Abstract
Aims
To determine if associations of alcohol consumption with all-cause mortality replicate in discordant monozygotic twin comparisons that control for familial and genetic confounds.
Design
A 30-year prospective follow-up.
Setting
Population-based older Finnish twin cohort.
Participants
Same-sex twins, aged 24–60 years at the end of 1981, without overt comorbidities, completed questionnaires in 1975 and 1981 with response rates of 89 and 84%. A total of 15 607 twins were available for mortality follow-up from the date of returned 1981 questionnaires to 31 December 2011; 14 787 twins with complete information were analysed.
Measurements
Self-reported monthly alcohol consumption, heavy drinking occasions (HDO) and alcohol-induced blackouts. Adjustments for age, gender, marital and smoking status, physical activity, obesity, education and social class.
Findings
Among twins as individuals, high levels of monthly alcohol consumption (≥ 259 g/month) associated with earlier mortality [hazard ratio (HR) = 1.63, 95% confidence interval (CI) = 1.47–1.81]. That association was replicated in comparisons of all informatively drinking-discordant twin pairs (HR = 1.91, 95% CI = 1.49–2.45) and within discordant monozygotic (MZ) twin pairs (HR = 2.24, 95% CI = 1.31–3.85), with comparable effect size. Smaller samples of MZ twins discordant for HDO and blackouts limited power; a significant association with mortality was found for multiple blackouts (HR = 2.82, 95% CI = 1.30–6.08), but not for HDO.
Conclusions
The associations of high levels of monthly alcohol consumption and alcohol-induced blackouts with increased all-cause mortality among Finnish twins cannot be explained by familial or genetic confounds; the explanation appears to be causal.
Keywords: Alcohol drinking, alcoholic intoxication, binge drinking, causality, confounding factors, follow-up studies, mortality, twins
INTRODUCTION
The global burden of morbidity and mortality attributable to alcohol consumption is documented [1–7], and sustained consumption of elevated levels of alcohol is linked to increased all-cause mortality [8,9]. Patterns of consumption, as well as average volume, are important [5,10,11], as reports from Finland [12,13] and elsewhere [14–17] associate binge drinking with earlier mortality.
However, apart from brief interventions for heavy alcohol users [18,19], research relating elevated alcohol-exposure to earlier mortality rests upon observational studies [5] with inadequate control for familial and genetic confounds.
These confounds are well documented in associations of alcohol use/abuse [20–22]. Variation in household environments, familial risks and genetic background contributes to both alcohol consumption and increased risk of early mortality; to evaluate causality in drinking-mortality associations, potential confounds from genetic liabilities and family history, familial status and structure, parental drinking and childhood neighbourhoods must be considered.
Informative research designs are required, and within-family comparisons of exposure-discordant twin pairs are especially informative [23]. In idealized fantasy experiments, individuals serve as their own controls; their outcomes observed when exposed, and at the same time, when not. Exposure-discordant monozygotic (MZ) twin pairs offer a feasible, yet powerful approximation. MZ twins are genetically identical at the sequence level, while dizygotic (DZ) twins share, on average, half their segregating genes. Nearly all twin siblings share their familial and neighbourhood environments into adolescence, when drinking is typically initiated. If associations between alcohol exposure and mortality are causal, within-family differences observed in discordant twin siblings will approach the between-family differences found among singletons from unrelated families.
In the absence of familial/genetic confounds, associations among exposure-discordant MZ twin pairs will show no attenuation of effect, while in the presence of complete confounding by genetic and familial background factors associations among discordant MZ pairs will disappear. Accordingly, our analyses focus upon comparisons of drinking-discordant MZ twin pairs [23–25].
Two earlier research reports associated drinking with mortality in drinking-discordant twins; each had limitations. One [26] lacked sufficient statistical power; the other [27] excluded heavy drinkers, comparing only moderate drinkers with abstainers.
We extended the Finnish twin sample by Kujala et al. [26], adding a decade of follow-up to gain cases and enhance power. Our analyses ask: will associations of increased all-cause mortality with (1) high levels of monthly alcohol consumption, (2) heavy drinking occasions (HDO) and (3) alcohol-induced blackouts replicate in paired comparisons of exposure-discordant monozygotic twin pairs that control for confounds from both shared familial environments and shared genes?
METHODS
Sample
The population-based Older Finnish Twin Cohort [28] was sent mailed questionnaires in 1975 and 1981, with response rates of 89% (1975) and 84% (1981). We studied same-sex twins aged 24–60 years at the end of 1981 (mean age 35.9 years), and then free of chronic diseases based on their questionnaires and medical register information as of 1 January 1983 [26]. Zygosity was determined by validated questionnaire methods [29]. Procedures were approved by Finland's National Board of Health and the Ethics Committee, University of Helsinki; participating twins were informed of record linkages to Finnish health registries and advised that they could withdraw. No financial incentives were provided. A total of 15 607 twins were available for mortality follow-up.
Assessment of alcohol exposure
The 1975 and 1981 questionnaires assessed average weekly consumption of beer (as bottles) and wine (as glasses) and monthly consumption of spirits (as bottles). Responses were converted [30] to monthly alcohol consumption with one drink (a 330-ml bottle of beer, a 12-cl glass of wine or a 4-cl portion of spirits) estimated to include 12 g of pure alcohol, and the mean from the two questionnaires was obtained.
HDO were defined as consuming, ‘at least once a month and on a single occasion’, more than five beers, a bottle of wine or a half-bottle of spirits (or a similar amount), to correspond to > five standard drinks or > 60 g of pure alcohol on a single occasion. Responses formed three categories: no HDO (neither in 1975 nor 1981), non-persistent HDO (either 1975 or 1981) and persistent HDO (both 1975 and 1981).
Blackouts, assessed only in the 1981 questionnaire, were defined by the frequency of alcohol-induced loss of consciousness (in Finnish: sammuminen) during the preceding year. Three response categories were formed for analysis: no blackouts in the past year, one, or two or more.
Potential confounders
Cigarette smoking status [31] was recorded in nine categories: never-smoker, ex-smoker, occasional smoker or a current smoker smoking fewer than five cigarettes per day and current smokers smoking five to nine, 10–14, 15–19, 20–24, 25–39 and ≥ 40 cigarettes per day.
Vigorous leisure-time physical activity [26], such as strenuous as ‘alternately walking and jogging’, yielded a three-category variable: persistently non-active (in both 1975 and 1981), non-persistently active and persistently active (in both 1975 and 1981).
Life satisfaction was assessed with four self-evaluations [32] of life ‘at present’ as ‘interesting/boring’, ‘happy/sad’, ‘easy/hard’ and ‘lonely/not’, yielding a range from four to 20, grouped into three categories.
Obesity [33] was defined as body mass index (body mass/length2) of ≥ 30 kg/m2 from self-reported weight and height.
Educational level was categorized from self-reports approximating primary, secondary and tertiary levels of education [34].
Social class formed six categories [35]: (1) upper white-collar workers and comparable entrepreneurs, (2) lower white-collar workers and small entrepreneurs, (3) skilled workers, (4) non-skilled workers, (5) farmers and fishermen and (6) unknown, including students, homemakers and pensioners.
Marital status [36] was categorized dichotomously: those single, divorced or separated and those married, divorced and remarried, cohabiting or widowed.
Information on educational level, life satisfaction, social class and marital status came from 1975 questionnaires; that for smoking habits and obesity was from 1981 questionnaires. Both questionnaires provided information on physical activity. Table 1 presents distributional data on potential confounders.
Table 1.
Distribution of drinking patterns and potential confounders from 1975 and 1981 surveys in the Older Finnish Twin Cohort among subjects with complete data, who were healthy and aged 24–60 years at the end of 1981; n = 14 787 individual twins.
| Men | Women | Total | % | ||
|---|---|---|---|---|---|
| Monthly alcohol consumption in grams | |||||
| 0 | 376 | 958 | 1334 | 9.0 | |
| 1–69 | 671 | 1885 | 2556 | 17.3 | |
| 70–139 | 1173 | 2273 | 3446 | 23.3 | |
| 140–209 | 1144 | 1104 | 2248 | 15.2 | |
| 210–419 | 1610 | 787 | 2397 | 16.2 | |
| 420–839 | 1573 | 317 | 1890 | 12.8 | |
| 840–1199 | 487 | 35 | 522 | 3.5 | |
| ≥ 1200 | 369 | 25 | 394 | 2.7 | |
| 0–258 | 3811 | 6502 | 10 313 | 69.7 | |
| ≥ 259 | 3592 | 882 | 4474 | 30.3 | |
| Heavy drinking occasions | |||||
| None | 3333 | 6222 | 9555 | 64.6 | |
| 1975 or 1981 | 1720 | 814 | 2534 | 17.1 | |
| 1975 and 1981 | 2350 | 348 | 2698 | 18.2 | |
| Alcohol-induced blackouts | |||||
| 0 | 5732 | 6892 | 12 624 | 85.4 | |
| 1 | 700 | 319 | 1019 | 6.9 | |
| ≥ 2 | 971 | 173 | 1144 | 7.7 | |
| Cigarette smoking status | |||||
| Never-smoker | 2425 | 4364 | 6789 | 45.9 | |
| Ex-smoker | 1905 | 1213 | 3118 | 21.1 | |
| Occasional smokera | 473 | 390 | 863 | 5.8 | |
| 5–9 cigarettes per day | 277 | 434 | 711 | 4.8 | |
| 10–14 cigarettes per day | 500 | 446 | 946 | 6.4 | |
| 15–19 cigarettes per day | 715 | 290 | 1005 | 6.8 | |
| 20–24 cigarettes per day | 772 | 192 | 964 | 6.5 | |
| 25–39 cigarettes per day | 296 | 52 | 348 | 2.4 | |
| ≥ 40 cigarettes per day | 40 | 3 | 43 | 0.3 | |
| Vigorous physical activity | |||||
| Non-active both 1975 and 1981 | 1822 | 2767 | 4589 | 31.0 | |
| Active either 1975 or 1981 | 2132 | 2427 | 4559 | 30.8 | |
| Active both 1975 and 1981 | 3449 | 2190 | 5639 | 38.1 | |
| Obesity | |||||
| BMI < 30 kg/m2 | 7146 | 7168 | 14 314 | 96.8 | |
| BMI ≥ 30 kg/m2 | 257 | 216 | 473 | 3.2 | |
| Educational level | |||||
| Primary | 5209 | 4567 | 9776 | 66.1 | |
| Secondary | 1262 | 1705 | 2967 | 20.1 | |
| Tertiary | 932 | 1112 | 2044 | 13.8 | |
| Social class | |||||
| (1) Upper white-collar | 713 | 371 | 1084 | 7.3 | |
| (2) Lower white-collar | 1484 | 2568 | 4052 | 27.4 | |
| (3) Skilled workers | 3516 | 2245 | 5761 | 39.0 | |
| (4) Non-skilled workers | 589 | 814 | 1403 | 9.5 | |
| (5) Farmers and fishermen | 508 | 450 | 958 | 6.5 | |
| (6) Unknown | 593 | 936 | 1529 | 10.3 | |
| Marital status | |||||
| Marriedb | 4202 | 4270 | 8472 | 57.3 | |
| Singlec | 3201 | 3114 | 6315 | 42.7 |
Monthly alcohol consumption is the mean of the 1975 and 1981 reports. Heavy drinking occasions, 1975 and 1981, were classified as persistent (1975 and 1981), non-persistent (1975 or 1981) or none. Alcohol-induced blackouts were reported only in 1981 in five categories but classified as none, one or ≥ 2. Cigarette smoking status in 1981.
Occasional smokers include current smokers smoking fewer than five cigarettes per day.
BMI = body mass index (body mass/length2) in 1981. Educational level in 1975, approximating primary, secondary and tertiary levels of education. Social class in 1975: (1) upper white-collar workers and comparable entrepreneurs, (2) lower white-collar workers and small entrepreneurs, (3) skilled workers, (4) non-skilled workers, (5) farmers and fishermen and (6) unknown, including students, homemakers and pensioners. Marital status in 1975.
One category comprises married, divorced and remarried, cohabiting or widowed.
The other comprises those single, divorced or separated.
Mortality follow-up
Follow-up of all-cause mortality began on the date of returned 1981 questionnaires and continued to 31 December 2011; median follow-up duration was 30.2 years (range 0.005–30.2), with end of follow-up mean age 64.6 years. Dates of death came from the Population Register Center of Finland; the final update was made on 29 February 2012.
Statistical analyses
Statistical analyses were performed with Stata software (release 12.1; Stata Corporation, College Station, TX, USA). All results were age-adjusted using time since birth as the time-scale in survival models and pairwise twin comparisons. Individual-based all-cause mortality risks were calculated by Cox proportional hazards models. In pairwise comparisons, the same models were stratified by family. In initial model-building [37,38], smoking status, sex, obesity, physical activity, social class, educational level, marital status and life satisfaction were included as potential confounders. In likelihood-ratio tests, all except life satisfaction yielded P < 0.10 to be retained in subsequent models. Secondly, we tested for interactions. Sex yielded no statistically significant interactions, so to enhance statistical power we analysed men and women together with sex as a covariate. Thirdly, the validity of the models was assessed with scaled Schoenfeld residuals, link test and graphical methods. Non-independence of observations within pairs was adjusted to yield appropriate confidence intervals (CI) [39]. All hazard ratios (HR) were adjusted for age, sex, smoking status, physical activity, obesity, educational level, social class and marital status. Tabular results also report the crude hazard ratios, adjusted only for age and sex. All confidence intervals and P-values were calculated at the 95% confidence level; all P-values are two-sided.
In supplementary analyses, the measures of HDO and blackouts were adjusted for monthly alcohol consumption, using eight categories ranging from persistent abstainers to those drinking, on average, ≥ 1200 g/month.
RESULTS
Follow-up sample
Complete data were available on 14 787 twins forming 4998 same-sex twin pairs (1569 monozygotic pairs, 3130 dizygotic pairs, 299 pairs of uncertain zygosity) and 4791 individual twins without data on co-twins. There were 421 153 person-years with 2203 deaths. Twins who moved abroad during follow-up (0.7%) were censored on the moving date. At the end of follow-up, 12 478 twins were alive and not censored.
We report results for different criteria of drinking discordance and for the full sample of all informatively discordant twins (including twins with unknown zygosity) and then, separately, for discordant MZ pairs contained within that full sample.
Descriptive results of drinking patterns
Nearly all twins (91.0%) reported some alcohol use, but there was wide variation in levels and patterns of drinking (Table 1). Abstinence was significantly less common among men than women and heavy drinking patterns were much more common among men. Drinking patterns were more concordant among monozygotic than dizygotic twin pairs. Co-twins in ~72% of all DZ pairs were discordant for their membership in the eight categories of monthly consumption levels shown in Table 1, more than a third (36.2%) were discordant for the three-level categorization of HDO and a fifth (21.8%) for categories of blackouts. Among MZ pairs, all corresponding percentages were significantly lower: 59.0, 28.7 and 18.6%, respectively.
Monthly alcohol consumption
We first studied monthly consumption levels in twins as individuals. The magnitude of between-family associations of monthly consumption with mortality offers the comparative standard for evaluating familial confounds from results obtained with discordant twins [23]. Individual-level results are shown in the upper portion of Table 2. Consumption levels for twins as individuals were dichotomized at the mean (258 g/month) of the full sample distribution, and associations with mortality were compared for individual twins at or below that mean to those above it. Given the highly skewed distribution of individual consumption levels, dichotomizing at the mean effectively split the sample at the 70th percentile for individual-level analyses, identifying 4474 highly exposed people from all 14 787 individual twins. The results were that 995 of the 4474 twins drinking above the overall mean (≥ 259 g/month) had died on follow-up (22.2%), compared to a lower mortality rate (11.7%) of twins drinking at or below that mean level (1208 deaths among 10 313 individuals). Adjusted for age and gender, the hazard ratio was twofold; further adjustment (for marital and smoking status, physical activity, obesity, education and social class) reduced that HR to 1.63, but it remained statistically highly significant (P = 2.2 × 10–20), with narrow 95% confidence intervals (1.47–1.81). This result, from a population-based sample of individual twins, replicates extensive previous research findings with non-twin singletons [4,8]. Consistent with earlier research, results from using the eight-level categorization of consumption levels shown in Table 1, available in Supporting information (Table S4), reveal a graded effect with hazard ratios increasing above 210 g/month and elevated substantially above consumption levels of 420 g/month.
Table 2.
Hazard ratios for all-cause mortality for level of monthly alcohol consumption in the Older Finnish Twin Cohort, defined by cutting the mean consumption reported in the 1975 and 1981 questionnaires at full sample mean (258 g/month). Those drinking at or below mean form the reference category.
| Individual-level analyses | |||||||
|---|---|---|---|---|---|---|---|
|
n Individuals
|
n Deaths
|
Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |||
| ≤ 258 g | ≥ 259 g | ≤ 258 g | ≥ 259 g | ||||
| 10313 | 4474 | 1208 | 995 | 2.03 (1.84–2.23) | 1.63 (1.47–1.81) | 2.2 × 10–20 | |
| Discordant twin analyses | ||||||
|---|---|---|---|---|---|---|
| Drinking-discordant pairs | Death-informative pairs | Drinking twin died first | Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |
| Full sample | 1227 | 320 | 217 | 2.11 (1.67–2.66) | 1.91 (1.49–2.45) | 2.7 × 10–7 |
| MZ sample | 309 | 72 | 50 | 2.27 (1.38–3.75) | 2.24 (1.31–3.85) | 0.0034 |
CI = confidence interval; HR = hazard ratio; MZ = monozygotic; drinking-discordant pairs = pairs where one co-twin drinks ≤ 258 g/month and the other one drinks ≥ 259 g/month; death-informative pairs = pairs where at least one of the twins died during follow-up; drinking twin = co-twin drinking ≥ 259 g/month; crude HR, adjusted for age and gender; adjusted HR and P-value = adjusted for age, gender, marital and smoking status, physical activity, obesity, education and social class; follow-up from 1981 to 2011.
We employed the same definition of drinking discordance described for individual twins to identify discordant pairs. Pairs where at least one of the twins died during follow-up (and the other co-twin was not censored at the time of the first death) were death-informative. A subset of 1227 twin pairs (24.5% of our sample) was identified as discordant for monthly consumption levels: one twin reported monthly consumption at or below 258 g/month, while the co-twin reported a level above that cut-off (≥ 259 g/month). Among them, 320 pairs were also death-informative, and in 217 of these 320 pairs the co-twin who had died first was the twin reporting a more elevated level of monthly consumption. The adjusted HR was 1.91 (95% CI = 1.49–2.45). One in four (309) of all twin pairs discordant for level of monthly consumption were monozygotic pairs, and 72 of these were death-informative. As Table 2 shows, in 50 of these 72 MZ twin pairs the co-twin to die first was the heavier-drinking twin. The smaller sample of MZ twins informatively discordant for both monthly drinking and mortality inflated CIs for the adjusted HR of 2.24, 95% CI = 1.31–3.85, but it was statistically significant, and the magnitude of effect was as large as that found for the full sample of discordant twins and not different from that observed in between-family analyses of all twins as individuals.
However, cutting at the mean level of consumption is not the only way to define discordance. Any dichotomy of the entire sample based on a single cutting score, as this was, will identify some pairs as ‘discordant’ in which the co-twins have a negligible difference in drinking—a small difference placing one twin at, or just below, the mean and the co-twin just above it. An alternative approach, specific to twin comparisons [25], is to employ intrapair difference scores and define discordance by a specified minimal intrapair difference in consumption levels, regardless of the actual level reported by either twin. Table 3 reports such a discordant twin analysis. Here, we defined discordance by an intrapair difference of ≥ 82 g/month—the median within-pair difference observed across all twin pairs. That definition yielded a drinking-discordant sample of nearly 2500 pairs, including 653 MZ pairs. The adjusted HR for the full sample was 1.60 (95% CI = 1.33–1.92); the HR for the smaller subsample of discordant MZs was nearly identical (1.62), but with wider confidence intervals (95% CI = 1.12–2.34). A less stringent cut-off used intrapair differences ≥ 50 g/month, corresponding to a difference of one standard drink per week; a sample of 3105 drinking-discordant twin pairs formed, including 738 death-informative pairs. Results were statistically significant both for the full sample and for the subsample of MZ twins; in 120 of the 193 informatively discordant MZ twin pairs, the heavier-drinking twin was the first to die (P = 0.0033, Supporting information, Table S1).
Table 3.
Hazard ratios for all-cause mortality for twin pairs with an intrapair difference of ≥ 82 g in monthly alcohol consumption regardless of level of consumption of either co-twin in the Older Finnish Twin Cohort. Co-twins drinking less form the reference category.
| Discordant twin analyses | ||||||
|---|---|---|---|---|---|---|
| Drinking-discordant pairs | Death-informative pairs | Drinking twin died first | Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |
| Full sample | 2499 | 633 | 401 | 1.73 (1.47–2.03) | 1.60 (1.33–1.92) | 6.6 × 10–7 |
| MZ sample | 653 | 159 | 99 | 1.65 (1.20–2.27) | 1.62 (1.12–2.34) | 0.011 |
CI = confidence interval; HR = hazard ratio; MZ = monozygotic; drinking-discordant pairs = pairs with an intra-pair difference of ≥ 82 g in monthly alcohol consumption; death-informative pairs = pairs where at least one of the twins died during follow-up; drinking twin = co-twin drinking ≥ 82 g/month in excess of his/her co-twin; crude HR = adjusted for age and gender; adjusted HR and P-value = adjusted for age, gender, marital and smoking status, physical activity, obesity, education and social class; alcohol consumption defined as mean of the consumptions reported in the 1975 and 1981 questionnaires; follow-up from 1981 to 2011.
Finally, the graded nature of hazard ratios identified for individual twins replicated both in the full sample and the MZ sample of twin pairs discordant for their membership in the eight categories of monthly consumption (Supporting information, Table S4).
Heavy drinking occasions
Our individual-level analyses (Table 4) compared mortality among 2698 individual twins who reported heavy drinking occasions in both the 1975 and 1981 questionnaires to the larger subsample who reported no HDO in either questionnaire. Twins reporting persistent HDO had significantly earlier mortality (adjusted HR = 1.68, 95% CI = 1.49–1.91) compared to twins reporting no heavy drinking occasions on either questionnaire.
Table 4.
Hazard ratios for all-cause mortality for persistent heavy drinking occasions versus no heavy drinking occasions in 1975 and 1981, defined as drinking more than five standard drinks on a single occasion at least monthly, in the Older Finnish Twin Cohort. Those reporting no heavy drinking occasions form the reference category.
| Individual-level analyses | |||||||
|---|---|---|---|---|---|---|---|
| n Individuals |
n Deaths |
Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |||
| no HDO | persistent HDO | no HDO | persistent HDO | ||||
| 9555 | 2698 | 1187 | 623 | 2.42 (2.16–2.71) | 1.68 (1.49–1.91) | 2.9 × 10–16 | |
| Discordant twin analyses | ||||||
|---|---|---|---|---|---|---|
| Drinking-discordant pairs | Death-informative pairs | Drinking twin died first | Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |
| Full sample | 458 | 148 | 108 | 2.70 (1.88–3.88) | 2.20 (1.49–3.26) | 7.9 × 10–5 |
| MZ sample | 99 | 30 | 20 | 2.00 (0.94–4.27) | 1.40 (0.60–3.23) | 0.43 |
CI = confidence interval; HDO = heavy drinking occasions; no HDO = no HDO neither 1975 nor 1981; persistent HDO = HDO both 1975 and 1981; HR = hazard ratio; MZ = monozygotic; drinking-discordant pairs = pairs where one co-twin has no HDO and the other one has persistent HDO; death-informative pairs = pairs where at least one of the twins died during follow-up; drinking twin = co-twin having persistent HDO; crude HR = adjusted for age and gender; adjusted HR and P-value = adjusted for age, gender, marital and smoking status, physical activity, obesity, education and social class; follow-up from 1981 to 2011.
Results from pairwise analyses of this dichotomous definition of persistent discordance for HDO are shown in the lower portion of Table 4: HRs obtained from the full twin sample mirrored corresponding HRs found in individual-based analyses. The adjusted HR for persistent HDO (heavy drinking occasions reported on both surveys versus none on either) was significant for all 148 informative twin pairs (HR = 2.20, 95% CI = 1.49–3.26), but only 30 of these doubly discordant pairs were MZs, and results from this small subsample failed to reach statistical significance.
Alcohol-induced blackouts
We classified all individual twins for alcohol-induced blackouts dichotomously, forming two groups, one from twins reporting no blackouts during the preceding year and the other comprising those who reported two or more. Table 5 (first row of numerical entries) presents results; deaths among individual twins who reported ≥ two blackouts (333 of 1144) doubled the rate for twins reporting no blackouts to yield an adjusted hazard ratio of 1.90 (95% CI = 1.66–2.16). Using that dichotomous definition of discordance, 425 blackout-discordant twin pairs were identified, 146 of whom were death-informative; in 112 of these informative pairs, the twin reporting multiple blackouts had died first: adjusted HR of 2.98 (95% CI = 1.99–4.47). The subset of MZ twins doubly informative for blackouts and death was limited to 40 pairs, but in 30 of them the co-twin reporting multiple blackouts had died first, yielding an adjusted HRof 2.82. The magnitude of effect was not attenuated from that found for the full twin sample, although with inflated confidence intervals (95% CI = 1.30–6.08).
Table 5.
Hazard ratios for all-cause mortality for alcohol-induced blackouts in 1981 in the Older Finnish Twin Cohort, dichotomously defined as none versus ≥ 2 during the past year. Those reporting no blackouts form the reference category
| Individual-level analyses | |||||||
|---|---|---|---|---|---|---|---|
| n Individuals |
n Deaths |
Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |||
| No blackouts | ≥ 2 Blackouts | No blackouts | ≥ 2 Blackouts | ||||
| 12 624 | 1144 | 1686 | 333 | 2.61 (2.30–2.97) | 1.90 (1.66–2.16) | 1. × 10–21 | |
| Discordant twin analyses | ||||||
|---|---|---|---|---|---|---|
| Drinking-discordant pairs | Death-informative pairs | Drinking twin died first | Crude HR (95% CI) | Adjusted HR (95% CI) | Adjusted P-value | |
| Full sample | 425 | 146 | 112 | 3.29 (2.24–4.84) | 2.98 (1.99–4.47) | 1.2 × 10–7 |
| MZ sample | 115 | 40 | 30 | 3.00 (1.47–6.14) | 2.82 (1.30–6.08) | 0.0084 |
CI = confidence interval; HR = hazard ratio; MZ = monozygotic; drinking-discordant pairs = pairs where one co-twin has no alcohol-induced blackouts during the past year and the other one has ≥ 2 alcohol-induced blackouts; death-informative pairs = pairs where at least one of the twins died during follow-up; drinking twin = co-twin having ≥ 2 blackouts during the past year; crude HR = adjusted for age and gender; adjusted HR and P-value = adjusted for age, gender, marital and smoking status, physical activity, obesity, education and social class; follow-up from 1981 to 2011.
The Supporting information provides results from analyses of HDO and blackouts that use all three categories (and all three different types of discordant pairs) of these drinking patterns (Supporting information, Tables S2 and S3). No HDO or no blackouts form the reference categories, the other two being non-persistent and persistent HDO; and one and ≥ two blackouts. Results parallel those from dichotomous definitions of discordance while providing evidence of gradient effects.
Excluding abstainers
All analyses reported above included twins reporting abstinence on both questionnaires. To evaluate effects of their exclusion, we deleted all persistently abstinent twins (1334 individuals); that deletion removed six MZ pairs from discordant pairwise comparisons for monthly consumption, and two MZ pairs from comparisons of HDO and blackouts. Effects of these modest deletions were negligible.
DISCUSSION
During a 30-year follow-up, we focused on drinking-discordant twin pairs to evaluate whether associations of drinking with all-cause mortality are due to familial/genetic confounds. Discordance was assessed for three measures of alcohol exposure: monthly consumption levels and heavy drinking occasions, both reported on two questionnaires administered 6 years apart and alcohol-induced blackouts reported in the second questionnaire.
The results permit three conclusions. First, analyses of twins as individuals confirmed significant associations of all three drinking measures with mortality; these results replicate earlier research with non-twins [4,8,12–17] and provide benchmark effects with which to compare results from drinking-discordant twins within our sample. Secondly, all associations found in individual-level analyses replicated robustly in our full samples of twin pairs discordant for each drinking measure (Fig. 1). Those results provide evidence that familial confounds cannot account fully for associations of heavy drinking with earlier mortality. Thirdly, associations of mortality with higher levels of monthly consumption, established in earlier between-family research, replicated within samples of drinking-discordant monozygotic pairs with little or no attenuation of effect. These results, the first of their kind, were found across different definitions of discordance for monthly consumption. They are informative findings, because they are fully consistent with an inference that the association of higher levels of consumption with mortality is causal in nature.
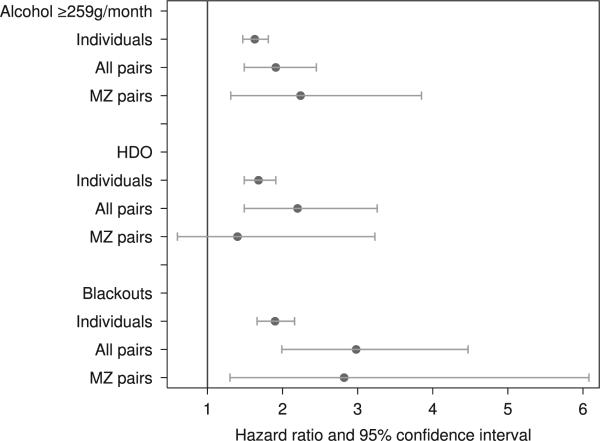
Figure 1.
Comparison of individual-based and pairwise hazard ratios for all-cause mortality for dichotomized measures of alcohol consumption in the Older Finnish Twin Cohort. MZ = monozygotic; alcohol ≥ 259 g/month = those reporting an average consumption of ≥ 259 g/month in the 1975 and 1981 questionnaires compared to those reporting ≤ 258 g/month; HDO = persistent heavy drinking occasions (heavy drinking occasions both 1975 and 1981) compared to no heavy drinking occasions; blackouts = ≥ 2 alcohol-induced blackouts during the past year in 1981 compared to no alcohol-induced blackouts during the past year; hazard ratios and confidence intervals adjusted for age, gender, marital and smoking status, physical activity, obesity, education and social class; follow-up from 1981 to 2011
Nor are these results dependent upon definitions of discordant drinking. Whether defined in an exposed/non-exposed dichotomy with a cut-off at the 70th percentile of consumption levels reported by the full sample of individual twins, or as intrapair differences greater than the median difference across all pairs, higher levels of monthly consumption associated with earlier mortality in comparisons of all discordant twin pairs and also in subsets of discordant monozygotic twin pairs.
Discordance for alcohol-induced blackouts, defined dichotomously (no blackouts versus two or more during the preceding year), also associated predictively with earlier mortality across all comparisons: in twins as individuals, in all discordant twin pairs and in the subsample of discordant MZ twins. Again, hazard ratios were not attenuated by controlling for familial/genetic confounds in within-family comparisons of drinking-discordant twins.
Our measure of heavy drinking occasions yielded no consistent association with mortality, but the sample of drinking-discordant and death-informative MZ twins for HDO was the smallest of any studied, and discordance for HDO, as we measured and defined it categorically, was insensitive to individual differences in the frequency of heavy drinking occasions. However, consistent with pairwise analyses of monthly consumption and blackouts, the difference in hazard ratios between MZ pairs and the rest of the pairs was not statistically significant.
An earlier report from our cohort [26] found comparable results for HDO, but a significant difference between MZ and DZ pairs was found. Another study [27] found increased mortality among abstainers, albeit without statistical significance, and in a small subset of the same cohort alcohol consumption was not associated with all-cause mortality [40].
Our analyses have strengths and limitations. Measurement issues are one limitation. All drinking measures were self-reported, and the precision with which different measures were assessed varied widely. Multiple questions on frequency/quantity of drinking beer, wine and spirits, each with alternative responses and reported on two occasions, permitted quasi-continuous assessment of individual differences in monthly consumption. Assessment of HDO and blackouts, in contrast, were limited. The measure of HDO was insensitive to its frequency, and dichotomous categorization of both HDO and blackouts ignored data from twins in middle categories of either. The level of consumption contributed substantially to the individual variation assessed by our measure of HDO; adjusting HDO for categories of consumption reduced its associations with mortality considerably (Supporting information, Tables S5 and S6). Blackouts were less affected (Supporting information, Tables S7 and S8), but some caution on the stability of our measure of blackouts is warranted, as the hazard associated with blackouts diminished towards the end of the follow-up period, and among the few non-smokers with blackouts the hazard ratios were increased.
Finnish drinking patterns are typical of Nordic cultures but differ from many others, and we caution generalization of our results; cultural contexts may modify effects. Our data did not permit separate analyses by gender, but gender differences in long-term associations of alcohol-exposure probably exist. Also, although our twin sample was large, MZ twin pairs discordant for heavy drinking occasions and alcohol-induced blackouts were of modest size. Co-twin concordance for alcohol exposure and life-course outcomes inevitably constrain the number of informatively discordant twin pairs identified [41].
Acknowledging these limitations, we emphasize our study's unusual strengths. These include the population-based twin sample, lengthy duration of follow-up and the power of the discordant twin design. Our results offer convincing evidence that drinking-mortality associations cannot be explained away by between-family confounds. Our results from drinking-discordant monozygotic twins are consistent with a causal explanation for the association of high levels of monthly alcohol consumption and alcohol-induced blackouts with earlier mortality. That novel finding should stimulate efforts to establish more definitively its meaning and generality.
Supplementary Material
Acknowledgements
We thank Janne Pitkäniemi PhD, Finnish Cancer Registry, for his consultation regarding the modelling of survival data, and Kauko Heikkilä PhLic, for data managing and software maintenance.
Declaration of interests
J.K. consulted for Pfizer from 2012 to 2014. J.K. was supported by the Academy of Finland (265240, 263278) and RJ.R. was supported by AA-12502. P.S. was supported by The Finnish Foundation for Alcohol Studies.
References
- 1.Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, et al. Alcohol use. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. World Health Organization; Geneva: 2004. pp. 959–1108. [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gowing LR, Ali RL, Allsop S, Marsden J, Turf EE, West R, et al. Global statistics on addictive behaviours: 2014 status report. Addiction. 2015;110:904–19. doi: 10.1111/add.12899. [DOI] [PubMed] [Google Scholar]
- 4.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 5.Rehm J, Baliunas D, Borges GLG, Graham K, Irving H, Kehoe T, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–43. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gawryszewski VP, Monteiro MG. Mortality from diseases, conditions and injuries where alcohol is a necessary cause in the Americas, 2007–09. Addiction. 2014;109:570–7. doi: 10.1111/add.12418. [DOI] [PubMed] [Google Scholar]
- 7.Rehm J, Dawson D, Frick U, Gmel G, Roerecke M, Shield KD, et al. Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res. 2014;38:1068–77. doi: 10.1111/acer.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayasekara H, English DR, Room R, MacInnis RJ. Alcohol consumption over time and risk of death: a systematic review and meta-analysis. Am J Epidemiol. 2014;179:1049–59. doi: 10.1093/aje/kwu028. [DOI] [PubMed] [Google Scholar]
- 9.Roerecke M, Rehm J. Alcohol use disorders and mortality: a systematic review and meta-analysis. Addiction. 2013;108:1562–78. doi: 10.1111/add.12231. [DOI] [PubMed] [Google Scholar]
- 10.Gmel G, Kuntsche E, Rehm J. Risky single-occasion drinking: bingeing is not bingeing. Addiction. 2011;106:1037–45. doi: 10.1111/j.1360-0443.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- 11.Naimi TS, Xuan Z, Brown DW, Saitz R. Confounding and studies of ‘moderate’ alcohol consumption: the case of drinking frequency and implications for low-risk drinking guidelines. Addiction. 2013;108:1534–43. doi: 10.1111/j.1360-0443.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 12.Kauhanen J, Kaplan GA, Goldberg DE, Salonen JT. Beer binging and mortality: results from the Kuopio ischaemic heart disease risk factor study, a prospective population based study. BMJ. 1997;315:846–51. doi: 10.1136/bmj.315.7112.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laatikainen T, Manninen L, Poikolainen K, Vartiainen E. Increased mortality related to heavy alcohol intake pattern. J Epidemiol Community Health. 2003;57:379–84. doi: 10.1136/jech.57.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehm J, Greenfield TK, Rogers JD. Average volume of alcohol consumption, patterns of drinking, and all-cause mortality: results from the US National Alcohol Survey. Am J Epidemiol. 2001;153:64–71. doi: 10.1093/aje/153.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Holahan CJ, Schutte KK, Brennan PL, Holahan CK, Moos RH. Episodic heavy drinking and 20-year total mortality among late-life moderate drinkers. Alcohol Clin Exp Res. 2014;38:1432–8. doi: 10.1111/acer.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle SH, Mortensen L, Grønbaek M, Barefoot JC. Hostility, drinking pattern and mortality. Addiction. 2008;103:54–9. doi: 10.1111/j.1360-0443.2007.02024.x. [DOI] [PubMed] [Google Scholar]
- 17.Molokhia M, Nitsch D, Patrick AL, McKeigue P. 30 Year patterns of mortality in Tobago, West Indies, 1976–2005: impact of glucose intolerance and alcohol intake. PLOS ONE. 2011;6:e14588. doi: 10.1371/journal.pone.0014588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev. 2011;8:CD005191. doi: 10.1002/14651858.CD005191.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99:839–45. doi: 10.1111/j.1360-0443.2004.00778.x. [DOI] [PubMed] [Google Scholar]
- 20.Dick DM, Prescott C, McGue M. The genetics of substance use and substance use disorders. In: Kim Y-K, editor. Handbook of Behavior Genetics. Springer New York; New York, NY: 2009. pp. 433–53. [Google Scholar]
- 21.Saraceno L, Munafó M, Heron J, Craddock N, van den Bree MBM. Genetic and non-genetic influences on the development of co-occurring alcohol problem use and internalizing symptomatology in adolescence: a review. Addiction. 2009;104:1100–21. doi: 10.1111/j.1360-0443.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- 22.McCambridge J, McAlaney J, Rowe R. Adult consequences of late adolescent alcohol consumption: a systematic review of cohort studies. PLOS Med. 2011;8:e1000413. doi: 10.1371/journal.pmed.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGue M, Osler M, Christensen K. Causal inference and observational research: the utility of twins. Perspect Psychol Sci J Assoc Psychol Sci. 2010;5:546–56. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rutter M. Proceeding from observed correlation to causal inference: the use of natural experiments. Perspect Psychol Sci. 2007;2:377–95. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 25.Rose RJ, Winter T, Viken RJ, Kaprio J. Adolescent alcohol abuse and adverse adult outcomes: evaluating confounds with drinking-discordant twins. Alcohol Clin Exp Res. 2014;38:2314–21. doi: 10.1111/acer.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all-cause mortality: the roles of genetics and childhood environment. Am J Epidemiol. 2002;156:985–93. doi: 10.1093/aje/kwf151. [DOI] [PubMed] [Google Scholar]
- 27.Carmelli D, Swan GE, Page WF, Christian JC. World War II-veteran male twins who are discordant for alcohol consumption: 24-year mortality. Am J Public Health. 1995;85:99–101. doi: 10.2105/ajph.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaprio J, Sarna S, Koskenvuo M, Rantasalo I. The Finnish Twin Registry: formation and compilation, questionnaire study, zygosity determination procedures, and research program. Prog Clin Biol Res. 1978;24:179–84. [PubMed] [Google Scholar]
- 29.Sarna S, Kaprio J, Sistonen P, Koskenvuo M. Diagnosis of twin zygosity by mailed questionnaire. Hum Hered. 1978;28:241–54. doi: 10.1159/000152964. [DOI] [PubMed] [Google Scholar]
- 30.Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic influences on use and abuse of alcohol: a study of 5638 adult Finnish twin brothers. Alcohol Clin Exp Res. 1987;11:349–56. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 31.Kaprio J, Koskenvuo M. A prospective study of psychological and socioeconomic characteristics, health behavior and morbidity in cigarette smokers prior to quitting compared to persistent smokers and non-smokers. J Clin Epidemiol. 1988;41:139–50. doi: 10.1016/0895-4356(88)90088-1. [DOI] [PubMed] [Google Scholar]
- 32.Koivumaa-Honkanen H, Honkanen R, Viinamäki H, Heikkilä K, Kaprio J, Koskenvuo M. Self-reported life satisfaction and 20-year mortality in healthy Finnish adults. Am J Epidemiol. 2000;152:983–91. doi: 10.1093/aje/152.10.983. [DOI] [PubMed] [Google Scholar]
- 33.Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M. Alcohol consumption and body weight: a systematic review. Nutr Rev. 2011;69:419–31. doi: 10.1111/j.1753-4887.2011.00403.x. [DOI] [PubMed] [Google Scholar]
- 34.Latvala A, Dick DM, Tuulio-Henriksson A, Suvisaari J, Viken RJ, Rose RJ, et al. Genetic correlation and gene–environment interaction between alcohol problems and educational level in young adulthood. J Stud Alcohol Drugs. 2011;72:210–20. doi: 10.15288/jsad.2011.72.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Statistics Finland . Use Since 1971 (Material of Year 1971) [in Finnish] Department of Population Statistics, Statistics Finland; 1972. Alphabetical Index of Occupations and Grouping of Social Status. [Google Scholar]
- 36.Leonard KE, Rothbard JC. Alcohol and the marriage effect. J Stud Alcohol Suppl. 1999;13:139–46. doi: 10.15288/jsas.1999.s13.139. [DOI] [PubMed] [Google Scholar]
- 37.Greenland S, Rothman KJ. Introduction to Stratified Analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd edn. Lippincott Williams & Wilkins; Philadelphia, USA: 2008. pp. 258–82. [Google Scholar]
- 38.Statistical Computing Seminars [30 June 2011];Survival Analysis with Stata. UCLA: Statistical Consulting Group. Available at: http://www.ats.ucla.edu/stat/stata/seminars/stata_survival/ (Archived by WebCite® at http://www.webcitation.org/6WYPYl0ho)
- 39.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–6. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 40.Dai J, Mukamal KJ, Krasnow RE, Swan GE, Reed T. Higher usual alcohol consumption was associated with a lower 41-y mortality risk from coronary artery disease in men independent of genetic and common environmental factors: the prospective NHLBI Twin Study. Am J Clin Nutr. 2015;102:31–9. doi: 10.3945/ajcn.114.106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madsen M, Osler M. Commentary: strengths and limitations of the discordant twin-pair design in social epidemiology. Where do we go from here? Int J Epidemiol. 2009;38:1322–3. doi: 10.1093/ije/dyp264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.