Abstract
In the present study, the agonists and antagonists of DP receptor were used to examine whether the PGD2-DP signaling pathway affects neuronal function. Primary cultured hippocampal neuron was prepared and treated with aluminum maltolate (100 μM) to establish the neuronal damage model. PGD2 and cAMP content was detected by ELISA. L-PGDS and DPs mRNA and protein expression were measured by RT-PCR and Western blotting, respectively. The aluminium-load neuron was treated with the DP1 agonist BW245C, the DP1 antagonist BWA868C, the DP2 agonist DK-PGD2, and the DP2 antagonist CAY10471, respectively. Neuronal pathomorphology was observed using H-E staining. The cell viability and the lactate dehydrogenase leakage rates of neurons were measured with MTT and LDH kit, respectively. Ca2+ level was detected by Fluo-3/AM. In the model group, the MTT values obviously decreased; LDH leakage rates and PGD2 content increased significantly; L-PGDS, DP1 mRNA and protein expressions increased, and DP2 level decreased. BW245C reduced the Ca2+ fluorescence intensity and protected the neurons. DK-PGD2 increased the intensity of Ca2+ fluorescence, while CAY10471 had the opposite effect. In conclusion, contrary to the effect of DP2, the PGD2-DP1 signaling pathway protects against the primary cultured rat hippocampal neuronal injury caused by aluminum overload.
Neurodegenerative diseases (NDDs) of the central nervous system (CNS), including Alzheimer’s disease(AD), Amyotrophic lateral sclerosis (ALS), and Parkinson’s disease (PD), have increased dramatically in recent years, comprising 30% of the total cases of disease in humans. Though the current medical treatments have significantly improved the quality and length of life for NDD patients, NDDs remain a significant unresolved societal burden that afflicts millions of people worldwide.
Many studies have shown that the pathogenesis of the NDDS includes ischemia, calcium overload, oxidative stress and inflammatory factors1,2,3,4,5,6. Among these factors, neuronal damage and apoptosis caused by inflammatory cytokines have been widely recognized.
Aluminum (Al), which is abundant in the crust, is omnipresent in everyday life and may enter the human body in many ways such as the environment, diet, or drugs. However, the physiological action of Al on humans is unclear. Since the first report of Al toxicity to humans at early 1970s, it has been identified that Al overload could cause severe brain damage and neurodegeneration7. In particular, Al was detected in senile plaques and neurofibrillary tangles in the brain tissues from AD patients8. Therefore, Al neurotoxicity could be involved in the degeneration of neurons and the production of Aβ peptide. As reported, the Al-induced neuronal injury is closely related to neuroinflammatory.
Inflammation is partially mediated by prostaglandins, which are mediated by the rate-limiting enzyme cyclooxygenase (COX). To date, studies on the significance of COX-2 and its metabolites in neural degenerative diseases suggest that Alzheimer’s disease is associated with the over-expression of COX-29,10,11. Thus, COX-2 inhibitors have been widely used. Unfortunately, COX-2 inhibitors cause many side effects, such as renal toxicity12, decreased ulcer healing13 and adverse cardiovascular reactions14. To avoid such side effects, it is a key to determine the significance of the COX-2 downstream signaling pathway in nerve injury.
Prostaglandins (PGs) are a type of unsaturated fatty acid derivative produced from arachidonic acid catalyzed by COX15. Prostaglandin D2 is one of the most abundant PGs synthesized by PGDS in the brain16. PGDS includes L-PGDS (lipocalintype prostaglandin D synthase) and H-PGDS (hematopoietic prostaglandin D synthase). L-PGDS is highly expressed in the central nervous system17. Several studies have suggested that PGD2 may protect against neuronal lesions caused by multiple factors18, but it has also been reported that PGD2 can cause hippocampal neuron apoptosis19,20. PGD2 plays a role via activating on prostaglandin D1 and prostaglandin D2 receptors. Focusing on the PGD2-DPs signaling pathway, this study aimed to evaluate the characteristics and significance of the changes of DP1 and DP2 in primary cultured hippocampal neuron treated with aluminum overload.
This experiment established the injury model of rat hippocampal neurons induced by aluminum overload in vitro and evaluated the characteristics of the PGDS-DP pathway by the methods of ELISA, PCR, Western blotting etc. Additionally, neurons were treated with the DP1 agonist BW245C, the DP1 antagonist BWA868C, the DP2 agonist DK-PGD2 and the DP2 antagonist CAY10471 to investigate the characteristics and significance of the changes of the PGDS-DP pathway. The results of our present study will provide a powerful experimental and theoretical basis for further research on the mechanism of the neuronal injury induced by aluminum overload, facilitating the development of drugs for the effective treatment of NNDS.
Results
Primary cultured and identification of neurons
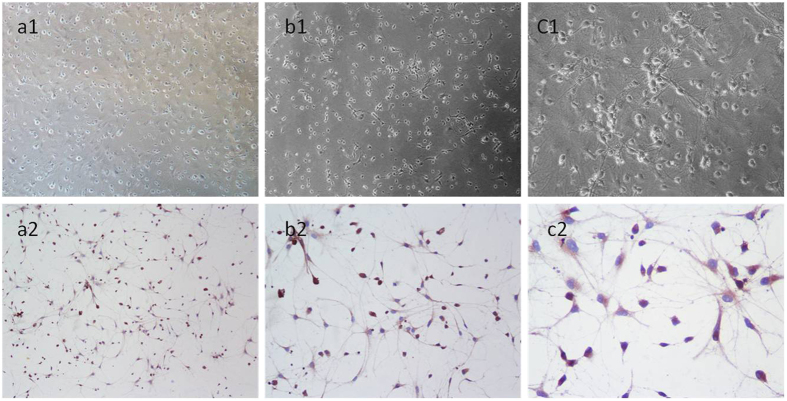
Observed under inverted microscope, the neuronal soma was plumped with short processes after cultured 1 day. The neurons appeared oval or vertebral after 3 days, and they had become stereoscopic. For 7 days, the cultured neuronal bodies enlarged and the nervous process eruption and extension into reticulation. The purity of the hippocampus neurons was detected by neuron specific enolase (NSE) after cultured for 7 d. The bodies and axons of the positive cells were stained to be brown by NSE and nuclei were stained to be blue after counterstained with hematoxylin. One hundred cells were selected randomly to evaluate the number and the percentage of positive cells. Our results showed that there were more than 95% of positive cells (Fig. 1).
Figure 1. The morphology of primary cultured hippocampal neurons and NSE staining of neurons.
(a1,b1,c1) Representative microscopic photographs show the morphology of hippocampal neurons after inoculation for 1d, 3d and 7d. Cultured 7 days later, adjacent neurons had developed to mutual cross-linked cell. Sections were pictured at 200× power. (a2,b2,c2) NSE staining showed that more than 95% of positive neurons existed. Sections were pictured at 100×, 200×, 400×, respectively.
Dose-dependent effect of Al (malt)3 and maltol on neuronal viability
The results showed that compared with the control group, the neuron viability decreased significantly in the Al3+-treated group at the concentration of 100, 200 and 400 μM with the neuron survival rates of 69.17%, 39.97% and 27.66%, respectively. However, the neuron survival rate was 95.47% at the concentration of 50 μM of Al (malt)3, which was no substantial difference compared with the control group. There was no considerable difference between the control group and the solvent control (maltol) (100–1200 μM) group. Considering each ion of Al is bound to three molecules of maltolate, 100 μM of Al (malt)3 and 300 μM of maltol were used in following experiments to evaluate the effects of PGD2-DP signaling on Al-load neuron (Fig. 2).
Figure 2. Dose-dependent effects of Al (malt)3 and maltol on neuronal viability detected by the method of MTT.
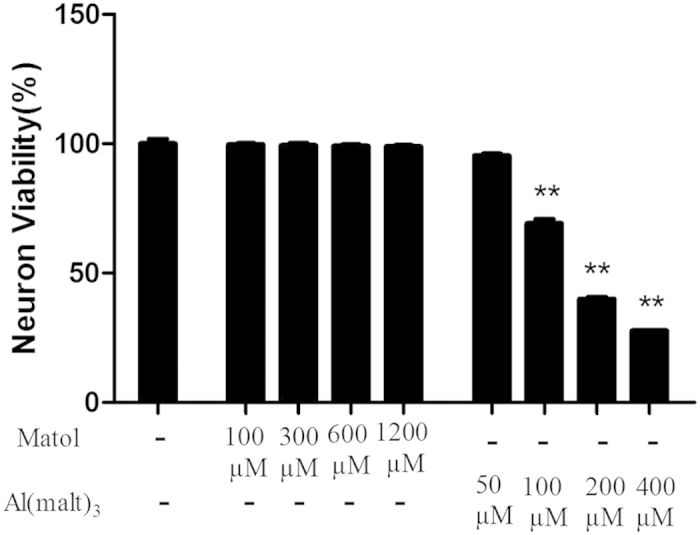
The 100, 200 and 400 M of Al (malt)3 decreased the neuron viability significantly whereas the neuron viability at 50 μM decreased slightly. The concentration of Al3+ at 100 μM was suitable for the damage model. There was no considerable difference between the control group and the solvent control (maltol) (100–1200 μM) group. Values were mean ± SD of six individual experiments (n = 6, **P < 0.01 vs. control group, one-way ANOVA with Dunnett’s multiple comparisons).
Expression of DP1, DP2 and L-PGDS mRNAs and proteins in primary cultured rat hippocampal neurons
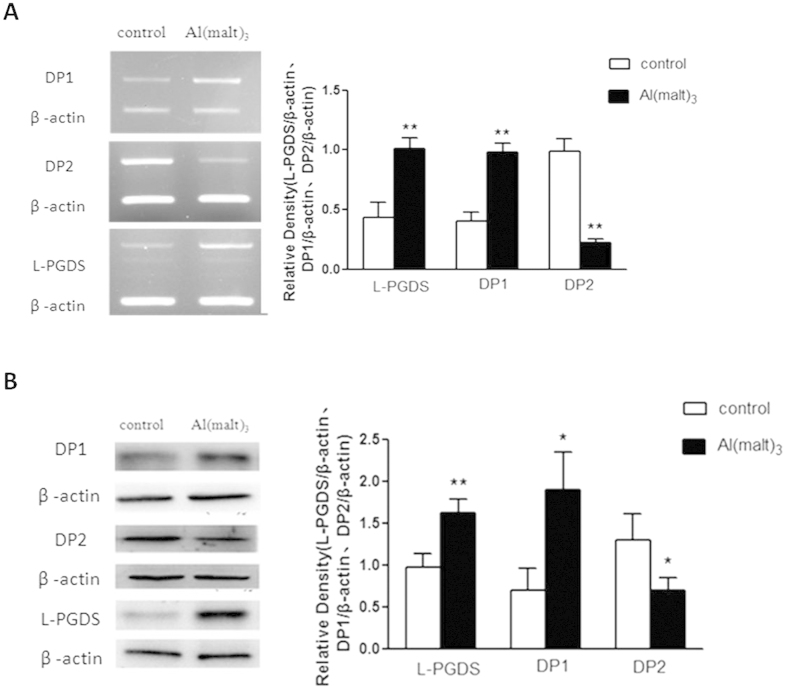
Compared with control group, the expression of L-PGDS and DP1 mRNA up-regulated 100% in the model group which was treated with Al (malt)3 (P < 0.01). By contrast, the expression of DP2 mRNA decreased significantly more than 70% in the model group (P < 0.01) (Fig. 3A).
Figure 3. Levels of DP1, DP2 and L-PGDS in primary cultured rat hippocampal neurons detected by reverse transcription polymerase chain reaction.
(A) The expressions of L-PGDS, DPs mRNA were measured by RT-PCR. The relative mRNA level of L-PGDS, DPs were standardized to endogenous β-actin mRNA for each sample. Al administration caused the significant increase of L-PGDS, DP1 levels and decrease of DP2 level compared with the control group. (B) The expressions of L-PGDS, DPs proteins were measured by WB. The relative protein levels of L-PGDS, DPs were standardized to endogenous β-actin protein for each sample. Al administration caused the significant increase of L-PGDS, DP1 levels and decrease of DP2 level compared with the control group. Values were mean ± SD (n = 3, **P < 0.01 vs. control group, one-way ANOVA with Dunnett’s multiple comparisons).
The results of WB showed that compared with control group, the expression of L-PGDS proteins increased 50% (P < 0.01), and that of DP1 proteins nearly doubled in the Al3+-treated group (P < 0.05), however the expression of the DP2 proteins decreased 50% in the Al3+-treated group (P < 0.05) (Fig. 3B).
The content of PGD2 was detected by Enzyme-linked Immunosorbent Assay
In control group, the concentration of PGD2 was about 4pg/mg whereas it was approximately 50 pg/mg in Al3+-treated group which showed nearly 12 times of that in the control group. Compared with the control group, the content of PGD2 increased significantly in model group (P < 0.01) (Fig. 4).
Figure 4. The content of PGD2 in each group detected by ELISA.
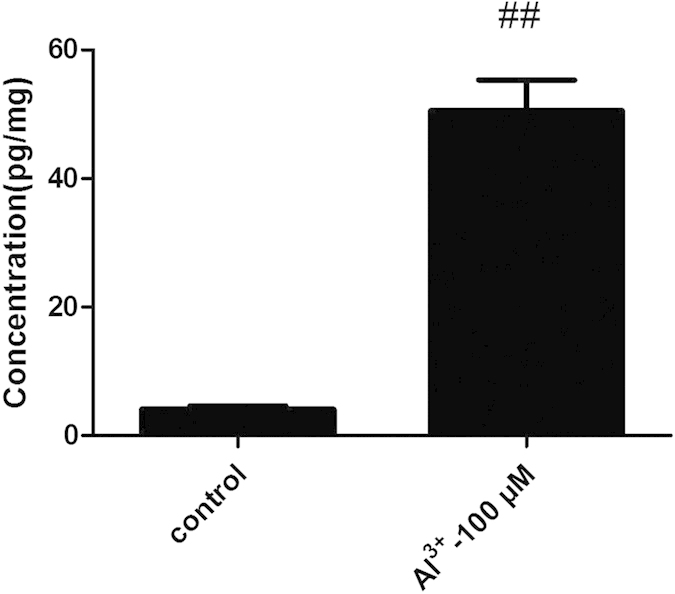
The content of PGD2 raised significantly in the Al3+-treated group. Values were mean ± SD (n = 6, ##P < 0.01 vs. control group, Student’s t test).
The change of neuron viability intervened by the DP agonists and antagonists
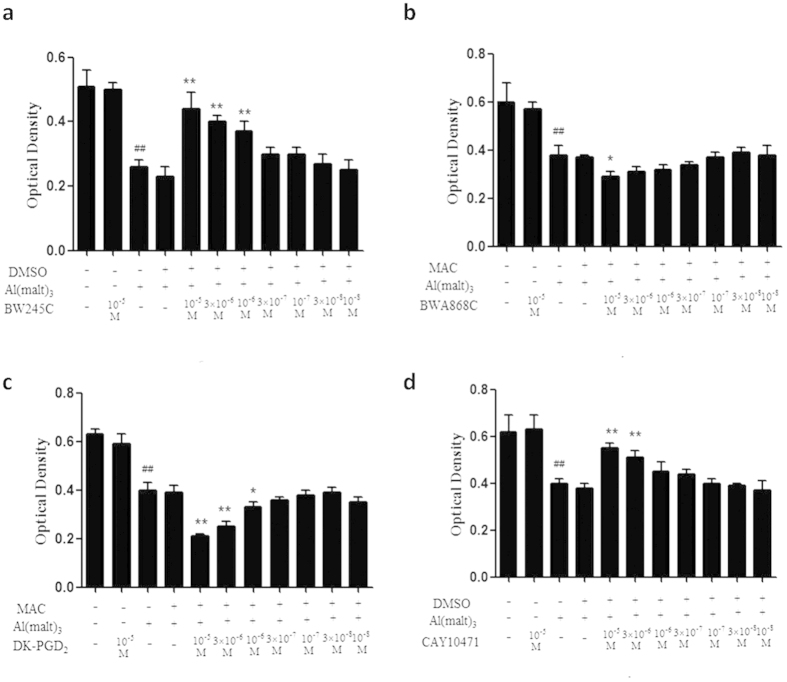
About the concentrations of the agonists and antagonists of DP, for the observation of neuronal viability, seven concentrations (10−5, 3 × 10−5, 10−6, 3 × 10−6, 10−7, 3 × 10−7, 10−8, 3 × 10−8 M) of each agonists and antagonists of DP were used. The results showed that compared with the control group, the neuron viability decreased significantly in model group (P < 0.01) and that treatment of DP1 agonists (BW245C) at a concentration of 10−6, 3 × 10−5 and 10−5 M increased significantly the neuron viability compared with model group, whereas the viability of neuron treated with DP1 antagonist (BWA868C) of 10−5 M was decreased significantly (P < 0.01). Compared with model group, the neuron viability in the DP2 antagonist (CAY10471) (10−6, 3 × 10−5 and 10−5 M) -treated group was increased substantial. The administration of DP2 agonists (DK-PGD2) at a concentration of 10−5 and 3 × 10−5 M decreased neuron viability significantly (P < 0.01). There was no considerable difference between the control group and the solvent control group (Fig. 5).
Figure 5. Effects of DP agonists and antagonists on the changes of neuronal viability caused by aluminium detected by the method of MTT.
(a) BW245C and (d) CAY10471 increased neuron viability in a concentration dependent manner in Al3+-treated group, whereas (b) BWA868C and (c) DK-PGD2 decreased neuron viability in Al3+-treated group. Values were mean ± SD of eight individual experiments (n = 8, ##P < 0.01 compared with control group, *P < 0.05 and **P < 0.01 compared with Al3+-treated group, respectively, one-way ANOVA with Dunnett’s multiple comparisons).
We found each agonist and antagonist of DP had significant effects on neuron viability at a concentration of 10−6, 3 × 10−5 and 10−5 M, so we used the three concentrations of 10−6, 3 × 10−5 and 10−5 M of each agonist and antagonist of DP in lactate dehydrogenase leakage rate test.
The change of LDH leakage rate intervened with the DP agonists and antagonists
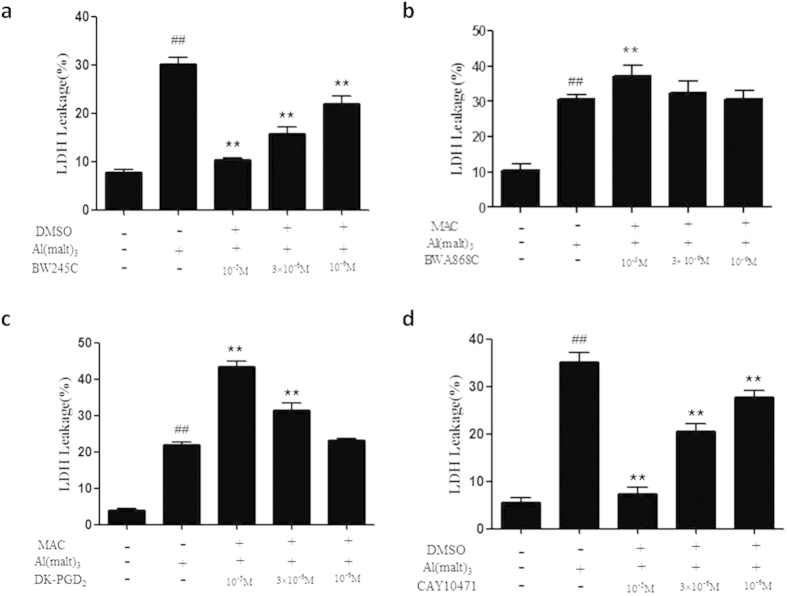
Compared with the control group, the solvent control group of primary cultured hippocampal neurons exhibited no considerable change in the LDH leakage rate, while the rate of LDH leakage rose significantly in the Al3+-treated group (P < 0.01). The DP1 agonist (BW245C) blunted the increase of leakage rate of LDH in Al (malt)3 –treated group in a concentration dependent manner (P < 0.01). The DP1 antagonist (BWA868C) of 10−5 M increased significantly the LDH leakage rate in Al (malt)3 –treated group (P < 0.01). The LDH leakage rate was increased significantly when treated with the DP2 agonists (DK-PGD2) at concentration of 10−5 M and 3 × 10−5 M (P < 0.01). The LDH leakage rate reduced significantly when treated with the DP2 antagonist (CAY10471) at different concentrations (10−5, 3 × 10−5 and 10−6 M) (P < 0.01) (Fig. 6).
Figure 6. The change of LDH leakage rate intervened with the DP agonists and antagonists.
(a) BW245C and (d) CAY10471 significantly decreased the LDH leakage rate in Al3+-treated groups. (b) BWA868C and (c) DK-PGD2 increased the LDH leakage rate in Al3+-treated groups. Values were mean ± SD of four individual experiments (n = 4. ##P < 0.01 compared with control group. **P < 0.01 compared with Al3+-treated group, one-way ANOVA with Dunnett’s multiple comparisons).
Considering that there were a dose-dependent effect of each agonist and antagonist of DP at concentration of 10−5, 3 × 10−5 and 10−6 M on neuron viability and LDH leakage by Al, so, only the 10−5 M of the agonists and antagonists of DP were used in the observation of change of the neuronal pathology, cAMP content and Ca2+ fluorescence intensity in Al3+-treated neurons.
The pathological change of primary cultured rat hippocampal neurons intervened with the DP agonists and antagonists
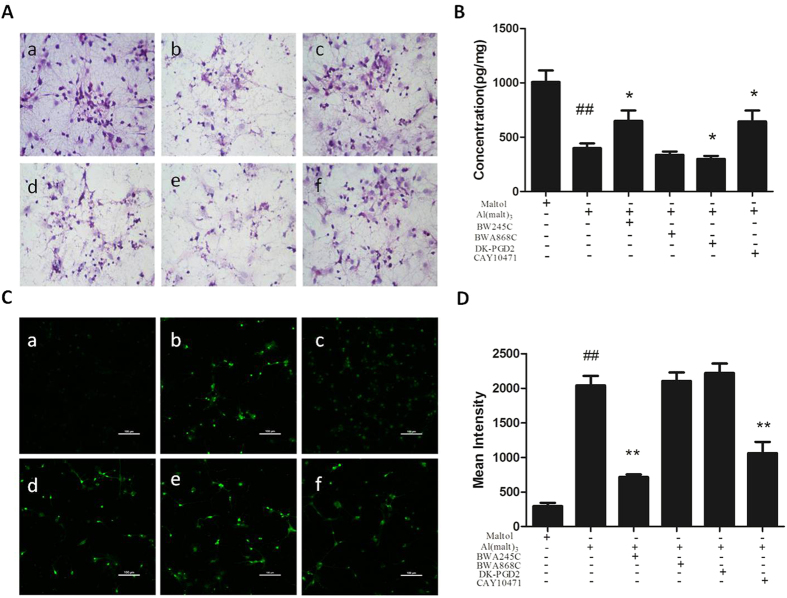
The structures of primary cultured hippocampal neurons were clear, complete and the protrusions of neurons were interwoven into the meshes in the control group. Compared with the control group, the number of neurons was substantial decreased and the protrusions were degenerated in the Al3+-treated group. Compared with the Al3+-treated group, the number of neurons increased and the structures recovered in BW245C-treated group. Treated with BWA868C, the neuronal injury was further aggravated compared with the Al3+-treated group. Treated with DK-PGD2, the hippocampal neurons almost exhibited karyopyknosis and disruption, whereas the bodies and nucleus of neurons were still clear and karyopyknosis compared with the Al3+-treated group. The neuronal injury in the Al3+-treated group was considerable reduced when treated with CAY10471 (Fig. 7A).
Figure 7. The effects of the DP agonists and antagonists on primary cultured rat hippocampal neurons.
(A)The picture showed the number and structure of neurons in (a) control group and (b) Al3+-treated group. (c) BW245C- treated group and (f) CAY10471-treated group raised the number and structure of neurons in model group, whereas (d) BWA868C-treated group and (e) DK-PGD2- treated group decreased the number and structure of neurons in the Al3+-treated group. Sections were pictured at ×400. (B) The intervention effect of DP on the cAMP level in the Al3+-treated group. Values were mean ± SD of six individual experiments. (n = 6, ##P < 0.01 vs. control group. *P < 0.05 vs. Al-treated group, one-way ANOVA with Dunnett’s multiple comparisons) (C) The picture showed the Ca2+ fluorescence of neurons in (a) control group and (b) model group, (c) BW245C and (f) CAY10471 decreased the Ca2+ fluorescence of neurons in model group. (d) BWA868C and (e) DK-PGD2 increased the Ca2+ fluorescence compared with model group (×400). (D) Summary graph showed the intensity of Ca2+ fluorescence relative levels in DP agonists and antagonists groups against model group. Values were mean ± SD of ten individual experiments (n = 10. ##P < 0.01 compared with control group. **P < 0.01 compared with Al3+-treated group, one-way ANOVA with Dunnett’s multiple comparisons).
The content of cAMP was detected by Enzyme-linked Immunosorbent Assay
The content of cAMP reduced significantly in the Al3+-treated group. Compared with the Al3+-treated group, the content of cAMP increased significantly when DP1 was activated or DP2 was inhibited (P < 0.05), whereas the content of cAMP showed a trend of decrease when DP1 was inhibited and the content of cAMP decreased significantly when DP2 was activated (P < 0.05) (Fig. 7B).
Effect of DP interventions on the Ca2+ fluorescence intensity of primary cultured rat hippocampal neurons
The intensity of Ca2+ fluorescence was very weak in the control group. However, the intensity of Ca2+ fluorescence was significantly increased in the Al3+-treated group compared with the control group (P < 0.01) (Fig. 7C). Compared with the Al3+-treated group, the Ca2+ fluorescence intensity was reduced significantly by treatment of BW245C (P < 0.01). Treated with BWA868C or DK-PGD2, the intensity of Ca2+ fluorescence slightly increased in primary cultured hippocampal neurons. In contrast, compared with the Al3+-treated group, treatment of CAY10471 decreased significantly the intensity of Ca2+ fluorescence (P < 0.01) (Fig. 7D).
Discussion
Aluminum accumulation may cause damage to cognitive function and central nervous system21. Maltol is a by-product of the hydrolysis of starch or sucrose, and it is also a common food additive22. Al (malt)3 can release Al3+ under physiological pH conditions eg in the gastrointestinal acid environment and facilitate the neurotoxicity23. Johnson et al. found that Al (malt)3 can cause apoptosis in primary cultured rat hippocampal neurons with time- and dose-dependent22. Our experiments showed that compared with the control group, MTT values were significantly decreased after treated with 100 μM Al (malt)3 for 24 hours in primary hippocampal neurons, while the LDH leakage rates were increased significantly. These results indicated that the injury model was successful.
COX and PGDS are the important rate-limiting enzymes for the synthesis of PGH2. PGDS has two subtypes, H-PGDS and L-PGDS. H-PGDS is mainly expressed in the placenta, lung, brain, and other tissues19,24. L-PGDS is the only trace protein in the lipocalin super family, which is mainly distributed in the brain and the reproductive organs and secreted into the cerebrospinal fluid, plasma and seminal fluid25,26. In recent years, it has been found that the expression of L-PGDS is related to cell apoptosis in the central nervous system27,28. The results of the present study showed that the expressions of L-PGDS mRNA and protein in the injury model induced by Al (malt)3 were significantly increased, suggested that L-PGDS may be involved in neuronal degeneration.
PGD2 is widely distributed and synthesized in the peripheral and central nervous systems. In the periphery, the main physiological functions of PGD2 contain the regulation of vascular diastolic and systolic pressure, and the inhibition of platelet aggregation29,30,31. In the brain, the main physiological functions of PGD2 contain the regulation of sleep, body temperature, olfactory function, sexual hormones and anti-anxiety effects32. In addition, PGD2 can significantly increase the contents of NGF (neuron growth factor) and BDNF (brain-derived neurotrophic factor), indicating that PGD2 may play a neuroprotective role in the CNS30. Our experimental results in this study showed that the PGD2 level increased significantly in the primary cultured rat hippocampal neurons, the main cause may be the intracellular calcium overload which sets out free radicals to activate phospholipase A2 (PLA2) which can produce more AA when Al is accumulated. At the same time, the expression of L-PGDS increased, and then the synthesis of PGD2 increased.
PGD2 acts as a potent positive modulator of DP receptors. There are two receptor subtypes for PGD2, DP1 receptor and DP2 receptor. DP1 is characterized by low expression, and it is mainly expressed in the hippocampus, cortex, hypothalamus and striatum of the brain tissue in addition to the peripheral circulation. Studies indicated that compared with the C57 mice, the susceptibility of C57 DP1 (−/−) mice to ischemia reperfusion injury was enhanced and the infarction area was significantly amplified33, suggesting that DP1 plays an obvious protective role in the ischemia reperfusion injury of brain. However, the protective mechanisms still unknown. DP2 is characterized by low expression, either, and it is widely distributed in the thalamus, cortex, hippocampus and other parts of the CNS. Studies reported that DP2 showed high expression and an excitotoxicity in normal hippocampal pyramidal neurons, and that DP2 agonist could significantly enhance the damage of the hippocampal CA1 and CA3 neurons caused by glutamate toxicity. In general, DP1 plays a protective role in the excitotoxicity and ischemic brain injury model, while DP2 accelerates the process of brain injury19.
In our study, we found that the content of PGD2 significantly increased after treated with aluminum in primary cultured rat hippocampal neurons as well as the expression of L-PGDS and DP1, whereas the expression of DP2 decreased. The results suggested that the PGD2-DP pathway may be involved in the injury of neurons. When DP1 was stimulated, the content of cAMP in the neurons and the release of Ca2+ increased, the barrier function of endothelial neurons enhanced34,35. However, research indicated that PGD2 promoted the aggregation of astrocytes in epileptic mice by activating DP136. Liang et al.19 reported that BW245C can significantly reduce the mortality of neurons caused by the treatment of primary hippocampal neurons and hippocampal slices with NMDA results in excitation injury. Ahmad et al.34 found that BW245C significantly enhanced the brain damage and magnified the cerebral infarction area caused by NMDA. Saleem et al.37 found that the viability of primary cultured mouse cortical neurons damage induced by glutamate was improved after treated with BW245C, whereas the viability of neurons showed non-significant changed after treated with BWA868C. However, these results indicated that the specific effects of DP1 and DP2 on neuronal damage are still unclear. Therefore, in our experiment, we intervened in the PGD2-DP pathway with DP agonists and antagonists to clarify the importance of this pathway in the injury progress of primary cultured rat hippocampal neurons induced by aluminum. In our study, compared with the Al (malt)3 treated groups, the number of hippocampal neurons significantly increased after treated with DP1 agonist (BW245C). At the same time, protrusions were interwoven into the mesh and the MTT values increased greatly, In addition, the LDH leakage rate and the intensity of Ca2+ fluorescence decreased significantly. In the DP1 antagonist (BWA868C)-treated group, hippocampal neuronal bodies ruptured and neuronal structures were incomplete, meanwhile MTT values reduced and the LDH leakage rate increased significantly although the changes of the intensity of Ca2+ fluorescence were not obvious in the BWA868C-treated group. Our experimental results are consistent with that of the study of Saleem et al.37. Moreover, it proclaimed that the survival rates of neurons can be increased by BW245C, while the opposite results were observed by BWA868C, suggesting that the expression and activation of DP1 could reduce the injury susceptibility of hippocampal neurons to aluminum toxicity.
Studies also reported that DP2 receptors were activated coupled to Gi protein and inhibited cAMP levels, increasing Ca2+ in neurons38. In our experiments, we found that all of the neurons in the DK-PGD2 (DP2 agonist)-treated group underwent death compared with model group. These data also showed that MTT value decreased, LDH leakage rate increased significantly and the intensity of Ca2+ fluorescence rose up. Karyopyknosis and disruption of neurons significantly reduced in the CAY10471 (DP2 antagonist) -treated group, while MTT values increased, and LDH leakage rates reduced. These results in the present study suggested that DP2 may mediate the neurotoxicity of PGD2.
However, it has also been reported that the DP2 antagonist BAY-u3405 does not effect on the injury model induced by PGD2, and it is speculated that DP2 may not mediate the neurotoxicity of PGD239. The contradictory effects of DP2 on neurons may be related to the neuron type and the degree of damage, and it needs further research in order to clarify its effect on neurons apoptosis in the over-expression DP2 or knockout mice. However, in this experiment, DK-PGD2 decreased the survival rates of neurons by activating DP2, whereas CAY10471 increased the survival rates by antagonizing DP2, indicating that the susceptibility of hippocampal neurons to aluminum neurotoxicity is increased by activating and expressing of DP2. This mechanism may be involved that its effect on DP receptor by regulating the Ca2+ signaling pathway, but the specific mechanism of the neural system is still unclear.
In conclusion, the expression of DP1 increased while DP2 decreased in the model induced by aluminum. DP1 expression and activation could decrease the injury susceptibility of hippocampal neurons to aluminum toxicity. The susceptibility of hippocampal neurons to aluminum neurotoxicity increased by activating and expressing of DP2. These results suggested that DP1 may protect the primary cultured hippocampal neuron from aluminum load damage, whereas DP2 may be harmful. DP can be considered as a potential candidate target for treatment of brain injury and neurodegenerative disease. However, considering the complex COX-2 downstream pathway, the complex regulation mechanism of DP in the central nervous system is worth our further study.
Methods
Animals
Rats were housed in the barrier housing facility, and it has in keeping with national standard “Laboratory Animal-Requirements of Environment and Housing Facilities”. The care of laboratory animal and the animal experimental operation have conforming to “Chongqing Administration Rule of Laboratory Animal”. The experimental procedures were approved by the animal laboratory administrative center and the institutional ethics committee of Chongqing Medical University (License number: SYXK YU 2012-0001) and also in accordance with the National Institutes of Health guidelines.
Chemicals
AlCl3·6H2O (Sinopharm Chemical Reagent Co., Ltd.,China) and maltol (Aladdin, USA) were of analytical grade. Aluminum maltol solution (20 mM) was prepared by adding 1.513 g maltol and 0.483 g of AlCl3 ·6H2O into 200 ml of autoclaved PBS. The 20 mM maltol aluminum was stored at 4 °C until used with the methods22,40. BW245C and CAY1047 (Cayman,USA) were dissolved in the DMSO (Sigma, USA) to be 10 mM reserve liquid. BWA868C and DK-PGD2 (Cayman,USA) were dissolved in methyl acetate to be 3.5 mM reserve liquid22.
Rat primary hippocampal neuron culture
Primary hippocampal neurons were prepared from E18 rat embryos and were immediately soaked with 75% ethanol. The hippocampus was isolated from the brain of each rat and the tissues were minced and digested with 0.125% trypsin at 37 °C for 20 min; digestion was stopped with the addition of 10% fetal bovine serum (FBS) (Gibco, USA). The neurons were centrifuged and suspended to a density of 1 × 106/L in DMEM (HyClone, USA) with 10% FBS in it. The different volumes of neuronal suspensions were inoculated in culture flasks and coated with L-poly lysine (Sigma, USA) and cultured in a humidified 5% CO2 atmosphere at 37 °C. When the neurons adhered, the medium was changed to neurobasal medium (Gibco, USA).
Neuron-specific enolase identification
Hippocampal neurons grown on glass cover slips were rinsed three times with PBS and fixed with 4% paraformaldehyde for 30 min at 4 °C and for 10 min at room temperature. The activity of endogenous peroxidase in the neurons was quenched with 3% H2O2 for 15 min. Goat serum (10%) was used as a blocking solution for 20 min at room temperature after three times washing. Then, neurons were incubated overnight at 4 °C with the appropriate dilutions of antibodies (NSE 1:50) (Boston, China). Afterward, the neurons were incubated with the second antibody (biotin-labeled goat anti-rabbit) for 30 min at 37 °C and with horseradish peroxidase-labeled avidin at 37 °C for 30 min. DAB (ZSGB, China) was used to analyze color development, and the samples were counterstained with hematoxylin, dehydrated with a series of graded alcohols, treated with xylene and sealed with neutral gum.
Establishment of models
On the seventh day, hippocampal neurons were divided into the control group, four solvent groups and four Al3+-treated groups. PBS was added into the control group, 100, 300, 600, 1200 μM of maltol was added into the solvent group, respectively. While 50, 100, 200 and 400 μM Al (malt)3 were added into the model group, respectively. After the experiment, the most suitable concentration of Al (malt)3 was selected in following experiments41.
PGD2 was detected by Enzyme-Linked Immunosorbent Assay (ELISA)
The rat hippocampal neurons were cultured in the flasks and divided into the control group and the Al3+-treated group until D7. After 24 h, neurons were collected after trypsin digestion. The levels of PGD2 was detected by ELISA kits (Cloud-Clone Corp, USA), following the manufacturer’s protocols.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
To determine the expressions of L-PGDS, DP1, and DP2 mRNA in the control and the model groups, total RNA was extracted from the neurons using RNAiso Plus reagent (Takara, China) to generate cDNA templates by reverse transcription (RT) kit (Takara, China) following the manufacturer’s instructions, and then amplified by using the MIX PCR kit (Cwbio, China). PCR products were separated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining. All the samples were normalized by the expression level of β-actin. The absorbance values of L-PGDS, DP1, DP2 and β-actin mRNA were measured with a Bio-Rad gel imaging analysis system (Bio-Rad, USA). The primer sequences for L-PGDS were: forward 5′-ATGTGCCAGACAGTGGTAGC-3′ and reverse 5′-TGGTCCTTGCTAAAGGTGATG-3′ (410 bp), DP1 (5′-TGAATGAGTCCTATCGCTGTC-3′ and 5′-GGTGATGTGCCTTTGGTAGAA-3′, 320 bp), DP2 (5′-CTTCCAAACCACAGCAACTC-3′ and 5′-CAGAGCATCAGGCAGACTC-3′, 326 bp), β-actin (5′-ACGGTCAGGTCATCACTATCG-3′ and 5′-GGCATAGAGGTCTTTACGGATG-3′, 155 bp).
Western blotting
Neurons from each group were homogenized within 10 volumes of ice-cold homogenization buffer and centrifuged at 12,000 × g for 10 min at 4 °C. The supernatant was collected, and the protein concentrations were determined with a BCA protein assay kit (Beyotime, China). Twenty micrograms of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membranes (Millipore, USA). The membranes were blocked with 5% non-fat dry milk for 1 h at room temperature and then probed with specific primary antibodies, including anti-L-PGDS, DP1, DP2 (1:200; Cayman, USA), and β-actin (1:1000; Boston, China) overnight at 4 °C. The membranes were washed for three times in TBST and incubated with HRP-conjugated secondary antibodies at room temperature for 1 h, and then washed four times in TBST, protein signals were visualized by ECL (Bio-Rad, USA). Quantification of data and subsequent statistical analyses were performed with Image Lab.
Detection of neuron viability
The primary cultured hippocampal neuronal viability was determined by 3-(4,5-Dimethyl-thiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) (Sigma, USA) assay. The neurons were plated in 96-well culture plates at a density of 1 × 105 neurons/ml. The neurons were divided into the control group, the solvent control group, the Al3+-treated group and the intervention group. Different drugs including DP1 agonist (BW245C), DP1 antagonist (BWA868C), DP2 agonist (DK-PGD2), and DP2 antagonist (CAY10471) were added up into the intervention group respectively with various concentrations (10−5, 3 × 10−5, 10−6, 3 × 10−6, 10−7, 3 × 10−7, 10−8, 3 × 10−8 M) for 24 hours at 37 °C and 5% CO2. Then, the supernatant was discarded and 100 μl of 5 mg/ml MTT was added to each well. The media was carefully removed and the color was developed after incubation with 150 μl DMSO for 4 hours. Finally, absorbance (OD) was read at 570 nm by a micro plate reader (BioTek, USA)42.
Lactate dehydrogenase (LDH) leakage rate measure
The cultured rat hippocampal neurons in the 24-well culture plate were cultured until D7 for drug treatment. The neurons were divided into the control group, the solvent control group, the Al3+-treated group and the intervention group. Different drugs including DP1 agonist (BW245C), DP1 antagonist (BWA868C), DP2 agonist (DK-PGD2), and DP2 antagonist (CAY10471) were added up into the intervention group respectively with various concentrations (10−5, 3 × 10−5, 10−6 M) for 24 hours at 37 °C and 5% CO2. Then, the LDH test kit was used according to the manufacturer’s instructions (Beyotime, China)42.
Observation of pathological morphology
Cover slips (10 mm × 10 mm) were applied to the 24 wells of rat hippocampal neurons from cultured until D7 for drug treatment. The neurons were divided into the control group, the solvent control group, the Al3+-treated group and the intervention group. 10−5 M of BW245C, BWA868C, DK-PGD2, and CAY10471 were added up into the intervention group, respectively. HE staining was performed on cultured cells after 24 h as previously described in detail. In brief, cells were rinsed with PBS, fixed with 4% paraformaldehyde (PFA) for 30 min and then washed by PBS. The neurons were stained with Hematoxylin-Eosin; afterwards, they were dehydrated in alcohols. Morphological changes of the neurons were observed under an optical microscope (Olympus, Japan) after mounted by neutral resins.
Content of cAMP detection by ELISA
The hippocampal neurons were divided into groups: the control group, the normal solvent group (300 μM of maltol), the normal intervention group (10−5 M BW245C, BWA868C, DK-PGD2, CAY10471) (Cayman, USA), the model group (100 μM Al(malt)3), the model solvent group (Al(malt)3 +10−3 M DMSO), and the model intervention group (Al(malt)3 + 10−5 M BW245C, BWA868C, DK-PGD2, CAY10471).
Concentration of Ca2+ detection
The cultured rat hippocampal neurons in the special culture dish were cultured until D7 for drug treatment. The neurons were divided into the control group, the solvent control group, the Al3+-treated group and intervention group, Different drugs including DP1 agonist (BW245C), DP1 antagonist (BWA868C), DP2 agonist (DK-PGD2), and DP2 antagonist (CAY10471) were added up into the intervention group at the concentration of 10−5 M, respectively for 24 hours at 37 °C and 5% CO2. The culture medium was removed, Fluo-3/AM was labeled with a fluorescent Ca2+ probe, the intensity of Ca2+ fluorescence was observed and measured with a laser scanning confocal microscope (Bio-Rad, USA). At the same time, 10 cells were randomly selected in the uniformity field of the fluorescence intensity to analyze the fluorescence intensity.
Statistical analysis
Data were presented as mean ± SD. All data were analyzed with SPSS 12.0 (SPSS Inc. Chicago, US) unless otherwise indicated. For the content of PGD2, statistical significance was determined by Student’s t test for pairwise comparisons. For RT-PCR, WB, LDH and MTT data, statistical significance was determined by one-way ANOVA with Dunnett’s multiple comparisons. p < 0.05 was considered statistically significant.
Additional Information
How to cite this article: Ma, J. et al. Effect of the PGD2-DP signaling pathway on primary cultured rat hippocampal neuron injury caused by aluminum overload. Sci. Rep. 6, 24646; doi: 10.1038/srep24646 (2016).
Acknowledgments
This reported study was supported by research grants from the Natural Science Foundation of China (No. 81070972 and No. 30672211).
Footnotes
Author Contributions J.Y. made substantial contribution to conception and design and performance of the study. Q.Y., Y.W., Y.Y., C.J., X.H., S.M., S.K., X.T., Y.L. and G.L. participated in performance of all in vivo experiments and carried out the data analysis. J.M. participated in performance of the study and in writing the manuscript. All authors read and approved the final manuscript.
References
- Amantea D., Nappi G., Bernardi G., Bagetta G. & Corasaniti M. T. Post-ischemic brain damage: pathophysiology and role of inflammatory mediators. FEBS J. 276, 13–26 (2009). [DOI] [PubMed] [Google Scholar]
- Chan P. H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 21, 2–14 (2001). [DOI] [PubMed] [Google Scholar]
- Fekete A., Vizi E. S., Kovacs K. J., Lendvai B. & Zelles T. Layer-specific differences in reactive oxygen species levels after oxygen-glucose deprivation in acute hippocampal slices. Free Radic Biol Med. 44, 1010–1022 (2008). [DOI] [PubMed] [Google Scholar]
- Kiewert C. et al. Role of glycine receptors and glycine release for the neuroprotective activity of bilobalide. Brain Res. 1201, 143–150 (2008). [DOI] [PubMed] [Google Scholar]
- Liu K., Yan M., Zheng X. & Yang Y. The dynamic detection of NO during the ischemic postconditioning against global cerebral ischemia/reperfusion injury. Nitric Oxide. 38, 17–25 (2014). [DOI] [PubMed] [Google Scholar]
- Oztanir M. N. et al. The beneficial effects of 18beta-glycyrrhetinic acid following oxidative and neuronal damage in brain tissue caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Neurol Sci. 35, 1221–1228 (2014). [DOI] [PubMed] [Google Scholar]
- Frisardi V. et al. Aluminum in the diet and Alzheimer’s disease: from current epidemiology to possible disease-modifying treatment. J Alzheimers Dis. 20, 17–30 (2010). [DOI] [PubMed] [Google Scholar]
- Shaw C. A. & Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 56, 304–316 (2013). [DOI] [PubMed] [Google Scholar]
- Dore S. et al. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 54, 155–162 (2003). [DOI] [PubMed] [Google Scholar]
- In t’ Veld B. A. et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 345, 1515–1521 (2001). [DOI] [PubMed] [Google Scholar]
- Yu L. et al. Time course change of COX2-PGI2/TXA2 following global cerebral ischemia reperfusion injury in rat hippocampus. Behav Brain Funct. 10, 42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J. Cyclooxygenase-2–specific inhibitors: are they safe? Am J Med. 110, 46S–49S (2001). [DOI] [PubMed] [Google Scholar]
- Whittle B. J. Mechanisms underlying intestinal injury induced by anti-inflammatory COX inhibitors. Eur J Pharmacol. 500, 427–439 (2004). [DOI] [PubMed] [Google Scholar]
- Jaturapatporn D., Isaac M. G., McCleery J. & Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev. 2, CD006378 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao C. M. & Breyer M. D. Physiologic and pathophysiologic roles of lipid mediators in the kidney. Kidney Int. 71, 1105–1115 (2007). [DOI] [PubMed] [Google Scholar]
- Narumiya S., Ogorochi T., Nakao K. & Hayaishi O. Prostaglandin D2 in rat brain, spinal cord and pituitary: basal level and regional distribution. Life Sci. 31, 2093–2103 (1982). [DOI] [PubMed] [Google Scholar]
- Medani M. et al. Prostaglandin D2 regulates human colonic ion transport via the DP1 receptor. Life Sci. 122, 87–91 (2015). [DOI] [PubMed] [Google Scholar]
- Chen C. P., Chen R. L. & Preston J. E. Age-related increase of prostaglandin D(2) synthase concentration and glycation in ovine cerebrospinal fluid. Exp Gerontol. 44, 639–645 (2009). [DOI] [PubMed] [Google Scholar]
- Liang X., Wu L., Hand T. & Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 92, 477–486 (2005). [DOI] [PubMed] [Google Scholar]
- Rohn T. T., Wong S. M., Cotman C. W. & Cribbs D. H. 15-deoxy-delta12,14-prostaglandin J2, a specific ligand for peroxisome proliferator-activated receptor-gamma, induces neuronal apoptosis. Neuroreport. 12, 839–843 (2001). [DOI] [PubMed] [Google Scholar]
- Molloy D. W. et al. Effects of acute exposure to aluminum on cognition in humans. J Toxicol Environ Health A. 70, 2011–2019 (2007). [DOI] [PubMed] [Google Scholar]
- Johnson V. J., Kim S. H. & Sharma R. P. Aluminum-maltolate induces apoptosis and necrosis in neuro-2a cells: potential role for p53 signaling. Toxicol Sci. 83, 329–339 (2005). [DOI] [PubMed] [Google Scholar]
- Bharathi et al. A new insight on Al-maltolate-treated aged rabbit as Alzheimer’s animal model. Brain Res Rev. 52, 275–292 (2006). [DOI] [PubMed] [Google Scholar]
- Wu D. C. et al. Protection against ischemic injury in primary cultured mouse astrocytes by bis(7)-tacrine, a novel acetylcholinesterase inhibitor [corrected]. Neurosci Lett. 288, 95–98 (2000). [DOI] [PubMed] [Google Scholar]
- Mohri I., Eguchi N., Suzuki K., Urade Y. & Taniike M. Hematopoietic prostaglandin D synthase is expressed in microglia in the developing postnatal mouse brain. Glia. 42, 263–274 (2003). [DOI] [PubMed] [Google Scholar]
- Blodorn B. et al. Expression of the beta-trace protein in human pachymeninx as revealed by in situ hybridization and immunocytochemistry. J Neurosci Res. 57, 730–734 (1999). [PubMed] [Google Scholar]
- Hoffmann A., Bachner D., Betat N., Lauber J. & Gross G. Developmental expression of murine Beta-trace in embryos and adult animals suggests a function in maturation and maintenance of blood-tissue barriers. Dev Dyn. 207, 332–343 (1996). [DOI] [PubMed] [Google Scholar]
- Fukuhara A. et al. Lipocalin-type prostaglandin D synthase protects against oxidative stress-induced neuronal cell death. Biochem J. 443, 75–84 (2012). [DOI] [PubMed] [Google Scholar]
- Taniguchi H. et al. Early induction of neuronal lipocalin-type prostaglandin D synthase after hypoxic-ischemic injury in developing brains. Neurosci Lett. 420, 39–44 (2007). [DOI] [PubMed] [Google Scholar]
- Matsugi T., Kageyama M., Nishimura K., Giles H. & Shirasawa E. Selective prostaglandin D2 receptor stimulation elicits ocular hypotensive effects in rabbits and cats. Eur J Pharmacol. 275, 245–250 (1995). [DOI] [PubMed] [Google Scholar]
- Angeli V. et al. Activation of the D prostanoid receptor 1 regulates immune and skin allergic responses. J Immunol. 172, 3822–3829 (2004). [DOI] [PubMed] [Google Scholar]
- Toyomoto M. et al. Prostaglandins are powerful inducers of NGF and BDNF production in mouse astrocyte cultures. FEBS Letters. 562, 211–215 (2004). [DOI] [PubMed] [Google Scholar]
- Gelir E., Arslan S. O., Sayan H. & Pinar L. Effect of rapid-eye-movement sleep deprivation on rat hypothalamic prostaglandins. Prostaglandins Leukot Essent Fatty Acids. 73, 391–396 (2005). [DOI] [PubMed] [Google Scholar]
- Ahmad A. S., Ahmad M., Maruyama T., Narumiya S. & Dore S. Prostaglandin D2 DP1 receptor is beneficial in ischemic stroke and in acute exicitotoxicity in young and old mice. Age (Dordr). 32, 271–282 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Kakizuka A., Aizawa M., Ushikubi F. & Narumiya S. Molecular characterization of a mouse prostaglandin D receptor and functional expression of the cloned gene. Proc Natl Acad Sci USA 91, 11192–11196 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A. N. & Breyer R. M. Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther. 103, 147–166 (2004). [DOI] [PubMed] [Google Scholar]
- Saleem S. et al. PGD(2) DP1 receptor protects brain from ischemia-reperfusion injury. Eur J Neurosci. 26, 73–78 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H. et al. Cutting edge: agonistic effect of indomethacin on a prostaglandin D2 receptor, CRTH2. J Immunol. 168, 981–985 (2002). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. U-shape suppressive effect of phenol red on the epileptiform burst activity via activation of estrogen receptors in primary hippocampal culture. PLoS One. 8, e60189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertholf R. L. et al. A long-term intravenous model of aluminum maltol toxicity in rabbits: tissue distribution, hepatic, renal, and neuronal cytoskeletal changes associated with systemic exposure. Toxicol Appl Pharmacol. 98, 58–74 (1989). [DOI] [PubMed] [Google Scholar]
- Chen T. J., Hung H. S., Wang D. C. & Chen S. S. The protective effect of Rho-associated kinase inhibitor on aluminum-induced neurotoxicity in rat cortical neurons. Toxicol Sci. 116, 264–272 (2010). [DOI] [PubMed] [Google Scholar]
- Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods. 96, 147–152 (2000). [DOI] [PubMed] [Google Scholar]