Abstract
Purpose
To characterize treatment patterns and oncologic outcomes in patients with low-volume lymph node metastasis (isolated tumor cells [ITCs] and micrometastasis [MM]) discovered during sentinel node (SLN) mapping for endometrial carcinoma.
Methods
We identified endometrial cancer cases treated surgically from 9/2005-4/2013 in which SLN mapping was performed. MM was defined as tumor within a lymph node measuring >0.2mm but <2.0mm. ITCs were those measuring ≤0.2mm.
Results
Eight hundred forty-four patients met inclusion criteria. Median age was 61 (range, 30-90). Histology was as follows: endometrioid, 724 (85.8%); serous, 104 (12.3%); and clear cell, 16 (1.9%). Median number of lymph nodes resected was 6 (range, 0-60); median number of SLNs was 2 (range, 0-15). Seven hundred fifty-three patients (89.2%) were node negative, 23 (2.7%) had ITCs only, 21 (2.5%) had MM only, and 47 (5.6%) had macrometastasis. Adjuvant chemotherapy was given to 106 (14%) of 753 node-negative patients, 19 (83%) of 23 patients with ITCs, 17 (81%) of 21 patients with MM, and 42 (89%) of 47 with macrometastasis. Median follow-up was 26 months (range, 0-108). Three-year recurrence-free survival was as follows: node-negative patients, 90% (± 1.5); ITCs only, 86% (± 9.4); MM only, 86% (± 9.7); and macrometastasis, 71% (± 7.2), (p<0.001).
Conclusion
Patients with ITCs and MM frequently received adjuvant chemotherapy, and had improved oncologic outcomes in comparison to those with macrometastasis to the lymph nodes. Further prospective study is needed to determine optimal post-resection management in patients with ITCs or MM alone.
Introduction
Endometrial cancer remains the most common gynecologic cancer diagnosed in the United States, with an estimated 54,870 new cases in 2015.1 The presence or absence of lymph node metastasis is a prognostic factor, as are surgical stage, grade, histology, lymphovascular space invasion, depth of myometrial invasion, cervical involvement, and extrauterine spread. As depth of invasion and tumor grade increase, so does the risk for nodal involvement.2 Despite this knowledge, significant controversy surrounds the staging of endometrial carcinoma and, specifically, the performance and extent of the lymph node dissection. Practice ranges from omission of the nodal dissection, to utilization of preoperative imagine or frozen section as a means of triage, to lymphadenectomy to the renal vessels for all patients.3-7 Two randomized controlled trials failed to demonstrate an improvement in oncologic outcomes with comprehensive lymphadenectomy.8,9 However, a recent CART analysis by Barlin et al. concluded that stage assignment significantly impacted overall survival, thus stressing the importance of surgical staging and lymph node assessment.10
Over the last decade, use of a sentinel lymph node (SLN) mapping algorithm for endometrial cancer has emerged as a middle ground in which all patients with endometrial cancer undergo lymph node evaluation, while limiting the morbidity associated with a full lymphadenectomy.11 Sentinel node biopsy has been studied extensively in breast cancer and in melanoma. Within the breast literature multiple randomized controlled trials have demonstrated an improved side effect profile and better quality of life outcomes for those patients who undergo SLN biopsy compared to those who undergo a full axillary lymph node dissection, without compromising regional disease control and survival endpoints.12-14 Lymph node metastasis remained an independent (and negative) prognostic factor in breast disease,15 but the prognostic significance of low-volume lymph node metastases remains an area of active investigation.
Ultrastaging of sentinel axillary nodes in breast cancer led to detection of small tumor deposits within the nodes. The American Joint Committee on Cancer (AJCC) defined micrometastasis (MM) as tumor within a lymph node measuring greater than 0.2 mm but less than 2.0 mm, and isolated tumor cells (ITCs) as tumor less than or equal to 0.2 mm.16 One prospective study of over 3,000 patients in Sweden demonstrated a decreased 5-year disease-specific survival (DSS) in patients with micrometastases compared to those with negative lymph nodes.17 There was no significant difference in survival between those with isolated tumor cells and those with negative lymph nodes; however, those with ITCs were more likely to undergo a completion axillary lymph node dissection than those without ITCs.
Use of an SLN algorithm with pathologic ultrastaging for endometrial carcinoma has led to increased detection of low-volume lymph node metastasis in the form of isolated tumor cells and micrometastases.18,19 However, the clinical significance of these findings in endometrial cancer is yet unknown. We sought to characterize treatment patterns and oncologic outcomes in those patients with endometrial cancer found to have low-volume lymph node metastasis compared to those patients with negative nodes and to patients with macrometastatic nodes. We hypothesized that there would be no statistically significant difference in recurrence-free survival (RFS) between those with low-volume lymph node disease (ITCs and micrometastases) compared to those with macrometastatic nodal involvement.
Materials and Methods
Approval was obtained from the Institutional Review board at Memorial Sloan Kettering Cancer Center. We then identified all cases of endometrial cancer treated surgically at our institution from September of 2005 through April of 2013 in which the Memorial Sloan Kettering (MSK) SLN mapping algorithm was utilized.11 At the time of surgery, blue dye or Indo Cyanine Green (ICG) was injected into the cervical stroma at the 3 o’clock and 9 o’clock positions to both deep and superficial levels totalling 4 mL; SLNs were excised and labeled as such, and then processed by the institution’s Pathology department per protocol.11,19
Lymph node status was defined as follows. Those lymph nodes without tumor present on pathologic evaluation were reported as negative. Isolated tumor cells were defined as those measuring less than or equal to 0.2 mm. Micrometastases were defined as tumor within a lymph node measuring greater than 0.2 mm but less than 2.0 mm. Notably, when the tumor measurement was not delineated in the pathology report and the terms “isolated tumor cells” and “micrometastasis” were not used, a determination was made based on the pathology report with clarification from a gynecologic pathologist when needed. For example, “rare scattered tumor cells” were classified as ITCs, whereas “diffuse clusters of cells” were defined as MM. This occurred in three cases classified as ITCs, in which the wording “scattered” or “single” cells was used, and in 10 cases classified as MMs, in which “clusters” of tumor were described within the node. Lymph nodes with a tumor burden greater than or equal to 2.0 mm were reported as metastatic lymph nodes without further delineation of number or cells or the size of the metastasis.
Medical records were reviewed, including but not limited to outpatient and inpatient notes, laboratory results and imaging if pertinent, operative reports, pathology reports, and records detailing postoperative treatment including chemotherapy and radiation therapy. All grades and histologic subtypes were included in the analysis. The stage at diagnosis was assigned based on the 1988 International Federation of Gynecology and Obstetrics (FIGO) staging for endometrial carcinoma, as the study period spanned the transition to the updated 2009 FIGO staging system. Those with intraperitoneal spread delineating stage IV disease were excluded; all other stages were included in the analysis. The primary outcome was recurrence-free survival.
Clinicopathologic data were reported using median values with a range for continuous variables, and number of patients (n) and percentage (%) for categorical variables. Recurrence-free survival was calculated using the Kaplan-Meier method. All statistical analyses were performed using IBM SPSS Statistics 22.0.
Results
Over the study period, 844 patients met the inclusion criteria and were analyzed (Table 1). The median age was 61 years (range, 30-90) and median BMI was 30 (range, 16-69). The majority of cases, 83%, were performed by a minimally invasive approach, with 443 (52.5%) robotic cases, and 257 (30.4%) laparoscopic cases. Of the 844 cases, 724 (85.8%) were endometrioid histology, 104 (12.3%) were serous cancers, and 16 (1.9%) were clear cell. The majority of specimens were low grade, with 479 patients, or 56.8%, with FIGO grade 1 tumors, and 177 patients, or 21%, with FIGO grade 2 tumors on final pathology. Lymphovascular invasion (LVSI) was present in 201 cases, or 23.8%, while 618 of tumors (73.2%) demonstrated no LVSI. Data on LVSI was unavailable for 25 patients (3%). Seven hundred thirty-two patients (86.7%) had no, or <50%, myometrial invasion. Finally, 717 patients, or 85%, had negative peritoneal washings, while 57 (6.8%) had positive washings, and 42 (5%) had washings noted to be suspicious. Data for peritoneal washings was unavailable for 28 patients (3.2%).
Table 1.
Clinicopathologic Patient Characteristics.
| Variable | N (%) |
|---|---|
|
| |
| Age, years | 61 (30-90) |
| Median (range) | |
|
| |
| BMI, kg/m2 | 30 (16-69) |
| Median (range) | |
|
| |
| Surgical Approach | |
| Laparotomy | 144 (17.1) |
| Laparoscopy | 257 (30.4) |
| Robotic | 443 (52.5) |
|
| |
| Histology | |
| Endometrioid | 724 (85.8) |
| Serous | 104 (12.3) |
| Clear cell | 16 (1.9) |
|
| |
| FIGO Grade | |
| 1 | 479 (56.8) |
| 2 | 177 (21.0) |
| 3 | 188 (22.2) |
|
| |
| Lymphovascular Invasion | |
| No | 618 (73.2) |
| Yes | 201 (23.8) |
| Unavailable | 25 (3.0) |
|
| |
| Depth of Myoinvasion | |
| None | 422 (50.0) |
| < 50% | 310 (36.7) |
| ≥ 50% | 112 (13.3) |
|
| |
| Peritoneal Washings | |
| Negative | 717 (85) |
| Positive | 57 (6.8) |
| Suspicious | 42 (5.0) |
| Unavailable | 28 (3.2) |
|
| |
| Stage I | |
| IA | 662 (78.4) |
| IB | 61 (7.2) |
| II | 20 (2.4) |
| IIIA | 9 (1.1) |
| IIIB | 1 (0.1) |
| IIIC | 89 (10.5) |
| IV | 2 (0.2) |
|
| |
| Nodal Status | |
| Node Negative | 753 (89.2) |
| Isolated Tumor Cells (ITCs) | 23 (2.7) |
| Micrometastasis (MMs) | 21 (2.5) |
| Macrometastasis | 47 (5.6) |
The median number of total lymph nodes collected was 6 (range, 0-60); the median number of pelvic lymph nodes was also 6 (range, 0-53); median paraaortic lymph nodes was 1 (range, 0-22).Fifty-one (6%) of 844 patients had no lymph nodes removed; these patients predominantly fell into two categories. In the first group, patients were injected for SLN mapping but failed to map, so frozen section was obtained and the surgeon used intraoperative uterine criteria to defer lymph node dissection. The second group consisted of patients in whom tissue was sent as nodal tissue, but no lymph nodes were identified on final pathology. The median number of SLNs was 2 (range, 0-15). Only one patient had 15 SLNs removed, and this was a case of serous endometrial cancer; it is unclear based on review of the chart whether 15 lymph nodes were mapped, or whether additional nodes were taken given the serous histology. Stage distribution was as follows: stage IA, 662 (78.4%); stage IB, 61 (7.2%); stage II, 20 (2.4%); stage IIIA, 9 (1.1); stage IIIB, 1 (0.1%); stage IIIC, 89 (10.5%); and stage IV, 2 (0.2%). On final pathology, 753 patients, or 89.2%, had negative lymph nodes. Isolated tumor cells were identified in 23 cases (2.7%), and micrometastases were found in 21 cases (2.5%). Forty-seven patients (5.6%) had macrometastases within the lymph nodes. Those patients with isolated tumor cells or micrometastases within the lymph nodes – also referred to as low-volume metastases – comprised our cohort of interest.
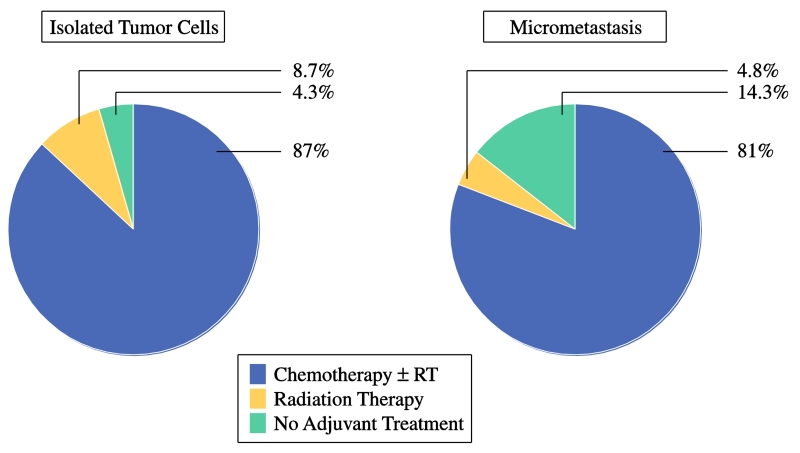
Patients with any evidence of nodal metastasis – whether ITCs, MM, or macrometastases – were considered stage IIIC, and evaluated for adjuvant therapy. The type of adjuvant therapy given to those patients with low-volume lymph node metastasis varied (Table 2). In the cohort of 23 patients with ITCs, 20 (87.0%) received chemotherapy postoperatively. Of those, 19 received chemotherapy in combination with radiotherapy; 12 (52.2%) received chemotherapy with intravaginal radiation therapy (IVRT), and 7 (30.5%) received combined chemotherapy and intensity-modulated radiation therapy (IMRT). In the group with micrometastases, 17 of 21 patients (81.0%) received chemotherapy in the adjuvant setting. Ten patients, or 47.6%, received chemotherapy plus IMRT, while 4 patients (19.0%) underwent chemotherapy and IVRT. As summarized in Figure 1, 87% of patients with positive lymph nodes by ITCs, and 81% of patients with positive lymph nodes by micrometastases, underwent adjuvant therapy that included chemotherapy. A percentage of patients in each group, 8.7 and 4.8%, respectively, received adjuvant radiation therapy alone. While postoperative treatment was recommended for all patients with ITCs and micrometastasis, 4 patients in total (9%) declined any adjuvant therapy. Of those 4 patients, all had FIGO grade 1 endometrioid adenocarcinoma, one had ITCs, and 3 had MM. One of the patients with MM has since recurred to the liver and peritoneal cavity after a disease-free interval (DFI) of 18 months. The remaining 3 patients are alive and well with no evidence of disease after DFIs of 10, 32, and 36 months.
Table 2.
Adjuvant treatment of those patients with positive lymph nodes by isolated tumor cells (ITCs) and micrometastasis (MMs).
| Treatment | ITCs (N=23) | MMs (N=21) |
|---|---|---|
| N (%) | N (%) | |
|
| ||
| Chemotherapy | 20 (87.0) | 17 (81.0) |
| Chemo Alone | 1 (4.3) | 3 (14.3) |
| Chemo + IVRT | 12 (52.2) | 4 (19.0) |
| Chemo + IMRT | 7 (30.5) | 10 (47.6) |
|
| ||
| Radiation Therapy (RT) | 2 (8.7) | 1 (4.8) |
| WPRT alone | ||
|
| ||
| No Adjuvant Treatment | 1 (4.3) | 3 (14.3) |
IVRT = Intravaginal radiation therapy
IMRT = Intensity-modulated radiation therapy
WPRT = Whole-pelvic radiation therapy
Figure 1.
Disease recurrence for the study population was as follows: of those 753 patients with negative lymph nodes, 47, or 6.2%, recurred; in those with ITCs, 2 patients of 23, or 8.7%, had a recurrence; in the micrometastases cohort, 2 patients of 21, or 9.5%, recurred; finally of the 46 patients with macrometastases in the nodes, there were 16 recurrences, or 34.8% of cases. The distribution of recurrences is displayed in Table 3.
Table 3.
Disease recurrence by lymph node status.
| Negative LNs | ITCs | MMs | Macrometastasis | |
|---|---|---|---|---|
| N (%) | N (%) | N(%) | N (%) | |
|
| ||||
| 47/753 | 2/23 | 2/21 | 16/46 | |
| Recurrences | (6.2%) | (8.7%) | (9.5%) | (34.8%) |
| Local | 13 | 1 | 0 | 1 |
| Nodal | 4 | 0 | 1 | 6 |
| Distant | 18 | 1 | 1 | 3 |
| Multi-site | 12 | 0 | 0 | 6 |
Abbreviations: LN, lymph node; ITC, isolated tumor cells; MM, micrometastasis.
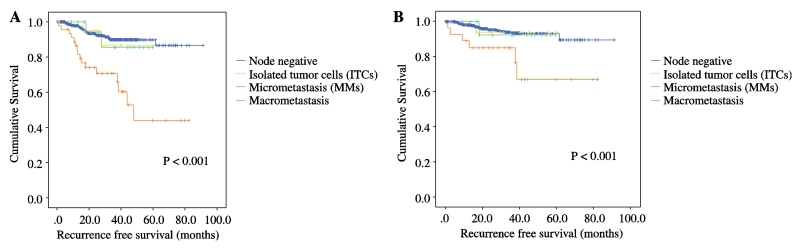
Figure 2a represents the Kaplan-Meier curve for RFS by nodal status. With a median follow-up of 26 months (range, 0-108), the 3-year RFS for those with negative lymph nodes was 90% (±1.5). For the ITC group the 3-year RFS was 86% (±9.4), and for the micrometastases patients 86% (±9.5). Those patients with macrometastatic lymph nodes had a recurrence-free survival at 3 years of 71% (±7.2), (p < 0.001). Given that the total cohort of 844 patients was composed of all histologies, we then looked at the RFS for those 724 cases with endometrioid endometrial cancers (Figure 2b). This is the group most likely to undergo SLN mapping with ultrastaging, and the patients whose adjuvant treatment may depend heavily on lymph node status. Here the 3-year recurrence-free survival was 93% (±1.4) for those with negative lymph nodes; 94% (±6.1) for those with ITCs; 92% (±7.4) for MMs; and 85% (±6.9) for macrometastases (p < 0.001). There were only 4 ITC and 4 MM cases within the non-endometrioid histology cohort. Meaningful survival outcome analysis was not possible in this specific cohort.
Figure 2.
Discussion
In our study population of over 800 patients with endometrial carcinoma treated surgically in which an SLN algorithm was utilized, the vast majority of patients found to have low-volume metastases received postoperative treatment including chemotherapy. When treated with surgery and adjuvant therapy, the oncologic outcomes for patients with ITCs and MM were improved over those with macrometastasis in our cohort, with a statistically significant improvement in 3-year RFS.
The literature regarding technique, feasibility, and detection rates for SLNs in endometrial cancer continues to grow.20-21 However, data regarding clinical outcomes in the setting of low-volume lymph node metastasis in endometrial cancer is lacking. One study by Todo et al. looked at 63 patients with FIGO stage I to II endometrial carcinoma with at least one adverse risk factor: grade 3 tumor, serous/clear cell histology, deep myoinvasion, cervical involvement, LVSI, or positive peritoneal washings.22 Within the cohort, ITCs and MM were identified in 9 patients, or 14.8%. They found that the presence of ITC/MM was an independent risk factor for recurrence (HR 17.9; 95% CI 1.4-232.2). The 8-year overall survival (OS) and RFS were lower in the ITC/MM group than in the node negative group (OS, 71.4% vs 91.9%; RFS, 55.6% vs 84.0%); however, given the small number of patients, statistical significance was not reached. Furthermore, with only 9 patients in total with low-volume metastases, the authors did not distinguish between those with ITCs versus MM.
With over 800 total patients and 44 with low-volume metastasis, our sample size is a strength of the study. Additionally, we benefit from dedicated gynecologic pathologists with over 10 years’ experience evaluating SLNs in gynecologic cancers. However, we acknowledge the limits of our data. Patients with ITCs and MM were treated as node positive. As such, the great majority of patients received adjuvant therapy, and in this study we are unable to comment on the natural history of patients with untreated low-volume metastasis. There is concern by some that we may be overtreating patients with ITCs and MM, and that they would do well without adjuvant therapy. While overtreatment should be avoided, it is important to note that only 5% of our study population of 844 patients was found to have low-volume lymph node metastasis, where adjuvant therapy may have been omitted had these positive lymph nodes gone undetected. Prospective study will be necessary to further inform the discussion regarding optimal post-resection management in this patient population.
Finally, the therapeutic benefit of lymphadenectomy to remove metastatic disease in non-SLNs remains unknown. In this cohort, patients with low-volume lymph node disease were treated postoperatively; however, none were brought back to the OR for completion lymph node dissection. Our study does not address the question of “debulking” of grossly normal appearing, but potentially metastatic, lymph nodes. Data recently published by Touhami et al looked at those factors that predict the presence of non-SLN metastasis when the SLN is positive.23 They evaluated 268 patients who underwent surgical staging for endometrial cancer including SLN mapping and pelvic lymphadenectomy, and found that the size of the metastasis within the SLN was highly predictive of non-SLN involvement. When the size of the SLN metastasis was ≤2.0 mm – ITCs or MM – the risk of having another positive lymph node was only 5%, with a negative predictive value of 95%. Those patients with SLN metastasis >2.0 mm had a risk of additional positive lymph nodes of 60.8% (p < 0.001). In our study, those patients with ITCs and MM at the time of SLN mapping had improved 3-year RFS over those with macrometastatic disease in the SLNs, which may corroborate this data.
In summary, our findings point to improved recurrence-free survival in endometrial cancer patients with low volume lymph node metastases treated in the adjuvant setting, in comparison to those with macrometastatic disease to the lymph nodes. Prospective study is needed to determine optimal postoperative treatment for patients with ITCs and MM alone. Until that time, our institution will continue to treat these patients as node-positive, recommending adjuvant therapy for them and fully recognizing that this may possibly be overtreatment in some patients. The MSK treatment algorithm for patients with stage IIIC disease calls for cisplatin 50 mg/m2 on days 1 and 29 plus volume-directed radiation therapy, followed by paclitaxel and carboplatin for four cycles. Alternatively, patients may be treated with chemotherapy (paclitaxel and carboplatin for six total cycles) with or without vaginal brachytherapy. The decision to proceed with one or the other depends upon additional clinicopathologic features as well as patient and provider preference. GOG 258 is comparing these two regimens and will hopefully provide information to help to support one regimen over the other. We will continue to closely follow those patients who have declined treatment in order to obtain additional data regarding the natural history of ITCs and MM in endometrial carcinoma.
Synopsis.
Our findings show improved recurrence-free survival in endometrial cancer patients with low-volume lymph node metastases treated in the adjuvant setting, in comparison to those with macrometastatic lymph nodes. Prospective study is needed to determine optimal postoperative treatment in this setting.
Acknowledgements
This work was supported in part by the Cancer Center core grant P30 CA008748. The core grant provides funding to institutional cores such as Pathology, which was used in this study.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Kang S, Kang WD, Chung HH, et al. Preoperative identification of a low-risk group for lymph node metastasis in endometrial cancer: a Korean Gynecologic Oncology Group study. J Clin Oncol. 2012;30:1329–1334. doi: 10.1200/JCO.2011.38.2416. [DOI] [PubMed] [Google Scholar]
- 4.Quinlivan JA, Petersen RW, Nicklin JL. Accuracy of frozen section for the operative management of endometrial cancer. BJOG. 2001;108:798–803. doi: 10.1111/j.1471-0528.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 5.Case AS, Rocconi RP, Straughn JM, Conner M, Novak L, Wang W, Huh WK. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gyncol. 2006;108:1375–1379. doi: 10.1097/01.AOG.0000245444.14015.00. [DOI] [PubMed] [Google Scholar]
- 6.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–1519. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 7.Dowdy SC, Aletti G, Cliby WA, Podratz KC, Mariani A. Extra-peritoneal laparoscopic para-aortic lymphadenectomy – a prospective cohort study of 293 patients with endometrial cancer. Gynecol Oncol. 2008;111:418–424. doi: 10.1016/j.ygyno.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK, ASTEC study group Efficacy of systemic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. Journal Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 10.Barlin JN, Zhou Q, St. Clair CM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: Seeing the forest for the trees. Gynecol Oncol. 2013;130:452–456. doi: 10.1016/j.ygyno.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlin JN, Khoury-Collado F, Kim CH, et al. The importance of applying a sentinel lymph node algorithm in endometrial cancer staging: beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–63. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]
- 13.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 14.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel RL, Ma J, Zou Z, Jemal A. Cancer Statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. Sixth Edition Springer; Philadelphia: 2002. [Google Scholar]
- 17.Andersson Y, Frisell J, Sylvan M, de Boniface J, Bergkvist L. Breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. J Clin Oncol. 2010;28:2868–2873. doi: 10.1200/JCO.2009.24.5001. [DOI] [PubMed] [Google Scholar]
- 18.Kim CH, Soslow RA, Park KJ, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–70. doi: 10.1097/IGC.0b013e3182954da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han G, Lim D, Leitao MM, Jr, Abu-Rustum NR, Soslow RA. Histological features associated with occult lymph node metastasis in FIGO clinical stage I, grade 1 endometrioid carcinoma. Histopathology. 2014;64:389–398. doi: 10.1111/his.12254. [DOI] [PubMed] [Google Scholar]
- 20.Jewell EL, Huang JJ, Abu-Rustum NR, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malingnancies. Gynecol Oncol. 2014;133:274–277. doi: 10.1016/j.ygyno.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plante M, Touhami O, Trihn XB, Renaud MC, Sebastianelli A, Grondin K, Gregoire J. Sentinel node mapping with indocyanine green and endoscopic near infrared fluorescence imaging in endometrial cancer. A pilot study and review of the literature. Gynecol Oncol. 2015;137:443–447. doi: 10.1016/j.ygyno.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Todo Y, Kato H, Okamoto K, Minobe S, Yamashiro K, Sakuragi N. Isolated tumor cells and micrometastases in regional lymph nodes in FIGO stage I to II endometrial cancer. J Gynecol Oncol. 2015 doi: 10.3802/jgo.2016.27.e1. epub ahead of print April 17, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touhami O, Trihn XB, Gregoire J, Sebastianelli A, Renaud MC, Grondin K, Plante M. Predictors of non-sentinel lymph node (non-SLN) metastasis in patients with sentinel lymph nodes (SLN) metastasis in endometrial cancer. Gynecol Oncol. 2015;138:41–45. doi: 10.1016/j.ygyno.2015.04.008. [DOI] [PubMed] [Google Scholar]