Brown adipose tissue (BAT) activation is considered as a potential treatment for obesity. Using in vitro and in vivo experiments in isolated brown adipocytes and mice, we demonstrate that β2-adrenoreceptor stimulation increased BAT blood flow and glucose uptake despite the absence of a direct effect on adipocytes, suggesting that an increase in BAT perfusion per se leads to an increase in BAT metabolic substrate uptake. Therefore, increasing BAT blood flow might represent an obesity-targeted therapy.
Keywords: brown adipose tissue, perfusion, blood flow, β2-adrenoreceptor agonist, glucose uptake
Abstract
Brown adipose tissue (BAT) activation increases glucose and lipid consumption; as such, it is been considered as a potential therapy to decrease obesity. BAT is highly vascularized and its activation is associated with a necessary increase in blood flow. However, whether increasing BAT blood flow per se increases BAT activity is unknown. To examine this hypothesis, we investigated whether an isolated increase in BAT blood flow obtained by β2-adrenoreceptor (β2-AR) stimulation with salbutamol increased BAT activity. BAT blood flow was estimated in vivo in mice using contrast-enhanced ultrasound. The absence of direct effect of salbutamol on the function of isolated brown adipocytes was assessed by measuring oxygen consumption. The effect of salbutamol on BAT activity was investigated by measuring BAT glucose uptake in vivo. BAT blood flow increased by 2.3 ± 0.6-fold during β2-AR stimulation using salbutamol infusion in mice (P = 0.003). β2-AR gene expression was detectable in BAT but was extremely low in isolated brown adipocytes. Oxygen consumption of isolated brown adipocytes did not change with salbutamol exposure, confirming the absence of a direct effect of β2-AR agonist on brown adipocytes. Finally, β2-AR stimulation by salbutamol increased BAT glucose uptake in vivo (991 ± 358 vs. 135 ± 49 ng glucose/mg tissue/45 min in salbutamol vs. saline injected mice, respectively, P = 0.046). In conclusion, an increase in BAT blood flow without direct stimulation of the brown adipocytes is associated with increased BAT metabolic activity. Increasing BAT blood flow might represent a new therapeutic target in obesity.
NEW & NOTEWORTHY
Brown adipose tissue (BAT) activation is considered as a potential treatment for obesity. Using in vitro and in vivo experiments in isolated brown adipocytes and mice, we demonstrate that β2-adrenoreceptor stimulation increased BAT blood flow and glucose uptake despite the absence of a direct effect on adipocytes, suggesting that an increase in BAT perfusion per se leads to an increase in BAT metabolic substrate uptake. Therefore, increasing BAT blood flow might represent an obesity-targeted therapy.
the most abundant adipose tissue, white adipose tissue, is white or yellow in color and stores energy as lipid droplets. In contrast, brown adipose tissue (BAT) is able to consume lipids and glucose to produce heat (thermogenesis) by increasing mitochondrial uncoupling using the uncoupling protein 1 (UCP1) (9). Until recently, it was assumed that in humans functional BAT was present in infants but disappeared later in life (14). However, functional BAT was recently detected in adult humans (17, 25, 34, 38), increasing the interest in investigating BAT stimulation and expansion as potential therapies to decrease or prevent obesity.
BAT is richly innervated both in mice (9) and humans (54) and is activated by norepinephrine, released by the sympathetic nervous system in conditions such as cold or food intake (7). Norepinephrine binds to BAT β-adrenoreceptors (β-AR), leading to an activation of UCP1 and to thermogenesis (40).
The search for therapies activating BAT currently relies on direct stimulation of brown adipocytes through β1- and β3-AR stimulation; however, this strategy is limited due to the significant side effects associated with β1-AR agonists and the disappointing results of β3-AR agonists as antiobesity drugs in humans (1). β2-AR are not present in isolated brown adipocytes (8, 53) and therefore, although β2-AR agonists are extensively used in clinical practice and have few side effects, they have not been studied as potential activators of BAT.
BAT is highly vascularized and the increase in BAT blood flow observed during cold-induced and/or β3-AR-induced BAT activation is thought to be necessary both to provide enough oxygen and substrates for thermogenesis and to avoid thermal injury (15, 36). However, the impact of an increase in BAT blood flow per se on BAT activation has never been studied.
We hypothesized that increasing BAT blood flow would lead to an increased BAT substrate availability and uptake and thus result in BAT activation. Stimulation of glucose uptake by an increase in muscle blood flow has been described in several studies in the skeletal muscle in vivo in humans (6, 12, 51). In these studies, investigators reported that physiological stimuli such as insulin or exercise induced an increase in muscle blood flow that was responsible, at least in part, for the muscle glucose uptake increase (6). In addition, increasing muscle blood flow with a nonphysiological stimulus such as methacholine infusion during either a high- or low-dose insulin clamp also enhanced glucose uptake (3).
Although they are not expressed in isolated brown adipocytes, β2-AR are detected in the BAT, leading to the hypothesis that β2-AR might only be present in BAT blood vessels (8). Despite the absence or low expression of β2-AR gene expression in isolated brown adipocytes, β2-AR gene expression levels in human BAT are higher than β1-AR and β3-AR gene expression levels (19).
The purpose of this study was to assess the effect of β2-AR stimulation on BAT blood flow using a novel in vivo contrast ultrasound method (5, 13) and to determine whether an increase in blood flow per se could increase BAT metabolic activity.
MATERIALS AND METHODS
Mice.
All animal studies were performed according to a written protocol approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (33).
Ten to twelve-week-old male C57BL6 mice (Jackson Laboratory, Bar Harbor, ME) were studied. All animals were fed a standard diet (11.8% kcal from fat; Prolab Isopro RMH 3000 5P75, LabDiet, Richmond, IN) and maintained on a standard 12:12-h light/dark cycle with food and water given ad libitum until the experiments.
β2-AR stimulation was obtained using acute administration of a widely used β2-AR selective agonist, salbutamol (27). Salbutamol was obtained from Sigma Aldrich (S8260) and diluted in sterile 0.9% saline before injection.
BAT tissue perfusion assessment by contrast ultrasound.
BAT perfusion was assessed by measuring BAT blood flow using contrast ultrasound as previously described (5, 13). Briefly, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). After orotracheal intubation, animals were mechanically ventilated at a respiratory rate of 110 to 120 breaths/min and a tidal volume of 10 μl/g body wt. To avoid any stimulation of BAT by cold, core body temperature was maintained constant at 37°C with a DC Temperature Control System (FHC, Bowdoin, ME). A left carotid catheter (fluid-filled PE-10 catheter) was surgically placed for continuous invasive measurement of mean blood pressure and heart rate. Similarly, catheters were placed in the left and right jugular veins and used for intravenous infusions of salbutamol and ultrasound contrast agent, respectively.
Ultrasound acquisition.
Acquisitions were performed in the prone position using a commercially available ultrasound system with a 14-MHz linear transducer (Acuson Sequoia C512; Siemens Medical Solutions, Mountain View, CA). Perflutren lipid microbubbles (Definity; Lantheus Medical Imaging, North Billerica, MA) were diluted in sterile 0.9% saline solution (1:10) and continuously infused intravenously at a rate of 20 μl/min into the right jugular vein. For each acquisition, images centered on the BAT were acquired during 10 s after a burst of 10 high-energy frames (mechanical index: 1.8). The first set of acquisitions was performed 10 min after the onset of microbubbles perfusion. An intravenous infusion of 0.2 μg·kg−1·min−1 of salbutamol was then initiated, and the second set of acquisitions was performed 10 min later.
BAT tissue perfusion analysis.
Regions of interest were traced manually within the two BAT interscapular lobes (5, 13). The average signal intensity within the region of interest was automatically measured for each frame (syngo ACQ; Siemens Medical Solutions). The relationship of the signal intensity over time after bubble destruction was obtained in each region of interest and fitted to an exponential function: y = A (1 − eβt) where y is the signal intensity, A is the plateau intensity, and β is the initial slope of the replenishment curve. Blood flow was estimated by calculating the product of A and β (52). A minimum of three measurements was averaged. A goodness-of-fit coefficient (R2 of the fit) was obtained for each curve, and an arbitrary threshold of 0.8 was set. All curves with a goodness-of-fit <0.8 were excluded.
Cardiac output measurement by echocardiography.
Estimation of cardiac output was based on M-mode echocardiographic measurements using a commercially available ultrasound system with a 10- to 14-MHz linear transducer (Vivid 7; GE Vingmed Ultrasound AS, Horten, Norway) (47). Mice were anesthetized with the same anesthetic regimen than for the contrast ultrasound experiments. Parasternal two-dimensional-guided M-mode acquisitions were performed in supine position before and 10 min after the onset of an intravenous infusion of 0.2 μg·kg−1·min−1 of salbutamol.
Left ventricular end-diastolic (LVEDD) and end-systolic (LVESD) diameters and heart rate (HR) were measured. Cardiac output (CO) was assessed using the following validated formula: CO = (LVEDD3 − LVESD3) × HR (47). Five measurements were averaged for each animal.
Brown adipocytes isolation.
Brown adipocytes were isolated by digestion of BAT interscapular lobes using collagenase as previously described (26). Mice (n = 8 for each experiment) were euthanized by an intraperitoneal injection of pentobarbital (200 mg/kg) and cervical dislocation. The interscapular BAT lobes were harvested immediately after death, and removal of white adipose tissue and muscles surrounding BAT lobes was performed using a dissecting microscope based on the color and consistence of the different tissues.
BAT lobes were digested in two steps using decreasing doses of collagenase (26). To that purpose, the buffer was changed to 3 ml with 1.3 mg/ml collagenase II, and the tissue was incubated in a slowly shaking water bath at 37°C with vortexing every minute. After 7 min, the buffer was discarded, and the tissue was placed in 6 ml of fresh buffer with 0.67 mg/ml collagenase II, minced with scissors, and incubated for 45 min in the water bath, vortexing every 5 min. The buffer with cells and tissue fragments was filtered through silk cloth, and the filtrate, containing the adipocytes, was centrifuged for 8 min at 30 g. The infranatant was discarded and fresh buffer was added. The remaining tissue was incubated for 45 min in 3 ml of buffer with 0.33 mg/ml collagenase, vortexed every 5 min, and collected. The two cell suspensions were pooled and washed by floating for 1 h. The buffer was removed. The cell suspension (1-4×106 cells/ml) was kept at room temperature during the experiment.
Measurement of mRNA expression levels in isolated brown adipocytes and BAT.
Total RNA was extracted either from isolated brown adipocytes or BAT using the Trizol (Ambion, Life Technologies, Austin, TX) method according to the manufacturer's instructions. One microgram of RNA was used in the Applied Bioscience Multiscribe Reverse Transcriptase cDNA Synthesis Kit (Applied Bioscience) for RT-PCR to produce cDNA using random hexameric primers. cDNA was subsequently used for relative expression quantitation using the Applied Bioscience Taqman FAST Advanced Master Mix or the FAST SYBR Green I Master mix (Applied Biosciences) in a Lightcycler 480 (Roche). To this end, subunit specific primer-probe sets were purchased for β2-AR and UCP1 (Mm02524224_s1 and Mm00494069_m1, respectively, Life Technologies). To normalize the data, the geometric mean of the two most stable housekeeping genes, 18s and beta-actin (ACTB) (Mm03928990_g1 and Mm00607939_s1, respectively, Life Technologies) was used as described (48). Each sample was measured in triplicate to determine the threshold cycle (Ct). For each sample, the normalization factor was calculated as the difference between the geometric mean Ct of the housekeeping genes of the sample and the mean Ct of the housekeeping genes of all samples. The level of target mRNA, relative to the mean of the reference housekeeping genes, was calculated by raising two to the power of [40 − (Ct of target − housekeeping gene normalization factor)].
Measurement of oxygen consumption rates from isolated brown adipocytes.
Oxygen consumption in freshly isolated brown adipocytes was measured at 37°C using a Clark-type oxygen electrode (Hansatech Oxygraph, Amesbury, MA) as previously described (26). Brown adipocytes (100,000 to 200,000 cells/experiment) were suspended in a Krebs-Ringer bicarbonate buffer (in mM: 145 Na+, 6.0 K+, 2.5 Ca2+, 1.2 Mg2+, 128 Cl−, 1.2 SO2−4, 25.3 HCO3−, and 1.2 mM HPO42−) with 10 mM glucose, 10 mM fructose, and 4% fatty-acid-free bovine serum albumin. A volume of 900 μl of the solution containing cells and buffer was placed in an oxygen electrode closed chamber, stirred continuously, and maintained at 37°C. The oxygen consumption rate was continuously recorded during the whole experiment. The basal oxygen consumption rate was determined after 4 min of incubation. Salbutamol was then added with a Hamilton syringe (100 μl) through the cover of the closed chamber in increasing concentrations (10 ng/ml, 100 ng/ml, 1 μg/ml, 10 μg/ml, and 100 μg/ml). At the end of the experiment, 100 μl of a 0.1 g/l norepinephrine solution was added as a positive control. Maximal oxygen consumption during the 120 s after the addition of salbutamol or norepinephrine in the closed chamber was recorded.
BAT glucose uptake in vivo.
Glucose uptake in vivo was measured as previously reported (43). Mice were fasted overnight (10 PM to 9 AM). Body weight was measured just before the beginning of the experiment. Thirty minutes after anesthesia with pentobarbital sodium (60 mg/kg mouse body wt, intraperitoneal injection), blood was taken from the tail to assess basal glucose concentrations and background radioactivity levels. Mice were injected intraperitoneally with either saline or salbutamol at a dose of 10 μg/g mouse body wt. Ten minutes later, 1 mg glucose in combination with 0.33 μCi [3H]2-deoxyglucose/g mouse body wt was administered via the retro-orbital sinus. After 45 min, animals were killed by cervical dislocation, and BAT, subcutaneous white adipose tissue, and visceral white adipose tissue were harvested and immediately frozen in liquid nitrogen. Accumulation of [3H]2-deoxyglucose was assessed in BAT, subcutaneous, and visceral white adipose tissues using a perchloric acid/Ba(OH)2/ZnSO4 precipitation procedure as previously described (43).
Statistical analysis.
Statistical analysis was performed using SPSS statistics software (version 17.0.0, SPSS, Chicago, IL). Values are expressed as means ± SE.
BAT blood flow, blood pressure, heart rate, and cardiac output before and during salbutamol infusion were compared using paired t-tests. Comparison of β2-AR gene expression in isolated brown adipocytes and BAT was performed using an unpaired t-test. Maximal oxygen consumption of isolated brown adipocytes at baseline, with salbutamol and with norepinephrine was compared using ANOVA for repeated measurements. If the effect of treatments (ANOVA) was significant, the treatments were compared with baseline conditions and to each other using paired t-tests. The difference in BAT and white adipose tissues glucose uptake between mice with salbutamol and saline injection was tested by unpaired t-test.
All tests were two-tailed and a P value <0.05 was considered significant.
RESULTS
Effect of β2-adrenoreceptor stimulation on BAT perfusion.
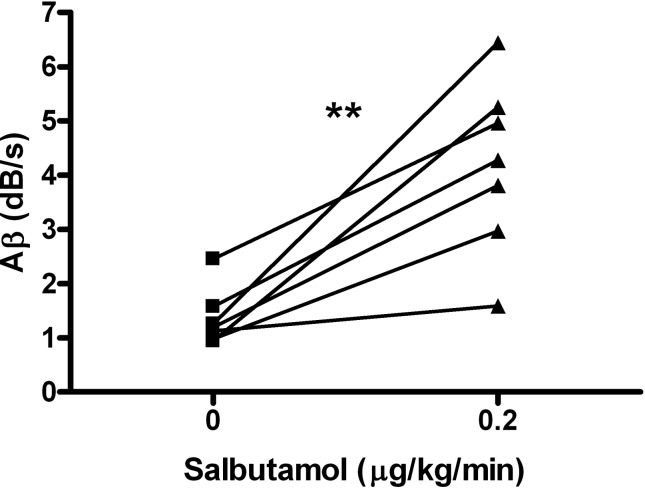
In thermoneutral conditions with core body temperature maintained at 37°C, salbutamol intravenous infusion (0.2 μg·kg−1·min−1) increased BAT blood flow by 2.3 ± 0.6-fold compared with baseline (Aβ product 4.2 ± 0.6 vs. 1.4 ± 0.2 dB/s, P = 0.003, Fig. 1). During salbutamol infusion, the mean blood pressure decreased from 79 ± 3 to 65 ± 4 mmHg (P = 0.008), whereas the heart rate remained stable (386 ± 13 and 387 ± 12 beats/min at baseline and during salbutamol infusion, respectively).
Fig. 1.
Brown adipose tissue (BAT) blood flow estimated by contrast-ultrasound before and during β2-adrenoreceptor stimulation. Individual data of Aβ product at baseline and 10 min after the onset of salbutamol infusion at 0.2 μg·kg−1·min−1 are presented (n = 7). BAT blood flow was significantly higher during β2-adrenoreceptor stimulation using salbutamol compared with baseline. **P < 0.001 vs. baseline.
To evaluate whether an increase in cardiac output was responsible for the BAT perfusion response, the effect of salbutamol infusion on cardiac output was tested in a separate set of mice (n = 6). A slight increase in cardiac output was observed (9.6 ± 0.8 vs. 11.5 ± 0.7 ml/min at baseline compared with salbutamol infusion; P = 0.04). The mean percentage change in cardiac output between baseline and salbutamol infusion was of 25 ± 10%.
β2-Adrenoreceptor expression in isolated brown adipocytes and BAT.
Expression of β2-AR and UCP1 was assessed in isolated brown adipocytes and total BAT by RT-PCR. As expected, β2-AR mRNA expression level was extremely low in isolated brown adipocytes, whereas a significant expression was found in total BAT (Fig. 2A). UPC1 gene expression was higher in isolated cells compared with BAT (Fig. 2B).
Fig. 2.
Gene expression of β2-adrenoreceptor and uncoupling protein 1 in isolated brown adipocytes and in the BAT. A: β2-adrenoreceptor gene expression level was extremely low in isolated brown adipocytes whereas mRNA level β2-adrenoreceptor was significantly higher in the tissue. B: uncoupling protein 1 (UCP1) mRNA gene expression level was higher in isolated brown adipocytes than in BAT. Data are presented as means ± SE, *P < 0.05 vs. isolated brown adipocytes.
Effect of β2-AR stimulation on oxygen consumption in isolated brown adipocytes.
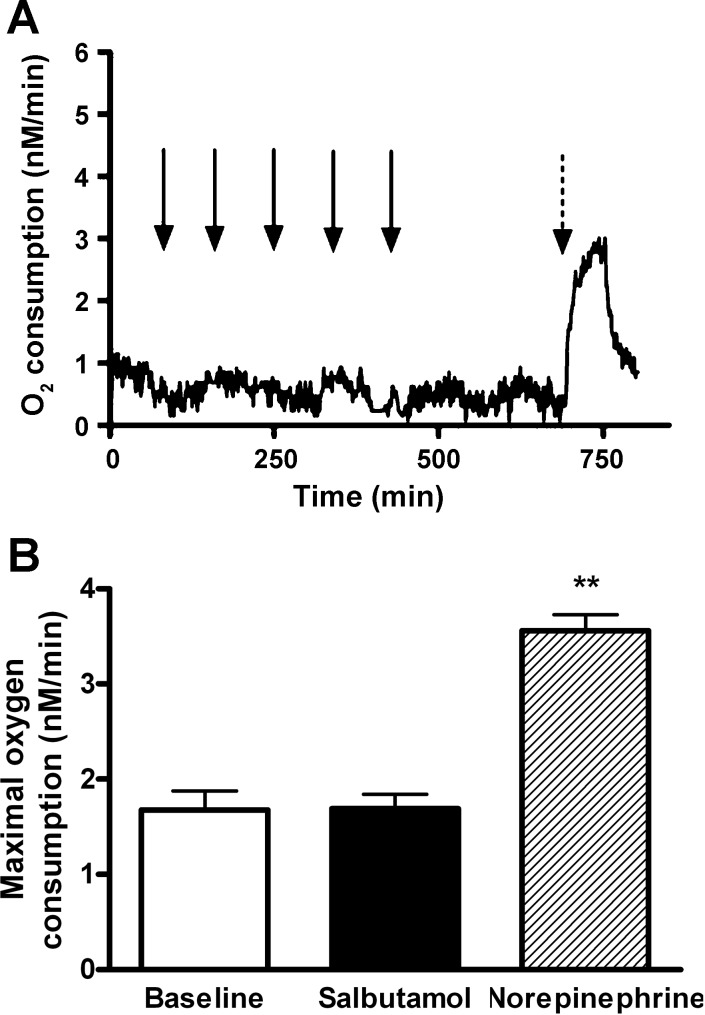
To test the hypothesis that β2-AR stimulation has no direct metabolic effect on brown adipocyte cells, oxygen consumption was measured in freshly isolated brown adipocytes at baseline, in the presence of increasing doses of salbutamol (ranging from 10−5 to 0.01 g/l) and in the presence of norepinephrine. No change in oxygen consumption was observed in presence of β2-AR agonist, confirming the absence of a direct effect of β2-AR stimulation on brown adipocytes (Fig. 3, A and B). In contrast, a marked increase in oxygen consumption was observed in presence of norepinephrine (2.1-fold increase of maximal oxygen consumption compared with baseline, P = 0.001).
Fig. 3.
In vitro measurements of oxygen consumption of isolated brown adipocytes at baseline in presence of selective β2-adrenoreceptor agonist and in presence of norepinephrine. A: oxygen consumption curve of isolated brown adipocytes measured using a Clark-type oxygen electrode. No change in oxygen consumption was observed in presence of increasing doses of salbutamol whereas a marked increase immediately followed addition of norepinephrine in the closed chamber. Black arrows represent addition of 100-μl salbutamol solution at the following concentrations (in g/l): 10−5, 10−4, 10−3, 0.01, and 0.1 g/ and the dotted arrow represents addition of 100 μl of a 0.01 g/l norepinephrine solution. B: maximal oxygen consumption in isolated brown adipocytes measured at baseline in presence of salbutamol and norepinephrine. **P < 0.01 vs. baseline. Measurement was repeated in 4 sets of cells during each experiment, and 4 independent experiments were performed.
Effect of β2-AR stimulation on BAT glucose uptake.
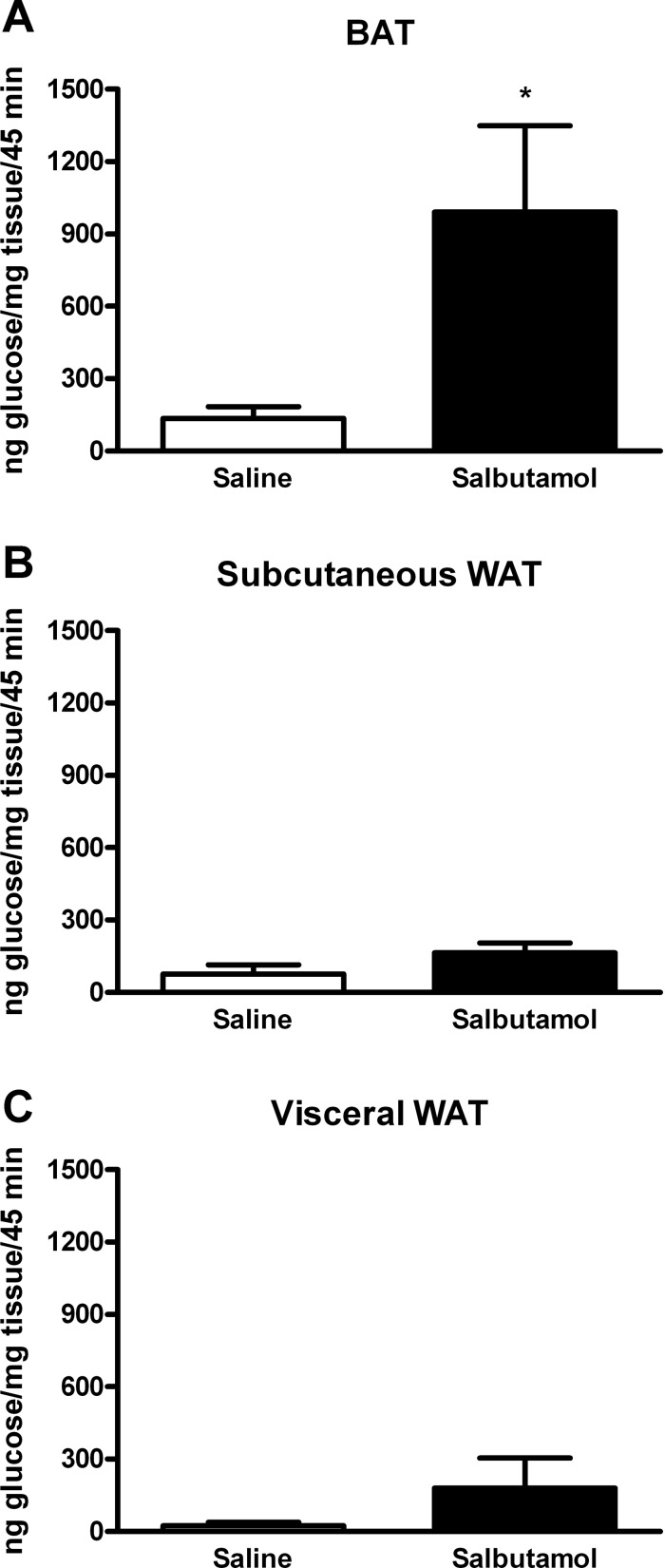
To establish whether β2-AR stimulation increased glucose uptake in vivo, mice were injected with labeled [3H]2-deoxyglucose and with either saline or salbutamol. β2-Adrenoreceptor stimulation by salbutamol resulted in a significant increase in BAT glucose uptake, whereas no change was observed in subcutaneous and visceral white adipose tissue (Fig. 4).
Fig. 4.
BAT (A) and visceral (B) and subcutaneous white adipose tissue (WAT; C) glucose uptake after β2-adrenoreceptor stimulation. Mice were treated with 10 μg/g mouse body wt of salbutamol intraperitoneally (n = 10), and their glucose uptake was compared with mice injected with saline (n = 8). An increase of glucose uptake after treatment with salbutamol was noted in BAT (991 ± 358 vs. 135 ± 49 ng glucose/mg tissue/45 min in salbutamol vs. saline injected mice respectively, P = 0.046) but not in subcutaneous or visceral WAT. *P < 0.05 vs. saline.
DISCUSSION
The present study demonstrates that β2-AR stimulation increases BAT glucose uptake despite the absence of a direct activation effect on brown adipocytes. β2-Adrenoreceptor stimulation, however, increases BAT blood flow, suggesting that an increase in BAT perfusion per se leads to an increase in BAT metabolic substrate uptake.
β2-Adrenoreceptor stimulation in vivo by salbutamol, a selective β2-AR agonist, increased BAT blood flow. β2-Adrenoreceptor gene expression by isolated brown adipocytes was extremely low. The absence of effect of β2-AR stimulation in vitro by salbutamol on the oxygen consumption of isolated brown adipocytes confirmed the absence of a direct effect of salbutamol on the metabolic activity of these cells. Interestingly, however, β2-AR stimulation in vivo increased BAT glucose uptake, suggesting an effect on the metabolic activity of BAT.
The critical role of BAT vascularization in the functionality of BAT was recently demonstrated (41). In a high-fat diet obesity-induced murine model, obesity caused capillary rarefaction and hypoxia in BAT, leading to BAT whitening with a diminished response to beta adrenergic signaling, accumulation of lipid droplets, and mitochondrial dysfunction and loss (41). Importantly, BAT revascularization led to an improvement in BAT function. Similarly, we reported a decreased BAT capillary density in a genetic murine model of obesity (db/db mice) (13). These data suggest that BAT vascularization and blood flow play a major role in the function of BAT and in the association between BAT dysfunction and obesity.
Vasculature in BAT might be different than that of other tissues. Our team (5) and others (21) previously reported that norepinephrine induces a significant increase in BAT blood flow. Vascular response to norepinephrine is dependent on the predominance of β- or α-AR (α1/β2 ratio) in the vessels. A norepinephrine-induced vasodilation is observed in specific tissues with β-AR predominance such as the iliac arteries (50), whereas an important norepinephrine-induced vasoconstriction is observed in the vast majority of tissues and is related to the α-AR predominance (49). Therefore, we can hypothesize that β2-AR might be predominant compared with α1-AR in the BAT vasculature.
The present study demonstrates the importance of BAT perfusion in rodents: the vascularity of BAT in humans may be less homogeneous because BAT depots are not as well delineated as in rodents (30, 31). Nonetheless, BAT perfusion in humans is significant, being twice that of muscle in warm conditions and increasing to four times that of muscle when cold-stimulated (36). Similarly, our data were obtained in rodents, in which the importance of BAT for glucose regulation is crucial (43, 45). In humans, several studies suggest that activated BAT has the ability to remove glucose from circulation, but whether this response is clinically relevant is still debated (42). Recent data suggest that this response may in certain cases at least be clinically relevant, because stimulation of BAT in human volunteers with detectable BAT (using 18FDG PET/CT scanning) increases insulin sensitivity (10).
We used salbutamol, a selective β2-AR agonist (27) routinely used in clinical practice (in pathologies such as asthma or preterm labor) to increase BAT perfusion. Using an in vivo technique recently validated by our team (5, 13), we demonstrated that a dose of 0.2 μg·kg−1·min−1 of salbutamol [equivalent to the dose of 5 to 20 μg/min used in adults in clinical practice (46)] increases BAT blood flow. The effect of β2-AR stimulation was significant but smaller than the effect of norepinephrine, with a two-fold increase in BAT blood flow with salbutamol compared with a 15-fold increase previously observed with norepinephrine (5). Of note, BAT blood flow was assessed in thermoneutral conditions with core body temperature maintained at 37°C to avoid BAT stimulation by cold.
Salbutamol infusion induced a small but significant decrease in mean blood pressure (46). A slight increase in cardiac output was observed, mainly due to an increase in stroke volume, possibly related to the decrease in left ventricular afterload. However, the increase in cardiac output was limited and lower than the increase in BAT blood flow. Therefore, it is unlikely that changes in cardiac output can fully account for the salbutamol-induced increase in BAT blood flow.
The absence of a direct effect of β2-AR stimulation on brown adipocytes was demonstrated by the extremely low β2-AR gene expression and the absence of effect of salbutamol on oxygen consumption in isolated brown adipocytes. The extremely low β2-AR gene expression in brown adipocytes confirms previous results (8, 9, 53). In addition, no effect of salbutamol on the oxygen consumption of isolated brown adipocytes was detected, in contrast to norepinephrine, which markedly increased oxygen consumption. Of note, the doses of salbutamol were chosen to reflect the plasma concentrations reported with clinical doses of salbutamol (28, 44).
Despite the absence of a direct effect of β2-AR stimulation on brown adipocytes, studies have suggested that β2-AR play an important role in BAT function and morphology. The morphological abnormalities of BAT in mice deficient in the three β-AR (with features of both brown and white adipose tissue) do not exist in mice deficient in both β1- and β3-AR, in which β2-AR are present (2). In addition, β2-AR have clinical relevance because they are the most abundant β-AR in human BAT, representing 63% of the total β-AR mRNA compared with 28 and 9% for β1-AR and β3-AR mRNA, respectively (19).
Selective β2-AR stimulation (using salbutamol) increased BAT glucose uptake, whereas no effect on WAT glucose uptake was observed. A dose of 10 μg/g body wt was chosen based on previous reports of active doses of salbutamol in other indications in murine model (11, 24). Salbutamol induced a 7-fold increase in BAT glucose uptake, whereas a previous study reported a 2-fold increase after 0.2 mg/kg norepinephrine intraperitoneal injection (22).
Assessment of BAT activity is challenging. BAT glucose uptake is imperfect but has been recognized as a marker of BAT activity in previous publications (17, 25, 34). In addition, direct assessment of thermogenesis is challenging and would not have reflected only BAT activity but also vasodilation induced by β2-AR stimulation in our study.
In humans, β2-AR agonists such as salbutamol increase both glucagon and insulin secretion and have effects on liver metabolism, which cause an overall increase in blood glucose level (37). In rat isolated islets of Langerhans, the β2-AR agonist clenbuterol induced a rise in glucagon secretion, whereas no direct effect on insulin secretion was observed (23). The potential impact of salbutamol on glucose homeostasis might be involved in the BAT increased glucose uptake that we observed. This hypothesis is counterbalanced by the fact that no increase in WAT glucose uptake was observed in the same conditions. Furthermore, it was recently shown that acute β2-AR stimulation by salbutamol has no effect on skeletal muscle glucose uptake (29).
An association between activation of BAT, either acute or chronic, and increase in BAT blood flow was previously reported (5, 15, 20, 21, 32, 36, 39). Stimuli that increase BAT metabolic activity such as cold exposure (21, 36), β3-AR agonist treatment (15), or norepinephrine treatment (5, 20, 32, 39) also increase BAT blood flow. In addition, a positive association between the whole body energy expenditure and BAT blood flow was reported in humans (36). Conversely, when the response of BAT blood flow to norepinephrine is inhibited, the thermogenic ability of BAT is suppressed (32). As in many other tissues, it is likely that blood flow increases in response to an increased metabolic demand. The present study demonstrates the distinct and reciprocal finding that an increase in BAT blood flow induces an increase in BAT metabolic activity.
The mechanism by which an increase in tissue blood flow stimulates glucose uptake is unknown; in muscles, it may involve an increase in glucose gradient between plasma and interstitium. This gradient in turn facilitates glucose delivery to the interstitium, resulting in an increased membrane transport into cells (4). Both glucose transporters GLUT 1 and GLUT 4 are present in brown adipocytes (18). Whereas insulin-stimulated glucose uptake is well characterized with the involvement of the rapid translocation of GLUT 4 from intracellular vesicles to cell membrane, insulin-independent BAT glucose uptake is less well understood. Recently, the mechanisms involved in the β3-AR stimulation-induced glucose uptake have been studied and involve a rapid de novo synthesis of GLUT 1 and the translocation of the newly synthesized GLUT 1 (35). Whereas β3-AR has a direct effect on brown adipocytes, we showed that β2-AR stimulation does not. Increased blood flow associated with β2-AR stimulation might result in an increase in glucose gradient between plasma and interstitium and a stimulation of GLUT 1 transporter.
There are several limitations to the present study. Although the salbutamol doses given in vivo were selected to produce relevant plasmatic doses, they may not reflect the doses used in clinical practice. In addition, salbutamol is a selective β2-AR agonist but a marginal effect on β1-AR cannot be excluded.
During in vivo experiments, salbutamol infusion was associated with a slight decrease in mean blood pressure (from 79 ± 3 to 65 ± 4 mmHg, P = 0.008). Therefore, the potential influence of the baroreflex, including a systemic increase in sympathetic activity that may stimulate BAT, cannot be completely excluded, although the decrease in blood pressure was small and no increase in heart rate was noted.
Despite the routine use of BAT glucose uptake as a marker of BAT activity in clinical studies using 18F-FDG-PET/CT, glucose is not the only substrate used for thermogenesis and may underestimate the thermogenic activity (16). In addition, glucose uptake by BAT endothelial cells (as opposed to uptake by brown adipocytes) cannot be excluded using this technique.
The kinetics of the increase in blood flow and glucose uptake were not assessed during the same experiment. Therefore, we cannot determine whether the glucose uptake increase lasted only as long as the blood flow increase.
Finally, BAT blood flow and glucose uptake measurement were not performed under the exact same conditions. This methodological difference was due to a technical issue. BAT perfusion assessment by contrast ultrasound required a complete anesthesia of the mice because of the need for microbubbles intravenous continuous injection. Because we aimed to perform experiments in the most physiological conditions and BAT glucose uptake experiment did not require intravenous injections, anesthesia was less profound and no intravenous catheter was implanted.
In conclusion, an increase in BAT blood flow without direct stimulation of brown adipocytes is associated with an increased BAT metabolic activity. Therefore, increasing BAT blood flow might represent an alternative or additional target in the development of therapies aimed at stimulating BAT.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R21-DK092909 (to M.S.-C.), R01-AR42238, R01-DK099511, and 5P30DK036836-27 (to L.J.G.); French Federation of Cardiology Grant (Fédération Française de Cardiologie, to L.E.); American College of Sports Medicine Research Endowment Grant (to K.I.S.) and Mary K. Iacocca Fellowship (to K.I.S.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.E. and M.S.-C. conception and design of research; L.E., K.I.S., R.T., H.Z., M.C., and M.F.H. performed experiments; L.E., K.I.S., R.T., H.Z., and M.C. analyzed data; L.E., K.I.S., R.T., E.S.B., and M.S.-C. interpreted results of experiments; L.E. and K.I.S. prepared figures; L.E. and M.S.-C. drafted manuscript; L.E., K.I.S., R.T., L.J.G., K.D.B., E.S.B., and M.S.-C. edited and revised manuscript; L.E., K.I.S., R.T., H.Z., M.C., M.F.H., L.J.G., K.D.B., E.S.B., and M.S.-C. approved final version of manuscript.
REFERENCES
- 1.Arch JRS. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol 378: 225–240, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. BetaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297: 843–845, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Baron A, Steinberg H, Brechtel G, Johnson A. Skeletal muscle blood flow independently modulates insulin-mediated glucose uptake. Am J Physiol Endocrinol Metab 266: E248–E253, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Baron A, Steinberg H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 96: 786–792, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron DM, Clerte M, Brouckaert P, Raher MJ, Flynn AW, Zhang H, Carter EA, Picard MH, Bloch KD, Buys ES, Scherrer-Crosbie M. In vivo noninvasive characterization of brown adipose tissue blood flow by contrast ultrasound in mice. Circ Cardiovasc Imaging 5: 652–659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett E, Eggleston E, Inyard A, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes 34: S36–S42, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bengtsson T, Cannon B, Nedergaard J. Differential adrenergic regulation of the gene expression of the beta-adrenoceptor subtypes beta1, beta2 and beta3 in brown adipocytes. Biochem J 347, Pt 3: 643–651, 2000. [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63: 4089–4099, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choucair-Jaafar N, Yalcin I, Rodeau J, Waltisperger E, Freund-Mercier M, Barrot M. Beta2-adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. Br J Pharmacol 158: 1683–1694, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark M. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 295: E732–E750, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerte M, Baron DM, Brouckaert P, Ernande L, Raher MJ, Flynn AW, Picard MH, Bloch KD, Buys ES, Scherrer-Crosbie M. Brown adipose tissue blood flow and mass in obesity: a contrast ultrasound study in mice. J Am Soc Echocardiogr 26: 1465–1473, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham S, Leslie P, Illingworth P, Jung R, Nicholls D, Peden N, Rafael J, Rial E. The characterization and energetic potential of brown adipose tissue in man. Clin Sci 69: 343–348, 1985. [DOI] [PubMed] [Google Scholar]
- 15.Cypess AM, Doyle AN, Sass CA, Huang TL, Mowschenson PM, Rosen HN, Tseng YH, Palmer EL, Kolodny GM. Quantification of human and rodent brown adipose tissue function using 99mTc-Methoxyisobutylisonitrile SPECT/CT and 18F-FDG PET/CT. J Nucl Med 54: 1896–1901, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab 20: 408–415, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny G, Kahn C. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallner OS, Chernogubova E, Brolinson KA, Bengtsson T. β3-Adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology 147: 5730–5739, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Deng C, Paoloni-Giacobino A, Kuehne F, Boss O, Revelli JP, Moinat M, Cawthorne MA, Muzzin P, Giacobino JP. Respective degree of expression of beta 1-, beta 2- and beta 3-adrenoceptors in human brown and white adipose tissues. Br J Pharmacol 118: 929–934, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster DO, Frydman ML. Nonshivering thermogenesis in the rat. II Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol 56: 110–122, 1978. [DOI] [PubMed] [Google Scholar]
- 21.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm-or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57: 257–270, 1979. [DOI] [PubMed] [Google Scholar]
- 22.Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes 54: 1385–1391, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Lacey RJ, Berrow NS, Scarpello JH, Morgan NG. Selective stimulation of glucagon secretion by β2-adrenoceptors in isolated islets of Langerhans of the rat. Br J Pharmacol 103: 1824–1828, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malfait A, Malik A, Marinova-Mutachieva L, Butler D, Maini R, Feldmann M. The beta2-adrenergic agonist salbutamol is a potent suppressor of established collagen-induced arthritis: mechanisms of action. J Immunol 162: 6278–6283, 1999. [PubMed] [Google Scholar]
- 25.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Matthias A, Ohlson K, Fredriksson J, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. J Biol Chem 275: 25073–25081, 2000. [DOI] [PubMed] [Google Scholar]
- 27.McCaffrey PM, Riddell JG, Shanks RG. Selectivity of xamoterol, prenalterol and salbutamol as assessed by their effects in the presence and absence of ICI 118 551. Eur Heart J 11, Suppl A: 54–55, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Morgan DJ, Paull JD, Richmond BH, Wilson-Evered E, Ziccone SP. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol 22: 587–593, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulder AH, Smits P, Tack CJ. Beta2-adrenoceptor stimulation has no effect on skeletal muscle glucose uptake. Clin Auton Res 24: 183–187, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat Using PET Imaging. Front Endocrinol 3: 15, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med 54: 523–531, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagashima T, Ohinata H, Kuroshima A. Involvement of nitric oxide in noradrenaline-induced increase in blood flow through brown adipose tissue. Life Sci 54: 17–25, 1994. [DOI] [PubMed] [Google Scholar]
- 33.National Research Council (US) Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press, 1996. [PubMed] [Google Scholar]
- 34.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Olsen JM, Sato M, Dallner OS, Sandstrom AL, Pisani DF, Chambard JC, Amri EZ, Hutchinson DS, Bengtsson T. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J Cell Biol 207: 365–374, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerbäck S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Philipson LH. β-Agonists and metabolism. J Allergy Clin Immunol 110: S313–S317, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Richard D, Carpentier AC, Doré G, Ouellet V, Picard F. Determinants of brown adipocyte development and thermogenesis. Int J Obes 34: S59–S66, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Rothwell N, Stock M. Influence of noradrenaline on blood flow to brown adipose tissue in rats exhibiting diet-induced thermogenesis. Pflugers Arch 389: 237–242, 1981. [DOI] [PubMed] [Google Scholar]
- 40.Satterfield MC, Wu G. Brown adipose tissue growth and development: significance and nutritional regulation. Front Biosci 16: 1589–1608, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, Maruyama S, Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest 124: 2099–112, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 125: 478–486, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan WC, Chan TB, Ang SM. Single-dose and steady-state effects of controlled-release salbutamol on drug level and airflow obstruction in patients with asthma. Singapore Med J 33: 371–374, 1992. [PubMed] [Google Scholar]
- 45.Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M. Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84: 202–211, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tobin A. Intravenous salbutamol: too much of a good thing? Crit Care Resusc 7: 119–127, 2005. [PubMed] [Google Scholar]
- 47.Tournoux F, Petersen B, Thibault H, Zou L, Raher MJ, Kurtz B, Halpern EF, Chaput M, Chao W, Picard MH, Scherrer-Crosbie M. Validation of noninvasive measurements of cardiac output in mice using echocardiography. J Am Soc Echocardiogr 24: 465–470, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vatner SF, Higgins CB, Braunwald E. Effects of norepinephrine on coronary circulation and left ventricular dynamics in the conscious dog. Circ Res 34: 812–823, 1974. [DOI] [PubMed] [Google Scholar]
- 50.Vatner S, Knight D, Hintze T. Norepinephrine-induced beta 1-adrenergic peripheral vasodilation in conscious dogs. Am J Physiol Heart Circ Physiol 249: H49–H56, 1985. [DOI] [PubMed] [Google Scholar]
- 51.Wasserman D, Kang L, Ayala J, Fueger P, Lee-Young R. The physiological regulation of glucose flux into muscle in vivo. J Exp Biol 214: 254–262, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J, Unelius L, Bengtsson T, Cannon B, Nedergaard J. Coexisting beta-adrenoceptor subtypes: significance for thermogenic process in brown fat cells. Am J Physiol Cell Physiol 267: C969–C979, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 23: 3113–3120, 2009. [DOI] [PubMed] [Google Scholar]