Abstract
Short periods of weightlessness are associated with reduced stroke volume and left ventricular (LV) mass that appear rapidly and are thought to be largely dependent on plasma volume. The magnitude of these cardiac adaptations are even greater after prolonged periods of simulated weightlessness, but the time course during and the recovery from bed rest has not been previously described. We collected serial measures of plasma volume (PV, carbon monoxide rebreathing) and LV structure and function [tissue Doppler imaging, three-dimensional (3-D) and 2-D echocardiography] before, during, and up to 2 wk after 60 days of 6° head down tilt bed rest (HDTBR) in seven healthy subjects (four men, three women). By 60 days of HDTBR, PV was markedly reduced (2.7 ± 0.3 vs. 2.3 ± 0.3 liters, P < 0.001). Resting measures of LV volume and mass were ∼15% (P < 0.001) and ∼14% lower (P < 0.001), respectively, compared with pre-HDTBR values. After 3 days of reambulation, both PV and LV volumes were not different than pre-HDTBR values. However, LV mass did not recover with normalization of PV and remained 12 ± 4% lower than pre-bed rest values (P < 0.001). As previously reported, decreased PV and LV volume precede and likely contribute to cardiac atrophy during prolonged LV unloading. Although PV and LV volume recover rapidly after HDTBR, there is no concomitant normalization of LV mass. These results demonstrate that reduced LV mass in response to prolonged simulated weightlessness is not a simple effect of tissue dehydration, but rather true LV muscle atrophy that persists well into recovery.
Keywords: echocardiography, cardiac atrophy, cardiovascular physiology, space flight
the removal of gravitational forces by exposure to actual or simulated weightlessness (e.g., head-down-tilt bed rest, HDTBR) causes a relative shift of blood from the lower portions of the body into the thoracic region, neck, and head. This central redistribution of fluid transiently increases venous return, elevating cardiac output and stroke volume (SV) (5, 60). A compensatory cardiovascular unloading, including plasma volume loss (13, 27), decreased red blood cell mass (1, 17), and reduced systemic vascular resistance (47) acts to reduce ventricular wall stress and work load of the heart. Although these initial adjustments likely help protect against pressure overload-induced damage, it is believed that the consequent decreased preload and altered left ventricular (LV) function contribute to orthostatic intolerance (6, 39) and reduced exercise capacity (32, 40, 59) in humans upon termination of bed rest or return to Earth's gravity (1 G) after space flight.
Evidence of altered cardiac performance has been extensively reported after bed rest and space flight. Most notable is a marked decrease in LV SV at rest in response to orthostatic stress, during exercise within hours of a space mission landing (6, 7, 9, 32), and immediately after bed rest (4, 52, 53, 62). For example, using two-dimensional (2D) echocardiography to evaluate systolic and diastolic function, researchers reported that resting supine SV was at least 17% lower after 4- to 8-day missions aboard the Space Shuttle and was due, in part, to a smaller LV end-diastolic volume (9, 44). Investigations examining the mechanisms responsible for the reduction in SV following short-duration space flight have focused largely on adaptive responses that affect cardiac preload (12, 34), such as reduced plasma volume (8, 19). Results from our laboratory have confirmed these findings and further suggest that the degree of cardiac impairment depends on mission length, with larger decrements after prolonged periods of weightlessness (37, 39, 51).
The functional deficits manifested in the human heart after weightlessness likely result not only of smaller ventricular volumes but also cardiac muscle atrophy. Results from early radiographic studies and from conventional 2D echocardiography suggested that cardiac mass in some astronauts and subjects undergoing bed rest was smaller after real and simulated weightlessness (20, 28). Unfortunately, given the poor spatial resolution and geometric assumptions that each method carries, these estimates of morphological changes are considered unreliable. More recently, cardiovascular magnetic resonance (CMR) imaging has proven useful in assessing space flight- and bed rest-induced changes to the heart (14, 24, 50, 54), but its use is currently restricted to pre- and post-space flight measurements and to use during ground-based analogs. Newer matrix-array 3D echocardiography is not burdened by geometric assumptions that are inherent to 2D ultrasound technology, and analysis of LV morphology and function are comparable to that of CMR imaging (43, 65). Investigations into the direct cardiac effects of bed rest using either 3D echocardiography or CMR imaging have reported similar reductions in LV volumes (10, 14, 49). Thus 3D echocardiography may be a useful method for investigating and monitoring cardiac mass during space flight.
Although previous studies have examined the effects of space flight and bed rest on LV mass and volumes, a clear time course of these adaptations has not been fully described and their recovery has been largely ignored. Serial measures of LV mass in the same subjects during bed rest have been conducted in only one study that examined five subjects after 2 and 6 wk but only three subjects after 12 wk of bed rest (49). Thus the primary objective of this study was to quantify the changes in LV volumes and mass in the same subjects during and after prolonged 6° HDTBR using 3D echocardiography. We hypothesized that without countermeasures, subjects would experience LV atrophy during 60 days of HDTBR that would partially recover during reambulation and reconditioning. Additionally, we sought to determine whether any relationship existed between changes in LV mass and changes in plasma volume during and after HDTBR. Plasma volume losses in response to short-duration space flight and pharmacologically induced diuresis may explain the majority of measured decreases in LV mass as a result of a reduction in the fluid compartment of cardiac tissue (55, 56), but reduced LV mass after prolonged bed rest and space flight likely result from the combined effects of cardiac muscle atrophy and decreased tissue fluid. Furthermore, although evidence from several studies investigating cardiac-specific consequences of short-duration bed rest and space flight suggest that most adaptations are resolved within hours of gravitational reloading, we hypothesized that LV mass would lag behind the rapid recovery of plasma volume following prolonged bed rest.
METHODS
Subject selection.
Subjects were recruited by the Johnson Space Center Human Test Subject Facility and passed health and psychological screenings in addition to a National Aeronautics and Space Administration (NASA)-modified Air Force Class III physical examination before they were accepted into the study. All subjects were without history of cardiovascular disease or metabolic disorders and had not smoked within 6 mo before the start of the study. Additionally, each subject was evaluated for recent substandard nutritional status and use of medication that might interfere with the study results. Subjects received verbal and written explanations of bed rest and test protocols before they provided written informed consent. The study protocols were reviewed and approved by the NASA Johnson Space Center Committee for the Protection of Human Subjects, the University of Texas Medical Branch (UTMB) Institutional Review Board, and the UTMB General Clinical Research Center Advisory Committee.
Study design.
The study design and general bed rest conditions were described in detail previously (38). Briefly, all subjects were housed and supervised in the Flight Analog Research Unit at the UTMB Children's Hospital. After an 11- to 14-day study lead-in for acclimation and dietary/fluid stabilization, subjects were maintained in strict 6° HDTBR for 60 days. Subjects were required to meet minimum daily water consumption (28.5 ml/kg), but allowed to drink ad libitum beyond the minimum. Each subject received a standardized diet of ∼2,500 kcal/day that averaged <3,500 mg of sodium/day. Caloric intake was adjusted as needed to maintain body weight within ±3% of the weight measured on the third day of HDTBR. Echocardiography, plasma volume, and hemodynamic data collection before bed rest and during recovery was performed with subjects in the supine position, whereas data collection during bed rest was performed in the 6° head-down position. These measurements were performed after at least 20 min of quiet rest.
After 60 days of HDTBR were completed, subjects remained in the hospital for an additional 14 days for physical reconditioning before exiting the study. During the post-HDTBR reconditioning period, subjects participated in a daily 1-h program of supervised ambulation and exercise beginning on the fourth day of recovery. The intensity, duration, and complexity of the exercises increased throughout the reconditioning period according to subject tolerance of foot tenderness and knee and ankle pain secondary to prolonged HDTBR.
Echocardiography.
Two- and three-dimensional echocardiograms (iE33; Philips Ultrasound, Bothell, WA) were performed by registered sonographers following the guidelines of the European Association of Echocardiography/American Society of Echocardiography (30) with subjects in the left lateral decubitus position while supine (before and after HDTBR) or −6° head-down (during HDTBR). Images were obtained before HDTBR (baseline), during HDTBR on bed rest day 7 (BR7), BR21, BR31, and BR60, and during recovery after 4 h after reambulation (BR+4 hr), on R+3, and on BR+13. Images were stored digitally for offline analysis using commercially available software. Each image was independently analyzed by two sonographers. For all ultrasound measures, a difference in analysis of greater than 10% between sonographers was flagged for reanalysis by a third sonographer. All cardiac measurements were collected after a standardized low-fat meal and within a 2- to 4-h window across days to minimize circadian variability.
Three-dimensional echocardiography data were acquired using an X3-1 broadband xMATRIX array transducer. The wide-angled acquisition was obtained from four consecutive cardiac cycles during held respiration with acquisition triggered to the R wave and harmonic mode off. Imaging was optimized to include the entire LV cavity within the pyramidal scan volume to ensure accurate assessment of volumes. The analysis of 3D images was performed offline using reconstructive analysis software (QLab Version 7.1; Phillips) (23). LV mass was calculated as the product of myocardial volume (the difference between the epicardial and endocardial borders) and myocardial specific density (1.05 g/ml). Additional parameters calculated from the LV volumes included SV, cardiac output (CO), and ejection fraction (EF). SV was calculated as LVEDV − LVESV, cardiac output = heart rate × SV, and EF = (LVEDV − LVESV)/LVEDV.
Tissue and pulsed-wave Doppler imaging.
Standard 2D imaging and tissue Doppler echocardiography was performed using an S5-1 transducer to assess LV function as previously performed by our laboratory (37, 54). Estimates of LV filling pressure were calculated as the ratio between early transmitral flow velocity (E) and early diastolic velocity of the mitral valve annulus (E′) (25, 45, 46).
Plasma volume.
Plasma volume was measured five times during the study (before the start of HDTBR [baseline]; at 21 [BR21], 31, [BR31], and 60 days of HDTBR [BR60], and after 3 days of reambulation [R+3]) using the carbon monoxide rebreathing technique as previously reported by our laboratory (51, 61). Briefly, the subjects breathed 100% oxygen for 2 min through a closed breathing circuit fitted with a two-way nonrebreathing valve (Model 2700; Hans Rudolph, Kansas City, MO). Carbon monoxide (60 ml) was then added to the system and was rebreathed for 10 min. Before and after injection of carbon monoxide, a 3-ml blood sample was collected for measurement of hemoglobin, carboxyhemoglobin, and hematocrit. Total blood volume, red blood cell volume, and plasma volume were calculated (35). Similar to echocardiography, plasma volume was measured in subjects while they were supine (before and after HDTBR) or −6° head-down (during HDTBR).
Cycle ergometry testing.
Peak exercise oxygen consumption (V̇o2 peak) was measured using upright cycle ergometry administered four times during the course of participation in this study. The first test performed served as a familiarization session and was performed 10-12 days before the start of HDTBR. V̇o2 peak was measured again 7 days before the start of HDTBR, which served as the baseline measurement. After HDTBR, testing was conducted either at the end of the first day or in the morning after 1 day of reambulation. The fourth test was conducted ∼10 days after the last day of HDTBR. The exercise test protocol consisted of 3-min stages at either 50, 75, and 100 W or 50, 100, and 150 W followed by 1-min stages increasing 25 W per stage until a subject reached volitional fatigue or he or she could no longer maintain the desired pedaling cadence.
Data analysis and statistics.
Our main dependent variables (except for plasma volume and V̇o2 peak) were assessed once before HDTBR, four times during HDTBR (BR7, BR21, BR31, and BR60), and three times after HDTBR (R+4 hr, R+3, and R+13). Separate mixed-effects linear regression models were used to evaluate the effects of HDTBR on each of our dependent variables of interest using dummy-coded beta coefficients for time allowing us to compare differences relative to baseline. Bootstrap resampling was performed to improve estimates of variance given the small sample size (16). Subject body surface area, calculated using the Mosteller formula (43), was included as a fixed covariate as needed to improve model precision for 3D echocardiography measures. We used the Holm (26) correction for multiple comparisons between baseline and each bed rest and recovery time point. Summary data are expressed as means ± SE unless otherwise stated. All statistical analyses were performed using Stata/IC software (v13.1; StataCorp LP, College Station, TX) and setting two-tailed alpha to reject the null hypothesis at 0.05.
RESULTS
Study population.
Eight subjects (4 men and 4 women, age 36 ± 8 yr) volunteered to participate in this study. One subject (a woman) was excluded from the analysis due to poor acoustic windows. Selected subject characteristics of the remaining seven subjects are presented in Table 1. Plasma volume (−16%) and V̇o2 peak (−31%) decreased following HDTBR (P < 0.0001 for both as measured on BR60). Mean body weight, although statistically lower than it was before bed rest, remained within ±3% of BR3 body weight.
Table 1.
Subject characteristics measured before, during, and after bed rest
| Baseline | 7 Days | 21 Days | 31 Days | 60 Days | R+4 hr | R+3 | R+13 | |
|---|---|---|---|---|---|---|---|---|
| Weight, kg | 71.2 ± 2.8 | 70.5 ± 2.9* | 70.0 ± 2.8* | 69.8 ± 2.9* | 69.9 ± 2.9* | 70.2 ± 2.9* | 71.2 ± 3.0* | 71.4 ± 3.0 |
| BMI, kg/m2 | 22.6 ± 0.9 | 22.4 ± 0.9* | 22.2 ± 0.9* | 22.2 ± 0.9* | 22.2 ± 0.8* | 22.3 ± 0.8* | 22.6 ± 0.9 | 22.6 ± 0.9 |
| SBP, mmHg | 114 ± 2 | 111 ± 2 | 114 ± 2 | 109 ± 2 | 114 ± 3 | 114 ± 3 | 114 ± 3 | 112 ± 3 |
| DBP, mmHg | 65 ± 2 | 64 ± 2 | 69 ± 2 | 66 ± 3 | 65 ± 2 | 65 ± 2 | 69 ± 3 | 67 ± 3 |
| MAP, mmHg | 81 ± 1 | 80 ± 2 | 84 ± 2 | 80 ± 2 | 82 ± 2 | 82 ± 2 | 84 ± 3 | 82 ± 2 |
| PV, liter | 2.7 ± 0.4 | 2.3 ± 0.1* | 2.3 ± 0.1* | 2.3 ± 0.1* | 2.7 ± 0.1 | |||
| V̇o2peak, l/min | 2.1 ± 0.1 | 1.4 ± 0.4* | 1.8 ± 0.4 |
Values are means ± SE. R+4 hr, 4 hours after reambulation; R+3, 3 days after reambulation; R+13, 13 days after reambulation;
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PV, plasma volume.
Statistically different than before bed rest and after correction for multiple comparisons.
LV morphologic and functional responses to bed rest deconditioning.
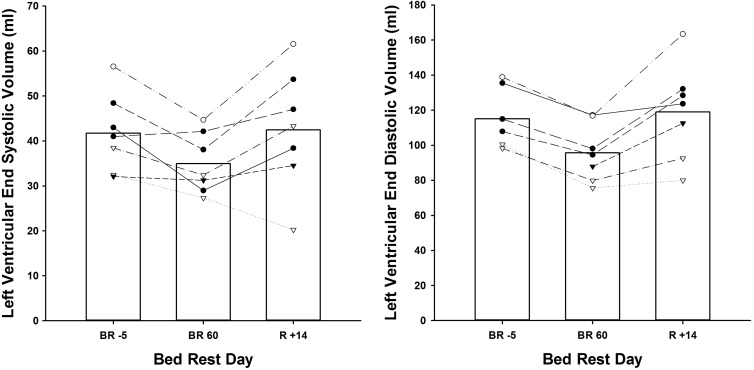
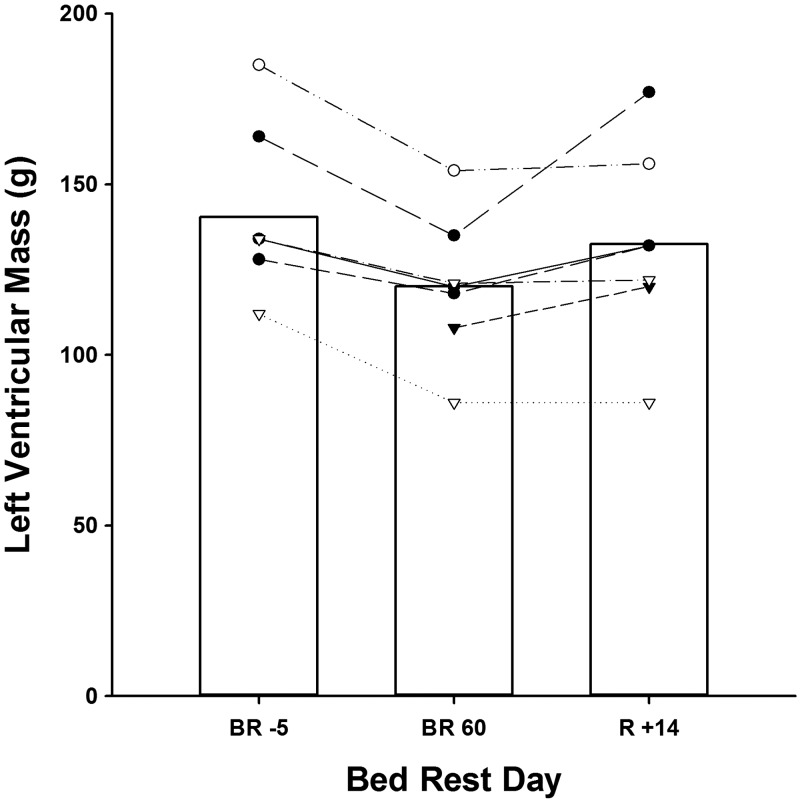
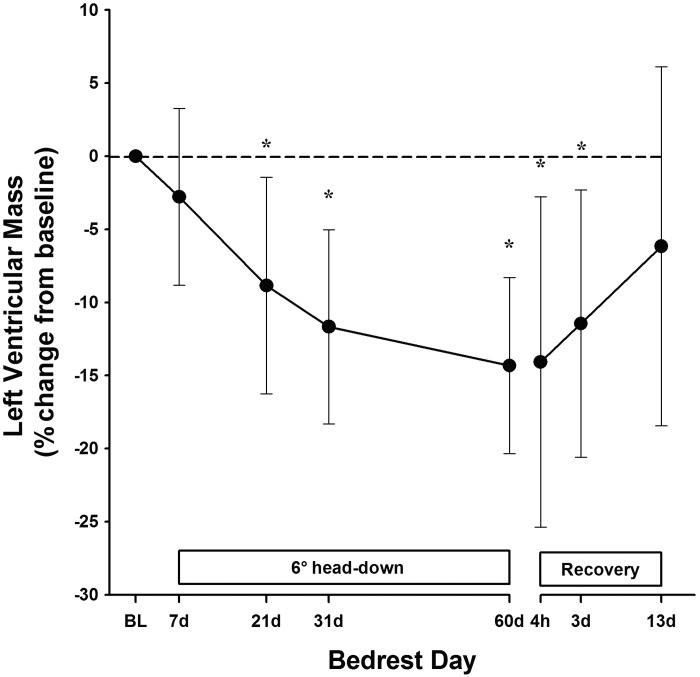
Before bed rest, 3D echocardiographic measurements of LV mass and LVEDV, but not LVESV, were statistically lower in women compared with men. However, changes in these cardiac indices with bed rest and adjusted for body surface area were similar between sexes (group data by sex not shown, Fig. 1). Compared with before bed rest, LVEDV was significantly reduced as early as BR21 (−6%; Table 2) and continued to decline throughout the duration of HDTBR. In contrast, LVESV was not significantly different than pre-HDTBR values until BR60, when both LVEDV and LVESV were reduced by ∼15% (P < 0.001 and P = 0.004, respectively). LV mass, like LVEDV, was significantly lower by BR21 (P = 0.007), reaching a nadir of ∼14% (range, −22.9 to −8.1%; P < 0.001) or a loss of ∼20 g by BR60 (Figs. 2 and 3).
Fig. 1.
Left ventricular (LV) end systolic and LV end-diastolic volume at baseline, after 60 days of head-down tilt bed rest (HDTBR), and after 14 days of active recovery in seven subjects. Bars represent mean group values; lines represent individual subject responses.
Table 2.
Resting hemodynamic and ventricular parameters before, during, and after bed rest
| Baseline | 7 Days | 21 Days | 31 Days | 60 Days | R+4 hr | R+3 | R+13 | |
|---|---|---|---|---|---|---|---|---|
| HR, bpm | 68 ± 5 | 66 ± 2 | 67 ± 3 | 72 ± 2 | 74 ± 2 | 86 ± 5* | 78 ± 3 | 77 ± 2 |
| SV, ml | 72.3 ± 3.8 | 72.5 ± 4.6 | 69.8 ± 3.3 | 67.6 ± 2.8 | 60.8 ± 4.4 | 61.7 ± 4.3 | 66.8 ± 3.3 | 76.6 ± 5.4 |
| CO, l/min | 5.0 ± 0.4 | 4.6 ± 0.4 | 4.7 ± 0.2 | 5.0 ± 0.2 | 4.5 ± 0.3 | 5.1 ± 0.3 | 5.2 ± 0.4 | 5.9 ± 0.4 |
| EF, % | 62.8 ± 1.4 | 64.9 ± 1.6 | 64.2 ± 0.8 | 64.3 ± 1.0 | 63.1 ± 1.9 | 62.8 ± 1.3 | 62.2 ± 1.3 | 64.7 ± 2.2 |
| LVESV, ml | 41.7 ± 2.6 | 38.7 ± 1.9 | 38.8 ± 1.7 | 37.4 ± 1.6 | 35.0 ± 2.1 | 36.2 ± 2.0 | 40.9 ± 2.6 | 42.5 ± 4.2 |
| LVEDV, ml | 115.1 ± 5.0 | 111.2 ± 5.4 | 108.7 ± 4.6* | 105.2 ± 3.8* | 95.8 ± 5.0* | 97.9 ± 5.6* | 107.7 ± 5.3 | 119.0 ± 8.7 |
| LVM, g | 140.4 ± 7.9 | 134.0 ± 6.9 | 126.6 ± 6.4* | 124.0 ± 5.5* | 120.1 ± 6.2* | 121.7 ± 7.2* | 124.6 ± 7.9* | 132.5 ± 9.2 |
Values are means ± SE.
HR, heart rate; bpm, beats per minute; SV, stroke volume; CO, cardiac output; EF, ejection fraction; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass.
Statistically different than before bed rest and after multiple comparisons correction.
Fig. 2.
Absolute LV mass at baseline, after 60 days of HDTBR, and after 14 days of active recovery in seven subjects. Bars represent mean group values; lines represent individual subject responses.
Fig. 3.
Percent change in LV mass from baseline with 95% confidence intervals, measured across 60 days of HDTBR and 14 days of physical reconditioning using three-dimensional echocardiography. *P < 0.01 compared with values before bed rest.
SV was unchanged during the first 31 days of HDTBR largely as a function of a preserved LVESV. By BR60, however, SV was reduced by −16 ± 4% (P < 0.001). Neither CO nor EF changed during bed rest. There was a marked impairment in early and late diastolic filling velocities resulting in a significantly reduced Doppler E/A ratio (−39 ± 11%, Table 3) by the end of HDTBR. Interestingly, the mitral peak Doppler E-wave, but not A-wave, was significantly lower at BR21 and steadily fell up to BR60. The reduction in the E/A ratio by BR60 coincided with a significant increase (14.2 ± 2.4 ms) in isovolumic relaxation time (IVRT). The ratio of transmitral flow velocity (E) vs. peak annulus velocity during early filling (E′) is an estimate of LV filling pressure. E/E′ was lower by ∼19% at BR60 (P = 0.019), although it did not reach statistical significance after adjustment for multiple comparisons.
Table 3.
Tissue Doppler parameters before, during, and after bed rest
| Baseline | 7 Days | 21 Days | 31 Days | 60 Days | R+4 hr | R+3 | R+13 | |
|---|---|---|---|---|---|---|---|---|
| IVCT, ms | 45.6 ± 2.4 | 45.8 ± 2.3 | 51.6 ± 1.4 | 46.6 ± 1.3 | 45.3 ± 2.9 | 50.9 ± 3.7 | 48.3 ± 3.1 | 44.9 ± 2.4 |
| IVRT, ms | 85.3 ± 2.8 | 93.7 ± 3.6 | 95.0 ± 4.4 | 93.8 ± 4.7 | 99.5 ± 1.8* | 94.0 ± 3.3 | 88.6 ± 2.8 | 86.1 ± 3.0 |
| E, cm/s | 76.0 ± 6.9 | 65.1 ± 5.8 | 61.4 ± 6.0* | 59.3 ± 3.4* | 53.3 ± 5.6* | 54.8 ± 6.7* | 73.7 ± 4.1 | 80.6 ± 5.0 |
| A, cm/s | 44.2 ± 2.3 | 43.3 ± 1.7 | 45.2 ± 1.6 | 52.2 ± 3.0 | 51.5 ± 1.8 | 52.1 ± 4.0 | 57.0 ± 2.3* | 54.5 ± 3.2* |
| E/A ratio | 1.72 ± 0.13 | 1.52 ± 0.14* | 1.35 ± 0.12* | 1.17 ± 0.09* | 1.05 ± 0.12* | 1.05 ± 0.10* | 1.30 ± 0.08* | 1.49 ± 0.08* |
| E′, cm/s | 15.5 ± 1.4 | 13.4 ± 1.0 | 13.2 ± 1.3 | 11.9 ± 0.6* | 13.6 ± 0.9 | 14.0 ± 0.8 | 13.7 ± 0.7 | 14.6 ± 0.8 |
| E/E′ ratio | 5.04 ± 0.32 | 4.97 ± 0.39 | 4.74 ± 0.32 | 5.02 ± 0.21 | 3.90 ± 0.24* | 3.92 ± 0.47* | 5.41 ± 0.17 | 5.54 ± 0.23 |
Values are means ± SE.
IVCT, isovolumic contraction time; IVRT, isovolumic relaxation time; E, peak mitral flow velocity during early filling; A, peak mitral flow during atrial systole; E′, peak annulus velocity during early filling.
Statistically different than before bed rest and after multiple comparisons correction.
LV morphological and functional responses to reconditioning after bed rest.
Bed rest-induced changes in LVESV and LVEDV were partially restored to pre-HDTBR volumes by R+3, coinciding with the restoration of plasma volume. Unlike the recovery of LV volumes, LV mass remained significantly lower at R+3 (P < 0.001) compared with pre-HDTBR values. By R+13, LV mass was no longer statistically different from pre-HDTBR levels (6.1 ± 4.1%, P = 0.18) (Figs. 2 and 3).
Resting heart rate increased gradually throughout HDTBR but it was not significantly higher until R+4 hr. Although CO was largely unchanged during HDTBR, it gradually increased across the recovery period. By R+13, although CO was not statistically different (P = 0.058) from before HDTBR, it was 19 ± 13% higher. At R+3, Doppler measures of peak E-wave velocity were similar to values before bed rest. Interestingly, however, peak A-wave velocity increased during recovery to the extent that the measure of late diastolic filling was highest at R+3 (P < 0.001) and remained significantly higher (difference of 10.4 ± 3.4 cm/s) at R+13. Estimates of LV filling pressure (E/E′ ratio) trended higher than before HDTBR throughout recovery but did not reach statistical significance (Table 3).
DISCUSSION
We examined the course of changes in LV volume and mass during 60 days of strict sedentary HDTBR and throughout 14 days of ambulatory reconditioning. The findings from this study 1) confirm previous reports of altered cardiac function and LV atrophy during prolonged bed rest and extend the methods of study to 3D echocardiography using serial measurements from the same subjects; 2) demonstrate that in this population of test subjects cardiac mass and function recover within 2 wk following HDTBR when performing a progressive reconditioning program; and 3) establish that the loss in LV mass, particularly after prolonged weightlessness, cannot be fully explained by plasma volume loss alone, which had been suggested following shorter periods of weightlessness (55, 56). Resting measures of LV volumes and function in our subjects were quickly restored with the normalization of plasma volume (by R+3), but readaptation of LV mass was much slower, indicating true cardiac muscle atrophy.
Long-duration cardiac deconditioning and LV adaptation.
An important feature of the current study design was the longitudinal assessment of cardiac structural and functional adaptations over the bed rest period in the same set of subjects studied under the same conditions. Using 2D and 3D echocardiography our data demonstrate that 60 days of sedentary HDTBR induced LV dysfunction (e.g., lower E/E′, E/A, and IVRT), which was accompanied by ventricular remodeling and progressive cardiac atrophy. The morphological adaptations observed in the current study are consistent with those of previous research (Table 4) using both CMR imaging and 3D echocardiography (10, 14, 24, 49, 54), although generally, those studies obtained only a single measure at the end of bed rest. In the only other bed rest study to obtain serial measurements, researchers found that in as little as 2 wk of horizontal bed rest, five male subjects displayed a significant decrease in LVEDV, which was accompanied by an 8% reduction in LV mass by week 6; an additional 8% loss was observed in three subjects who remained in bed until week 12 (49). The role that those structural adaptations contribute to changes in LV function remains unclear. However, it has been hypothesized that these atrophic alterations underlie changes that affect LV chamber filling characteristics, including a less distensible ventricle resulting in a leftward shift on the pressure-volume curve (33), impaired diastolic suction (15), and abnormalities of myocardial deformation (29).
Table 4.
Studies quantifying changes in ventricular mass and function during bed rest without countermeasures
| Bed Rest Days | Subjects | LVM | LVEDV | SV | Bed Rest Posture | Test Posture | |
|---|---|---|---|---|---|---|---|
| Perhonen et al., 2001 (49) | 14 | 5 men | MRI | MRI | RB | Horizontal | Horizontal |
| Levine et al., 1997 (33) | 14 | 11 men, 1 woman | 2D | 2D | RB | −6° HDT | Horizontal |
| Shibata et al., 2010 (53) | 14 | 6 men, 1 woman | MRI | 2D | RB | −6° HDT | Horizontal |
| Dorfman et al., 2008 (15) | 18 | 14 | MRI | MRI | NA | −6° HDT | Horizontal |
| Stenger et al., 2014 (54) | 21 | 7 men | 2D | 2D | PWV | −6° HDT | Horizontal/HDT |
| Hastings et al., 2012 (24) and Carrick-Ranson et al., 2013 (10) | 35 | 8 men, 1 woman | MRI | 3D | RB | −6° HDT | Horizontal |
| Kozakova et al., 2011 (29) | 35 | men | 2D | 2D | PWV | −6° HDT | Not described |
| Perhonen et al., 2001 (49) | 42 | 5 men | MRI | MRI | RB | Horizontal | Horizontal |
| Dorfman et al., 2007 (14) | 60 | 8 women | MRI | MRI | NA | −6° HDT | Horizontal |
| Perhonen et al., 2001 (49) | 84 | 3 men | MRI | MRI | RB | Horizontal | Horizontal |
2D, two-dimensional echocardiography; 3D, three-dimensional echocardiography; MRI, magnetic resonance imaging; PWV, pulse-wave Doppler; RB, rebreathing technique; NA, not applicable.
In general, reductions in LV volume during bed rest, particularly diastolic volume, occur rapidly and precede cardiac remodeling. Thereafter, LVEDV appears to decrease at a slower rate as bed rest duration increases, although this has not been a consistent finding. For example, Perhonen et al. (49) reported that LVEDV decreased in the first 2 wk of bed rest but did not decrease further by 6 wk. In contrast, LVEDV decreased progressively throughout 60 days of bed rest in our subjects and was paralleled by a progressive reduction in the mitral E wave. Subjects studied by Perhonen et al. (49) were not in head-down tilt during bed rest and testing, in contrast to the positioning of our subjects. Although this likely resulted in differences in ventricular filling during bed rest between the two groups of subjects and confounds direct comparisons, we had previously adopted the head-down posture for in- and end-of-bed rest tests with the assumption that this more closely represented the inflight condition of astronauts (51). Differences in the positioning of subjects during data acquisition in these two longitudinal studies may contribute to the disparate findings. Alternatively, differences in LVEDV in response to bed rest may have been related to plasma volume status. It has previously been shown that plasma volume is significantly reduced after a 1-wk period of dietary stabilization (62). Subjects who participated in our study followed the NASA standard 2-wk fluid and dietary stabilization period before entering HDTBR. Although we did not assess plasma volume loss during the stabilization period, it is likely that our subjects already had a significant loss in plasma volume at the start of HDTBR and therefore the reduction in LVEDV in the initial stages of bed rest were not as large as previously reported (3, 49).
Largely on the basis of a single study in female bed rest subjects, it has been assumed that the rate of LV atrophy in men and women during bed rest are not different from each other (14). Dorfman and colleagues (14) examined CMR measures of LV mass and volume in women after 60 days of HDTBR and found that both were significantly lower after bed rest compared with pre-bed rest values. Although the authors did not directly compare cardiac atrophy in men and women, the percent decrease in LV mass measured after 60 days of HDTBR appears to agree with the rate of loss reported in men by Perhonen et al. during 84 days of bed rest (49). The current study, in which both men and women were tested in the same bed rest conditions, appears to confirm this assertion, but further studies with a larger number of female participants may further strengthen these conclusions.
Recovery from bed rest.
Subjects participated in a progressive reconditioning program that was intended to return them to a state of conditioning in which they could be safely released from the bed rest facility. Although the overall objective of reconditioning was to allow for successful performance of activities of daily living and was the same for all subjects, the program was individualized for each subject on the basis of their own levels of strength, endurance, aerobic fitness, and neurovestibular disturbances during the recovery period. Subjects progressed from simple ambulation, balance, and body weight exercises to mild stair climbing and weightlifting exercises until the time that they were discharged. The reconditioning program was not designed specifically to promote cardiac hypertrophy, and release from the facility was not determined on the basis of achieving a particular score on any of the physiological tests. However, it is clear that recovery to pre-bed rest levels of LV mass in most subjects is not prolonged when participating in a moderate-intensity programmed reconditioning plan; LV mass after 2 wk of reambulation and reconditioning was not different than it was before bed rest even though the deconditioning during HDTBR was almost four times longer. Regaining cardiac mass likely is predicated on the restoration of cardiac work associated with normal upright activities. However, recovery of LV mass and function after bed rest might be hastened by more intense or more frequent reconditioning (2), but this has not been specifically studied in a systematic manner (31).
Plasma volume and cardiac structure and function after bed rest.
The reduction in cardiac preload, presumably caused by hypovolemia, is considered to be one of the contributing factors behind cardiovascular deconditioning during weightlessness. To examine the independent contribution of plasma volume loss to the observed reduction in LV mass, Summers and colleagues (55) used a low-salt diet combined with an intravenous infusion of furosemide to mimic the dehydration experienced during short periods of weightlessness. The authors reported a significant and positive correlation between plasma volume and LV mass, which was then used to explain the rapid improvements in 2D echocardiographic measurements of LV volume and LV wall thickness observed in Shuttle astronauts 3 days after landing (55). However, in a separate study comparing cardiac adaptations between acute furosemide-induced hypovolemia and bed rest, Perhonen and colleagues (50) showed that changes in both LVEDV and SV were significantly greater after bed rest than with hypovolemia alone. These data demonstrate that the reductions in LV volume and mass that occur as a result of weightlessness only in part are the result of tissue dehydration. Myocardial mass is responsive to changes in loading conditions associated with the transition from normal ambulatory activities to space flight and bed rest, which include reduced cardiac work as a result of decreased physical activity, lack of orthostatic stress, and lower blood pressures (47, 48). Additionally, cardiac remodeling might be induced by reduced regional wall stress resulting from an increased ventricular sphericity during weightlessness (55, 57).
The rate of LV volume and mass recovery has not been compared with that of plasma volume restoration after prolonged bed rest until now. In the present study, resting SV, along with LVEDV and LVESV, increased within 3 days following HDTBR, a rate similar to plasma volume repletion. Although LV volumes were normal soon after HDTBR ended, LV mass remained significantly lower than baseline. The prognostic significance of this slow-resolving atrophy is not apparent in the resting functional measures obtained in this study, but may contribute to the delayed recovery of V̇o2 peak (31, 40).
Space flight applications.
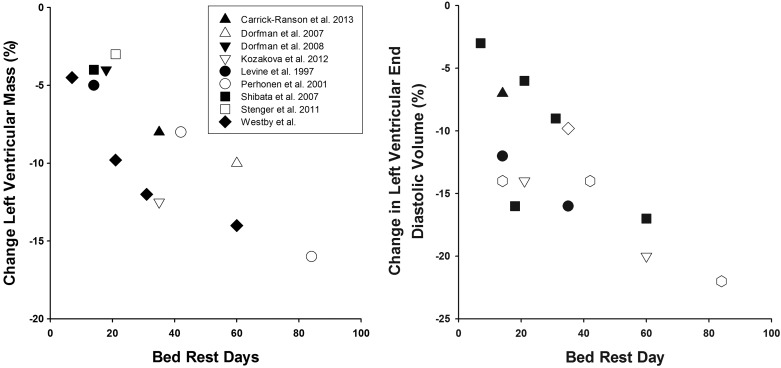
Understanding the cardiovascular changes associated with long-duration space flight to design and implement better countermeasure strategies that preserve astronaut cardiac structure and function are of paramount importance to NASA and its partnering space agencies. Given that structural and functional changes of the myocardium likely contribute to the decrease in orthostatic tolerance (6, 33, 39) and exercise capacity (32, 40) after both short- and long-duration space flight, preventing cardiac atrophy may have profound implications for the performance of mission-critical tasks during exploration missions, on extraterrestrial surfaces, and upon return to Earth. The aggregate of results from this study and others [(14, 24, 49, 53), see Fig. 4] suggest that cardiac functional and morphological adaptations to space flight likely are progressive, and thus countermeasures should be regularly applied throughout the mission. Although it is clear that LV volume and mass can be maintained with prescription of appropriate countermeasures during bed rest (14, 24, 53), and from this study that reambulation and reconditioning can contribute to recovery, it has yet to be proven that LV mass and function can be restored during space flight after a period of reduced participation in countermeasures or inactivity due to mission-specific constraints, failure of exercise hardware, or injury that precludes regular participation in exercise countermeasures.
Fig. 4.
Mean percent change in LV mass and LV end-diastolic volume in bed rest studies of varying duration. See Table 4 for details about each study with regard to bed rest duration, sex of subjects, and measurement methodology.
Additionally, the results from the current study support the use of high-resolution 3D echocardiography for future in-flight medical evaluations and research needs. Although 3D echocardiography is not currently available on the International Space Station, unlike 2D echocardiography (11), it is not burdened by geometric assumptions and therefore provides more accurate estimates of LV mass and volumes, which are comparable with CMR imaging (42, 63). Three-dimensional echocardiography in a compact portable form will overcome many of the technical and cost issues (e.g., volume of space needed, electric power consumption) associated with flying a CMR imager on the International Space Station or during space exploration beyond low Earth orbit (36). Moreover, use of this technology will make it possible to track cardiac adaptations during space flight, to modify or refine countermeasures, and to obtain meaningful results from studies involving fewer subjects, which is desirable for space flight research. Three-dimensional echocardiography has the advantage of having lower intraobserver and interobserver variability than traditional 2D echocardiography (21), and guidance by an ultrasound expert on the ground communicating directly with the astronaut in orbit as they step through an ultrasound examination has been used successfully to acquire on-orbit ultrasound vascular and cardiac images (18, 22).
Study limitations.
First, the number of subjects was relatively small, primarily because of the cost of performing an HDTBR study. To improve our power to detect differences over time, we used multilevel mixed-effects linear regression. Multilevel modeling allows the detection of fixed group differences (analogous to standard regression) while accounting for the variation of within-subject (random) effects such as differences in intercepts and slopes. Second, our study population consisted of both men and women who, on average, were not well conditioned according to their initial low absolute V̇o2 peak values. Although 60 days of HDTBR resulted in further deconditioning and significant losses of LV mass in these subjects, it is likely that even greater remodeling would be observed in more fit subjects, as has been observed in other physiological measures (31). As such, reductions in LV mass observed in this study may not be representative of the expected changes in astronauts, who typically are more fit (40, 41), if they were unable to perform exercise countermeasures during a space flight mission.
Conclusions.
Reductions in cardiac mass and function are progressive during long-duration bed rest in subjects who are not performing countermeasures, and there are no apparent differences in the responses between women and men. Recovery of LV volumes and function are rapid, and appear to be linked to plasma volume recovery, while restoration of LV mass lags and is not recovered in normal healthy subjects until 2 wk of reambulation.
GRANTS
This work was funded by National Aeronautics and Space Administration Human Research Program Grant BRC-2003-0000-0543 and in part by National Center for Advancing Translational Sciences Grant 1UL-1RR-029876-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.W., D.S.M., S.M.C.L., M.B.S., and S.H.P. conception and design of research; C.M.W., D.S.M., S.M.C.L., M.B.S., and S.H.P. performed experiments; C.M.W. and D.S.M. analyzed data; C.M.W., D.S.M., S.M.C.L., M.B.S., and S.H.P. interpreted results of experiments; C.M.W. and S.M.C.L. prepared figures; C.M.W. drafted manuscript; C.M.W., D.S.M., S.M.C.L., M.B.S., and S.H.P. edited and revised manuscript; C.M.W., D.S.M., S.M.C.L., M.B.S., and S.H.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We express our sincere gratitude and appreciation to our subject volunteers and to the staff from both the NASA Johnson Space Center Cardiovascular Laboratory and NASA's Flight Analogs Program for their countless hours of support.
REFERENCES
- 1.Alfrey CP, Udden MM, Huntoon CL, Driscoll T. Destruction of newly released red blood cells in space flight. Med Sci Sports Exerc 28: S42–S44, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 130: 2152–2161, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbeille P, Fomina G, Roumy J, Alferova I, Tobal N, Herault S. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur J Appl Physiol 86: 157–168, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bringard A, Pogliaghi S, Adami A, De Roia G, Lador F, Lucini D, Pizzinelli P, Capelli C, Ferretti G. Cardiovascular determinants of maximal oxygen consumption in upright and supine posture at the end of prolonged bed rest in humans. Respir Physiol Neurobiol 172: 53–62, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Buckey JC, Gaffney FA, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Yancy CW, Meyer DM, Blomqvist CG. Central venous pressure in space. J Appl Physiol 81: 19–25, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Buckey JC, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG. Orthostatic intolerance after spaceflight. J Appl Physiol 81: 7–18, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Buderer MC, Rummel JA, Michel EL, Mauldin DG, Sawin CF. Exercise cardiac output following Skylab missions: the second manned Skylab mission. Aviat Space Environ Med 47: 365–372, 1976. [PubMed] [Google Scholar]
- 8.Bungo MW, Charles JB, Johnson PC. Cardiovascular deconditioning during space flight and the use of saline as a countermeasure to orthostatic intolerance. Aviat Space Environ Med 56: 985–990, 1985. [PubMed] [Google Scholar]
- 9.Bungo MW, Goldwater DJ, Popp RL, Sandler H. Echocardiographic evaluation of space shuttle crewmembers. J Appl Physiol 62: 278–283, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Carrick-Ranson G, Hastings JL, Bhella PS, Shibata S, Levine BD. The effect of exercise training on left ventricular relaxation and diastolic suction at rest and during orthostatic stress after bed rest. Exp Physiol 98: 501–513, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Chuang ML, Beaudin RA, Riley MF, Mooney MG, Mannin WJ, Douglas PS, Hibberd MG. Three-dimensional echocardiographic measurement of left ventricular mass: comparison with magnetic resonance imaging and two-dimensional echocardiographic determinations in man. Int J Card Imaging 16: 347–357, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Convertino VA, Doerr DF, Stein SL. Changes in size and compliance of the calf after 30 days of simulated microgravity. J Appl Physiol 66: 1509–1512, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Diedrich A, Paranjape SY, Robertson D. Plasma and blood volume in space. Am J Med Sci 334: 80–85, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman TA, Levine BD, Tillery T, Peshock RM, Hastings JL, Schneider SM, Macias BR, Biolo G, Hargens AR. Cardiac atrophy in women following bed rest. J Appl Physiol 103: 8–16, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Dorfman TA, Rosen BD, Perhonen MA, Tillery T, McColl R, Peshock RM, Levine BD. Diastolic suction is impaired by bed rest: MRI tagging studies of diastolic untwisting. J Appl Physiol 104: 1037–1044, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall, 1993. [Google Scholar]
- 17.Fischer CL, Johnson PC, Berry CA. Red blood cell mass and plasma volume changes in manned space flight. JAMA 200: 579–583, 1967. [PubMed] [Google Scholar]
- 18.Foale CM, Kaleri AY, Sargsyan AE, Hamilton DR, Melton S, Martin D, Dulchavsky SA. Diagnostic instrumentation aboard ISS: just-in-time training for non-physician crewmembers. Aviat Space Environ Med 76: 594–598, 2005. [PubMed] [Google Scholar]
- 19.Gaffney FA, Buckey JC, Lane LD, Hillebrecht A, Schulz H, Meyer M, Baisch F, Beck L, Heer M, Maass H. The effects of a 10-day period of head-down tilt on the cardiovascular responses to intravenous saline loading. Acta Physiol Scand Suppl 604: 121–130, 1992. [PubMed] [Google Scholar]
- 20.Goldstein MA, Edwards RJ, Schroeter JP. Cardiac morphology after conditions of microgravity during COSMOS 2044. J Appl Physiol 73: 94S–100S, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Gopal AS, Schnellbaecher MJ, Shen Z, Sciacca RR, Keller AM, Sapin PM, King DL. Serial assessment of left ventricular mass regression by 3D echocardiography requires three-fold fewer subjects compared to conventional 1D and 2D echocardiography. J Am Coll Cardiol 27: 150–150, 1996. [Google Scholar]
- 22.Hamilton DR, Sargsyan AE, Garcia K, Ebert DJ, Whitson PA, Feiveson AH, Alferova IV, Dulchavsky SA, Matveev VP, Bogomolov VV, Duncan JM. Cardiac and vascular responses to thigh cuffs and respiratory maneuvers on crewmembers of the International Space Station. J Appl Physiol 112: 454–462, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Hascoët S, Brierre G, Caudron G, Cardin C, Bongard V, Acar P. Assessment of left ventricular volumes and function by real time three-dimensional echocardiography in a pediatric population: a TomTec versus QLAB comparison. Echocardiography 27: 1263–1273, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Hastings JL, Krainski F, Snell PG, Pacini EL, Jain M, Bhella PS, Shibata S, Fu Q, Palmer MD, Levine BD. Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol 112: 1735–1743, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillis GS, Møller JE, Pellikka PA, Gersh BJ, Wright RS, Ommen SR, Reeder GS, Oh JK. Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 43: 360–367, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979. [Google Scholar]
- 27.Johansen LB, Gharib C, Allevard AM, Sigaudo D, Christensen NJ, Drummer C, Norsk P. Haematocrit, plasma volume and noradrenaline in humans during simulated weightlessness for 42 days. Clin Physiol 17: 203–210, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Johnson RL, Hoffler GW, Nicogossian A, Bergman SA. Skylab experiment M-092: results of the first manned mission. Acta Astronaut 2: 265–296, 1975. [DOI] [PubMed] [Google Scholar]
- 29.Kozàkovà M, Malshi E, Morizzo C, Pedri S, Santini F, Biolo G, Pagani M, Palombo C. Impact of prolonged cardiac unloading on left ventricular mass and longitudinal myocardial performance: an experimental bed rest study in humans. J Hypertens 29: 137–143, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, Faletra FF, Franke A, Hung J, de Isla LP, Kamp O, Kasprzak JD, Lancellotti P, Marwick TH, McCulloch ML, Monaghan MJ, Nihoyannopoulos P, Pandian NG, Pellikka PA, Pepi M, Roberson DA, Shernan SK, Shirali GS, Sugeng L, Ten Cate FJ, Vannan MA, Zamorano JL, Zoghbi WA; American Society of Echocardiography, European Association of Echocardiography. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 25: 3–46, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Lee SM, Moore AD, Everett ME, Stenger MB, Platts SH. Aerobic exercise deconditioning and countermeasures during bed rest. Aviat Space Environ Med 81: 52–63, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Levine BD, Lane LD, Watenpaugh DE, Gaffney FA, Buckey JC, Blomqvist CG. Maximal exercise performance after adaptation to microgravity. J Appl Physiol 81: 686–694, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Levine BD, Zuckerman JH, Pawelczyk JA. Cardiac atrophy after bed-rest deconditioning: a nonneural mechanism for orthostatic intolerance. Circulation 96: 517–525, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Louisy F, Schroiff P, Guell A. Changes in leg vein filling and emptying characteristics and leg volumes during long-term head-down bed rest. J Appl Physiol 82: 1726–1733, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Maas AH, Hamelink ML, de Leeuw RJ. An evaluation of the spectrophotometric determination of HbO2, and Hb in blood with the co-oximeter IL 182. Clin Chim Acta 29: 303–309, 1970. [DOI] [PubMed] [Google Scholar]
- 36.Martin DS, Caine TL, Matz T, Lee SM, Stenger MB, Sargsyan AE, Platts SH. Virtual guidance as a tool to obtain diagnostic ultrasound for spaceflight and remote environments. Aviat Space Environ Med 83: 995–1000, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Martin DS, South DA, Wood ML, Bungo MW, Meck JV. Comparison of echocardiographic changes after short- and long-duration spaceflight. Aviat Space Environ Med 73: 532–536, 2002. [PubMed] [Google Scholar]
- 38.Meck JV, Dreyer SA, Warren LE. Long-duration head-down bed rest: project overview, vital signs, and fluid balance. Aviat Space Environ Med 80: A1–A8, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Meck JV, Reyes CJ, Perez SA, Goldberger AL, Ziegler MG. Marked exacerbation of orthostatic intolerance after long- vs. short-duration spaceflight in veteran astronauts. Psychosom Med 63: 865–873, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Moore AD, Downs ME, Lee SM, Feiveson AH, Knudsen P, Ploutz-Snyder L. Peak exercise oxygen uptake during and following long-duration spaceflight. J Appl Physiol 117: 231–238, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Moore AD, Lee SM, Stenger MB, Platts SH. Cardiovascular exercise in the U.S. space program: past, present and future. Acta Astronaut 66: 974–988, 2010. [Google Scholar]
- 42.Mor-Avi V, Sugeng L, Weinert L, MacEneaney P, Caiani EG, Koch R, Salgo IS, Lang RM. Fast measurement of left ventricular mass with real-time three-dimensional echocardiography: comparison with magnetic resonance imaging. Circulation 110: 1814–1818, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987. [DOI] [PubMed] [Google Scholar]
- 44.Mulvagh SL, Charles JB, Riddle JM, Rehbein TL, Bungo MW. Echocardiographic evaluation of the cardiovascular effects of short-duration spaceflight. J Clin Pharmacol 31: 1024–1026, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30: 1527–1533, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Nagueh SF, Mikati I, Kopelen HA, Middleton KJ, Quiñones MA, Zoghbi WA. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation 98: 1644–1650, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Norsk P, Asmar A, Damgaard M, Christensen NJ. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J Physiol 593: 573–584, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to Earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol 101: 143–194, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol 91: 645–653, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest “cardiovascular deconditioning” or hypovolemia? Circulation 103: 1851–1857, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Platts SH, Martin DS, Stenger MB, Perez SA, Ribeiro LC, Summers R, Meck JV. Cardiovascular adaptations to long-duration head-down bed rest. Aviat Space Environ Med 80: A29–A36, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Saltin B, Blomqvist G, Mitchell JH, Johnson RL Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation 38: VII1–VII78, 1968. [PubMed] [Google Scholar]
- 53.Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol 108: 1177–1186, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenger MB, Lee SM, Ribeiro LC, Phillips TR, Ploutz-Snyder RJ, Willig MC, Westby CM, Platts SH. Gradient compression garments protect against orthostatic intolerance during recovery from bed rest. Eur J Appl Physiol 114: 597–608, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Summers RL, Martin DS, Meck JV, Coleman TG. Mechanism of spaceflight-induced changes in left ventricular mass. Am J Cardiol 95: 1128–1130, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Summers RL, Martin DS, Meck JV, Coleman TG. Computer systems analysis of spaceflight induced changes in left ventricular mass. Comput Biol Med 37: 358–363, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Summers RL, Martin DS, Platts SH, Mercado-Young R, Coleman TG, Kassemi M. Ventricular chamber sphericity during spaceflight and parabolic flight intervals of less than 1 G. Aviat Space Environ Med 81: 506–510, 2010. [DOI] [PubMed] [Google Scholar]
- 58.Trappe T, Trappe S, Lee G, Widrick J, Fitts R, Costill D. Cardiorespiratory responses to physical work during and following 17 days of bed rest and spaceflight. J Appl Physiol 100: 951–957, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Verbanck S, Larsson H, Linnarsson D, Prisk GK, West JB, Paiva M. Pulmonary tissue volume, cardiac output, and diffusing capacity in sustained microgravity. J Appl Physiol 83: 810–816, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Watenpaugh DE, O'Leary DD, Schneider SM, Lee SM, Macias BR, Tanaka K, Hughson RL, Hargens AR. Lower body negative pressure exercise plus brief postexercise lower body negative pressure improve post-bed rest orthostatic tolerance. J Appl Physiol 103: 1964–1972, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Waters WW, Platts SH, Mitchell BM, Whitson PA, Meck JV. Plasma volume restoration with salt tablets and water after bed rest prevents orthostatic hypotension and changes in supine hemodynamic and endocrine variables. Am J Physiol Heart Circ Physiol 288: H839–H847, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Williams WJ, Schneider SM, Gretebeck RJ, Lane HW, Stuart CA, Whitson PA. Effect of dietary sodium on fluid/electrolyte regulation during bed rest. Aviat Space Environ Med 74: 37–46, 2003. [PubMed] [Google Scholar]
- 63.Yap SC, van Geuns RJ, Nemes A, Meijboom FJ, McGhie JS, Geleijnse ML, Simoons ML, Roos-Hesselink JW. Rapid and accurate measurement of LV mass by biplane real-time 3D echocardiography in patients with concentric LV hypertrophy: comparison to CMR. Eur J Echocardiogr 9: 255–260, 2008. [DOI] [PubMed] [Google Scholar]