Abstract
Limited options exist for efficiently and effectively treating water runoff from agricultural fields and landfills. Traditional treatments include excavation, transport to landfills, incineration, stabilization, and vitrification. In general, treatment options relying on biological methods such as bioremediation have the ability to be applied in situ and offer a sustainable remedial option with a lower environmental impact and reduced long-term operating expenses. These methods are generally considered ecologically friendly, particularly when compared to traditional physicochemical cleanup options. Phytoremediation, which relies on plants to take up and/or transform the contaminant of interest, is another alternative treatment method which has been developed. However, phytoremediation is not widely used, largely due to its low treatment efficiency. Endophytic phytoaugmentation is a variation on phytoremediation that relies on augmenting the phytoremediating plants with exogenous strains to stimulate associated plant-microbe interactions to facilitate and improve remediation efficiency. In this review, we offer a summary of the current knowledge as well as developments in endophytic phytoaugmentation and present some potential future applications for this technology. There has been a limited number of published endophytic phytoaugmentation case studies and much remains to be done to transition lab-scale results to field applications. Future research needs include large-scale endophytic phytoaugmentation experiments as well as the development of more exhaustive tools for monitoring plant-microbe-pollutant interactions.
Introduction
Pollutants associated with industrial and agricultural runoffs are of concern to human and ecological health as they can be challenging to efficiently and effectively treat. For example, wood treatment and petroleum wastes can contain high levels of carcinogenic polycyclic aromatic hydrocarbons (PAHs); livestock wastes contain high levels of nitrates associated with “baby blue syndrome” and algal blooms; and urban runoffs contain heavy metals such as zinc and lead that are known to be toxic to humans and animals.1–4 Plant-based remediation, or phytoremediation, directly uses plants and their associated microbes in situ for the stabilization or reduction of contaminants. Phytoremediation has been used for remediation in soil, sludge, sediment, surface water, and groundwater for a diverse range of contaminants.5–7 This wide remedial ability is owed in part to the multifarious levels of contaminant treatment (Fig. 1). Plants have the ability to control, degrade, or remove contaminants. Below-ground techniques rely on transformation, stabilization, or degradation stimulation. Once absorbed into the plant, transformation, stabilization or immobilization, and degradation can also occur. Methods of phytoremediation are thoroughly reviewed in Salt et al. and Ali et al.1,5
Fig. 1.
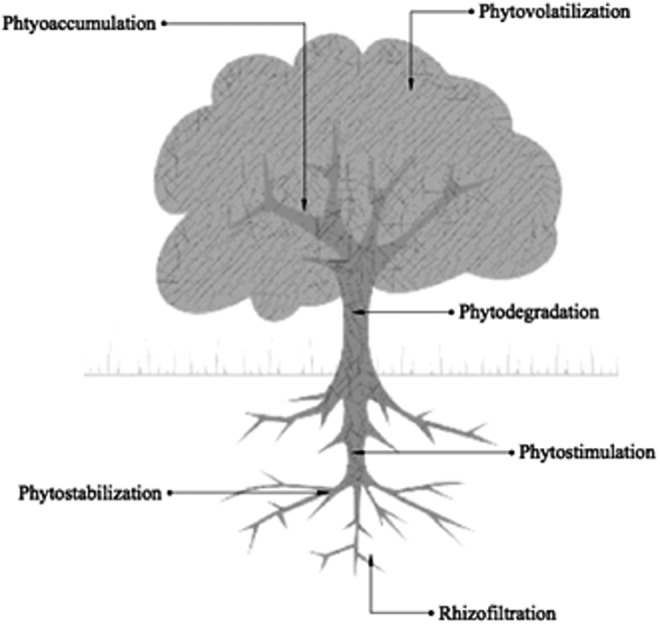
Methods of contaminant removal through phytoremediation. The driving force behind phytotechnology is the plant-microbe interactions. Plants and their associated microbial communities influence the control and degradation of contaminants.
Phytoremediation has been used to remediate numerous chemicals including metals, radionuclides, pesticides and herbicides, excessive nutrients, and organic pollutants.1,7–12 Depending on the location and desired treatment outcome, there are several types of phytoremediation planting schemes and applications that have been shown to be successful. The most common phytoremediation applications are riparian buffer strips, which consist of a strip of plantings along a wetland, stream, river, or lake, or a vegetation filter, which is used more commonly for managing municipal wastes and landfill leachates.13,14
Along with phytoremediation, in situ bioremediation is another in situ treatment option that is more ecologically friendly than traditional remediation technologies.15 Bioaugmentation is a common bioremediation strategy that consists of adding exogenous microorganisms such as endophytes to remediate contaminated sediments and soils. However, in this context, bioremediation may be ineffective or inefficient if the bioaugmented strain is unable to thrive under the specific physical site conditions and local microbial ecology. In endophytic phytoremediation, endophytes interact and exchange genes with both the rhizospheric and phyllospheric bacterial communities.7 In doing so, the overall microbial community develops degradation capacities without requiring survival of the donor strain. Thus, the combined use of endophytic augmentation and phytoremediation, or endophytic phytoaugmentation, may offer an effective option for in situ treatment of runoff and waste systems.
Endophytic phytoaugmentation is a promising area of research, with numerous direct and indirect benefits. For instance, endophytes are known to help the growth and health of various bioenergy- and biofiber-related crops, including poplars, willows, and cotton.6,16–20 Primary and secondary wood products from poplar and willow trees, including pulp and paper, lumber, veneer and plywood, composite panels, structural composite lumber and pallets, furniture, containers and utensils, and animal feed, are expected to increase.21 Furthermore, phytosystems also help aid in carbon dioxide (CO2) sequestration and may be useful for reducing greenhouse gases.22 Endophytic phytoaugmentation may also be useful for agricultural systems for applications such as healthier tomato crops or grapevine growth.14,23,24 This approach may also be used for biocontrol systems to provide enhanced aesthetics, to act as soil stabilizers, and to reduce dust dispersal.16,25,26
Biology and Function of Endophytic Augmentation in Phytoremediation
Endophytic Biology
Plants are colonized by a range of microflora such as bacteria, fungi, yeasts, viruses, and protists, as well as epiphytes including algae and nematodes. Plant-microbe populations are dynamic, and variations in microbial communities that are influenced by the large fluctuations in the physical and nutritional conditions, as well as other biotic and abiotic influences, have been observed.2,27–32 Some microorganisms, predominantly bacteria and fungi, are recruited to enter the plant locally and systemically as endophytes, establishing asymptomatic or mutualistic relationships.2,20,33,34 Endophytes are found systemically in roots, stems, leaves, seeds, fruits, tubers, ovules, and some nodules.2,35,36 Endophytes may be recruited to their host through chemotaxis, electrotaxis, or simply accidental encounter and are most commonly recruited from the roots.31,37,38 Roots have been shown to have the highest localized concentration of endophytes, and endophytic densities tend to decrease from stem to leaf.39,40 The most commonly reported endophytic locations are the intercellular spaces and xylem vessels.41 Endophytic communities depend on the taxa within a given community, host genotype and corresponding host developmental stage, inoculum density, temporal and seasonal conditions, plant location, and environmental conditions.14,17,42,43 Though dynamic, many endophytic communities have been shown to contain common soil taxa such as Pseudomonas, Burkholderia, Bacillus, and Azospirillua.37,44
Diversity and Function of Endophytes
Endophytes have been isolated from a diversity of plants, yet their exact function and associations remain unclear.38 For instance, it is unclear how endophytes interact with each other in the plant, and little is known about the complex endophyte-host molecular interactions or their gene regulation and expression.28,41 Endophytes seem to form diverse and complex associations with their hosts, including mutualistic and symbiotic relationships.5,45–48 In many cases, endophytes are believed to be beneficial to their plant hosts through nitrogen fixation, accelerated seedling emergence, protection from environmental stressors, enhanced nutrient availability and vitamin supply, and contaminant protection and removal.9,28,31,37 Endophytes are capable of producing bioactive compounds associated with increased plant growth and health, and offer protection from abiotic and biotic stresses.7,8,37,38,49–55 Endophytes may also offer their plant host protection and defense against microbial diseases, insects, and nematodes.19,25,33,38,56 For example, endophytic actinobacteria offer defense against the pathogenic fungus Gaeumannomyces graminis in wheat and potatoes, and the endophyte Curtobacterium flaccumfaciens protects citrus plants from the pathogen Xylella fastidiosa.34 Interestingly, even some mycorrhizal fungi themselves have endosymbiotic bacteria for protection.52,57–60 More in-depth endophyte reviews are provided by Newman and Reynolds, Sturz et al., Strobel and Daisy, Mercado-Blanco, Tadych and White, and Lodewyckx et al.32,44,61–64
Endophytic Augmentation
Many endophytes have shown a natural capacity for xenobiotic degradation.9 Further, plant-associated microorganisms capable of direct degradation are more abundant among endophytes than in the rhizosphere at contaminated sites.65 This may be because the plants themselves selectively enrich degraders inside and around the phytoremediating host plants. Siciliano et al. found that plants grown in soil contaminated with xenobiotics naturally recruit endophytes with contaminant-degrading genes.30 This selective enrichment suggests a potential against a wide-range of contaminants and sites.26,30,31,65–67 The natural ability of some endophytes to degrade xenobiotics has been investigated with regard to improving phytoremediation efficiency (Table 1).7–9,18,20,27,38,51–53,65,67–79
Table 1.
Previously Published Endophytic Phytoaugmentation Systems for Remediation
| ENDOPHYTIC SPECIES | PHYTOAUGMENTATION SYSTEM | WASTE COMPONENT | REFERENCE |
|---|---|---|---|
| Remediation of Heavy Metals | |||
| Neotyphodium | Festuca arundinacea (tall grass); Festuca pratensis (meadow grass) | Cd | 69 |
| Pseudomonas putida PD1 | Salix alba (willow tree) | Cd | 53 |
| Pseudomonas sp. Lk9 | Solanum nigrum (black nightshade) | Cd | 70 |
| *Burkholderia sp. HU001; Pseudomonas sp. HU002 | Salix viminalis cv Tora (willow tree) | Cd, toluene | 71 |
| Sphingomonas SaMR12 | Sedum alfredii (perennial herb) | Zn | 72,73 |
| Pseudomonas sp. M6; Pseudomonas jessenii M15 | Ricinus communis (castor oil plant) | Ni, Cu, Zn | 18 |
|
*Burkholderia cepacia L.S.2.4:: ncc-nre *H. seropedicaeLMG2284::ncc-nre |
Lupinus leteus (yellow lupine); Lolium perenne (perennial ryegrass) |
Ni | 74 |
| *Pseudomonas putida W619-TCE, Ni-resistant | Populus trichocarpa (poplar tree) | Ni,TCE | 75 |
| Remediation of Chlorinated Contaminants | |||
| *Pseudomonas putida VM1441 (pNAH7) or VM1450 | Pisum sativum (pea plant) | 2,4-D | 27,76 |
| *Pseudomonas putida W619-TCE | Populus trichocarpa (poplar tree) | TCE | 77 |
| Enterobacter sp. Strain PDN3 | Populus trichocarpa (poplar tree) | TCE | 78 |
| *Burkholderia vietnamiensis BU61 (MMO) | Salix alba (willow tree) | TCE | 79 |
| *Pseudomonas putida W619-TCE (ncc-enre) | Populus tremula (poplar tree) | TCE, Ni | 77 |
| *Pseudomonas putida W619-TCE (tomA4) | Populus deltoids (poplar tree) | TCE | 7 |
| Burkholderia xenovorans LB400 | Panicum virgatum (switch grass) | Organic pesticides; PCBs | 8,79 |
| Pseudomonas aeruginosa R75; Pseudomonas savastanoi CB35 | Elymus dauricus (wild rye) | Chloro-benzoic acids | 67 |
| Remediation of Aromatic Compounds | |||
| *Burkholderia cepacia G4, BU0072 and VM1330 | Lupinus leteus (yellow lupine) | Toluene | 74 |
| *Burkholderia cepacia VM1468 (pTOM-Bu61) | Populus trichocarpa × deltoids (hybrid Poplar tree) | Toluene | 51 |
| *P. putida VM1441 (pNAH7) | Pisum sativum (pea plant) | Naphthalene | 52 |
| *Burkholderia cepacia G4 (nre) | Lupinus luteus (yellow lupine) | Toluene | 68 |
| Staphylococcus sp. BJ06 | Lolium (rye grass) | Pyrene | 38 |
*indicates engineered endophyte.
An advantage of using endophytic degraders in remediation is toxic xenobiotics may be degraded in planta, reducing phytotoxic effects and eliminating any toxic effects on herbivorous fauna residing on or near contaminated sites.4,9,15 More specifically, some highly water-soluble and volatile organic xenobiotic compounds are quickly absorbed and may remain in the xylem for up to two days, allowing endophytic detoxification.6,8,37,63,80 When plant species and environmental conditions are relatively constant, contaminant uptake depends on the lipophilicity, or the log of the octanol-water partition coefficient (log Kow).7 Relatively hydrophilic compounds, or compounds with a log Kow between 0.5–3.5, such as benzene and toluene, tend to be absorbed rapidly into the plant.7,45,48,81,82 Once absorbed into the plant, there are several kinetic processes for phytoremediation that may occur, including uptake, transformation, and stabilization via immobilization, which are thoroughly reviewed elsewhere.1,5,7,13 When the log Kow is outside of the uptake range, it may still interact with the plant-microbe system through other methods like adsorption or volatilization. Further, mixed inoculations consisting of more than one endophytic bacterium that inhibit plant growth individually may result in plant-growth promotion, suggesting some important but poorly understood in planta interactions.38 Consequently, full characterization of the plant-microbe interactions and their effects on pollutant degradation is needed.
Candidates for Endophytic Phytoaugmentation
In general, phytoremediation-based treatment approaches have gained attention for their ecological and economic benefits. Along with site remediation, phytoremediation provides the additional benefits of a high level of public acceptance, pleasing aesthetics, use of naturally occurring plant processes, enhancement of soil and plant health, and improved wildlife habitats.1,5,7,53
Several plant species have been tested in phytoaugmentation applications. Poplars (Populus spp.) and willows (Salix spp.) are commonly used because of their rapid growth, deep roots, and large uptake of water.83,84 Along with trees, recent reports suggest that hyperacculumators (plants that can accumulate high levels of toxins) and their associated microbes may play a key role in phytoremediation and can support organized endophytic communities.1,5,12,45,64,85 Hyperaccumulators may be used to accumulate heavy metals often found in wastes and at contaminated sites such as As, Cu, Pb, Se, and Zn.86,87 This is critical as metals are difficult to remove using traditional techniques and are often only controlled or immobilized. Phytoremediation offers the advantage of metal extraction without disturbing the site. For example, Thlaspi caerulescens (Alpine pennycress) is commonly used to accumulate large amounts of zinc and cadmium, and Thlaspi goesingense is able to accumulate large amounts of nickel.88–90 The metals may then be reclaimed and reused.23,91
Along with hyperaccumulators, some other plants are capable of producing root exudates that enhance contaminant desorption, which may make some compounds more bioavailable for the subsequent degradation by microbial communities.39,79 In addition, when augmented with endophytes, some plants have been shown to have improved health and growth, increased drought resistance, reduced transplanting shock, increased resistance to pathogens, and lower mortality.5,8,23,25,40,52,76,79 It has been reported that several endophytic taxa from contaminant-exposed poplar trees have the potential to enhance phytoremediation of volatile organics and herbicides.17,52,76,92,93 A review of phytoremediation plant species and their respective endophytes may be found in Mastretta et al.31
Phytoaugmentation has proven feasible for several endophytic species (Table 1). One key example is the inoculation with a Pseudomonas endophyte capable of degrading the organochlorine herbicide 2,4-dichlorophenoxyacetic (2,4-D) to pea plants, reported by Germaine et al.76 The phytoaugmented plants demonstrated increased removal of 2,4-D and showed no 2,4-D acid accumulation in aerial tissues.76 There is potential to phytoaugment with naturally engineered endophytes, or those who have been genetically altered via natural gene transfer or recombinant DNA technology.8 For example, naturally engineered endophytic Burkholderia cepacia strains with a nickel-resistance operon improved phytoremediation and promoted plant tolerance to toluene.51,68 Lodewyckx et al. demonstrated that this same engineered B. cepacia endophytic strain increased the nickel accumulation and tolerance of inoculated plants when added to yellow lupine.74 Transgenic, or genetically engineered, trees may also be designed to increase remediation.94,95
Though several studies have reported positive findings such as increased remediation potential or improved plant health with pollutant exposure, there are still research and technology gaps to fill before widespread use can be adopted. Many of the current studies have been conducted at lab scale and with controlled parameters; it is unclear how they may translate to the field or large scale use with dynamic environmental conditions. For instance, it is unknown what metabolites will be produced from pollutant degradation during endophytic phytoaugmentation in the field, nor the ecological impact of long-term endophytic colonization. In addition, though some engineered endophytes are transformed naturally, public education and assurance of safety will need to be demonstrated prior to large-scale use.32 Poorly understood plant-microbe-pollutant interactions before, during, and after endophytic phytoaugmentation are another obstacle, as are the limited tools and techniques for monitoring these interactions.
Propagation of Beneficial Genes Through Horizontal Gene Transfer
Interest in genetically modifying endophytes is increasing because of their enhanced ability to degrade or resist targeted contaminants. The advantages and obstacles to using bioengineered endophytes have been clearly discussed by Weyens et al.46 Recently, there has been a focus on naturally modified endophytes, through horizontal gene transfer (HGT). Taghavi et al. found the degradative plasmid pTOM-BU61 transferred naturally to a number of different endophytes in planta, resulting in more efficient degradation of toluene in poplar plants.51 It has also been reported that HGT in planta is likely to be widespread.9 Further, endophytes have been shown to interact and exchange genes with the rhizopheric and phyllospheric bacterial communities through HGT.7 For instance, genetic transfer to enhance phenol degradation in planta and in the rhizosphere has been demonstrated.96 Similar to genetic bioaugmentation, endophytic phytoaugmentation strategies relying on genetic transfer between the exogenous and indigenous strains have an increased likelihood of success, as they do not require the survival of the donor strains.60,97–100 Though promising, targeted genetic transfer to increase degradation potential is novel, and little has been done at field scale. From a regulatory standpoint, guidelines for endophytic phytoaugmentation are needed, particularly with respect to engineered endophytes.
Potential Field-Scale Treatment Systems
The translation of endophytic phytoaugmentation from lab scale to the field scale is an area of interest, but there are a number of challenges. Optimization of contamination removal in field-level phytosystems, including artificial wetlands, riparian buffers, and vegetative filters, has been a topic of ongoing research. For example, endophytic phytoaugmentation in vegetative buffer systems may be used to treat industrial wastewaters, or wastewaters that may contain high levels of dyes, phenolic compounds, and metals. Using endophytic phytoaugmentation, Shehzadi et al. were able to treat effluent from a textile plant in a field-scale constructed wetland reactor.29,66 They reported significant reductions in chemical oxygen demand (79%), biological oxygen demand (77%), total dissolved solids (59%) and total suspended solids (27%) in the constructed wetlands phytoaugmented with Typha domingensis and the textile effluent-degrading endophytes Microbacterium arborescens TYSI04 and Bacillus pumilus PIRI30.
Willow vegetative filters may be used at field scale for municipal wastewater and sludge treatment.12,56,83,101 When filtering wastewater effluents through willow buffers, there is selective uptake of heavy metals, removal of excessive nutrients, and possible treatment of emerging contaminants. Willows may offer an economical and efficient solution for removing micropollutants such as endocrine-disrupting chemicals (e.g, estrogenic compounds) that are of concern in wastewater effluent.102 Although much has been done with willows in traditional phytoremediation, little has been done with endophytic phytoaugmentation of willow systems. Riparian buffers can also help reduce excessive nutrient runoff and capture some of the pesticide/herbicide and heavy metal runoff.10,65,103 They have been shown to be effective at field scale in channelized runoff, agricultural watersheds and drainage waters, and landfill leachate.13,65,69,80,92,96,104,105 The treatment of wastewater effluent containing nonylphenols compounds from a tannery has also been investigated, as have hydrocarbons, persistent organic pollutants, and polychlorinated biphenyls.6,11,26,59,78,80,81,93,106
Challenges
Complex and poorly understood community-plant interactions, natural microbial community dynamics, and variations in environmental conditions may limit the application of endophyte inoculation or waste treatments. When immediate or emergency action is required, endophytic phytoaugmentation may not be as appropriate as it is typically slower and only seasonally effective.8,9,107 All of these considerations would require optimization in design and removal, which would require further research and understanding of the plant-microbe and plant-pollutant interactions.
Weyens et al. emphasized that although successfully applied in several laboratory-scale experiments, the large-scale field application of endophytic phytoaugmentation is limited by a number of issues including the levels of contaminants tolerated by plants; the limited bioavailability of organic contaminants; and the unacceptable levels of evapotranspiration of volatile organic compounds into the atmosphere.7
Furthermore, there is no clear best method for monitoring endophytic phytoaugmentation. Endophytic colonization has been measured using fluorescently tagged endophytes.52,77 This approach may be helpful for future model development but is unreasonable for field-scale studies. Monitoring a metabolite such as peroxidase may be a potential way of monitoring markers of plant health, while others have suggested using duckweed to monitor pollutant levels.2,42 Unfortunately, these methods do not monitor plant-endophyte interaction. Preferably, a reproducible method would be developed to monitor three-way plant-microbe-contaminant interactions in the field.
Another challenge is that phytosystems occasionally absorb enough contaminants that leaves and stems may be classified as toxic waste, thereby making waste management difficult. In some cases, such as with metals, the contaminants may be reclaimed and reused, but for other contaminants the plants may need to be disposed of as biohazard waste.13 Still, disposal of contaminant plant biomass may be simpler than full-scale site remediation using traditional methods such as excavation or chemical oxidation. Thus, it is critical that in-depth analyses be carried on a site-by-site basis.
Conclusions
Endophytic phytoaugmentation with naturally occurring xenobiotic-degrading endophytes have the advantage of reduced competition in the internal plant tissue and do not require re-inoculation, but there is still a need to determine what conditions help support a successful augmentation event. Additionally, natural endophytes have the potential to be isolated and genetically enhanced to degrade target compounds once reintroduced to the host, though the ecological implications of genetically altered microbes will need to be fully characterized.63
A more in-depth understanding of the three-way plant-microbe-contaminant interactions is needed to capitalize on the potential benefits. In the future, tools will be needed to determine three-way interactions before, during, and after endophytic phytoaugmentation. Though green fluorescent protein tagging has been effective in monitoring endophyte-associated HGT at small scale (as previously discussed), more environmentally relevant, exhaustive, and field-level techniques and tools are needed. To do this, improved metagenomic, metatranscriptomic, and metaproteomic work on plant-microbe relationships is needed to fully understand and thereby optimize the augmented phytoremediation system. As methods of genomic and proteomic analysis become cheaper and faster, it has become more feasible to determine the relationship between the biotic systems and the pollutant systems. For example, targeting pollutant catabolic genes within endophyte communities using quantitative gene expression may be a useful tool for assessing colonization and remediation.28 Other protein-associated techniques, such as modified enzyme-linked immunosorbent assay (ELISA test) and chromatin immunoprecipitation sequencing (ChIP-Seq), may help accurately describe the DNA-protein interactions between the plant and microbial community. Given the large numbers of environmental variables and parameters, these tools need to be applied to more field-scale endophytic phytoaugmentation studies to fully characterize the remediation potential. With a more complete understanding of the “-omics” associated with remediation and of the changes in field-level parameters, a system for reproducible and reliable endophytic phytoaugmentation may be established. Simple biomarkers or indicators of remediation (e.g., monitoring levels of a specific gene or protein) along with more exhaustive tools and techniques to monitor colonization and communications, would be valuable for large-scale or long-term projects. Though many research gaps remain, the use of endophytic phytoaugmentation may provide an economical and environmentally friendly alternative to traditional remediation techniques.
Acknowledgments
Duke's Superfund Research Center and the National Institute for Environmental Health Sciences under the grant NIEHS P42-ES010356 provided support for this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013;91(7):869–881 [DOI] [PubMed] [Google Scholar]
- 2.Hegazy AK, Kabiel HF, Fawzy M. Duckweed as heavy metal accumulator and pollution indicator in industrial wastewater ponds. Desal Water Treat 2009;12(1-3)400–406 [Google Scholar]
- 3.Frith GR. Methemoglobinemia caused by nitrate pollution in drinking water. J Med Assoc Ga 1950;39(6):258–259 [PubMed] [Google Scholar]
- 4.Davis AP, Shokouhian M, Ni S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 2001;44(5):997–1009 [DOI] [PubMed] [Google Scholar]
- 5.Salt DE, Blaylock M, Kumar NP, et al. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Nat Biotechnol 1995;13(5):468–474 [DOI] [PubMed] [Google Scholar]
- 6.Trapp S, Karlson U. Aspects of phytoremediation of organic pollutants. J Soil Sediments 2001;1(1):37–43 [Google Scholar]
- 7.Weyens N, van der Lelie D, Taghavi S, Vangronsveld J. Phytoremediation: Plant-endophyte partnerships take the challenge. Curr Opin Biotechnol 2009;20(2):248–254 [DOI] [PubMed] [Google Scholar]
- 8.McGuinness M, Dowling D. Plant-associated bacterial degradation of toxic organic compounds in soil. Int J Environ Res Public Health 2009;6:2226–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan RP, Germaine K, Franks A, et al. Bacterial endophytes: Recent developments and applications. FEMS Microbiol Lett 2008;278:1–9 [DOI] [PubMed] [Google Scholar]
- 10.Arora K, Mickelson SK, Baker JL. Effectiveness of vegetated buffer strips in reducing pesticide transport in simulated runoff. Transactions of the ASABE 2003;46(3):635 [Google Scholar]
- 11.Arslan M, Imran A, Khan QM, Afzal M. Plant–bacteria partnerships for the remediation of persistent organic pollutants. Environ Sci Pollut Res Int 2015;1–15 [DOI] [PubMed] [Google Scholar]
- 12.Janssen J, Weyens N, Croes S, et al. Phytoremediation of metal contaminated soil using willow: Exploiting plant-associated bacteria to improve biomass production and metal uptake. Int J Phytoremediation 2015;17(11):1123–1136 [DOI] [PubMed] [Google Scholar]
- 13.Martínez M, Bernal P, Almela C, et al. An engineered plant that accumulates higher levels of heavy metals than Thlaspi caerulescens, with yields of 100 times more biomass in mine soils. Chemosphere 2006;64(3):478–485 [DOI] [PubMed] [Google Scholar]
- 14.Pillay VK, Nowak J. Inoculum density, temperature, and genotype effects on in vitro growth promotion and epiphytic and endophytic colonization of tomato (Lycopersicon esculentum L.) seedlings inoculated with a pseudomonad bacterium. Can J Microbiol 1997;43(4):354–361 [Google Scholar]
- 15.van Cauwenberghe L, Roote DS. In situ bioremediation. (1998) Available at: https://clu-in.org/download/toolkit/insbio_o.pdf (Last accessed March2016)
- 16.Kloepper JW, Ryu CM. Bacterial endophytes as elicitors of induced systemic resistance. In: Schulz B, et al., eds. Microbial Root Endophytes. Springer-Verlag, Berlin, Heidelberg, Germany: 2006:33–52 [Google Scholar]
- 17.Müller H, Berg C, Landa BB, et al. Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front Microbiol 2015;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajkumar M, Freitas H. Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 2008;71:834–842 [DOI] [PubMed] [Google Scholar]
- 19.Quadt-Hallmann A, Hallmann J, Kloepper JW. Bacterial endophytes in cotton: Location and interaction with other plant-associated bacteria. Can J Microbiol 1997;43(3):254–259 [Google Scholar]
- 20.Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol 1997;43:895–914 [Google Scholar]
- 21.Balatinecz JJ, Kretschmann , Leclercq A. Achievements in the utilization of poplar wood—guideposts for the future. Forest Chron 2001;77(2):265–269 [Google Scholar]
- 22.Stepniewska Z, Kuźniar A. Endophytic microorganisms—Promising applications in bioremediation of greenhouse gases. Appl Microbiol Biotechnol 2013;97(22):9589–9596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barka EA, Gognies S, Nowak J, et al. Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol Control 2000;24(2):135–142 [Google Scholar]
- 24.M'piga P, Belanger R, Paulitz T, et al. Increased resistance to Fusarium oxysporumf. sp. radicis-lycopersiciin tomato plants treated with the endophytic bacterium Pseudomonas fluorescens strain 63-28. Physiol Mol Plant Pathol 1997;50(5):301–320 [Google Scholar]
- 25.Compant D, Duffy B, Nowak J, et al. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl Eviron Microbiol 2005;71:4951–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan Z, Doty S. Endophyte-assisted phytoremediation. Plant Biol 2011;12:97–105 [Google Scholar]
- 27.Germaine KJ, Keogh E, Garcia-Cabellos G, Borremans B, et al. Colonisation of poplar trees by gfp expressing bacterial endophytes. FEMS Microbiol Ecol 2004;48:109–118 [DOI] [PubMed] [Google Scholar]
- 28.Ryan RP, Ryan DJ, Dowling DN. Plant protection by the recombinant, root-colonising Pseudomonas fluorescens F113rifPCB strain expressing arsenic resistance: Improving rhizoremediation. Lett Appl Microbiol 2007;45(5):668–674 [DOI] [PubMed] [Google Scholar]
- 29.Shehzadi M, Afzal M, Khan MU, et al. Enhanced degradation of textile effluent in constructed wetland system using Typha domingensis and textile effluent-degrading endophytic bacteria. Water Res 2014;58:152–159 [DOI] [PubMed] [Google Scholar]
- 30.Siciliano SD, Fortin N, Mihoc A, et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl Environ Microbiol 2001;67:2469–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastretta C, Barac T, Vangronsveld J, et al. Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotech Genet Eng Rev 2006;23(1):175–188 [DOI] [PubMed] [Google Scholar]
- 32.Newman LA, Reynolds CM. Bacteria and phytoremediation: New uses for endophytic bacteria in plants. Trends Biotechnol 2005;23:6–8 [DOI] [PubMed] [Google Scholar]
- 33.Azevedo JL, Maccheroni J, Jr, Pereira O, Ara WL. Endophytic microorganisms: A review on insect control and recent advances on tropical plants. Electr J Biotechnol 2000;3:40–65 [Google Scholar]
- 34.Araujo WL, Marcon J, Maccheroni W Jr, et al. Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 2002;68:4906–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benhizia Y, Benhizia H, Benguedouar A, et al. Gamma proteobacteria can nodulate legumes of the genus Hedysarum. Syst Appl Microbiol 2004;27(4):462–468 [DOI] [PubMed] [Google Scholar]
- 36.Sturz A, Christie B, Matheson B, et al. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol Fertil Soils 1997;25(1):13–19 [Google Scholar]
- 37.McGrady JK, McFarlene C, Lindstrom F. The transport and affinity of substituted benzenes in soybean stems. J Exp Bot 1987;38:1875–1890 [Google Scholar]
- 38.Sun K, Liu J, Jin L, Gao YZ. Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination. Plant Soil 2014;347:251–263 [Google Scholar]
- 39.LeFevre GH, Hozalski RM, Novak PJ. Root exudate enhanced contaminant desorption: An abiotic contribution to the rhizosphere effect. Environ Sci Technol 2013;47(20):11545–11553 [DOI] [PubMed] [Google Scholar]
- 40.Benhamou N, Kloepper JW, Quadt-Hallman A, Tuzun S. Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol 1996;112(3):919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenblueth M, Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact 2006;19(8):827–837 [DOI] [PubMed] [Google Scholar]
- 42.Naffaa W, Ravel C, Boyer N, Guillaumin JJ. Peroxidase activity of perennial ryegrass and tall fescue seedlings artificially infected with endophytes. Agronomie 1999;19(7):611–619 [Google Scholar]
- 43.Ping L, Boland W. Signals from the underground: Bacterial volatiles promote growth in Arabidopsis. Trends Plant Sci 2004;9(6):263–266 [DOI] [PubMed] [Google Scholar]
- 44.Lodewyckx C, Vangronsveld J, Porteous F, et al. Endophytic bacteria and their potential applications. Crit Rev Plant Sci 2002;21(6):583–606 [Google Scholar]
- 45.Ikuma K, Gunsch CK. Effect of carbon source addition on toluene biodegradation by an Escherichia coli DH5α transconjugant harboring the TOL plasmid. Biotechnol Bioeng 2010;107(2):269–277 [DOI] [PubMed] [Google Scholar]
- 46.Weyens N, Van Der Lelie D, Artois , et al. Bioaugmentation with engineered endophytic bacteria improves contaminant fate in phytoremediation. Environ Sci Technol 2009;43(24):9413–9418 [DOI] [PubMed] [Google Scholar]
- 47.Schardl CL, Leuchtmann A, Spiering MJ. Symbioses of grasses with seedborne fungal endophytes. Annu Rev Plant Biol 2004;55:315–340 [DOI] [PubMed] [Google Scholar]
- 48.Ijaz A, Imran A, ul Haq MA, Khan QM, Afzal M. Phytoremediation: Recent advances in plant-endophytic synergistic interactions. Plant Soil 2015;1–17 [Google Scholar]
- 49.Krutz LJ, Senseman SA, Zablotowicz RM, Matocha MA. Reducing herbicide runoff from agricultural fields with vegetative filter strips: A review. Weed Sci 2005;53(3) [Google Scholar]
- 50.Seghers D, Wittebolle L, Top EM, et al. Impact of agricultural practices on the Zea mays L. endophytic community. Appl Environ Microbiol 2004;70:1475–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taghavi S, Barac T, Greenberg B, et al. Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl Environ Microbiol 2005;71:8500–8505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Germaine KJ, Keogh E, Ryan D, Dowling DN. Bacterial endophyte-mediated naphthalene phytoprotection and phytoremediation. FEMS Microbiol Lett 2009;296:226–234S [DOI] [PubMed] [Google Scholar]
- 53.Khan Z, Roman D, Kintz T, et al. Degradation, phytoprotection, and phytoremediation of phenoanthrene by endophyte Pseudomonas putida, PD1. Environ Sci Technol 2014;48(20):12221–12228 [DOI] [PubMed] [Google Scholar]
- 54.Achari GA, Ramesh R. Diversity, biocontrol, and plant growth promoting abilities of xylem residing bacteria from solanaceous crops. Int J Microbiol 2014;(12);doi: 10.1155/2014/296521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weyens N, Truyens S, Dupae J, et al. Potential of the TCE-degrading endophyte Pseudomonas putida W619-TCE to improve plant growth and reduce TCE phytotoxicity and evapotranspiration in poplar cuttings. Environ Pollut 2010;158(9):2915–2919 [DOI] [PubMed] [Google Scholar]
- 56.Perttu KL. Environmental and hygienic aspects of willow coppice in Sweden Biomass Bioenerg 1999;16(4):291–297 [Google Scholar]
- 57.Cunningham SD, Berti WR. Remediation of contaminated soils with green plants: An overview. In Vitro Cell Dev Biol Plant 1993;29(4):207–212 [Google Scholar]
- 58.Berg G, Krechel A, Ditz M, et al. Endophytic and ectophytic potato-associated bacterial communities differ in structure and antagonistic function against plant pathogenic fungi. FEMS Microbiol Ecol 2005;51(2):215–229 [DOI] [PubMed] [Google Scholar]
- 59.Jha P, Panwar J, Jha PN. Secondary plant metabolites and root exudates: Guiding tools for polychlorinated biphenyl biodegradation. Int J Environ Sci Technol 2015;12(2):789–802 [Google Scholar]
- 60.Jargeat P, Cosseau C, Ola'h B, et al. Isolation, free-living capacities, and genome structure of Candidatus Glomeribacter gigasporarum, the endocellular bacterium of the mycorrhizal fungus Gigaspora margarita. J Bacteriol 2004;186(20):6876–6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sturz AV, Christie BR, Nowak J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit Rev Plant Sci 2000;19:1–30 [Google Scholar]
- 62.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 2003;67:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mercado-Blanco J. Life of microbes inside the plant. In: Lugtenberg B, eds. Principles of Plant-Microbe Interactions. Switzerland, Heidelberg: Springer International Publishing; 2015:25–32 [Google Scholar]
- 64.Tadych M, White JF, Moselio S. Endophytic microbes. In: Encyclopedia of Microbiology. Academic Press, Oxford: 2009;431–442 [Google Scholar]
- 65.Smith CM. Riparian pasture retirement effects on sediment, phosphorus, and nitrogen in channellised surface run-off from pastures. New Zealand J Mar Freshwater Res 1989;23(1):139–146 [Google Scholar]
- 66.Shehzadi M, Fatima K, Imran A, et al. Ecology of bacterial endophytes associated with wetland plants growing in textile effluent for pollutant-degradation and plant growth-promotion potentials. Plant Biosyst 2015;1–10 [Google Scholar]
- 67.Siciliano SD, Goldie H, Germida JJ. Enzymatic activity in root exudates of Dahurian wild rye (Elymus dauricus) that degrades 2-chlorobenzoic acid. J Agric Food Chem 1998;46:5–7 [DOI] [PubMed] [Google Scholar]
- 68.Barac T, Taghavi S, Borremans B, et al. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat Biotechnol 2004;22:583–588 [DOI] [PubMed] [Google Scholar]
- 69.Soleimani M, Hajabbasi MA, Afyuni M, et al. Effect of endophytic fungi on cadmium tolerance and bioacculumation by Festuca arundinacea and Festuca pratensis. Int J Phytorem 2010;12(6):535–49 [DOI] [PubMed] [Google Scholar]
- 70.Chen L, Luo S, Xiao X, et al. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl Soil Ecol 2010;46(3):383–389 [Google Scholar]
- 71.Weyens N, Schellingen K, Beckers B, et al. Potential of willow and its genetically engineered associated bacteria to remediate mixed Cd and toluene contamination. J Soil Sediments 2013; (1), 176–188 [Google Scholar]
- 72.Chen L, Luo S, Li X, et al. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol Biochem 2014;68:300–308 [Google Scholar]
- 73.Chen B, Shen J, Zhang X, et al. The endophytic bacterium, Sphingomonas SaMR12, improves the potential for zinc phytoremediation by its host, Sedum alfredii. PloS One 2014;9(9): e106826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lodewyckx C, Taghavi S, Mergeay M, et al. The effect of recombinant heavy metal-resistant endophytic bacteria on heavy metal uptake by their host plant. Int J Phytorem 2001;3(2):173–187 [Google Scholar]
- 75.Weyens N, Beckers B, Schellingen K, et al. The potential of the Ni-resistant TCE-degrading Pseudomonas putida W619-TCE to reduce phytotoxicity and improve phytoremediation efficiency of poplar cuttings on A Ni-TCE co-contamination. Int J Phytom 2015;17(1):40–48 [DOI] [PubMed] [Google Scholar]
- 76.Germaine KJ, Liu X, Cabellos GG, et al. Bacterial endophyte-enhanced phytoremediation of the organochloride herbicide 2,4-dichlorophenoxyacteric acid. FEMS Microbiol Ecol 2006;57:302–310 [DOI] [PubMed] [Google Scholar]
- 77.Weyens N, Croes S, Dupae J, et al. Endophytic bacteria improve phytoremediation of Ni and TCE co-contamination. Environ Pollut 2010;158(7):2422–2427 [DOI] [PubMed] [Google Scholar]
- 78.Kang JW, Khan Z, Doty SL. Biodegradation of trichloroethylene by an endophyte of hybrid poplar. App Environ Microbiol 2012;78:3504–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang Y, Meggo R, Dingei H, et al. Enhanced polychlorinated biphenyl removal in a switchgrass rhizospheres by bioaugmentation with Burkholderia xenovorans LB400. Ecol Eng 2014;71:215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trapp S, Köhler A, Larsen LC, et al. Phytotoxicity of fresh and weathered diesel and gasoline to willow and poplar trees. J Soil Sediments 2001;1(2):71–76 [Google Scholar]
- 81.Di Gregorio S, Giorgetti L, Castiglione MR. Phytoremediation for improving the quality of effluents from a conventional tannery wastewater treatment plant. Int J Environ Sci Technol 2015;12(4):1387–1400 [Google Scholar]
- 82.Wang XH, Yin CQ, Shan BQ. The role of diversified landscape buffer structures for water quality improvement in an agricultural watershed, North China. Agric Ecosyst Environ 2005;107(4):381–396 [Google Scholar]
- 83.Aronsson P, Perttu K. Willow vegetation filters for wastewater treatment and soil remediation combined with biomass production. Forest Chron 2001;77(2):293–299.S [Google Scholar]
- 84.Isebrands J, Karnosky DF. Environmental benefits of poplar culture. In: Dickmann DI, et al. (eds). Poplar culture in North America. Ontario, Canada: NRC Research Press, 2001:207–218 [Google Scholar]
- 85.Sura-de Jong M, Reynolds RJ, Richterova K, et al. Selenium hyperaccumulators harbor a diverse endophytic bacterial community characterized by high selenium resistance and plant growth promoting properties. Front Plant Sci 2015;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajkumar M, Ae N, Freitas H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009;77(2):53–160 [DOI] [PubMed] [Google Scholar]
- 87.Cappa JJ, Pilon-Smits EAH. Evolutionary aspects of elemental hyperaccumulation. Planta 2014;239:267–275 [DOI] [PubMed] [Google Scholar]
- 88.Brown S, Angle J, Chaney R, et al. Zinc and cadmium uptake by hyperaccumulator Thlaspi caerulescens grown in nutrient solution. Soil Sci Soc Amer J 1995;59(1)125–133 [DOI] [PubMed] [Google Scholar]
- 89.Lombi E, Zhao F, Dunham S, et al. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol 2000;145(1):11–20 [Google Scholar]
- 90.Idris R, Trifonova R, Puschenreiter M, et al. Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Environ Microbiol 2004;70(5):2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaney RL, Malik M, Li YM, et al. Phytoremediation of soil metals. Curr Opinion Biotechnol 1997;8(3):279–284 [DOI] [PubMed] [Google Scholar]
- 92.Moore FP, Barac T, Borremans B, et al. Endophytic bacterial diversity in poplar trees growing on a BTEX-contaminated site: The characterisation of isolates with potential to enhance phytoremediation. Syst Appl Microbiol 2006;29(7):539–556 [DOI] [PubMed] [Google Scholar]
- 93.Khan S, Afzal M, Iqbal S, Khan QM. Plant–bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 2013;90(4):1317–1332 [DOI] [PubMed] [Google Scholar]
- 94.Elowson S. Willow as a vegetative filter for cleaning of polluted drainage water from agricultural land. Biomass Bioenerg 1999;16:281–290 [Google Scholar]
- 95.Doty SL, Oakley B, Xin G, et al. Enhanced phytoremediation of volatile environmental pollutants with transgenic trees. Proc Natl Acad Sci USA 2007;104:16816–16821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y, Xiao M, Geng X, Liu J, Chen J. Horizontal transfer of genetic determinants for degradation of phenol between the bacteria living in plant and its rhizosphere. Appl Microbiol Biotechnol 2007;77(3):733–739 [DOI] [PubMed] [Google Scholar]
- 97.Ikuma K, Gunsch CK. Genetic bioaugmentation as an effective method for in situ bioremediation: Functionality of catabolic plasmids following conjugal transfers. Bioeng 2012;3(4):236–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ikuma K, Holzem RM, Gunsch CK. Impacts of organic carbon availability and recipient bacteria characteristics on the potential for TOL plasmid genetic bioaugmentation in soil slurries. Chemosphere 2012;89(2):158–163 [DOI] [PubMed] [Google Scholar]
- 99.Ikuma K, Gunsch CK. Functionality of the TOL plasmid under varying environmental conditions following conjugal transfer. Appl Microbiol Biotechnol 2013;97(1):395–408 [DOI] [PubMed] [Google Scholar]
- 100.Ikuma K, Gunsch CK. Successful genetic bioaugmentation with Pseudomonas putida for toluene degradation in soil columns. Environ Chem Lett 2013;11(4):365–370 [Google Scholar]
- 101.Algreen M, Trapp S, and Rein A. Phytoscreening and phytoextraction of heavy metals at Danish polluted sites using willow and poplar trees. Environ Sci Pollut Res Int 2014;21(15):8992–9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Franks CG. 2006. Phytoremediation of pharmaceuticals with Salix exigua [thesis]. [Lethbridge, Alberta: ]: University of Lethbridge [Google Scholar]
- 103.Lamb TG, Tonkyn DW, Kluepfel DA. Movement of Pseudomonas aureofaciens from the rhizosphere to aerial plant tissue. Can J Microbiol 1996;42(11):1112–1120 [Google Scholar]
- 104.Fuentes-Ramírez LE, Caballero-Mellado J, Sepúlveda J, Martínez-Romero E. Colonization of sugarcane by Acetobacter diazotrophicus is inhibited by high N-fertilization. FEMS Microbiol Ecol 1999; (2), 117–128 [Google Scholar]
- 105.Rosenqvist H, Ness B. An economic analysis of leachate purification through willow-coppice vegetation filters. Bioresour Technol 2004;94(3):321–329 [DOI] [PubMed] [Google Scholar]
- 106.Dickey EC, Vanderholm DH. Vegetative filter treatment of livestock feedlot runoff. J Environ Quality 1981;10(3):279–284 [Google Scholar]
- 107.Sahay NS, Varma A. Piriformospora indica: A new biological hardening tool for micropropagated plants. FEMS Microbiol Lett 1999;181(2):297–302 [DOI] [PubMed] [Google Scholar]


