Contributions of foot sole tactile input to posture have been examined extensively; similarly, proprioceptive signals from the ankle have received attention. Our experiment examined the indirect influence of fast-adapting cutaneous input from the foot on proprioception at the ankle. Our results demonstrate that there is interplay between tactile (external) and proprioceptive (internal) signals in the lower limb. These data suggest the functions of cutaneous afferents in proprioception and tactile perception may not operate independently.
Keywords: proprioception, ankle joint, cutaneous afferent, mechanoreceptor, sensory
Abstract
It has previously been shown that cutaneous sensory input from across a broad region of skin can influence proprioception at joints of the hand. The present experiment tested whether cutaneous input from different skin regions across the foot can influence proprioception at the ankle joint. The ability to passively match ankle joint position (17° and 7° plantar flexion and 7° dorsiflexion) was measured while cutaneous vibration was applied to the sole (heel, distal metatarsals) or dorsum of the target foot. Vibration was applied at two different frequencies to preferentially activate Meissner's corpuscles (45 Hz, 80 μm) or Pacinian corpuscles (255 Hz, 10 μm) at amplitudes ∼3 dB above mean perceptual thresholds. Results indicated that cutaneous input from all skin regions across the foot could influence joint-matching error and variability, although the strongest effects were observed with heel vibration. Furthermore, the influence of cutaneous input from each region was modulated by joint angle; in general, vibration had a limited effect on matching in dorsiflexion compared with matching in plantar flexion. Unlike previous results in the upper limb, we found no evidence that Pacinian input exerted a stronger influence on proprioception compared with Meissner input. Findings from this study suggest that fast-adapting cutaneous input from the foot modulates proprioception at the ankle joint in a passive joint-matching task. These results indicate that there is interplay between tactile and proprioceptive signals originating from the foot and ankle.
NEW & NOTEWORTHY
Contributions of foot sole tactile input to posture have been examined extensively; similarly, proprioceptive signals from the ankle have received attention. Our experiment examined the indirect influence of fast-adapting cutaneous input from the foot on proprioception at the ankle. Our results demonstrate that there is interplay between tactile (external) and proprioceptive (internal) signals in the lower limb. These data suggest the functions of cutaneous afferents in proprioception and tactile perception may not operate independently.
afferent nerves innervating receptors in joints, muscle, and skin provide information about the position and movement of body segments, termed proprioception (2, 3, 6, 15, 16, 18, 38). Compromised feedback from these large-diameter sensory afferents has been shown to result in severe impairments to movement accuracy (26, 44), indicating that this proprioceptive feedback has a critical function in motor control. Proprioceptive feedback from the ankle joint, in particular, has an important contribution to the control of posture and gait (1, 32). Single-afferent recordings from human peripheral nerves provide evidence that both cutaneous and muscle spindle afferents innervating the anterior lower leg code for the direction and magnitude of ankle joint rotation (2, 3, 39). Additionally, these cues contribute to position and movement awareness; the activation of muscle spindles through tendon vibration has been shown to create the illusion of ankle joint movement (4), and the removal of skin feedback from the anterior ankle and foot dorsum has been shown to impair joint position sense (35).
Cutaneous afferents innervating skin on the feet also provide exteroceptive information; contact with the ground or objects. This sensory information also contributes to the control of posture and gait. For example, the activation of cutaneous mechanoreceptors in the soles of the feet using vibration has been shown to evoke characteristic postural sway responses during stance (31, 32, 36), and electrical activation of cutaneous afferents innervating the foot has been shown to alter lower limb trajectory during gait (50).
In the upper limb, it is known that cutaneous input generated by the stretch and compression of skin surrounding joints directly contributes to proprioception (16, 17). Interestingly, it has also been shown that the manipulation of cutaneous input from regions more remote to the test joint can influence proprioception; the ability to detect movement at the index finger has been found to be impaired by suprathreshold vibration, as well as electrical stimulation or light brushing of skin on adjacent digits or the thenar eminence (37, 47, 48). Conversely, subthreshold vibratory stimuli applied to various regions of the hand and wrist has been shown to improve tactile detection at the index finger and thumb in stroke survivors (21). Altogether, these results suggest tactile input even from remote skin regions can affect both proprioception and tactile perception; this demonstrates that there are interconnections between sensory inputs across the hand and wrist.
While this previous research has investigated how cutaneous input from regions across the hand interacts with proprioception in the upper limb (37, 47, 48), it is unknown whether cutaneous input from skin across different regions of the foot can influence proprioception in the lower limb. The importance of this question lies in the different functions of the hand and foot; cutaneous feedback from the hand is important for haptic perception and object manipulation, whereas, in the foot, skin input is critical for signaling ground contact for the control of posture and gait. The current experiment was thus devised to test whether cutaneous input from different regions of the foot can influence proprioception at the ankle joint. We assessed proprioception using a passive joint-matching task while applying cutaneous vibration to two different foot sole regions (heel and distal metatarsals) and one dorsal foot region (proximal aspect). Vibration was applied at two different frequencies intended to preferentially target Meissner's corpuscles [fast-adapting type I (FAI) afferents; 45 Hz] or Pacinian corpuscles [fast-adapting type II (FAII) afferents; 255 Hz]. The aim was to determine if proprioception was influenced predominantly by Pacinian input (47). It was hypothesized that only cutaneous input from high-frequency vibration would influence ankle proprioception. It was also hypothesized that vibration applied to the foot dorsum would have the most pronounced effect, since afferents innervating dorsal skin are directly involved in coding ankle position (2, 3).
METHODS
Subjects.
Twenty healthy young adults (10 men) [mean age (±SD) 22.6 ± 2.0 yr, height 1.73 ± 0.08 m, weight 70.8 ± 10.0 kg] participated in the study. One subject was left-foot dominant, and the remaining subjects were right-foot dominant, determined by the Waterloo Footedness Questionnaire (19). Subjects were free from neurological and musculoskeletal disorders. Subjects provided written, informed consent. All procedures conformed to the declaration of Helsinki and were approved by the University of Guelph Research Ethics Board.
Experimental setup.
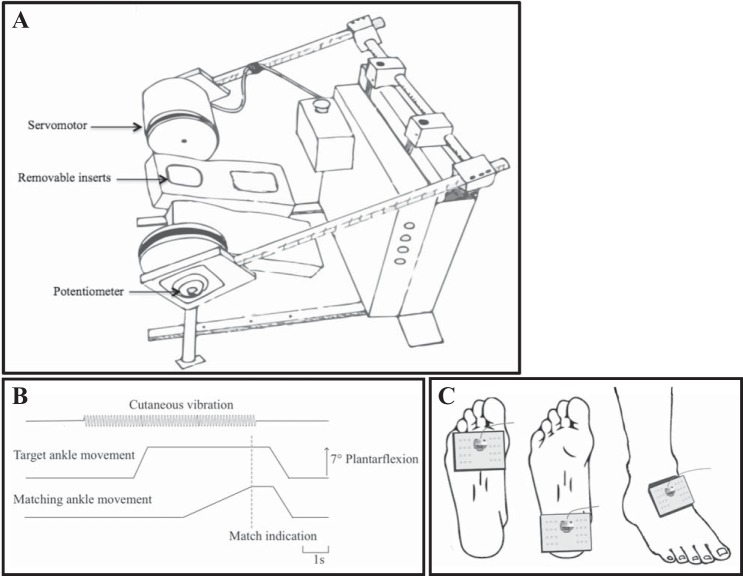
Subjects were seated with both feet secured to individual foot pedals using compliant elastic Velcro around the midfoot. Each foot pedal had 1 degree of freedom to rotate about the mediolateral axis in the sagittal plane; potentiometers in each pedal provided angular position information (sampled at 100 Hz) (Fig. 1A). The axes of rotation were aligned with the malleoli, and subjects' knees were restrained using Velcro straps to prevent movement. The foot pedals were lined with high-density foam to minimize cues from pressure changes under the foot during ankle movement. The left pedal had removable inserts under the heel and metatarsal regions to accommodate the custom-made vibration pads used to activate cutaneous mechanoreceptors. Rotation of the left pedal was controlled by a servomotor (Yokogawa Electric), while rotation of the right pedal was controlled by an experimenter. Calibrated electrogoniometers (Biometrics, SG110) were attached across the lateral borders of the ankles running just posterior to the malleoli to provide angular position information (sampled at 100 Hz).
Fig. 1.
A: experimental apparatus used to assess ankle proprioception using a passive joint-matching task. Subjects were seated with their feet secured to each foot pedal. Removable sections allowed the vibration pads to be inserted to stimulate the sole of the target foot. B: illustration of the timing of cutaneous vibration and rotation of the target and matching ankles for an example trial with a 7° plantar flexion (PF) target angle. C: custom-made vibration pads used to activate cutaneous afferents innervating skin on the target foot sole (heel or metatarsals) or foot dorsum. At each location, vibration was applied at 45 Hz (80 μm) and 255 Hz (10 μm).
All movements about the ankle joint were imposed passively to limit the contribution of efferent commands and active muscle force feedback to position sense, thereby increasing reliance on cutaneous and muscle spindle feedback. In addition, target angles within natural range of motion (∼60°) were chosen to limit joint receptor feedback, since evidence suggests joint receptors primarily respond at end ranges of motion (7, 8).
Subjects were instructed to relax their lower limb muscles, since the presence of voluntary muscle activity has been shown to bias perceived joint position (41, 46). To ensure the absence of muscle activity, surface electromyography (EMG) was measured from the tibialis anterior (TA) and soleus (SOL) muscles bilaterally using silver/silver-chloride (Ag/AgCl) electrodes placed on skin overlying the muscle bellies in bipolar arrangement. EMG signals were amplified (gain ∼1,000), band-pass filtered 10-1,000 Hz, and sampled at 2,048 Hz (Bortek AMT-8). EMG data were monitored online using Spike2 software (version 6, CED). In the case of noticeable muscle activity, subjects were given verbal feedback to reduce the activity, and the trial was repeated. In some cases, subjects were unable to fully relax their lower limb muscles; therefore, EMG data were recorded for offline analysis to ensure any low level of activity was consistent across conditions.
Ankle joint-matching task.
Before the joint-matching task, passive range of motion was determined independently for each ankle by rotating the ankle into full dorsiflexion (DF) and full plantar flexion (PF). Each matching trial began with the ankles at subjects' perceived neutral position (i.e., where they perceived they were neither dorsi- nor plantar flexed); this neutral position was ∼14° plantar flexed, on average, relative to a standard 90° position (i.e., foot perpendicular to the shank). Subjects had their eyes closed to eliminate visual cues and wore headphones to minimize auditory cues from the servomotor and vibration pads. The left (target) ankle was passively rotated by the servomotor to a target angle at a velocity of 10°/s and held. Then the right (matching) ankle was rotated by an experimenter at a velocity of ∼2°/s until the subject verbally indicated that both ankles were aligned (Fig. 1B). The purpose of moving each ankle at a different angular velocity was to prevent the use of timing to estimate joint position. Subjects were allowed to make up to two adjustments by giving verbal feedback to ensure they were confident in their final position. Three different target angles were assessed: 17° PF, 7° PF, and 7° DF. The order of target angles was randomized anew for each subject. Subjects were given six practice trials (two of each angle) before beginning the experiment. No feedback was given regarding matching accuracy.
The experiment began with a block of 12 control trials comprised of four repeats of each target angle to measure initial ankle proprioception. This initial control block was always performed first to ensure there were no contamination effects from trials with cutaneous vibration. One block of trials was then presented (in randomized order) for each cutaneous vibration condition. There were six vibration blocks total: a combination of three locations (heel, metatarsal, and dorsum) and two vibration frequencies (45 and 255 Hz; see below). Each vibration block was composed of 12 trials with cutaneous vibration and 6 trials without vibration. At the end of the experiment, six more control trials were performed to determine whether any learning effects or drift in perceived position occurred throughout the course of the experiment. There were 132 trials total (including practice). Subjects were given breaks between blocks to minimize fatigue.
Cutaneous stimuli.
Cutaneous afferents were mechanically activated using vibration applied through custom made pads molded to the foot using silicone rubber (Fig. 1C). Each vibration pad covered a surface area of 7 × 8 cm. Two different vibration frequencies (45 Hz, 80 μm; 255 Hz, 10 μm) were applied to target the activation of Meissner's corpuscles and Pacinian corpuscles, respectively. At each frequency, these amplitudes of vibration are ∼3 dB above mean perceptual thresholds measured from the heel and metatarsal regions of the foot sole from a separate sample of 18 healthy young adults during pilot testing (heel thresholds at 40 Hz, 31 μm and 250 Hz, 6 μm; metatarsal thresholds at 40 Hz, 27 μm and 250 Hz, 5.5 μm). These threshold data corroborated previous measurements at the foot sole using similar vibration frequencies (33). Vibration was applied to one of three locations (heel, distal metatarsals, and proximal dorsum) on the target foot, beginning ∼1 s before the onset of pedal movement and ending after the subject indicated both ankles were aligned.
Data analyses.
For each trial, root mean square (RMS) EMG was calculated for each TA and SOL muscle bilaterally during a time period beginning with the onset of target pedal movement and ending with the offset of matching pedal movement. Additionally, since an experimenter controlled rotation of the matching foot pedal, mean absolute angular velocity of the matching pedal was calculated from potentiometer data to ensure velocity was consistent across vibration and control conditions.
Using electrogoniometer data, directional error in ankle matching was calculated as the target minus the matching angle, such that positive values indicate that the target angle was undershot. This measure represents whether vibration induced a bias toward the perception of larger or smaller movement magnitudes. Absolute error was calculated as the absolute value of the directional error to indicate overall error, regardless of direction; this measure represents accuracy in calibrating sensory feedback to discern ankle position. Finally, variable error was calculated as the standard deviation in directional error among the four repeats of each angle. Increased variable error represents inconsistent integration and interpretation of the sensory feedback generated with movement and static position.
Statistical analyses.
Data were checked for normality and sphericity; a Greenhouse-Geisser correction was applied with violations of Mauchly's test of sphericity. A one-way repeated measures analysis of variance (ANOVA) was conducted on RMS amplitude of each muscle (TA and SOL bilaterally) to determine whether background muscle activity differed across vibration and control conditions. A one-way repeated-measures ANOVA was also conducted to determine whether the velocity of the matching pedal differed across vibration and control conditions.
To determine the effect of the vibration frequency on each dependent variable (absolute, directional, and variable error), a three-way repeated-measures ANOVA was conducted with the factors vibration “frequency” (45 Hz, 255 Hz), vibration “location” (dorsum, heel, metatarsals), and target “angle” (17° PF, 7° PF, 7° DF). Since there were no significant main or interaction effects of vibration frequency for any of the dependent variables, data were pooled across frequencies (45 and 255 Hz). To determine whether there were differences between the initial control trials and trials with vibration applied to different locations of the foot, a two-way repeated-measures ANOVA was conducted with the factors “location” (control, heel, dorsum, metatarsals) and “angle” (17° PF, 7° PF, 7° DF). Significant main or interaction effects of location were followed up with comparisons between each vibration location and the initial control condition, with a Bonferroni correction for multiple comparisons applied to the α-level of 0.05. Significant main effects of angle were also followed up with comparisons between each angle with a Bonferroni correction for multiple comparisons applied.
Since the control block was always performed before the cutaneous vibration blocks, six additional control trials were added at the end of the experiment to determine whether there were any order effects. For directional and absolute error, paired t-tests were performed comparing the initial and final control blocks.
RESULTS
There was a strong overall tendency to undershoot PF target angles with the matching ankle, particularly for the 17° PF position (seen as positive directional error of 4.0° for the control condition). In general, vibration of the heel and metatarsals further increased directional error by ∼1.5° from control (see Fig. 3A), and absolute error was increased during foot sole vibration by a similar margin (see Fig. 3B). Overall, the most pronounced effects of skin input on the perception of ankle joint position were observed with heel stimulation. Vibration of all three foot regions (dorsum, heel, and metatarsals) had a strong effect on variable error for the 7° PF position; where initial control error level was 0.7°, vibration increased error by ∼0.5° from control (see Fig. 3F). Subjects had the lowest baseline error (∼1.8°) and variability (∼0.8°) when matching the 7° DF target angle, and, interestingly, a decrease in absolute error of ∼0.5° was observed during dorsum and heel vibration conditions.
Fig. 3.
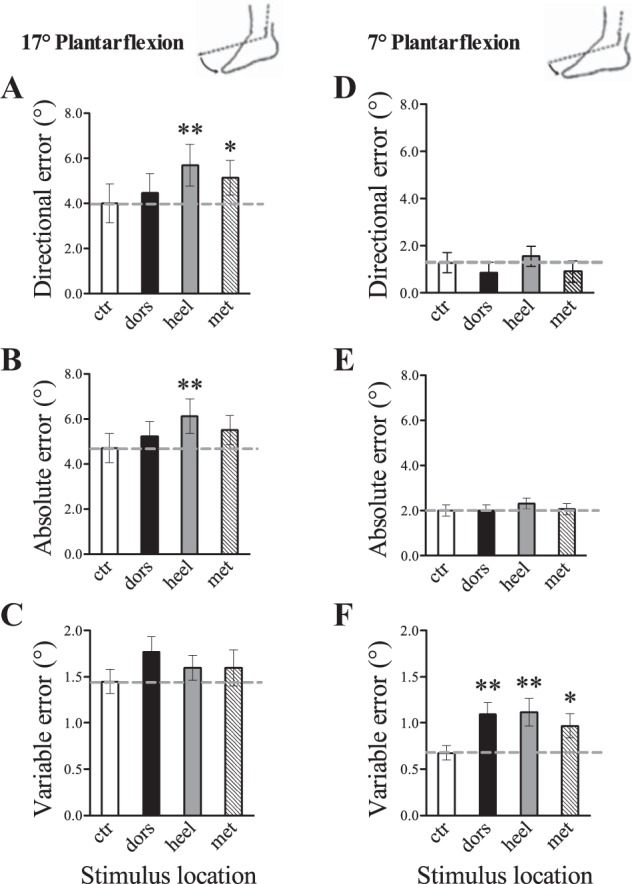
Effects of the location of cutaneous vibration on directional (A and D), absolute (B and E), and variable (C and F) error during a passive ankle joint-matching task. A–C: 17° PF. D–F: 7° PF. The dashed line represents initial control error level. Each bar represents the control condition or vibration location (ctr, control; dors, dorsum; heel, heel; met, metatarsal). Compared with control, vibration applied to the heel significantly increased directional and absolute error for the 17° PF angle, and increased variable error for the 7° PF angle. Vibration applied to the metatarsals significantly increased directional error for the 17° PF angle and variable error for the 7° PF angle. Vibration applied to the foot dorsum significantly increased variable error for the 7° PF angle. Values are means ± SE. *P < 0.05 and **P < 0.01 compared with control.
Mean (±SD) passive ranges of motion for the right and left ankles were 59.1 ± 10.3° and 57.3 ± 10.5°, respectively; therefore, ankle proprioception was tested at angles within ∼41% of full range of motion (24° testing range). RMS EMG amplitudes were not significantly different between conditions for the right SOL [F(6,114) = 1.217, P = 0.303], left SOL [F(6,114) = 1.140, P = 0.344], right TA [F(6,114) = 0.521, P = 0.792], or left TA [F(6,114) = 0.864, P = 0.524]. Mean matching pedal velocity (controlled by an experimenter) was 2.06 ± 0.35°/s; this velocity was consistent across vibration and control conditions [F(6,114) = 0.126, P = 0.993].
Effect of 45 vs. 255 Hz cutaneous vibration.
Two different vibration frequencies were used to target different cutaneous afferent subtypes. No significant main effects of vibration frequency were found for the dependent variables directional, absolute, or variable error (statistics reported in Table 1). In addition, there were no significant frequency by location interaction effects for any of the dependent variables, as well as no frequency by angle interaction effects (Table 1; Fig. 2). Therefore, since there was no evidence of a difference between the effects of low- and high-frequency vibration when applied at normalized intensities, data were pooled across the 45- and 255-Hz vibration frequencies for subsequent analyses.
Table 1.
Main and interaction effects of vibration frequency on ankle-matching direction, absolute, and variable error
| Directional Error | Absolute Error | Variable Error | |
|---|---|---|---|
| Frequency | F(1,19) = 0.009, P = 0.924 | F(1,19) = 0.867, P = 0.364 | F(1,19) = 0.159, P = 0.695 |
| Frequency by location | F(2,38) = 2.181, P = 0.322 | F(2,38) = 0.091, P = 0.913 | F(2,38) = 1.943, P = 0.159 |
| Frequency by angle | F(2,38) = 1.166, P = 0.322 | F(2,38) = 1.220, P = 0.306 | F(2,38) = 1.295, P = 0.286 |
Fig. 2.
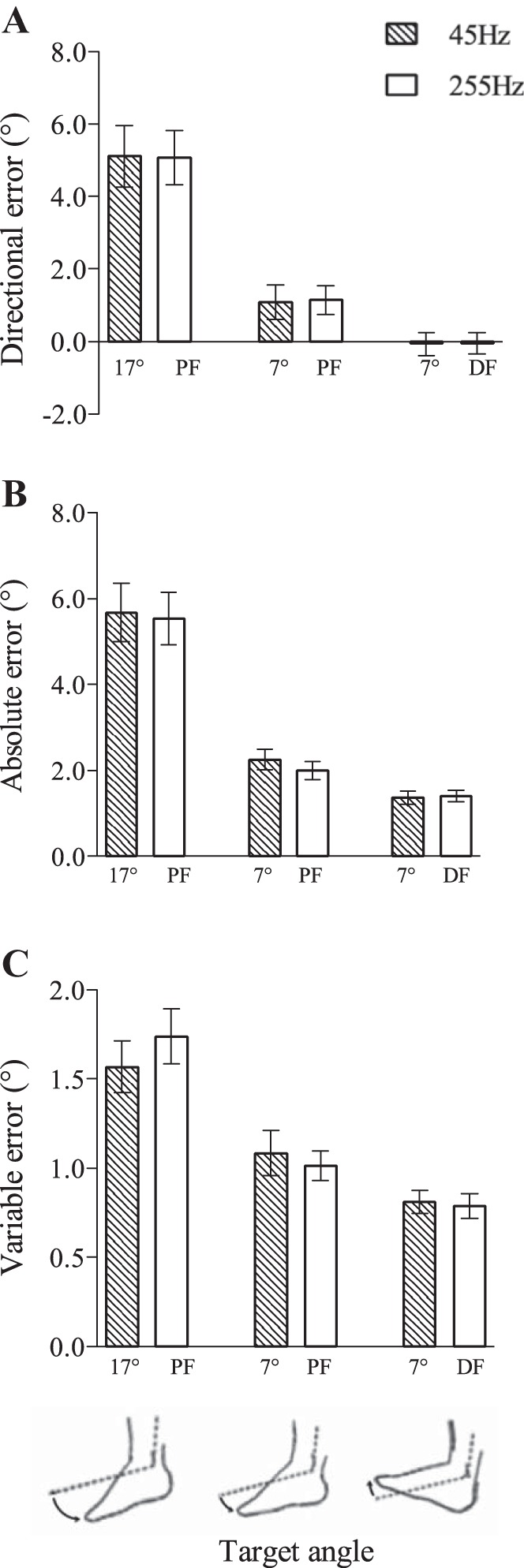
Frequency effects of cutaneous vibration applied to the foot on directional (A), absolute (B), and variable (C) error during a passive ankle joint-matching task. There were no significant differences between 45- and 255-Hz vibration on ankle-matching directional, absolute, or variable error. Values are means ± SE. DF, dorsiflexion.
Effect of heel, dorsum, and metatarsal vibration on ankle proprioception.
To assess how the location of vibration affected ankle proprioception, vibration was applied to the heel, metatarsals, or dorsum of the foot following the initial control (no vibration) condition. Our results showed a trend toward a main effect of vibration location on directional error [F(3,57) = 2.459, P = 0.072]; in general, across all angles it was observed that heel vibration caused the perception of smaller movement magnitudes (i.e., more undershoot error) relative to the control and other vibration conditions (Figs. 3 and 4). For the 17° PF angle, for example, directional error was 5.7° during heel vibration compared with 4.0° during control (Fig. 3A). There was a significant location by angle interaction effect for directional error [F(6,114) = 6.838, P < 0.001], indicating that the effect of the cutaneous vibration was modulated by joint angle. For example, in the large 17° PF angle, dorsum and metatarsal vibration caused greater target undershoot (directional error increased to 4.5° for dorsum and 5.1° for metatarsal vibration) relative to control (directional error = 4.0°), but not in the smaller 7° PF or DF angles. For the dependent variable absolute error, there was no significant main effect of vibration location [F(3,57) = 1.927, P = 0.136]; however, there was a significant location by angle interaction effect [F(6,114) = 6.114, P < 0.001]. It was observed that heel, metatarsal, and dorsum vibration increased absolute error in the larger 17° PF angle relative to control (Fig. 3B), had no influence at the smaller 7° PF angle (Fig. 3E), and actually reduced absolute error in the small 7° DF angle (Fig. 4B). Specifically during the 17° PF angle, absolute error was increased to 6.1° for heel, 5.5° for metatarsal, and 5.2° for dorsum vibration from the control error level of 4.7°. Finally, for variable error, there was a significant main effect of vibration location [F(3,57) = 3.801, P = 0.015], seen as an increase in variable error primarily during heel and dorsum vibration. Notably for the 7° PF angle, variable error was increased during dorsum (error = 1.1°), heel (error = 1.1°), and metatarsal (error = 1.0°) vibration relative to control (error = 0.7°). There was no significant location by angle interaction effect for variable error [F(6,114) = 1.512, P = 0.181].
Fig. 4.
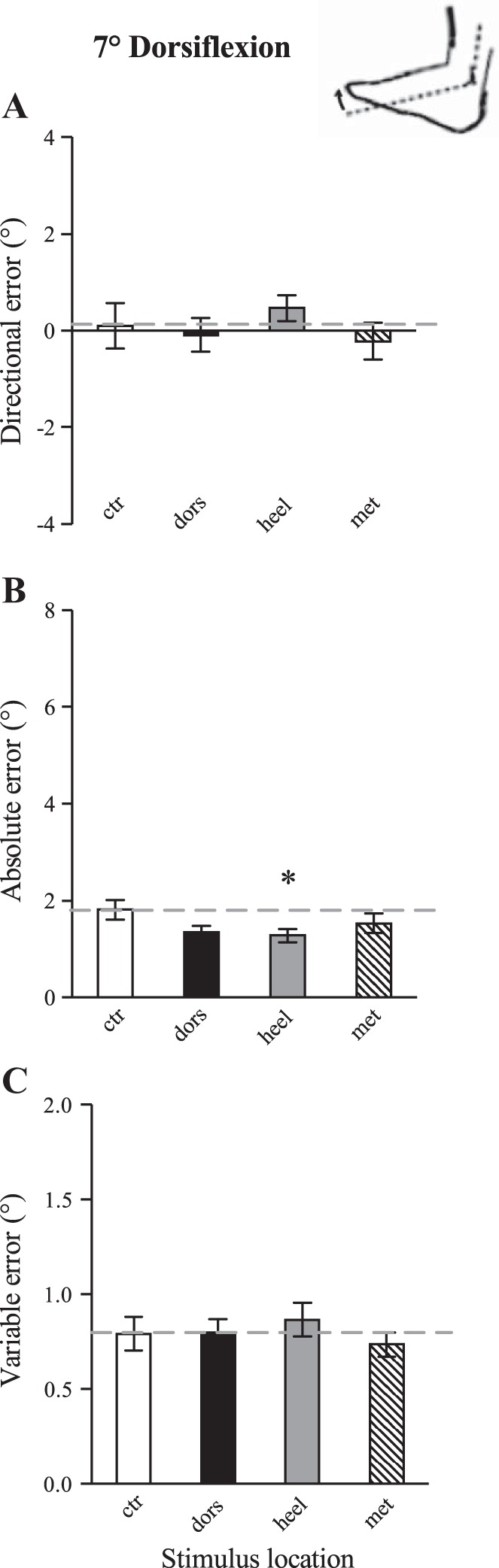
Effects of the location of cutaneous vibration on directional (A), absolute (B), and variable (C) error during a passive ankle joint-matching task for the 7° DF target angle. The dashed line represents initial control error level. Each bar represents the control condition or vibration location. Vibration applied to the heel resulted in significantly decreased absolute error compared with control. Values are means ± SE. *P < 0.05 compared with control.
Post hoc analyses confirmed that, compared with the initial control block, vibration applied to the heel resulted in significantly increased directional (P = 0.001) and increased absolute (P = 0.003) error for the 17° PF angle (Fig. 3); this indicates heel vibration caused more undershoot of the target angle and less accurate perception of joint position. Heel vibration also resulted in significantly increased variable error (P = 0.002) for the 7° PF angle, indicating lower matching consistency. Vibration applied to the metatarsals resulted in significantly increased directional error compared with initial control for the 17° PF angle (P = 0.014), and increased variable error compared with initial control for the 7° PF angle (P = 0.014). Vibration applied to the foot dorsum had no influence on directional or absolute error across all target angles; however, dorsum vibration resulted in significantly increased variable error compared with initial control for the 7° PF angle (P = 0.002). Cutaneous vibration had a limited influence on the perception of joint position in the 7° DF position; statistically heel vibration significantly decreased absolute error in 7° DF (P = 0.015) (Fig. 4).
Proprioception at different ankle positions.
There were significant main effects of target angle on directional error [F(1.06,20.17) = 22.838, P < 0.001], absolute error [F(1.07,20.29) = 37.512, P < 0.001], and variable error [F(2,36) = 26.717, P < 0.001], whereby the lowest error values were always observed at the 7° DF angle and the highest values at the 17° PF angle (Fig. 5). Pairwise comparisons confirmed directional error was significantly different between all three target angles (all P values < 0.01). For PF target angles, directional error was positive, indicating a tendency to undershoot the target angle, while for the DF target angle directional error was close to zero (Fig. 5A). Absolute error was significantly higher at the 17° PF angle compared with the 7° PF and 7° DF angles (P values < 0.001) (Fig. 5B). Variable error was also significantly higher at the 17° PF angle compared with the 7° PF and 7° DF angles (P values < 0.001) (Fig. 5C).
Fig. 5.
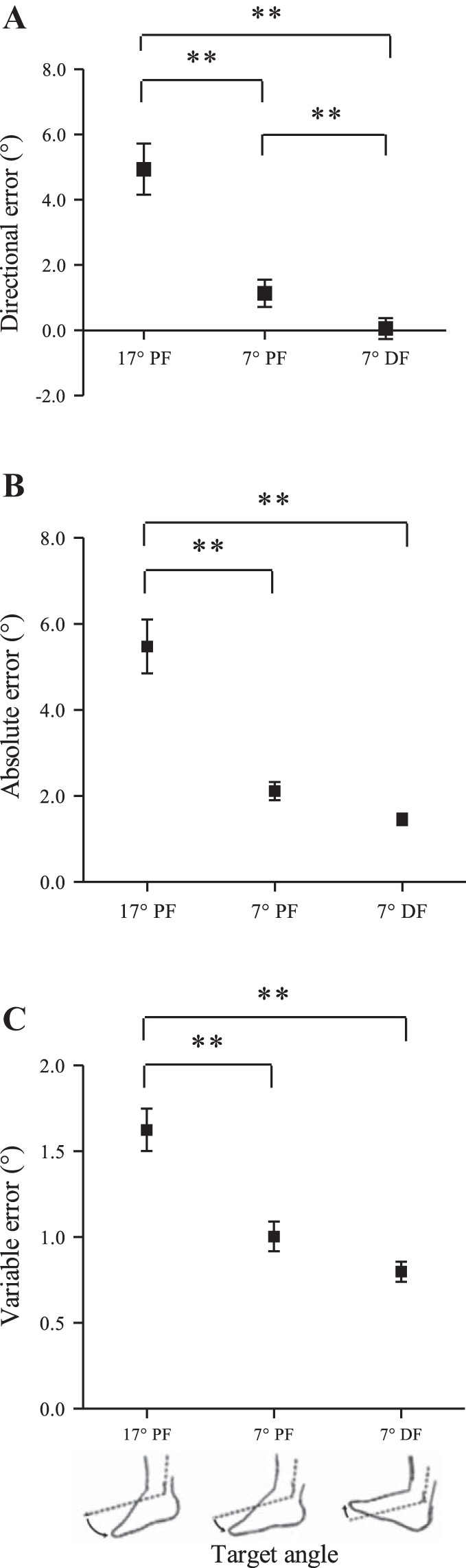
Overall mean (±SE) for directional (A), absolute (B), and variable (C) error measured for three different target angles during a passive ankle joint-matching task. Directional, absolute, and variable error were significantly higher at the 17° PF angle compared with the other angles. Directional error was significantly lower at the 7° DF angle compared with the same angle (7°) in PF. **P < 0.01.
Change in position bias from the initial to final control block.
Since the control block used to determine initial ankle proprioception was always performed before the cutaneous vibration blocks, control trials were also performed at the end of the experiment to determine whether there was a learning effect or drift in perceived ankle position following the vibration blocks. Directional error was significantly lower in the final control trials compared with the initial control block for the 7° PF angle (P = 0.019) (Fig. 6A). This indicates subjects undershot target angles less in the final control trials. Similarly, absolute error was significantly lower in the final control trials compared with the initial control block for the 17° PF angle (P = 0.004) and the 7° DF angle (P = 0.034) (Fig. 6B). This indicates that overall subjects were closer to the target angle in the final control trials.
Fig. 6.
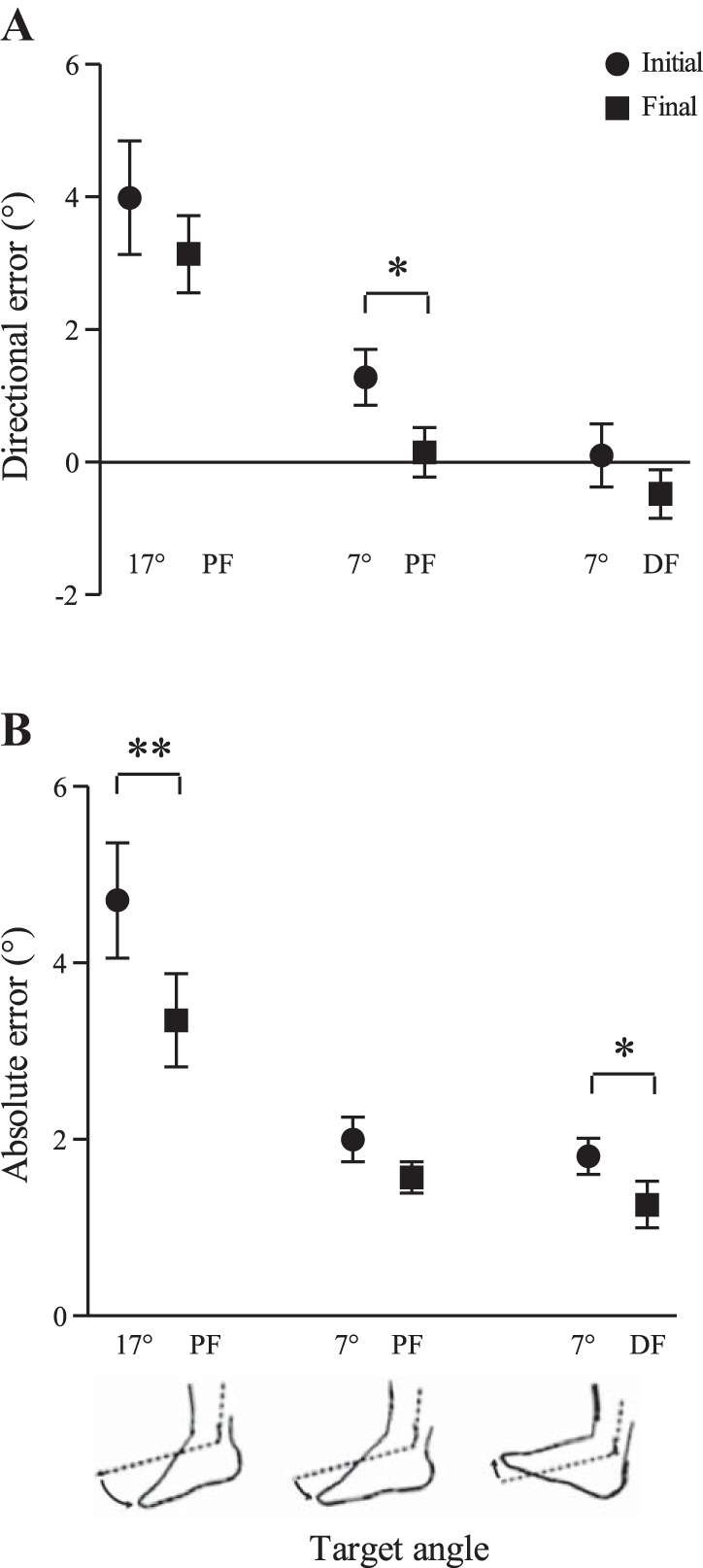
Comparisons between the initial and final control blocks for directional (A) and absolute (B) error in passive ankle joint matching. Directional error was significantly lower in the final control block for the 7° PF angle. Absolute error was significantly lower in the final control block for the 17° PF and 7° DF target angles. This indicates a drift toward less undershoot of the target angle and improved overall matching accuracy by the end of the experiment. Values are means ± SE. *P < 0.05; **P < 0.01.
DISCUSSION
The primary objective of this experiment was to determine whether cutaneous input from different regions of the foot could influence proprioception at the ankle joint. We showed that the perception of ankle joint position was indeed influenced by the activation of dorsal and plantar skin using mechanical vibration. Furthermore, the degree of skin interaction was related to the location of vibration and joint angle, where greater effects were seen with heel vibration and in PF ankle positions. Heel vibration was found to increase errors in matching ankle joint position by up to 1.7° (Fig. 3). In contrast, the specific frequency of vibration (low, 45 Hz; high, 255 Hz) had no distinct influence on the perceptual outcomes, arguing against a receptor-specific response. The observation of significant tactile interplay with proprioceptive information could have implications for functional control of the ankle joint.
Frequency of cutaneous vibration.
In the upper limb, it has been shown that cutaneous input from adjacent skin regions generated by high-frequency vibration (300 Hz; to target FAII afferents), but not lower frequency vibration (30 Hz; to target FAI afferents) interferes with proprioception (47, 48). In contrast, our results showed cutaneous input from the foot generated by both low (45 Hz) and high (255 Hz) frequency vibration induced a similar influence on proprioception at the ankle joint, with no evidence of a distinction between the two frequencies. In our study, the amplitudes of vibration were ∼3 dB above perceptual threshold; therefore, the relative stimulus intensities were similar for each frequency. In the upper limb work, vibration amplitudes have not been normalized to perceptual or afferent firing thresholds, making it difficult to determine whether the observed effects were specific to the cutaneous afferent subtype or the overall volume of cutaneous input. However, previous work in the hand has shown that, when the 30-Hz vibration was applied at a matched subjective intensity to the 300-Hz vibration, it still failed to influence proprioception (47). The ability of both FAI and FAII afferents in the foot to modulate proprioception at the ankle might suggest a similar importance of FAI and FAII afferents in sensing dynamic events at the foot. Despite subtle distinctions between the types of dynamic stimuli coded by FAI and FAII afferents, these fast-adapting afferents may share a similar ability to interact with other sensory information.
Activation of different skin regions.
Our results showed that, in particular, input from cutaneous mechanoreceptors in the heel had the strongest influence on ankle proprioception. Compared with the initial control trials, heel vibration increased directional and absolute error by 1.7° and 1.4°, respectively, when matching the larger PF angle and increased variable error by 0.4° when matching the smaller PF angle (Fig. 3). Overall, this suggests there are robust interactions between cutaneous input from the heel of the foot sole and proprioceptive signals from the ankle. Our results also showed evidence that cutaneous input from the metatarsal region influenced proprioceptive acuity; metatarsal vibration increased directional error by 1.1° when matching the larger PF angle and increased variable error by 0.3° when matching the smaller PF angle. It is possible that vibration of the metatarsals had a lesser effect compared with the heel because this skin region is more remote to the ankle joint, perhaps making this information less able to interact with ankle proprioceptive signals that are farther in proximity. However, the finding that stimulation of metatarsal skin still had an effect suggests fast-adapting cutaneous input, even from quite remote regions of the foot sole, has an ability to influence proprioception at the ankle. Although the observed effects were small, they are sufficient to have functional significance. For example, the toe clears the ground during gait by only ∼1.3 cm; thus precise awareness and control of the ankle joint is critical (49). Additionally, ankle proprioceptive feedback naturally provides the most sensitive means for detecting postural sway while standing, compared with the vestibular and visual systems (24). As a result, we believe the observed small increases in error with skin vibration are physiologically important. Finally, proprioceptive acuity is generally higher at the ankle joint compared with the arm, where detection thresholds have been found to be ∼10 times lower at the ankle relative to the elbow (24). High sensitivity to ankle movement likely reflects the functional relevance of this joint in posture and gait, where even minor positional errors are consequential (49).
Stimulation of skin on the foot dorsum was hypothesized to have the greatest influence on ankle proprioception due to the activation of cutaneous afferents either directly involved or in close proximity to those coding ankle movement. Our results showed dorsum vibration primarily increased variable error by 0.4° in both PF positions (Fig. 3); this indicates a poorer ability to integrate sensory feedback to determine ankle position. Since cutaneous mechanoreceptors in the foot dorsum and anterior shin have previously been shown to preferentially respond to movement that induces stretch of their receptive field (2), it was expected that cutaneous vibration would evoke a PF position bias. In contrast, our results showed that the manipulation of skin on the dorsum had no effect on directional error. The absence of a change in position bias following the activation of fast-adapting afferents from the foot dorsum might suggest that these afferents provide information about skin changes related to both stretch and compression, creating a confounding signal resulting in increased variable error. In support of this idea, in joints of the hand, local FAI afferents have been shown to respond to both flexion and extension movements (16). Importantly, there was a limitation to the current protocol with regard to vibration of the foot dorsum; vibration amplitudes were not normalized to thresholds at each skin location. It is known that threshold levels for perception are higher on the nonglabrous skin of the foot dorsum compared with the glabrous foot sole (45). As a result, the calculated vibration intensity may have been lower on the foot dorsum relative to the glabrous skin of the foot sole, resulting in a reduced influence of dorsum vibration on directional and absolute matching error.
Of note, in our experiment, the absence of a change in directional error with dorsum vibration suggests that spindles in dorsiflexor muscles were not activated, since this would create the illusion of muscle lengthening in PF (4) (whereas skin vibration produced undershoot of PF targets). In addition, the vibration amplitudes chosen (45 Hz, 80 μm; 255 Hz, 10 μm) are below the threshold of activating spindle afferents, both at rest and during a low-level background contraction (22). Even with vibration positioned deliberately over tendon (extensor digitorum longus or TA), it requires 100- to 300-μm amplitudes at frequencies between 40 and 120 Hz to modulate spindle activity (22). Thus spindle thresholds are generally higher than the amplitudes used in our experiment and substantially higher than skin receptor thresholds (where foot sole Pacinian corpuscles can respond to high-frequency vibration at ∼1 μm). Therefore, overall, we are confident that the vibration primarily affected cutaneous input.
Ankle proprioception at different angles.
The greatest ankle-matching accuracy was observed in the 7° DF position; this was demonstrated by smaller directional, absolute, and variable error compared with both PF positions (Fig. 5). Since subjects' neutral position was slightly plantar flexed, DF movements brought the ankle closer to 90° (i.e., foot perpendicular to the shank). Evidence in the upper limb suggests improved proprioception with the arms closer to the body midline (40), perhaps due to more experience manipulating objects close to the body. Similarly, ankle proprioception might be optimized at angles close to natural ankle position during stance (90°). Our results showed that cutaneous vibration of the foot sole and dorsum did not impair proprioception in the 7° DF position; in contrast, vibration actually decreased absolute matching error in 7° DF (improved position sense). While this decrease in absolute error is of interest to note, it is possible that this finding was a result of the learning effect observed over the course of the experiment. Alternatively, the lack of disturbance to proprioception during movements into DF could suggest humans are adapted to appropriately filter proprioceptive and tactile sensory feedback from the lower limb in this ankle position.
Sensory learning effect.
Ankle-matching error was lower in the control trials at the end of the experiment relative to the initial control block. It is interesting that this improvement occurred without experimenter feedback and without the involvement of a motor program (to allow for peripheral reafference mechanisms). Lower matching error in the final control trials was due to reduced undershooting of the target angles. Repeated exposure to the matching task may have led to the development of a better representation of ankle position within the limited range of target angles tested. This adaptation could have resulted from using information during the return back to the neutral position following the undershooting caused by the vibration stimulus. It is important to note that, even with this drift toward lower matching error over the course of the experiment, results were able to show that cutaneous vibration increased error and variability in the PF positions relative to the initial control block.
Potential mechanisms.
We did not include vibration at a remote skin site as a control condition to determine whether the change in position sense is due to attentional distraction by cutaneous vibration. However, the differences between the effects of heel, dorsum, and metatarsal vibration suggest that it is unlikely that our results are simply due to attentional distraction. In addition, previous work in the upper limb has shown that vibration of more remote skin locations has no influence on proprioception (47).
We assessed ankle proprioception using a passive joint-matching task to increase the reliance on cutaneous and spindle feedback. Therefore, we believe tactile input could be interacting with either spindle or cutaneous proprioceptive feedback. There is strong evidence supporting the contribution of both cutaneous (2, 35) and spindle (4) cues to the awareness of ankle position and movement. In our experiment, cutaneous vibration of the foot sole resulted in increased error due to undershooting the target angle. This undershoot bias could be due to an inhibition of cutaneous or spindle proprioceptive feedback, causing the perception of smaller movement magnitudes.
Inhibitory interactions have been demonstrated among cutaneous afferents innervating neighboring skin regions (10, 23, 34). For example, it has previously been shown that cutaneous input from one skin region increases tactile detection thresholds at neighboring regions (23). Additionally, recordings from dorsal column nuclei in cats have shown cutaneous input from one skin region inhibits the transmission of cutaneous information from neighboring regions (10). Therefore, in the present experiment, the decreased awareness of ankle position could be due to interactions between the cutaneous tactile input (from vibration) and cutaneous proprioceptive input (from skin surrounding the ankle). In the hand, it was shown that cutaneous vibration of surrounding regions interfered with movement detection when cutaneous and joint information were available (with muscle spindle feedback disengaged); however, cutaneous vibration did not significantly interfere with movement detection when only muscle spindle afferent information was available (with a digital nerve block to remove local skin feedback from the joint) (48). Therefore, it was suggested that, in the hand, cutaneous input from remote regions acts to impair movement detection primarily through interactions with other cutaneous afferent classes that signal movement of the test joint (48).
There is also evidence that cutaneous input can interact with muscle spindle input. Cutaneous and spindle afferents have been shown to provide similar population vector coding of ankle movement (2, 3). It has been suggested that this similarity facilitates coprocessing (3), making it likely muscle and skin information influence each other. Burke et al. (8) showed that the transmission of both cutaneous and spindle information to the cortex could be altered by conditioning stimuli applied to either muscle or skin; which provides evidence that cutaneous and spindle afferents interact within the ascending somatosensory pathway. These cutaneous-spindle interactions occurred whether the inputs were from the same or different nerves, with the degree of suppression primarily dependent on the magnitudes of the two stimuli (8). In addition, it has previously been shown that cutaneous input can modify spindle feedback specifically at the spinal level (5, 14, 20, 29). In reduced animal preparations; stimulation of skin on the foot pad was found to exert potent, short-latency effects on fusimotor drive to spindles, thereby changing spindle background firing and stretch responses (14, 20, 29). Admittedly, we are limited in our ability to interpret any cutaneous-fusimotor interactions since spindle thixotropy was not controlled for in this experiment. Future studies could exploit the thixotropic properties of spindles as a way to identify whether cutaneous-fusimotor interactions are present in humans under these conditions. To date, limited cutaneous-fusimotor interactions have been demonstrated in humans and only in a standing posture (5), not in a prone posture (25). Overall, this makes it unlikely cutaneous-fusimotor interactions at the spinal level were the mechanism for changes in proprioception in our experiment. However, there is still the possibility that other central mechanisms involving cutaneous-spindle interactions could be at play (8).
The results of our experiment suggest that tactile input can impair proprioception. Interestingly, it has also been demonstrated that proprioceptive signals generated by movement can impair tactile detection (11, 13, 28). Proprioceptive input generated by passive and active arm movement has been shown to interfere with the detection of cutaneous stimuli applied to the hand (11, 13), and the transmission of cutaneous information to the cortex (12). In combination with our results, this demonstrates that there is interplay between proprioceptive and tactile information that results in reduced input from both sources. This interplay might function to limit the sum of sensory feedback to be processed and interpreted centrally.
Conclusions and significance.
Cutaneous input from the sole and dorsum of the foot can affect proprioception at the ankle joint. The strongest effect was found with stimulation of the heel, suggesting more robust interactions between tactile input from the heel of the foot sole and proprioceptive signals from the ankle. Cutaneous input from the foot had a limited influence on proprioception at the ankle angle closest to its natural position during stance, which suggests humans are adapted to have heightened proprioception in this ankle position even in the presence of cutaneous sensory “noise” from the foot.
The contribution of tactile information from the foot sole to posture has been well established (31, 32, 36); likewise, proprioceptive signals from the ankle have received considerable attention (2, 3, 4, 35). Our results suggest there is interplay between tactile and proprioceptive signals in the lower limb. As recent work has emphasized the important roles of cutaneous feedback in both tactile perception and proprioception, it is important to understand that these functions may not work independently.
GRANTS
This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada Discovery Grant to L. R. Bent, and an Alexander Graham Bell Canada Graduate Scholarship to R. L. Mildren.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L.M. and L.R.B. conception and design of research; R.L.M. performed experiments; R.L.M. analyzed data; R.L.M. and L.R.B. interpreted results of experiments; R.L.M. prepared figures; R.L.M. drafted manuscript; R.L.M. and L.R.B. edited and revised manuscript; R.L.M. and L.R.B. approved final version of manuscript.
ACKNOWLEDGMENTS
Thank you to Cathy M. Hare for assistance with data collection and Dan Rose for technical assistance.
REFERENCES
- 1.af Klint R, Nielsen JB, Cole J, Sinkjaer T, Grey MJ. Within-step modulation of leg muscle activity by afferent feedback in human walking. J Physiol 586: 4643–4648, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol 580: 649–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aimonetti JM, Roll JP, Hospod V, Ribot-Ciscar E. Ankle joint movements are encoded by both cutaneous and muscle afferents in humans. Exp Brain Res 221: 167–176, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Albert F, Bergenheim M, Ribot-Ciscar E, Roll JP. The Ia afferent feedback of a given movement evokes the illusion of the same movement when returned to the subject via muscle tendon vibration. Exp Brain Res 172: 163–174, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Aniss AM, Diener HC, Hore J, Burke D, Gandevia SC. Reflex activation of muscle spindles in human pretibial muscles during standing. J Neurophysiol 64: 671–679, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans. I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res 134: 301–310, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Burgess PR, Clark FJ. Characteristics of knee joint receptors in the cat. J Physiol 203: 317–335, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol 402: 347–361, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke D, Gandevia SC, McKeon B, Skuse NF. Interactions between cutaneous and muscle afferent projections to cerebral cortex in man. Electroencephalogr Clin Neurophysiol 53: 349–360, 1982. [DOI] [PubMed] [Google Scholar]
- 10.Bystrzycka E, Nail BS, Rowe M. Inhibition of cuneate neurones: its afferent source and influence on dynamically sensitive “tactile” neurones. J Physiol 268: 251–270, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman CE, Beauchamp E. Differential controls over tactile detection in humans by motor commands and peripheral reafference. J Neurophysiol 96: 1664–1675, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Chapman CE, Jiang W, Lamarre Y. Modulation of lemniscal input during conditioned arm movements in the monkey. Exp Brain Res 72: 316–334, 1988. [DOI] [PubMed] [Google Scholar]
- 13.Cybulska-Klosowicz A, Meftah E, Raby M, Lemieux ML, Chapman CE. A critical speed for gating of tactile detection during voluntary movement. Exp Brain Res 210: 291–301, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Davey NJ, Ellaway PH. Facilitation of individual gamma-motoneurones by the discharge of single slowly adapting type 1 mechanoreceptors in cats. J Physiol 411: 97–114, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol 531: 289–297, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edin BB, Abbs JH. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol 65: 657–670, 1991. [DOI] [PubMed] [Google Scholar]
- 17.Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol 487: 243–251, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edin BB, Vallbo AB. Muscle afferent responses to isometric contractions and relaxations in humans. J Neurophysiol 63: 1307–1313, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Elias LJ, Bryden MP, Bulman-Fleming MB. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 36: 37–43, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Ellaway PH, Davey NJ, Ljubisavljevic M. Organization of the sural cutaneous input regulating the discharge of triceps surae gamma-motoneurones in the cat. Exp Physiol 82: 121–138, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Enders LR, Hur P, Johnson MJ, Seo JJ. Remote vibrotactile noise improves light touch sensation in stroke survivors' fingertips via stochastic resonance. J Neuroeng Rehabil 10: 105, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve 36: 21–29, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Ferrington DG, Nail BS, Rowe M. Human tactile detection thresholds: modification by inputs from specific tactile receptor classes. J Physiol 272: 415–433, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick R, McCloskey DI. Proprioceptive, visual, and vestibular thresholds for the perception of sway during standing in humans. J Physiol 478: 173–186, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandevia SC, Miller S, Aniss AM, Burke D. Reflex influences on muscle spindle activity in relaxed human leg muscles. J Neurophysiol 56: 159–170, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Hermsdorfer J, Elias Z, Cole JD, Quaney BM, Nowak DA. Preserved and impaired aspects of feed-forward grip force control after chronic somatosensory deafferentation. Neurorehabil Neural Repair 22: 374–384, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Inglis JT, Kennedy PM, Wells C, Chua R. The role of cutaneous receptors in the foot. Adv Exp Med Biol 508: 111–117, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Jiang W, Lamarre Y, Chapman CE. Modulation of cutaneous cortical evoked potentials during isometric and isotonic contractions in the monkey. Brain Res 536: 69–78, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Johansson H, Sjolander P, Sojka P, Wadell I. Effects of electrical and natural stimulation of skin afferents on the gamma-spindle system of the triceps surae muscle. Neurosci Res 6: 537–555, 1989. [DOI] [PubMed] [Google Scholar]
- 30.Johansson RS, Landstrom U, Lundstrom R. Responses of mechanoreceptive afferent units in the glabrous skin of the human hand to sinusoidal skin displacements. Brain Res 244: 17–25, 1982. [DOI] [PubMed] [Google Scholar]
- 31.Kavounoudias A, Roll R, Roll JP. Specific whole-body shifts induced by frequency-modulated vibrations of human plantar soles. Neurosci Lett 266: 181–184, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol 532: 869–878, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kekoni J, Hamalainen H, Rautio J, Tukeva T. Mechanical sensibility of the sole of the foot determined with vibratory stimuli of varying frequency. Exp Brain Res 78: 419–424, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Lipton ML, Liszewski MC, O'Connell MN, Mills A, Smiley JF, Branch CA, Isler JR, Schroeder CE. Interactions within the hand representation in primary somatosensory cortex of primates. J Neurosci 30: 15895–15903, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowrey CR, Strzalkowski ND, Bent LR. Skin sensory information from the dorsum of the foot and ankle is necessary for kinesthesia at the ankle joint. Neurosci Lett 485: 6–10, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Maurer C, Mergner T, Bolha B, Hlavacka F. Human balance control during cutaneous stimulation of the plantar soles. Neurosci Lett 302: 45–48, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Refshauge KM, Collins DF, Gandevia SC. The detection of human finger movement is not facilitated by input from receptors in adjacent digits. J Physiol 551: 371–377, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roll JP, Albert F, Ribot-Ciscar E, Bergenheim M. “Proprioceptive signature” of cursive writing in humans: a multi-population coding. Exp Brain Res 157: 359–368, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Roll JP, Bergenheim M, Ribot-Ciscar E. Proprioceptive population coding of two-dimensional limb movements in humans. II. Muscle-spindle feedback during “drawing-like” movements. Exp Brain Res 134: 311–321, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Schaap TS, Gonzales TI, Janssen TW, Brown SH. Proprioceptively guided reaching movements in 3D space: effects of age, task complexity and handedness. Exp Brain Res 233: 631–639, 2015. [DOI] [PubMed] [Google Scholar]
- 41.Smith JL, Crawford M, Proske U, Taylor JL, Gandevia SC. Signals of motor command bias joint position sense in the presence of feedback from proprioceptors. J Appl Physiol 106: 950–958, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. [DOI] [PubMed] [Google Scholar]
- 43.Toma S, Nakajima Y. Response characteristics of cutaneous mechanoreceptors to vibratory stimuli in human glabrous skin. Neurosci Lett 195: 61–63, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Torres EB, Cole J, Poizner H. Motor output variability, deafferentation, and putative deficits in kinesthetic reafference in Parkinson's disease. Front Hum Neurosci 8: 823, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trulsson M. Mechanoreceptive afferents in the human sural nerve. Exp Brain Res 137: 111–116, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Walsh LD, Proske U, Allen TJ, Gandevia SC. The contribution of motor commands to position sense differs between elbow and wrist. J Physiol 591: 6103–6114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weerakkody NS, Mahns DA, Taylor JL, Gandevia SC. Impairment of human proprioception by high-frequency cutaneous vibration. J Physiol 581: 971–980, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weerakkody NS, Taylor JL, Gandevia SC. The effect of high-frequency cutaneous vibration on different inputs subserving detection of joint movement. Exp Brain Res 197: 347–355, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Winter DA. Foot trajectory in human gait: a precise and multifactorial motor control task. Phys Ther 72: 45–53, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Zehr EP, Nakajima T, Barss T, Klarner T, Miklosovic S, Mezzarane RA, Nurse M, Komiyama T. Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sports Sci Med Rehabil 6: 33–1847, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]