Abstract
Cerebral blood flow (CBF) is temporally related to exercise-induced changes in partial pressure of end-tidal carbon dioxide (PetCO2); hyperoxia is known to enhance this relationship. We examined the hypothesis that preventing PetCO2 from rising (isocapnia) during submaximal exercise with and without hyperoxia [end-tidal Po2 (PetO2) = 300 mmHg] would attenuate the increases in CBF. Additionally, we aimed to identify the magnitude that breathing, per se, influences the CBF response to normoxic and hyperoxic exercise. In 14 participants, CBF (intra- and extracranial) measurements were measured during exercise [20, 40, 60, and 80% of maximum workload (Wmax)] and during rest while ventilation (V̇e) was volitionally increased to mimic volumes achieved during exercise (isocapnic hyperpnea). While V̇e was uncontrolled during poikilocapnic exercise, during isocapnic exercise and isocapnic hyperpnea, V̇e was increased to prevent PetCO2 from rising above resting values (∼40 mmHg). Although PetCO2 differed by 2 ± 3 mmHg during normoxic poikilocapnic and isocapnic exercise, except for a greater poikilocapnic compared with isocapnic increase in blood velocity in the posterior cerebral artery at 60% Wmax, the between condition increases in intracranial (∼12-15%) and extracranial (15–20%) blood flow were similar at each workload. The poikilocapnic hyperoxic increases in both intra- and extracranial blood-flow (∼17–29%) were greater compared with poikilocapnic normoxia (∼8–20%) at intensities >40% Wmax (P < 0.01). During both normoxic and hyperoxic conditions, isocapnia normalized both the intracranial and extracranial blood-flow differences. Isocapnic hyperpnea did not alter CBF. Our findings demonstrate a differential effect of PetCO2 on CBF during exercise influenced by the prevailing PetO2.
Keywords: carbon dioxide, cerebral blood flow, exercise, hyperoxia, hyperpnea
steady-state increases in exercise up to 60–80% of maximum achieved workload (Wmax) are reflected in elevations of extracranial blood flow (1) and intracranial cerebral blood velocities (CBV) (1–4). At intensities greater than 80% of V̇o2 peak, blood flow in the internal carotid artery (QICA) and CBV through the middle and posterior cerebral arteries (MCAv and PCAv) decrease from their peak toward baseline values (1, 5–7). These responses are similar to the exercise response of global CBF (gCBF) when quantified using positron emission tomography (2, 8). In contrast, vertebral artery flow (QVA) continues to rise at 80% of Wmax (25), whereas PCAv at 80 and 100% Wmax has been reported to decrease from the peak response at 60% (6) or plateau (9) at maximal intensities. Furthermore, we have recently identified a larger increase, from rest to exercise, in MCAv compared with PCAv at submaximal intensities (i.e., 20 and 40% Wmax) (6). It is unclear if these regional differences (MCAv vs. PCAv) or the contrasting responses in proximal vs. distal vessels in the posterior circulation (PCAv vs. QVA) during maximal exercise (i.e., 80% Wmax) are manifested by technical issues (e.g., velocity vs. flow), or differential sensitivities to arterial blood gases in the different regions/vessels (10) during incremental exercise (11), or both.
Regulation of gCBF during exercise is likely influenced by four main factors: metabolic demand, perfusion pressure, redistribution of cardiac output, and the partial pressure of arterial carbon dioxide (PaCO2; reviewed in Ref. 12). Of these factors, PaCO2 appears to be the most important. For example, changes in CBF and CBV during incremental exercise parallel changes in PaCO2 and are further influenced by an enhanced CBF reactivity to PaCO2 (∼5% per mmHg change in PaCO2; Ref. 13) compared with an ∼4% per mmHg at rest (10). Therefore, the paralleled elevations (e.g., +15–25%) in CBF and PaCO2 (e.g., +3–5 mmHg PaCO2 at ∼70% V̇o2 peak), combined with the paralleled return to baseline values of both CBF and PaCO2 at maximal exercise intensities, largely implicate PaCO2 as the primary regulator of CBF during exercise. In support, Lambertsen et al. (11) elevated end-tidal PCO2 (PetCO2) by 10 mmHg at maximal exercise intensities (80–100% V̇o2 peak) and were able to increase MCAv by +15%. The lack of hypocapnic vasoconstriction and subsequent increases in MCAv at maximal exercise supports the concept that PaCO2 plays a primary role in regulating CBF during incremental exercise (11). While these findings are pertinent to CBF at maximal exercise, what remains to be established is if maintaining PaCO2 at isocapnic levels at the onset of exercise would inhibit the typical exercise induced increases in CBF. It is also unknown if preventing the changes in PaCO2 may influence the previously reported regional differences in QVA compared with QICA and MCAv (1).
Under resting conditions, normobaric poikilocapnic hyperoxia (FiO2 >300 mmHg) is a mild respiratory stimulant in adults, resulting in a hyperventilatory drop in PaCO2 ranging from 1 to 4 mmHg (14). In contrast, during exercise while breathing [i.e., end-tidal partial pressure of oxygen (PetO2) >95 mmHg] leads to a reduced min ventilation (V̇e) and elevated PetCO2 compared with V̇e during normoxic exercise with similar absolute workloads (15). Recently, we have demonstrated that during submaximal exercise (∼40% Wmax) the increase in PCAv was significantly greater during hyperoxia (+43%) compared with normoxic exercise (+20%) at the same relative intensity (29). In this study, the resting PetCO2 was reduced by ∼5 mmHg following 10 min of breathing hyperoxia (PetO2 = ∼600 mmHg) (29). In the same study, the increase in PetCO2 from rest to exercise during hyperoxia (+10 mmHg) was significantly greater than the increase observed during normoxia (+7 mmHg). The larger relative increase in PetCO2 during hyperoxic trial was speculated as the primary reason for the larger increase in PCAv compared with MCAv. It remains unknown if the greater rise in PetCO2 from rest to exercise is responsible for the increased PCAv during submaximal exercise intensities or if hyperoxia has a direct influence on CBF during exercise.
In addition to the regulatory influence of PaCO2 on CBF during exercise, the action of V̇e per se may also influence CBF. For example, Neubauer et al. (19) observed a 22% increase in ventral medullary (i.e., brainstem) blood flow in cats, following a specialized hindlimb electrical stimulation that increased V̇e by 2.5-fold while maintaining arterial blood gas tensions and pH (isocapnic hyperpnea). This model effectively showed that, despite no difference in the gCBF during isocapnic hyperpnea, regional blood flow through the respiratory control centers (which are located in the posterior circulation) was significantly increased. Although such changes in V̇e may explain, in part, previously reported regional changes in CBF during exercise (1), this possibility has yet to be explored in humans.
Considering the above background and rationale, the aims of this study were to address the following questions: 1) is the increase in CBF during incremental exercise primarily mediated by PaCO2; 2) are the regional differences in CBF during hyperoxia also explained by differential sensitivities to PaCO2; and 3) what are the independent effects of V̇e on regional and gCBF during hyperoxic and normoxic exercise. The following three hypotheses were examined: 1) maintaining isocapnia during submaximal exercise would attenuate the rise in gCBF, as well as any regional differences observed during normoxic poikilocapnic exercise; 2) the enhanced posterior CBF, as well as any gCBF or intra- vs. extracranial blood flow differences observed during poikilocapnic hyperoxic exercise, would be attenuated by maintaining isocapnia throughout exercise; and 3) isocapnic hyperpnea will increase posterior CBF but to a lesser extent than that observed during exercise.
METHODS
Participants
Seventeen healthy (11 male), nonsmoking participants (18–26 yr; body mass index <30 kg/m2) were screened before participation in the current investigation to ensure they were free from cardiovascular, cerebrovascular, and respiratory disorders. Fourteen subjects completed the protocol. The three female participants were tested in the early follicular phase (days 1–7) of their menstrual cycle. All subjects avoided exercise, caffeine, and alcoholic beverages for 12 h and fasted for 4 h before each session. All testing was approved by the Clinical Research Ethical Review Board of the University of British Columbia and conformed to the standards set forth by the Declaration of Helsinki; participants provided written informed consent.
Study Desgin
Following baseline screening and familiarization, participants visited the laboratory on three separate occasions. At the beginning of the first two visits participants performed one incremental exercise protocol (+20 W every 3 min beginning at 40 W) on a recumbent cycle ergometer (Lode Ergometer; Lode, Groningen, The Netherlands) until exhaustion was achieved [maximum achieved workload (Wmax)]. Participants were asked to maintain their cadence between 60 and 70 rpm throughout the protocol. Each test was performed in either normoxia (PetO2 = ∼95 mmHg) or hyperoxia (PetO2 = ∼300 mmHg), respectively. Visits 1 and 2 were randomly ordered (separated by a minimum of 48 h), and all participants were blinded to the specific PetO2. During the final visit, participants completed the isocapnic hyperpnea trial (details below).
Visits 1 and 2 (normoxia or hyperoxia).
Following the maximal exercise test (∼1 h) when baseline heart rate (HR), blood pressure, and CBF had returned to resting values, participants were asked to perform one submaximal exercise protocol with relative workloads corresponding to 20, 40, 60, and 80% of the maximum achieved workload (% Wmax) in the exhaustive trial while breathing room air without an PetCO2 clamp in that order. The duration of each workload was ∼5 min with data sampling occurring within the final 3 min of the stage once steady state had been achieved. Once cardiorespiratory and cerebrovascular values had returned to baseline (∼20 min), participants performed a final submaximal exercise protocol at 20, 40, and 60% of Wmax with a similar duration and sampling period as the previous submaximal test; however, during this test PetCO2 was maintained at basline values (isocapnic) using dynamic end-tidal forcing (16). The PetCO2 was maintained at baseline values by asking participants to increase V̇e by ∼20% above that achieved during the corresponding poikilocapnic exercise stages (i.e., 20, 40, and 60% Wmax). This level of hyperventilation was adequate to reduce PetCO2 slightly below baseline values irrespective of exercise intensity while the forcing system “fine-tuned” and stabilized PetCO2 at resting values by supplying a small fraction of CO2 on each breath (10, 16). To achieve the necessary rate of gas delivery during exercise, two gas delivery systems were connected in parallel to a gas humidification chamber and a 6-liter capacity inspiratory breathing reservoir. In this configuration, the end-tidal forcing system can supply the necessary gas mixtures to achieve a ventilatory capacity in excess of 220 l/min. Visit 2 was identical to visit 1; however, all exercise was performed while breathing the alternate inspirate (depending on the experimental randomization). Experimentally preventing the rise in PetCO2 during isocapnia enabled the quantification of the magnitude that CO2 influences CBF during submaximal exercise by comparing this with poikilocapnic exercise conditions.
Visit 3 (isocapnic hyperpnea).
Following the completion of visits 1 and 2 (>48 h), 11 participants returned to the laboratory to perform both a normoxic and hyperoxic isocapnic hyperpnea intervention. The order of normoxia and hyperoxia was randomly assigned. During each test, subjects were asked to breathe at similar ventilations achieved during the respective submaximal poikilocapnic exercise tests at 20, 40, 60, and 80% Wmax, while PetCO2 was maintained at basal values using the end-tidal forcing system. The duration of each hyperpnea stage was ∼5 min with data sampling occurring within the final 3 min of each stage once steady state had been achieved. Normoxic and hyperoxic hyperpnea trials were separated by 20 min once cardiorespiratory and cerebrovascular values had returned to baseline values. Comparisons between poikilocapnic exercise and isocapnic hyperpnea served to address the second hypothesis of our study; that was to quantify the role that increases in V̇e per se might play in CBF regulation.
Instrumentation
During each visit, with the exception of volumetric vascular ultrasound measures (i.e., ICA and VA), the measurements listed below were continuously and concurrently monitored using a 16-channel digital to analog data acquisition system (Powerlab/16SP ML 880; ADInstruments, Colorado Springs, CO).
Intracranial and extracranial blood flow.
Intracranial blood flow velocity [i.e., CBV (MCAv and PCAv)] responses to exercise were assessed using a 2-MHz transcranial Doppler ultrasound (TCD; ST3, Spencer Technologies, Seattle, WA). Identification and location of the MCA and PCA were determined using standardized procedures (17). Bilateral TCD probes were fixed and held in place over the temporal window via a headband fixation device (Mark600; Spencer Technologies). Our coefficient of determination for between day MCAv and PCAv measurements is 3 and 2%, respectively (6). Concurrent to the TCD measurements, QICA and QVA were measured simultaneously on either side of the neck using two high-resolution linear array vascular ultrasound systems (Terrason t3000, Burlington, MA). The left ICA and right VA were insonated in all but two subjects, with MCA and PCA scans occurring ipsilateral to their respective confluent neck artery. As per recently published guidelines, the ICA was insonated ∼1–1.5 cm distal to the carotid bifurcation, while the VA was insonated between the transverse processes of the C3 and subclavian artery (34). Synchronous measurement of velocity and diameter at 30 Hz was achieved using customized edge-detection software (19). Test-retest reliability for baseline measures of QICA and QVA are ∼5 and 4%, respectfully. Application of the offline analysis of ICA and VA measurements has been discussed elsewhere (10, 34, 20). Global CBF was calculated by combing (QICA·2) and (QVA·2) and assuming symmetrical flow volumes in the respective contralateral arteries (9). It is important to note that each of the volumetric measurements were made on the same side throughout the entire experiment (i.e., QICA right side and QVA left side); thus all gCBF and regional disparities discussed are assuming a perfect symmetry in vascular response in the contralateral vessels. Despite no differences between the resting contralateral QICA a previous study identified a regional disparity (∼20%) between contralateral vertebral vessels at rest (21); however, a recent study investigating the individual contribution of the extracranial arteries (QICA and QVA; n = 94) using high-resolution magnetic resonance imaging did not observe a significantly different contribution between the contralateral vessels (34, 22). Nevertheless, should a disparity between contralateral VAs exist, we would still expect the stimulus-response data would be similar during exercise.
Respiratory gas exchange.
Ventilation, PetO2, and PetCO2 were measured continuously using a two-way pneumotach (Series 3813; Hans Rudolph, Shawnee, KS) and gas analyzer, respectively, which were connected to our data acquisition device (Powerlab). These data were interfaced with commercially available software (Labchart 7; AdInstruments) and stored for offline analysis. Invasive arterial blood gases were sampled from two individuals during both normoxic and hyperoxic exercise in normo- and poikilocapnia to confirm effectiveness of the end-tidal forcing system. A 20-gauge catheter (Arrow, Markham, ON, Canada) was placed in the radial artery under local anesthesia (Lidocaine, 1%) and connected to an in-line wasteless sampling system (Edwards Lifesciences VAMP, Irvine, CA). Blood gas samples were drawn into preheparinized syringes and analyzed immediately (ABL-90 CO-Ox; Radiometer, Copnehagen, Denmark).
Arterial blood pressure.
Automated manual blood pressure (SunTech Tango; SunTech Medicals, Morrisville, NC) was intermittently measured at the brachial artery, while continuous beat-by-beat blood pressure (Finometer Pro; Finapres Medical Systems, Amsterdam, The Netherlands) was measured using finger photo-plethysmography. Mean arterial pressure (MAP) was derived using manual pressure measurements [(systolic × 0.333) + (diastolic × 0.667)] when continuous monitoring was unable to obtain a pressure reading. Cerebrovascular resistance index in the intracranial (CVCiMCA and PCA) and extracranial (CVCiICA and VA) vessels was calculated by dividing the respective cerebral CBV or CBF values by MAP (i.e., CBV/MAP). In two volunteers, intra-arterial blood pressure observations (TruWave transducer; Edwards Lifesciences) were also obtained to confirm the accuracy of non-invasive assessment of arterial pressures.
Statistical Analysis
Two-way repeated-measures ANOVA was performed to investigate changes in all variables from baseline during normoxic vs. hyperoxic exercise in conditions of poikilocapnia vs. isocapnia. Repeated-measures ANOVA was also performed to investigate absolute and relative differences from baseline values between anterior and posterior arteries (MCA vs. PCA; ICA vs. VA), as well as proximal and distal cerebral arteries (ICA vs. MCA; VA vs. PCA) during both PetO2 and PetCO2 manipulations. P < 0.05 was considered statistically significant. Sidak's correction for multiple comparisons was applied when ANOVA identified significant differences or interactions.
RESULTS
Tables 1 and 2 summarize the collective findings during normoxic and hyperoxic exercise in conditions of poikilocapnia and isocapnia. In three subjects volumetric blood flow metrics were unattainable; therefore, comparisons among ICA, VA, and gCBF were based on an n = 14. The mean maximum achievable wattage during recumbent cycling was significantly elevated during hyperoxic (240 ± 55 W) compared with normoxic (200 ± 50 W) exercise (P < 0.05). During poikilocapnic exercise V̇e increased linearly with exercise intensity Table 1. Additionally, because of the nature of the isocapnic exercise protocol, V̇e was 20% elevated at each stage of exercise compared with poikilocapnic exercise (Table 1).
Table 1.
Cardiorespiratory measurements during poikilocapnic and isocapnic exercise while breathing either normoxia or hyperoxia
| Stage |
||||||
|---|---|---|---|---|---|---|
| Measurement/Condition | BL1 | BL2 | 20 % | 40% | 60% | 80% |
| Poikilocapnic | ||||||
| V̇e, l/min | ||||||
| Normoxia | 13.5 ± 1.7* | 12.4 ± 2.4 | 22.0 ± 4.0* | 30.3 ± 6.3* | 41.0 ± 9.5* | 57.0 ± 12.0* |
| Hyperoxia | 13.4 ± 2.2* | 13.7 ± 3.4 | 21.3 ± 5.0* | 30.4 ± 5.8* | 42.0 ± 8.2* | 55.0 ± 13.0* |
| HR, beats/min | ||||||
| Normoxia | 75 ± 13 | 74 ± 12 | 100 ± 15* | 113 ± 15* | 131 ± 13* | 150 ± 13* |
| Hyperoxia | 72 ± 16 | 68 ± 15 | 93 ± 17* | 110 ± 18* | 126 ± 19* | 142 ± 22* |
| MAP, mmHg | ||||||
| Normoxia | 88 ± 8 | 89 ± 10 | 97 ± 6* | 103 ± 7* | 108 ± 9* | 112 ± 11* |
| Hyperoxia | 89 ± 8 | 91 ± 6 | 97 ± 8* | 99 ± 8* | 106 ± 9* | 110 9* |
| PetO2, mmHg | ||||||
| Normoxia | 98 ± 13 | 99 ± 13 | 94 ± 14 | 93 ± 6 | 96 ± 5 | 100 ± 5 |
| Hyperoxia | 96 ± 3* | 299 ± 8† | 298 ± 5† | 297 ± 6† | 294 ± 7† | 294 ± 6† |
| PetCO2, mmHg | ||||||
| Normoxia | 40 ± 2 | 40 ± 2 | 41 ± 2 | 42 ± 3* | 42 ± 3* | 41 ± 3 |
| Hyperoxia | 40 ± 3* | 36 ± 4§ | 39 ± 3*§ | 42 ± 3*§ | 42 ± 4*§ | 42 ± 4*§ |
| Isocapnia | ||||||
| V̇e, l/min | ||||||
| Normoxia | 13.5 ± 1.8* | 16.5 ± 3.7‡ | 29.6 ± 4.7*‡ | 39.7 ± 7.2*‡ | 56.3 ± 13.4*‡ | — |
| Hyperoxia | 13.9 ± 2.2* | 18.8 ± 4.8‡ | 30.0 ± 6.4*‡ | 39.4 ± 7.4*‡ | 53.8 ± 13.6*‡ | — |
| HR, beats/min | ||||||
| Normoxia | 73 ± 14 | 77 ± 13 | 101 ± 12* | 116 ± 133* | 133 ± 17* | — |
| Hyperoxia | 71 ± 18 | 73 ± 20 | 97 ± 19* | 113 ± 18* | 128 ± 19* | — |
| MAP, mmHg | ||||||
| Normoxia | 88 ± 6.7 | 89 ± 6.2 | 96 ± 6.0* | 101 ± 8.0* | 108 ± 11* | — |
| Hyperoxia | 89 ± 7.5 | 91 ± 7.7 | 99 ± 7.8* | 102 ± 9.1* | 112 ± 13* | — |
| PetO2, mmHg | ||||||
| Normoxia | 95 ± 4.2 | 99 ± 1.9 | 97 ± 5.7 | 97 ± 4.8 | 94 ± 4.2 | — |
| Hyperoxia | 96 ± 6.7 | 298 ± 5.3 | 298 ± 3.5 | 299 ± 5.2 | 310 ± 34 | — |
| PetCO2, mmHg | ||||||
| Normoxia | 40 ± 1.6 | 40 ± 1.7 | 40 ± 1.8 | 40 ± 1.9‡ | 41 ± 2.6‡ | — |
| Hyperoxia | 39 ± 3.8 | 39 ± 3.7‡ | 39 ± 3.5 | 39 ± 3.4‡ | 40 ± 4.0‡ | — |
>Values are means ± SD. BL, baseline; V̇e, ventilation; HR, heart rate; MAP, mean arterial pressure; PetO2 and PetCO2, partial pressure of arterial oxygen and carbon dioxide.
P < 0.05, from BL2;
P < 0.05, from normoxia;
P < 0.05, from poikilocapnia;
P < 0.05, interaction effect between normoxia and hyperoxia.
Table 2.
Cerebrovascular measurements during poikilocapnic and isocapnic exercise while breathing either normoxia or hyperoxia
| Stage |
||||||
|---|---|---|---|---|---|---|
| Measurement/Condition | BL1 | BL2 | 20 % | 40% | 60% | 80% |
| Poikilocapnia | ||||||
| MCAv,† cm/s | ||||||
| Normoxia | 61 ± 8.4 | 60 ± 9.2 | 65 ± 11* | 67 ± 12* | 70 ± 12* | 75 ± 16* |
| Hyperoxia | 60 ± 8.4* | 55 ± 8.5 | 61 ± 8.7* | 66 ± 14* | 69 ± 12* | 71 ± 14* |
| CVCiMCA, cm·s−1 ·mmHg−1 | ||||||
| Normoxia | 0.71 ± 0.12 | 0.70 ± 0.11 | 0.67 ± 0.10 | 0.66 ± 0.1 | 0.66 ± 0.1 | 0.69 ± 0.1 |
| Hyperoxia | 0.67 ± 0.12* | 0.58 ± 0.09 | 0.63 ± 0.08 | 0.67 ± 0.1* | 0.65 ± 0.12* | 0.64 ± 0.14* |
| QICA, ml/min | ||||||
| Normoxia | 234 ± 64 | 232 ± 66 | 249 ± 76 | 277 ± 79* | 288 ± 80* | 279 ± 97* |
| Hyperoxia | 226 ± 52 | 223 ± 53 | 257 ± 62 | 282 ± 79* | 305 ± 106* | 295 ± 81* |
| ICAdiam, cm | ||||||
| Normoxia | 0.50 ± 0.06 | 0.50 ± 0.06 | 0.50 ± 0.06 | 0.49 ± 0.06 | 0.49 ± 0.06 | 0.47 ± 0.06 |
| Hyperoxia | 0.49 ± 0.06 | 0.48 ± 0.04 | 0.48 ± 0.06 | 0.49 ± 0.06 | 0.49 ± 0.07 | 0.49 ± 0.06 |
| ICAvel, cm/s | ||||||
| Normoxia | 40 ± 9.3 | 39.8 ± 8.7 | 43.4 ± 9.2* | 49.0 ± 10* | 50.5 ± 10* | 53.2 ± 11* |
| Hyperoxia | 39.4 ± 8.1 | 40.0 ± 7.0 | 47.8 ± 7.4* | 50.2 ± 8.3* | 53.2 ± 11* | 53.8 ± 13* |
| CVCiICA, ml·min−1·mmHg−1 | ||||||
| Normoxia | 2.6 ± 0.1 | 2.6 ± 0.7 | 2.5 ± 0.7 | 2.6 ± 0.7 | 2.6 ± 0.7 | 2.4 ± 0.8 |
| Hyperoxia | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.5 ± 0.5 | 2.8 ± 0.6 | 2.7 ± 0.7 | 2.6 ± 0.7 |
| PCAv,† cm/s | ||||||
| Normoxia | 43 ± 6.4 | 42 ± 6.8 | 45 ± 7.4* | 47 ± 7.4* | 48 ± 7.6* | 49 ± 6.8* |
| Hyperoxia | 42 ± 10* | 39 ± 9.7 | 43 ± 13* | 45 ± 13* | 48 ± 16* | 50 ± 17* |
| CVCiPCA,† cm·s−1·mmHg−1 | ||||||
| Normoxia | 0.52 ± 0.12 | 0.51 ± 0.12 | 0.50 ± 0.14 | 0.49 ± 0.14 | 0.48 ± 0.13 | 0.48 ± 0.13 |
| Hyperoxia | 0.48 ± 0.14*† | 0.42 ± 0.12† | 0.45 ± 0.17† | 0.47 ± 0.17* | 0.46 ± 0.18 | 0.46 ± 0.18 |
| QVA, ml/min | ||||||
| Normoxia | 86 ± 31 | 85 ± 34 | 87 ± 35 | 92 ± 38 | 96 ± 44* | 94 ± 38 |
| Hyperoxia | 80 ± 28 | 75 ± 27 | 82 ± 28 | 93 ± 33* | 102 ± 38* | 101 ± 44* |
| VAdiam, cm | ||||||
| Normoxia | 0.36 ± 0.7 | 0.36 ± 0.07 | 0.36 ± 0.07 | 0.36 ± 0.07 | 0.36 ± 0.07 | 0.35 ± 0.07 |
| Hyperoxia | 0.35 ± 0.06 | 0.34 ± 0.06 | 0.34 ± 0.06 | 0.34 ± 0.06 | 0.35 ± 0.06 | 0.34 ± 0.06 |
| VAvel,† cm/s | ||||||
| Normoxia | 29 ± 6.2 | 28 ± 5.6 | 29 ± 5.4 | 30 ± 7.0 | 31 ± 7.2* | 33 ± 8.8* |
| Hyperoxia | 27 ± 5.2 | 27 ± 5.8 | 30 ± 5.7* | 33 ± 6.9* | 35 ± 8.8* | 36 ± 11* |
| CVCiVA,† ml·min−1·mmHg−1 | ||||||
| Normoxia | 1.0 ± 0.4 | 1.0 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.5 | 0.9 ± 0.4 |
| Hyperoxia | 0.9 ± 0.4*† | 0.8 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.4* | 1.0 ± 0.4* | 1.0 ± 0.5* |
| gCBF,† ml/min | ||||||
| Normoxia | 640 ± 140 | 634 ± 146 | 673 ± 179 | 738 ± 188* | 770 ± 204* | 773 ± 256* |
| Hyperoxia | 611 ± 109 | 596 ± 108 | 677 ± 132 | 751 ± 190* | 814 ± 238* | 792 ± 184* |
| Isocapnia | ||||||
| MCAv, cm/s | ||||||
| Normoxia | 59 ± 6.6 | 59 ± 7.8 | 61 ± 9.2 | 65 ± 12 | 71 ± 16* | — |
| Hyperoxia‡ | 56 ± 8.9 | 55 ± 7.9 | 57 ± 10.6 | 57 ± 9.8 | 64 ± 15* | — |
| CVCiMCA, cm·s−1·mmHg−1 | ||||||
| Normoxia | 0.67 ± 0.1 | 0.68 ± 0.1 | 0.65 ± 0.1* | 0.65 ± 0.1* | 0.65 ± 0.1* | — |
| Hyperoxia | 0.64 ± 0.1 | 0.62 ± 0.1 | 0.60 ± 0.1 | 0.57 ± 0.1* | 0.59 ± 0.1* | — |
| QICA, ml/min | ||||||
| Normoxia | 241 ± 58 | 240 ± 58 | 255 ± 57 | 269 ± 76* | 282 ± 78* | — |
| Hyperoxia‡ | 229 ± 57 | 237 ± 52 | 255 ± 64 | 262 ± 51* | 278 ± 74* | — |
| ICAdiam, ml | ||||||
| Normoxia | 0.49 ± 0.06 | 0.49 ± 0.06 | 0.49 ± 0.07 | 0.49 ± 0.07 | 0.48 ± 0.06 | — |
| Hyperoxia | 0.50 ± 0.06 | 0.49 ± 0.05 | 0.48 ± 0.06 | 0.48 ± 0.06 | 0.48 ± 0.06 | — |
| ICAvel, cm/s | ||||||
| Normoxia | 42 ± 9.9 | 42 ± 10 | 45 ± 10* | 47 ± 10* | 51 ± 9* | — |
| Hyperoxia | 40 ± 9.4 | 42 ± 6.1 | 46 ± 7.0* | 49 ± 7.8* | 50 ± 8.3* | — |
| CVCiICA | ||||||
| Normoxia | 2.7 ± 0.7 | 2.7 ± 0.7 | 2.6 ± 0.6 | 2.6 ± 0.7 | 2.5 ± 2.7 | — |
| Hyperoxia | 2.5 ± 0.6 | 2.6 ± 0.6 | 2.6 ± 0.7 | 2.5 ± 0.7 | 2.5 ± 0.7 | — |
| PCAv, cm/s | ||||||
| Normoxia‡ | 42 ± 7.0 | 41 ± 6.5 | 43 ± 6.0 | 46 ± 7.0* | 49 ± 6.8* | — |
| Hyperoxia‡ | 43 ± 11 | 43 ± 12 | 44 ± 13 | 45 ± 13* | 50 ± 18* | — |
| CVCiPCA, cm·s−1 ·mmHg−1 | ||||||
| Normoxia | 0.48 ± 0.07 | 0.46 ± 0.06 | 0.44 ± 0.07 | 0.45 ± 0.07 | 0.46 ± 0.07 | — |
| Hyperoxia | 0.48 ± 0.1 | 0.46 ± 0.1 | 0.44 ± 0.1 | 0.43 ± 0.4 | 0.44 ± 0.1 | — |
| QVA, ml/min | ||||||
| Normoxia | 81 ± 26 | 80 ± 28 | 86 ± 36 | 91 ± 40 | 95 ± 47* | — |
| Hyperoxia‡ | 76 ± 25 | 76 ± 25 | 82 ± 27 | 85 ± 34‡ | 93 ± 38*‡ | — |
| VAdiam, cm | ||||||
| Normoxia | 0.35 ± 0.06 | 0.36 ± 0.07 | 0.35 ± 0.07 | 0.35 ± 0.07 | 0.35 ± 0.07 | — |
| Hyperoxia | 0.35 ± 0.06 | 0.35 ± 0.06 | 0.35 ± 0.06 | 0.34 ± 0.07 | 0.34 ± 0.06 | — |
| VAvel, cm/s | ||||||
| Normoxia | 28 ± 5.0 | 27 ± 5.4 | 29 ± 7.1 | 31 ± 7.3* | 33 ± 7.2* | — |
| Hyperoxia | 26 ± 3.6 | 27 ± 4.1 | 29 ± 4.6 | 30 ± 5.0* | 33 ± 7.8* | — |
| CVCiVA | ||||||
| Normoxia | 0.93 ± 0.3 | 0.90 ± 0.3 | 0.89 ± 0.4 | 0.90 ± 0.4 | 0.84 ± 0.4 | — |
| Hyperoxia | 0.86 ± 0.3 | 0.84 ± 0.3 | 0.84 ± 0.3 | 0.83 ± 0.4 | 0.82 ± 0.3 | — |
| gCBF, ml/min | ||||||
| Normoxia | 644 ± 128 | 641 ± 123 | 682 ± 124 | 719 ± 181* | 755 ± 214* | — |
| Hyperoxia‡ | 609 ± 120 | 626 ± 115 | 675 ± 148 | 693 ± 132* | 741 ± 180* | — |
Values are means ± SD. MCAv and PCAv, middle and posterior cerebral arteries; CVCiMCA and PCA and CVCiICA and VA, cerebrovascular resistance in intracranial and extracranial vessels; QICA and QVA, blood flow in the internal carotid artery and vertebral artery; VAvel and VAdiam, vertebral artery velocity and diameter; ICAvel and ICAdiam, internal carotid artery velocity and diameter; gCBF, global cerebral blood flow.
P < 0.05, from BL2.
P < 0.01, interaction normoxia vs. hyperoxia.
P < 0.017, interaction poikilocapnia vs. isocapnia.
Cardiorespiratory Measurements
As outlined in Table 1, the main goal of controlling PetCO2 was effectively achieved. For example, the progressive elevations in PetCO2 during poikilocapnic normoxic and hyperoxic exercise were prevented during the isocapnic conditions. Likewise, the isocapnic interventions prevented the ∼4-mmHg reductions in PetCO2 that were observed at rest during poikilocapnic hyperoxia. As expected, PetCO2 was also effectively maintained at hyperoxic levels in the related interventions. During both the normoxic and hyperoxic isocapnic exercise, the changes in PaCO2 and PetCO2 were comparable (i.e., within 1–2 mmHg). Additionally, despite a slightly lower intra-arterial MAP at each intensity, the relative changes in the intra-arterial blood pressure measurements were similar (i.e., within 3–6 mmHg) to the noninvasive estimates of MAP during isocapnic exercise, regardless of inspired O2. Both HR and MAP were not influenced by the hyperoxic or isocapnic interventions. Likewise, the progressive elevations in HR and MAP during exercise were similar during exercise between the poikilocapnia and isocapnic interventions.
Cerebrovascular Measurements
Normoxia and hyperoxic poikilocapnic exercise.
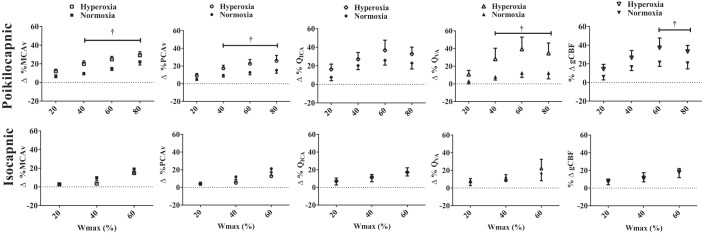
All cerebrovascular variables were unchanged following 10 min of normal breathing; however, during poikilocapnic hyperoxia, MCAv and PCAv were significantly lower compared with initial baseline (−4.4 ± 1.3 and −3.0 ± 1.3 cm/s, respectively) owing to the ∼4-mmHg reduction in PetCO2 (Tables 1 and 2; Fig. 1). Incremental poikilocapnic normoxic exercise generated progressive absolute increases in MCAv and PCAv at 20, 40, 60, and 80% Wmax. Similarly, QICA was increased at 40, 60, and 80% Wmax, and QVA was increased at 60% Wmax (Table 2; P < 0.05). A similar time course of cerebrovascular changes occurred during the incremental poikilocapnic hyperoxic exercise (Table 1; P < 0.05). Incremental poikilocapnic normoxic exercise resulted in progressive increases in gCBF from rest during 40–80% Wmax (∼Δ104–138 ml/min; P < 0.05); while poikilocapnic hyperoxic exercise also evoked progressive increases in gCBF from rest during 20–60% Wmax (∼Δ155–218 ml/min; P < 0.05). Compared with the relative increases in CBF (QVA) and CBV (MCAv and PCAv) values during poikilocapnic normoxic exercise at 40, 60, and 80% Wmax (∼Δ5–20%), the increases during the hyperoxic poikilocapnic exercise (∼Δ17–30%) were significantly greater (Fig. 1; P < 0.01 for all), with no significant difference in the QICA response (P > 0.01). Likewise, the relative increase in gCBF was significantly greater at 60% (37 vs. 21%; P < 0.01) and 80% Wmax (33 vs. 20%; P < 0.05) during hyperoxic vs. normoxic poikilocapnic exercise, respectively.
Fig. 1.
The percent change in the middle (%ΔMCAv) and posterior (%ΔPCAv) cerebral artery velocities, internal (%ΔQICA) and vertebral (%ΔQVA) artery blood flows, and global cerebral blood flow (%ΔgCBF) during hyperoxia compared with normoxia with (isocapnic) or without (poikilocapnic) PetCO2 controlled at basal values. †P < 0.05, significant differences between normoxia and hyperoxia.
Normoxia and hyperoxic isocapnic exercise.
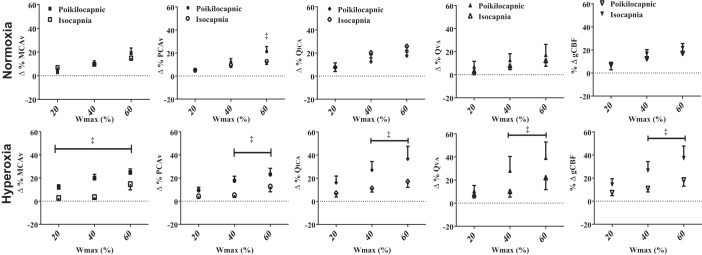
No differences in any cerebrovascular responses were observed between room air and hyperoxia during resting isocapnia (Table 2; Fig. 2). Regardless of the inspired O2, isocapnic exercise generated an elevated MCAv and QVA at 60% Wmax (Δ10.2 ± 1.6 cm/s and Δ16.1 ml/min, respectively) from basal values (P < 0.05; Table 2). While, PCAv and QICA were elevated both at 40 and 60% Wmax (P < 0.05; Table 2). The gCBF during incremental exercise was also elevated (P < 0.05) at 40 (+13%) and 60% Wmax (+20%) similarly in isocapnic normoxia and hyperoxia (Table 2; Fig. 2). Figure 2 illustrates that maintaining isocapnia during normoxic exercise (20–60% Wmax) did not significantly alter the cerebrovascular response to incremental exercise compared with poikilocapnia, except for reducing PCAv at 60% Wmax (P < 0.05). However, CBF and CBV through all vessels and gCBF were significantly reduced during hyperoxic isocapnia compared with poikilocapnia (Fig. 2; P < 0.017).
Fig. 2.
Comparing the percent change in the middle (%ΔMCAv) and (%ΔPCAv) posterior cerebral artery blood flow velocities, internal (%ΔQICA) and vertebral (%ΔQVA) artery blood flows, and global cerebral blood flow (%ΔgCBF) during isocapnic or poikilocapnic in both normoxia and hyperoxia. ‡P < 0.05, significant differences between isocapnic and poikilocapnic.
Comparisons between vessels.
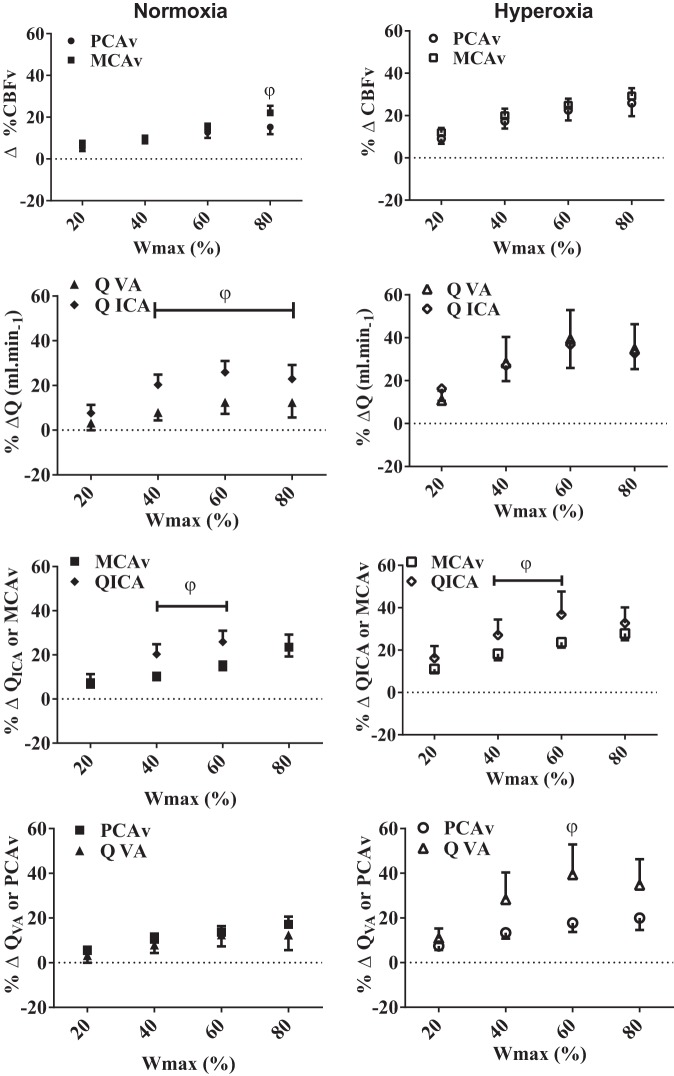
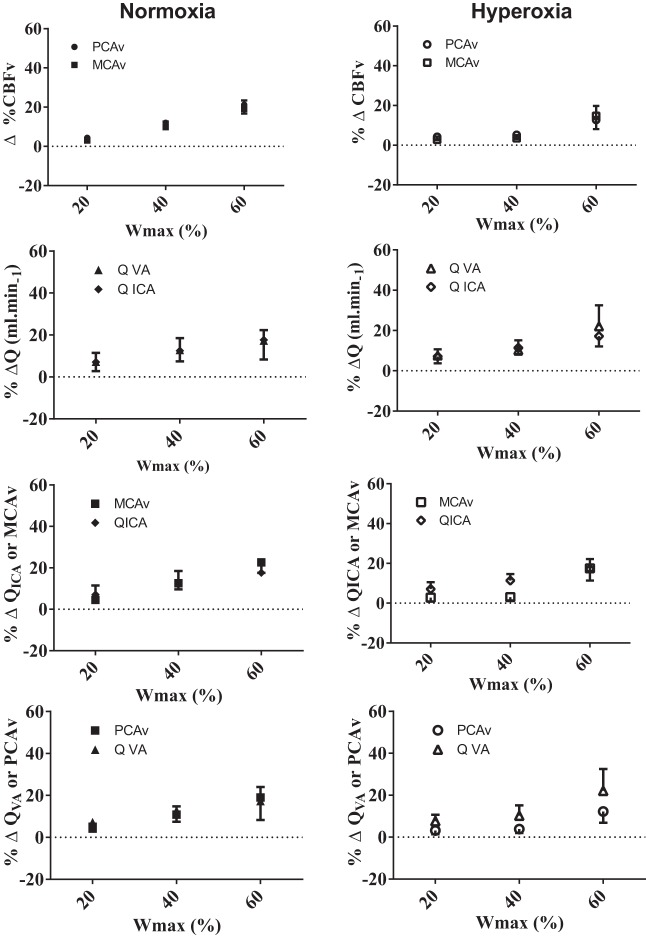
There were no relative CBV or extracranial blood flow differences observed while resting during poikilocapnia and isocapnia in normoxia and hyperoxia (Table 2; Figs. 3 and 4). During normoxic, but not hyperoxic, poikilocapnic exercise, the relative increase in MCAv at 80% Wmax was greater (+6.8 ± 1.7%) compared with the increases in PCAv (P < 0.05; Fig. 3). Similarly, during normoxic, but not hyperoxic, poikilocapnic exercise elevations in QVA were significantly lower compared with QICA at 40, 60, and 80% Wmax (∼45–61% lower; Fig. 3). Additionally, during both normoxic and hyperoxic poikilocapnic exercise the relative increases in QICA were ∼48 and 55.3% greater compared with MCAv increases during 40 and 60% Wmax, respectively (Table 2; Fig. 3; P < 0.05). Except for 60% Wmax during hyperoxia (%ΔQVA equals 21% > than %ΔPCAv), no significant differences were observed between the relative QVA and PCAv changes during normoxic and hyperoxic poikilocapnic exercise (Fig. 3). There were no differences between any of the intra- and extracranial or regional flows during normoxic or hyperoxic exercise when isocapnia was maintained (Fig. 4).
Fig. 3.
Comparisons of the regional intracranial velocities (MCAv vs. PCAv) and extracranial blood flows (QICA vs. QVA), as well as relative velocity and blood flow changes in the proximal intra-cranial vs. extracranial (MCAv vs. QICA; PCAv vs. QVA) vessels during poikilocapnic normoxia and hyperoxia. φP < 0.05, significance between vessels.
Fig. 4.
Comparisons of the regional intracranial velocities (MCAv vs. PCAv) and extracranial blood flows (QICA vs. QVA), as well as relative velocity and blood flow changes in the proximal intracranial vs. extracranial blood flow (MCAv vs. QICA and PCAv vs. QVA) vessels during isocapnic normoxia and hyperoxia. No significant differences were observed between vessels in normoxia and hyperoxia, P < 0.05.
Influence of PetCO2.
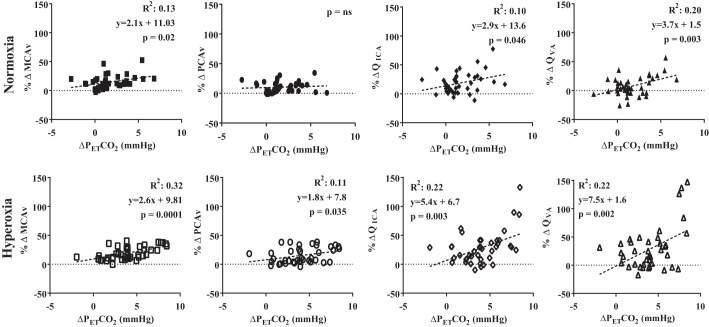
During normoxic poikilocapnic exercise the relative change in MCAv, QICA, and QVA were positively correlated (P < 0.05) with the absolute change in PetCO2 (R2 = 0.13, 0.10, and 0.20, respectively; Fig. 5). During hyperoxic poikilocapnic exercise the relative change in MCAv, PCAv, QICA, and QVA was positively (P < 0.05) correlated with the absolute change in PetCO2 (R2 = 0.32, 0.11, 0.22, and 0.22, respectively; Fig. 5). Since PetCO2 was maintained during isocapnia, no correlations were ran during these trials.
Fig. 5.
The relationship between the percent change in intracranial velocities (%ΔMCAv and %ΔPCAv) and extracranial blood flow (%ΔQICA and %ΔQVA) with the unit change in the partial pressure of end-tidal carbon dioxide (ΔPetCO2) during poikilocapnic normoxic and hyperoxic exercise.
Isocapnic hyperpnea.
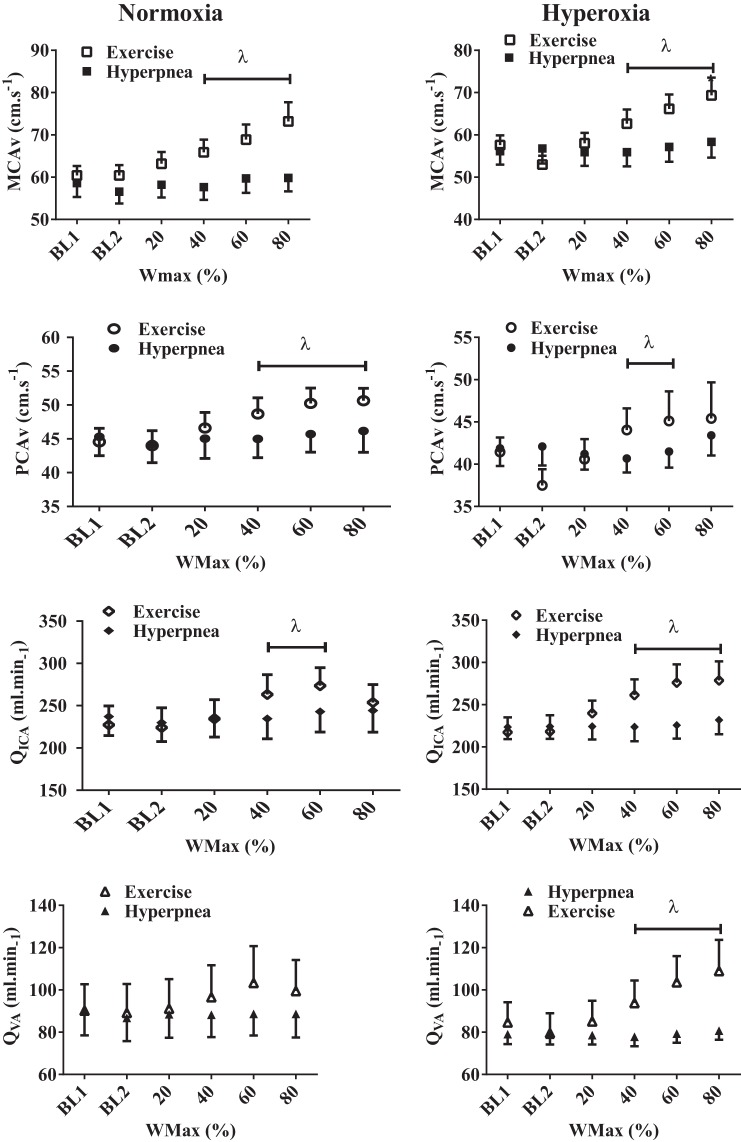
There were no changes in CBV or extracranial blood flow during isocapnic hyperpnea when compared with the changes observed with poikilocapnic exercise (Fig. 6).
Fig. 6.
Comparison between the absolute intracranial velocities (MCAv; PCAv) and extracranial blood flow (QICA; QVA) during poikilocapnic exercise and isocapnic hyperpnea. λP < 0.05, significant differences between poikilocapnic exercise and isocapnic hyperpnea.
DISCUSSION
There are three novel findings from the current study: first, although maintaining isocapnia during normoxic exercise does not attenuate the gCBF response to pokilocapnic exercise conflicts with our first hypothesis, it did attenuate the regional (QICA vs. QVA and MCAv vs PCAv) and intra- vs. extracranial (MCAv vs. QICA) CBF differences observed during poikilocapnic normoxic exercise. Alternately, in support of our second hypothesis, maintaining isocapnia during hyperoxic exercise not only attenuates the gCBF response, but also the subtle intra- vs. extracranial (MCAv vs. QICA and PCAv vs. QVA) CBF differences observed during hyperoxic poikilocapnic exercise. Finally, contrary to our third hypothesis, isocapnic hyperpnea per se did not alter CBF. Cumulatively, our findings demonstrate a differential effect of PetCO2 on CBF during exercise influenced by the prevailing PetO2.
CBF During Exercise
Normoxia.
The CBF response to incremental exercise has traditional been described as being driven primarily by the hypercapnic and hypocapnic consequences of hypo- and hyperventilation during submaximal and maximal exercise, respectively (4, 11, 23–25). The current findings demonstrate that MCAv increases to the same extent (i.e., ∼20%) during exercise up to 60% Wmax with and without PetCO2 clamped at baseline values (Figs. 1 and 2). Furthermore, the similar response throughout all exercise intensities, regardless of PetCO2, was also observed in both of the proximal neck vessels (QICA and QVA), and gCBF. The PCAv response at 60%, however, was slightly elevated during poikilocapnia compared with isocapnia. Thus, except for the PCAv, the vessel responses were contrary to our hypothesis. These findings indicate that the magnitude of the cerebrovascular response to submaximal semirecumbent normoxic exercise is not necessarily influenced by PetCO2. Since both MAP and cardiac output were similar between conditions, the mechanism(s) elevating CBF are therefore likely related to compensatory increases in cerebral metabolism and/or shear stress-mediated vasodilation. However, although our findings of a similar MAP and cardiac output throughout the study are broadly consistent with previous data demonstrating a linearity between elevations in cardiac output and CBF during submaximal exercise and a preserved pressure-flow dynamic relationship (26), the ∼20% increase in CBF falls short of the nearly fourfold increase in cardiac output at 60% Wmax, which suggests that neither PetCO2 or cardiac output independently dictates the magnitude of CBF regulation during submaximal exercise. It remains to be established what mechanism(s) is facilitating similar rises in gCBF during isocapnic exercise; cerebral metabolism or shear stress, or both, seem plausible factors.
Hyperoxia.
During isocapnic hyperoxia, experimentally preventing the rise in PetCO2 above resting values attenuated the gCBF response compared with poikilocapnic hyperoxic exercise. Furthermore, isocapnic exercise negated the regional differences in the intracranial (MCAv vs. PCAv) and neck (QICA vs. QVA) vessels, present during the poikilocapnic hyperoxic trial. The relative underestimation of volumetric extracranial observed in the intracranial vessels (i.e., %ΔQICA > %ΔMCAv; %ΔQVA > %ΔPCAv) during poikilocapnic hyperoxia was inhibited through isocapnia. These findings support previous speculation, which identified an elevated PetCO2 stimulus as a potential mechanism responsible for the enhanced PCAv response (6). Overall, the discrepancy between intra- extracranial flows highlight the technical issues associated using CBV measures to index CBF during poikilocapnic hyperoxic exercise.
Lambertsen et al. (11) first attempted to address the influence that hyperoxia had on gCBF regulation during constant load using normobaric normoxia (FiO2 = 0.21) and hyperbaric (2 atm) hyperoxia (FiO2 = 1.0). Likely because of the small sample size (n = 2), absolute workloads, and lack of baseline, hyperoxic exercise did not result in a different absolute gCBF compared with normoxic values despite a significantly elevated PetCO2 (+3.5 mmHg). Thus this is the first study to quantify the volumetric enhancement of gCBF during hyperoxic exercise, while demonstrating the importance that PetCO2 plays in regulating gCBF and regional CBF distribution. Future studies investigating CBF during exercise solely using TCD or volumetric flow in one region of the brain need to consider the potential underestimation of the true CBF response, specifically when dealing with variations in PetO2 and PetCO2.
In an earlier study, we found a strong and significant relationship (R2 = 0.8) between the elevations in PetCO2 with PCAv during upright hyperoxic exercise (6). In the current study, however, despite all of the cerebrovascular variables being linearly related to the change in PetCO2 (Fig. 5), the strength of the relationships were less (R2 = 0.11 to 0.32) compared with our previous findings. One possible explanation regarding the differences between PetCO2 relationships in these two studies is that upright hyperoxic exercise had a greater PetCO2 increase from basal values compared with semirecumbent exercise (e.g., 9 vs. 6 mmHg, respectively). Therefore, the postural effect of upright vs. recumbent cycling in hyperoxia will have likely reduced the PetCO2 response to submaximal exercise; however, this remains to be established and posture did not seem to have affected the overall cerebrovascular response to hyperoxic exercise.
Cerebrovascular Response to Isocapnic Hyperoxic Exercise
Hyperoxia heightened the cerebrovascular response to exercise compared with normoxia (Figs. 1 and 3). When isocapnia was maintained during exercise, the hyperoxic cerebrovascular response was attenuated, demonstrating the critical importance of PaCO2 in mediating this response (Fig. 4). At rest, 1-mmHg elevation in PaCO2 results in a 3–5% increase in CBV (i.e., MCAv; reviewed in Ref. 41). During exercise, however, this CBV reactivity is increased to 4–6% per mmHg elevation in PetCO2 (13). Based on these latter findings, the hyperoxic induced 6-mmHg rise in PetCO2 from baseline values could explain the majority of the elevated intra- and extracranial CBF during poikilcapnic hyperoxic compared with isocapnic hyperoxic exercise. The abolishment of the observed differences during poikilocapnic hyperoxia further supports this finding (Fig. 4).
Regional, intracranial, and proximal artery vascular responses to exercise.
To date only five studies have investigated the regional cerebrovascular response to exercise (1, 6, 7, 28, 29). The first study to investigate regional CBF during exercise, using a xenograph (i.e., γ-radiation detection following injections of xenon) reported no differences in the blood flow response between various cortical regions (frontal, precentral, postcentral, parietal, temporal, and occipital) during recumbent cycling exercise at 25 or 100 W (28). Three of the remaining four studies observed larger relative increases in posterior compared with anterior intracranial (PCAv vs. MCAv; Ref. 29) velocities and extracranial blood flow (QVA vs. QICA; Refs. 1, 7) flows during normoxic exercise. The final study, consistent with the current findings, observed a regional difference in the CBV response that was selective to hyperoxic exercise (i.e., hyperoxia only exacerbated the PCAv response not the MCAv; Ref. 6). In the current study we have identified a greater relative increase in QICA vs. MCAv in the anterior circulation during normoxia and greater relative increases in extracranial blood flow compared with the corresponding distal intracranial arteries (e.g., QICA > MCAv and QVA > PCAv) during hyperoxia. Given that clamping PetCO2 (isocapnia) during exercise in both normoxia and hyperoxia attenuates any of the observed regional and intracranial vs. extracranial responses, it appears that PaCO2 is primarily responsible for the variation (i.e., regional, intracranial vs. proximal arteries and FiO2) in the cerebrovascular response to exercise.
PetCO2, CBF, and Exercise
Collectively, the results from this study are the first to indicate that CBF regulation during exercise, at least during submaximal intensities, is not solely dependent on PetCO2. Furthermore, the relationship between CBF and PetCO2 during poikilocapnic exercise was lower than previously reported (6, 25, 30). Nonetheless, studies previously reporting an elevated CBF and PetCO2 reactivity, observed the relationship during upright exercise, which is reported to enhance the MCA/PetCO2 reactivity (13). Thus our findings may indicate that PetCO2 and CBF reactivity during recumbent exercise is either reduced or unaltered from resting reactivity. Unfortunately, the influence that posture may have on the PaCO2 and CBF relationship remains unknown. Regardless, the increases in CBV and extracranial blood flow observed during poikilocapnic and isocapnic recumbent exercise in the current study were similar to the rises observed in the previous studies, which utilized upright (6, 25, 30). Therefore, the lack of an attenuated CBF response during isocapnic normoxic exercise strongly suggests that, although PetCO2 is a known regulator of CBF at rest and during maximal exercise (25), it is not an essential component of the hyperemic CBF response observed during submaximal recumbent exercise. Thus it seems plausible that the magnitude of gCBF response during submaximal exercise, in the absence of a vasodilatory CO2 stimulus, relies more on appropriate distribution in cardiac output, cerebral metabolism and cerebral perfusion pressure; however, the regional distribution is at least partially dictated by PetCO2.
Isocapnic Hyperpnea
The observations by Subudhi et al. (31) indicating an increase in ventral medullary blood flow (∼22%) in anesthetized cats during isocapnic hyperpnea were suggested to indicate that increases in V̇e lead to local increases in brain metabolism sufficient to elevate local blood flow. Given that no changes were observed in any of the intra- or extracranial blood flow measures during the isocapnic hyperpnea trial, our findings are not consistent to these well-controlled animal data. Further investigations with more localized resolution (i.e., magnetic resolution imaging) are needed to precisely determine if local cortical blood flow in the medulla is altered during isocapnic hyperpnea.
Methodological Considerations and Limitations
Although the current study was performed in a controlled laboratory setting, some limitations are apparent. For instance, because of the difficulty associated with measuring volumetric flow in the proximal neck vessels during exercise we utilized a recumbent ergometer to minimize potential motion artifacts. Nevertheless, despite the 17 participants from whom we attempted to collect extracranial blood flow in during exercise, we were unable to obtain adequate measures in 3 subjects. Additionally, because of the difficulties associated with measuring beat-to-beat volumetric flow during exercise, we chose to utilize a protocol that provided sufficient time (i.e., 5-min stages) to achieve an adequate time window with which to collect the volumetric measures during steady state exercise. Thus our protocol limited our ability to measure volumetric flow during exercise intensities above 80% Wmax. Regardless, the maximal exercise tests performed at the beginning of each trial allowed for accurate determination of the absolute wattages required for controlling the relative intensity. Additionally, volumetric extracranial assessment was not achievable at 80% Wmax in the isocapnic trials due to excess movement. During maximal exercise (i.e., >60% Wmax) hyperventilation induces a reduction in PaCO2 from its peak value consequently mitigating any further rise in CBF (4, 25). We also observed this response characteristic at 80% Wmax during poikilocapnic exercise (Fig. 1). Mitigating this reduction in PaCO2 at maximal exercise intensities, using a modified rebreathing system, eliminates the reduction in peak CBF typically observed during poikilocapnic maximal exercise (25, 32).
It has traditionally been thought that the changes in the TCD measurements of MCAv were valid indexes of the CBF response to exercise, when compared with gCBF measured using the Kety-Schmidt technique (8, 23). However, only three studies have successfully quantified both the MCAv and QICA responses during exercise (1, 7, 33), and no studies have compared the PCAv and QVA responses during exercise. The previous studies investigating the MCAv and QICA responses to exercise showed almost identical responses. The previous findings are in contrast with the current results, which observed greater proximal CBF vs. CBV responses to incremental exercise in both normoxia and hyperoxia (Fig. 3). The discrepancies between studies may be a reflection of differences in exercise duration and the use of manual (1, 7, 33) measures of blood flow rather than our automated approach. Using edge-detection software not only provides a more robust and sensitive assessment of vessels diameter and velocity (34), it limits subjectivity and bias during data analysis (34). Whether dilation of the MCA occurs during exercise is unknown, although contrasting reports have recently surfaced demonstrating either a stable MCA diameter (35) or a subtle MCA dilation (36) during modest (>8 mmHg) elevations in PetCO2, albeit at rest, using high-resolution MRI. Future studies are required to establish whether or not there are changes in MCA diameter during exercise.
Conclusion
Our findings demonstrate the absence of a V̇e influence per se and a differential effect of PetCO2 on CBF during exercise that is influenced by the prevailing PetO2.
GRANTS
K.J.S is a Doctoral Research Award holder for the Heart and Stroke Foundation of Canada. K.J.S. and N.C.S hold NSERC post Doctoral fellowships; R.L.H. is funded by a NSERC Post Graduate Scholarship; P.N.A holds a Canada Research Chair.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.J.S., K.W.W., N.C.L., G.E.F., and P.N.A. conception and design of research; K.J.S., K.W.W., R.L.H., H.H., N.C.L., A.P., S.L.S., and T.K. performed experiments; K.J.S., A.P., S.L.S., and T.K. analyzed data; K.J.S., H.H., A.P., G.E.F., and P.N.A. interpreted results of experiments; K.J.S. and S.L.S. prepared figures; K.J.S. drafted manuscript; K.J.S., R.L.H., N.C.L., G.E.F., and P.N.A. edited and revised manuscript; K.J.S., R.L.H., and P.N.A. approved final version of manuscript.
REFERENCES
- 1.Asmussen E, Nielsen M. Studies on the regulation of respiration in heavy work. Acta Physiol Scand 12: 171–188, 1946. [Google Scholar]
- 2.Bannister RG, Cunningham DJ. The effects on the respiration and performance during exercise of adding oxygen to the inspired air. J Physiol 125: 118–137, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker HF, Polo O, McNamara SG, Berthon-Jones M, Sullivan CE. Effect of different levels of hyperoxia on breathing in healthy subjects. J Appl Physiol 81: 1683–1690, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow-mediated dilatation in humans. Hypertension 51: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Coverdale NS, Gati JS, Opalevych O, Perrotta A, Shoemaker JK. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 117: 1090–1096, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Evans DH. On the measurement of the mean velocity of blood flow over the cardiac cycle using Doppler ultrasound. Ultrasound Med Biol 11: 735–741, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Fisher JP, Hartwich D, Seifert T, Olesen ND, McNulty CL, Nielsen HB, Van Lieshout JJ, Secher NH. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiol 591: 1859–1870, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellström G, Wahlgren NG. Physical exercise increases middle cerebral artery blood flow velocity. Neurosurg Rev 16: 151–156, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Herholz K, Buskies W, Rist M, Pawlik G, Hollmann W, Heiss WD. Regional cerebral blood flow in man at rest and during exercise. J Neurol 234: 9–13, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol 72: 1123–1132, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Lambertsen CJ, Owen SG, Wendel H, Stroud MW, Lurie AA, Lochner W, Clark GF. Respiratory and cerebral circulatory control during exercise at 21 and 20 atmospheres inspired Po2. J Appl Physiol 14: 966–982, 1959. [DOI] [PubMed] [Google Scholar]
- 12.Linkis P, Jorgensen LG, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. J Appl Physiol 78: 12–16, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiødtz G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. J Appl Physiol 74: 245–250, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Marsden KR, Haykowsky MJ, Smirl JD, Jones H, Nelson MD, Altamirano-Diaz LA, Gelinas JC, Tzeng YC, Smith KJ, Willie CK, Bailey DM, Ainslie PN. Aging blunts hyperventilation-induced hypocapnia and reduction in cerebral blood flow velocity during maximal exercise. Age (Dordr) 34: 725–735, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateika JH, Duffin J. The ventilation, lactate and electromyographic thresholds during incremental exercise tests in normoxia, hypoxia and hyperoxia. Eur J Appl Physiol Occup Physiol 69: 110–118, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Moraine JJ, Lamotte M, Berré J, Niset G, Leduc A, Naeije R. Relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol 67: 35–38, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Neubauer JA, Strumpf DA, Edelman NH. Regional medullary blood flow during isocapnic hyperpnea in anesthetized cats. J Appl Physiol 55: 447–452, 1983. [DOI] [PubMed] [Google Scholar]
- 20.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 107: 1370–1380, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol 569: 697–704, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olin JT, Dimmen AC, Subudhi AW, Roach RC. Cerebral blood flow and oxygenation at maximal exercise: the effect of clamping carbon dioxide. Respir Physiol Neurobiol 175: 176–180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen P, Nielsen J, Overgaard M, Krogh Madsen R, Gjedde A, Secher NH, Petersen NC. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol 588: 1985–1995, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol 96: 299–304, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Sato K, Ogoh S, Hirasawa A, Oue A, Sadamoto TA. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 589: 2847–2856, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato K, Sadamoto T. Different blood flow responses to dynamic exercise between internal carotid and vertebral arteries in women. J Appl Physiol 109: 864–869, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Schöning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke 25: 17–22, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Smith KJ, MacLeod D, Willie CK, Lewis NCS, Hoiland RL, Ikeda K, Tymko MM, Donnelly J, Day TA, MacLeod N, Lucas SJ, Ainslie PN. Influence of high altitude on cerebral blood flow and fuel utilization during exercise and recovery. J Physiol 592: 5507–5527, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith KJ, Wong LE, Eves ND, Koelwyn GJ, Smirl JD, Willie CK, Ainslie PN. Regional cerebral blood flow distribution during exercise: influence of oxygen. Respir Physiol Neurobiol 184: 97–105, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Subudhi AW, Lorenz MC, Fulco CS, Roach RC. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: effect of oxygenation on maximal performance. Am J Physiol Heart Circ Physiol 294: H164–H171, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Subudhi AW, Miramon BR, Granger ME, Roach RC. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J Appl Physiol 106: 1153–1158, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subudhi AW, Olin JT, Dimmen AC, Polaner DM, Kayser B, Roach RC. Does cerebral oxygen delivery limit incremental exercise performance? J Appl Physiol 111: 1727–1734, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas KN, Lewis NC, Hill BG, Ainslie PN. Technical recommendations for the use of carotid duplex ultrasound for the assessment of extracranial blood flow. Am J Physiol Regul Integr Comp Physiol 309: R707–R720, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Trangmar SJ, Chiesa ST, Stock CG, Kalsi KK, Secher NH, Gonzalez-Alonso J. Dehydration affects cerebral blood flow but not its metabolic rate for oxygen during maximal exercise in trained humans. J Physiol 592: 3143–3160, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tymko MM, Ainslie PN, Macleod DB, Willie CK, Foster GE. End-tidal-to-arterial CO2 and O2 gas gradients at low- and high-altitude during dynamic end-tidal forcing. Am J Physiol Regul Integr Comp Physiol 308: R895–R906, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Verbree J, Bronzwaer AS, Ghariq E, Versluis MJ, Daemen MJ, van Buchem MA, Dahan A, Van Lieshout JJ, van Osch MJ. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra high-field MRI. J Appl Physiol 117: 1084–1089, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol 42: 385–390, 1977. [DOI] [PubMed] [Google Scholar]
- 39.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Willie CK, Cowan EC, Ainslie PN, Taylor CE, Smith KJ, Sin PY, Tzeng YC. Neurovascular coupling and distribution of cerebral blood flow during exercise. J Neurosci Methods 198: 270–273, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA, Ainslie PN. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590: 3261–3275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodman RJ, Playford DA, Watts GF, Cheetham C, Reed C, Taylor RR, Puddey IB, Beilin LJ, Burke V, Mori TA, Green D. Improved analysis of brachial artery ultrasound using a novel edge-detection software system. J Appl Physiol 91: 929–937, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Methods 35: 648–654. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]