 nA, respectively. (c) Fluorescence intensity of oocytes injected with α4S336D mutant. The initial response at 300 µM ACh for the incubated oocyte was 1,830 and 1,876 nA for the control oocyte. (d) Fluorescence intensity of oocyte not injected but treated with primary and secondary antibodies (left). Oocyte injected with wild-type α4β2 (335 nA) but treated with primary antibody (right). (e) The average n-fold values are presented as bar graphs for wild-type (black), α4S336A (red), and α4S336D (blue)
Discussion
Neuronal nAChR subunits are subject to phosphorylation by cyclic AMP-dependent PKA and PKC. Such chemical modifications may regulate agonist-induced desensitization (Downing and Role 1987; Vijayaraghavan et al. 1990). Activation of protein kinases, such as PKA and PKC, has been demonstrated to promote recovery from desensitization (Fenster et al. 1999a; Khiroug et al. 1998). The α4 subunit contains at least nine serine phosphorylation consensus sites. Several in vitro studies have demonstrated that some of these sites are directly phosphorylated by PKA or PKC (Moss et al. 1996; Nakayama et al. 1993; Vijayaraghavan et al. 1990; Wecker et al. 2001). In the present study, one of the putative phosphorylation consensus sites in the α4 nAChR subunit, S336, has been replaced with either alanine or aspartic acid. The alanine substitution removed the potential phosphorylation consensus site and aspartic acid was used to mimic the negative charge at position α4S336. We used both ACh and nicotine as agonists to determine the effect of these mutations on the activation, desensitization, and nicotine-induced up-regulation of α4β2 nAChRs expressed in Xenopus oocytes. The results presented here demonstrate the contribution of the α4S336 residue to the functional regulation of α4β2 nAChRs expressed in Xenopus oocytes.
Functional Implications
The electrophysiological data revealed differences, both in macroscopic peak current and in activation properties, between mutant and wild-type α4β2 nAC-hRs when using ACh as agonist (Fig. 1 and Table 1). Mutant receptors exhibited a threefold increase in the ACh-induced macroscopic current. The observed increment in macroscopic current could be caused by an increase in the fraction of functional receptors assembled in the plasma membrane of oocytes or a large unitary conductance compared to the wild-type receptor. Epibatidine binding confirmed that, when compared to wild-type, both mutations result in an increase in the number of α4β2 receptors in the oocyte membrane (Fig. 1d). Both mutations displayed a significant increase in the EC50 value for ACh (Fig. 1 and Table 1). However, the dose–response curve generated for the α4S336D mutant was best fitted using a two-component Hill equation, whereas the α4S336A mutation results in a less sigmoidal dose–response curve (Fig. 1b). These observations suggest that elimination of the serine consensus site results in a less allosteric nAChR, while addition of a negative charge in the S336 residue, as with the α4S336D mutation, results in a more allosteric nAChR. The present data suggest that residue S336 plays a crucial role in the functional state of the α4β2 receptor.
It is noteworthy that, even though the mutants showed distinct activation properties induced by ACh, the EC50 and Hill coefficient values for nicotine activation and the nicotine-induced macroscopic peak current remained unaffected (Fig. 1 and Table 1). Consistent with our previous study (López-Hernán-dez et al. 2004), the marked differences between ACh and nicotine effects seen in the data presented here suggest that the properties of agonist binding for α4β2 channel activation have very distinct dynamic and/or structural requirements for ACh and nicotine.
Desensitization and Nicotine-induced Up-regulation
Following repetitive applications of ACh, the α4S336D mutant displayed more resistance to ACh-induced desensitization (Fig. 2a – c), as evidenced by its lowest percent current loss, when compared to wild-type and α4S336A. The agonist-induced desensitization was more evident when consecutive pulses of nicotine were applied to the oocytes expressing mutant or wild-type α4β2 receptors (Fig. 2a – c). The α4S336A mutant displayed the largest nicotine-induced desensitization, whereas the α4S336D mutant showed no significant difference, compared to wild-type, in the percent current loss following consecutive applications of 30 µM nicotine (Fig. 2a – c). Compared to wild-type α4β2 nAChR, both mutants exhibited a significant decrease in the percent current recovered after nicotine-induced desensitization. Specifically, α4S336A and α4S336D mutants recovered <40% of their original macroscopic current following consecutive applications of 30 µM nicotine (Fig. 2d). It is noteworthy that although the mutants did not differ from wild-type in the activation properties induced by nicotine (Fig. 1c), there were changes with respect to the percentage of current lost and in the recovery from desensitization after consecutive nicotine applications. This supports our previous hypothesis, which proposes that the site responsible for nicotine-induced activation of the α4β2 nAChR has different dynamic and/or structural requirements from the site that promotes channel desensitization (López-Hernández et al. 2004).
Both mutants exhibited different patterns of functional regulation, in control condition as well as after chronic nicotine exposure. For example, the ACh dose–response curve for the α4S336D mutant was best fitted using a two-component Hill equation, whereas the α4S336A mutant and the wild-type receptor were fitted using a single Hill equation. Moreover, dose–response relationships obtained from chronic nicotine exposure experiments revealed differences in the allosteric transitions of the receptor between wild-type and both α4S336 mutants. That is, the α4S336A mutant appears to be a less allosteric receptor before chronic nicotine exposure (Fig. 3b). The latter finding may account for the dramatic inactivation observed in this mutant after chronic nicotine exposure, i.e., a 14-fold increase in EC50 after chronic nicotine incubation (Table 2). Alternatively, the two component Hill equation fit for the α4S336D data might suggest that this mutation results in two distinct populations of nAChRs in the membrane, one with high sensitivity to ACh (EC50 = 17.4 µM; Table 2) and the other with lower sensitivity to ACh (EC50 = 137.7 µM; Table 2). Overall, these results demonstrated that the PKC consensus α4S336 position might play a remarkable role in the allosteric properties of the α4β2 nAChR.
Both S336 mutants failed to display a significant up-regulation after chronic nicotine exposure. This finding could be explained by the fact that both mutants display a remarkable enhance on the expression of the α4β2 nAChR, therefore, it seems that chronic nicotine exposure cannot up-regulate a receptor that is already over expressed compared to wild-type. Based on these results, we hypothesize that phosphorylation at multiple consensus sites in this domain induces a conformational change that triggers an intracellular signal responsible for the desensitization of α4β2 nAChRs and consequently the nicotine-induced up-regulation process. However, the present data suggest that adding or removing a negative charge at this phosphorylation site cannot be explained by a simple straightforward on-and-off mechanism; rather a more complex mechanism(s) may govern the functional expression of the α4β2 nAChR. Along the same line, our data support the idea that phosphorylation at multiple consensus sites in the α4 subunit has a critical role on the regulation of the functional expression of the α4β2 nAChR. One appealing hypothesis is that phosphorylation at multiple consensus sites in the α4 subunit might lead to receptors with altered stoichiometry. For instance, the activation profile of the α4S336D mutation is very similar to the parameters of the 4(α4)1(β2) nAChR reported in our previous study (López-Hernández et al. 2004).
Finally, our results indicate the importance of the α4S336 residue in the functional regulation of the α4β2 nAChR. The α4S336 position could provide a way to fine-tune synaptic transmission of α4β2 receptors and could be of great physiological relevance given the critical role of neuronal nAChRs in presynaptic membranes where they modify the excitability of neurons and facilitate neurotransmitter release.
Acknowledgments
We thank Mr. José Serrano, Dr. Janice Salas, and Dr. Amelia Rivera for valuable technical assistance. This research was supported by National Institutes of Health Grants NIH 2RO1GM56371-11, SNRP-U54N54301, and GM08102-27. The Research Initiative for Scientific Enhancement-Minority Biomedical Research Support (RISE-MBRS)-NIH program (5R25GM61151) supported Gretchen Y. López-Hernández. Nilza M. Biaggi-Labiosa was supported by the RISE-MBRS-NIH program (2R25GM061151). Alexis Torres-Cintrón was supported by the Minority Access to Research Careers (MARC)-MBRS-NIH program.
Contributor Information
Gretchen Y. López-Hernández, Department of Pharmacology and Therapeutics, University of Florida, 100267, Gainesville, FL 32610-0267, USA
Nilza M. Biaggi-Labiosa, Department of Biology, University of Puerto Rico, JGD-114, 23360, San Juan, PR 00931-3360, USA
Alexis Torres-Cintrón, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA.
Alejandro Ortiz-Acevedo, Department of Natural Sciences, University of Puerto Rico, 2500, Utuado, PR 00641-2500, USA.
José A. Lasalde-Dominicci, Department of Biology, University of Puerto Rico, JGD-114, 23360, San Juan, PR 00931-3360, USA joseal@coqui.net; jlasalde@gmail.com
References
- Benwell ME, Balfour DJ, Anderson JM. Evidence that tobacco smoking increases the density of (−)-[3H]nicotine binding sites in human brain. J Neurochem. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- Downing JE, Role L. Activators of protein kinase C enhance acetylcholine receptor desensitization in sympathetic ganglion neurons. Proc Natl Acad Sci USA. 1987;84:7739–7743. doi: 10.1073/pnas.84.21.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers H, Schaeffer E, Bickler PE, Forsayeth JR. Functional deactivation of the major neuronal nicotinic receptor caused by nicotine and a protein kinase C-dependent mechanism. Mol Pharmacol. 1997;52:1105–1112. [PubMed] [Google Scholar]
- Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, et al. Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol. 1999a;55:432–443. [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA. Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999b;19:4804–4814. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- Huganir RL, Delcour AH, Greengard P, Hess GP. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986;321:774–776. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- Ke L, Eisenhour CM, Bencherif M, Lukas RJ. Effects of chronic nicotine treatment on expression of diverse nic-otinic acetylcholine receptor subtypesI. Dose- and time-dependent effects of nicotine treatment. J Pharmacol Exp Ther. 1998;286:825–840. [PubMed] [Google Scholar]
- Khiroug L, Sokolova E, Giniatullin R, Afzalov R, Nistri A. Recovery from desensitization of neuronal nico-tinic acetylcholine receptors of rat chromaffin cells is modulated by intracellular calcium through distinct second messengers. J Neurosci. 1998;18:2458–2466. doi: 10.1523/JNEUROSCI.18-07-02458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchack PA, Araujo DM, Quirion R, Collier B. Effect of chronic nicotine treatment on nicotinic autoreceptor function and N-3H–Mcc binding sites in the rat brain. J Neurochem. 1989;52:483–491. doi: 10.1111/j.1471-4159.1989.tb09146.x. [DOI] [PubMed] [Google Scholar]
- López-Hernández GY, Sánchez-Padilla J, Ortiz-Acevedo A, Lizardi-Ortiz J, Salas-Vincenty J, Rojas V, et al. Nicotine-induced up-regulation and desensitization of α4β2 neuronal nicotinic receptors depend on subunit ratio. J Biol Chem. 2004;279:38007–38015. doi: 10.1074/jbc.M403537200. [DOI] [PubMed] [Google Scholar]
- Lukas RJ. Effects of chronic nicotinic ligand exposure on functional activity of nicotinic acetylcholine receptors expressed by cells of the PC12 rat pheochromocytoma or the TE671/RD human clonal line. J Neurochem. 1991;56:1134–1145. doi: 10.1111/j.1471-4159.1991.tb11403.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Moss SJ, McDonald BJ, Rudhard Y, Schoepfer R. Phosphorylation of the predicted major intracellular domains of the rat and chick neuronal nicotinic acetylcholine receptor alpha 7 subunit by cAMP-dependent protein kinase. Neuropharmacology. 1996;35:1023–1028. doi: 10.1016/s0028-3908(96)00083-4. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Okuda H, Nakashima T. Phosphorylation of rat brain nicotinic acetylcholine receptor by cAMP-dependent protein kinase in vitro. Brain Res Mol Brain Res. 1993;20:171–177. doi: 10.1016/0169-328x(93)90123-7. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Swope SL, Moss SJ, Blackstone CD, Huganir RL. Phosphorylation of ligand-gated ion channels: a possible mode of synaptic plasticity. FASEB. 1992;6:2514–2523. [PubMed] [Google Scholar]
- Vijayaraghavan S, Schmid HA, Halvorsen SW, Berg DK. Cyclic AMP-dependent phosphorylation of a neuronal acetylcholine receptor alpha-type subunit. J Neurosci. 1990;10:3255–3262. doi: 10.1523/JNEUROSCI.10-10-03255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viseshakul N, Figl A, Lytle C, Cohen BN. The alpha4 subunit of rat alpha4beta2 nicotinic receptors is phosphorylated in vivo. Brain Res Mol Brain Res. 1998;59:100–104. doi: 10.1016/s0169-328x(98)00128-4. [DOI] [PubMed] [Google Scholar]
- Wecker L, Guo X, Rycerz AM, Edwards SC. Cyclic AMP-dependent protein kinase (PKA) and protein kinase C phosphorylate sites in the amino acid sequence corresponding to the M3/M4 cytoplasmic domain of alpha4 neuronal nicotinic receptor subunits. J Neurochem. 2001;76:711–720. doi: 10.1046/j.1471-4159.2001.00041.x. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CG, Wonnacott S. Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol. 1998;53:950–962. [PubMed] [Google Scholar]