Physical inactivity compromises skeletal muscle fitness. Gross changes in lean muscle mass and function following 14 days of bed rest in healthy middle-aged adults were associated with reduced satellite cell and capillary content and fiber atrophy. Satellite cell loss was correlated with muscle fiber atrophy, highlighting their interplay during mechanical unloading. Efforts and awareness of the need to protect muscle health during enforced inactivity should be expanded to include middle-aged, phenotypically healthy adults.
Keywords: Pax7, mechanical unloading, spaceflight, myonuclei, capillary
Abstract
Bed rest, a ground-based spaceflight analog, induces robust atrophy of skeletal muscle, an effect that is exacerbated with increasing age. We examined the effect of 14 days of bed rest on skeletal muscle satellite cell content and fiber type atrophy in middle-aged adults, an understudied age demographic with few overt signs of muscle aging that is representative of astronauts who perform long-duration spaceflight. Muscle biopsies were obtained from the vastus lateralis of healthy middle-aged adults [n = 7 (4 male, 3 female); age: 51 ± 1 yr] before (Pre-BR) and after (Post-BR) 14 days of bed rest. Immunohistochemical analyses were used to quantify myosin heavy chain (MyHC) isoform expression, cross-sectional area (CSA), satellite cell and myonuclear content, and capillary density. Peak oxygen consumption, knee extensor strength, and body composition were also measured Pre-BR and Post-BR. Post-BR MyHC type 2a fiber percentage was reduced, and mean CSA decreased in all fiber types (−24 ± 5%; P < 0.05). Satellite cell content was also reduced Post-BR (−39 ± 9%; P < 0.05), and the change in satellite cell content was significantly correlated with the change in mean fiber CSA (r2 = 0.60; P < 0.05). A decline in capillary density was observed Post-BR (−23 ± 6%; P < 0.05), and Post-BR capillary content was significantly associated with Post-BR peak aerobic capacity (r2 = 0.59; P < 0.05). A subtle decline in myonuclear content occurred during bed rest (−5 ± 1%; P < 0.05). The rapid maladaptation of skeletal muscle to 14 days of mechanical unloading in middle-aged adults emphasizes the need for robust countermeasures to preserve muscle function in astronauts.
NEW & NOTEWORTHY
Physical inactivity compromises skeletal muscle fitness. Gross changes in lean muscle mass and function following 14 days of bed rest in healthy middle-aged adults were associated with reduced satellite cell and capillary content and fiber atrophy. Satellite cell loss was correlated with muscle fiber atrophy, highlighting their interplay during mechanical unloading. Efforts and awareness of the need to protect muscle health during enforced inactivity should be expanded to include middle-aged, phenotypically healthy adults.
mechanical unloading induces a myriad of detrimental changes in skeletal muscle, including rapid atrophy and decreases in strength and functional capacity in younger and older adults (14, 27, 34, 38, 40, 74). Traditionally, studies of physical inactivity have targeted young (18-40 yr) or older (65+ yr) adult populations with middle-aged adults (45–60 yr) remaining largely unexamined (21, 71). It is unknown whether middle-aged adults experience accelerated bed rest-induced muscle loss similar to an older population. The study of middle-aged adults is particularly relevant to spaceflight, as the average age of the International Space Station crewmember is 48 yr (75).
Satellite (muscle stem) cells are integral to the regeneration and recovery of skeletal muscle. Satellite cell population and proliferative capacity are known to decline during the aging process (60, 65), which may impair the ability of the muscle to recover from an injury. Following injury or during adaptation, myonuclear addition within muscle fibers is accomplished solely through the activation and fusion of satellite cells (29, 43, 47). In an extreme model of skeletal muscle disuse (e.g., spinal cord injury), a reduction in satellite cell content is observed in conjunction with robust muscle fiber atrophy (68). However, in healthy younger adults, a 2-wk period of lower-limb immobilization induces fiber atrophy that is not accompanied by changes in satellite cell content (64).
Although middle-aged adults maintain an outwardly youthful phenotype, during mechanical unloading such as bed rest they may be at an increased risk of rapid, negative skeletal muscle alterations with responses more characteristic of older, not younger, adults (12, 20, 55). In the present study, we hypothesized that muscle fiber atrophy would be associated with a decline in satellite cell content in middle-aged adults during 14 days of bed rest.
METHODS
Subjects.
Seven healthy, community-dwelling, middle-aged adults (4 males, 3 females) participated in this study. Subjects were 51.0 ± 0.6 yr old (range: 49–54) with a BMI of 24.8 ± 1.5 kg/m2. All subjects were healthy and physically active but were not currently engaged in an exercise-training or weight-loss program. All study protocols and procedures were approved by the University of Texas Medical Branch (UMTB) Institutional Review Board (IRB), which is in compliance with the third revision of the Declaration of Helsinki. Subjects provided written, informed consent and were screened for participation in the UTMB Institute for Translational Sciences-Clinical Research Center (ITS-CRC).
Study design.
Following screening, subjects were admitted to the ITS-CRC. Peak aerobic capacity (V̇o2 peak) was assessed 2 days (day −2) before beginning bed rest using a metabolic cart (VMax Encore 29; Care Fusion, Yorba Linda, CA) with a graded exercise test on a cycle ergometer (Monark Ergomedic 828E; Monark Exercise, Vansbro Sweden). V̇o2 peak data were expressed in relative terms (ml·kg body mass−1·min−1). Unilateral knee extensor strength (peak torque, Nm) and knee muscle endurance (total work, J) were assessed using isokinetic dynamometry pre-bed Rest (Pre-BR) and post-bed rest (Post-BR) (Biodex System 4; Biodex Medical Systems, Shirley, NY) 2 days (day −2) before beginning bed rest and 1 day following completion of bed rest (day 15). Familiarization sessions were conducted upon admission. Peak torque was assessed via five maximal repetitions at 180°/s, whereas knee total work/muscular endurance was assessed using 20 repetitions at 180°/s. One day before (day −1) beginning bed rest, subjects completed a baseline dual energy X-ray absorptiometry (DXA, Lunar iDXA; GE Medical Systems, Madison, WI) scan to assess body composition. To standardize and minimize the effects of fluid shifts, subjects were required to lie supine for 10 min before the scan. After baseline testing, subjects began 14 days of bed rest (days 1–14). The horizontal bed rest model provided constant subject monitoring, safety, and comfort consistent with our previous studies (49, 50, 51). All bathing and toiletry activities were performed without weight bearing. To facilitate eating, bed backs were raised to a 5° incline during three, 2-h periods each day that corresponded with daily meals. Subjects received controlled, isoenergetic diets (55% carbohydrate, 30% fat, and 15% protein) evenly distributed across three daily meals (0800, 1300, and 1800); snacking was not permitted. Daily energy requirements were estimated using the Harris-Benedict equation. An activity factor of 1.3 was used during the bed rest period (49, 50, 51). Caloric intake was sufficient to maintain body weight, as subjects lost an average of 627 ± 217 g (0.8 ± 0.3% body wt) during the bed rest period. Total lean mass declined by 711 ± 239 g (1.3 ± 0.5%), and total fat mass increased by 84 ± 100 g (0.2 ± 0.2%). Body composition (DXA) was reassessed on day 13 of the bed-rest period, and Post-BR aerobic testing and knee extensor strength occurred 1 day following completion of bed rest (day 15). Posttesting was performed using the same protocols utilized during Pre-BR testing.
Muscle biopsies.
At 0900 on days 1 (Pre-BR) and 14 (Post-BR) of bed rest, with subjects in the postabsorptive state, a muscle biopsy was obtained from the vastus lateralis muscle (incision site located 15–25 cm proximal to the midpatella) using a 5-mm Bergström biopsy needle under sterile conditions and local anesthesia (1% lidocaine) (6). Pre- and Post-BR muscle biopsies were obtained from contralateral limbs. The dominant leg was randomized for muscle collection Pre- or Post-BR. The position of the biopsy site Pre-BR was marked with a permanent marker, so that the Post-BR sample was taken at the same position proximal to the midpatella. Muscle tissue samples (∼50 mg) were oriented and mounted in Tissue Tek (optimal cutting temperature compound; Sakura Finetek, Torrance CA) media on cork immediately after the biopsy and frozen in liquid nitrogen-cooled isopentane for immunohistochemical analysis.
Immunohistochemistry.
Seven-micron-thick sections were cut in a cryostat (HM525X; ThermoFisher, Waltham, MA), and sections were allowed to air dry for 1 h. For fiber typing, unfixed slides were incubated overnight at room temperature with antibodies against anti-myosin heavy chain (MyHC) isoforms type 1 (BA.D5; IgG2b), type 2a (SC.71; IgG1), and type 2x (6H1; IgM) from Developmental Studies Hybridoma Bank (DSHB, Iowa City, IA). The next day, slides were incubated with immunoglobulin-specific secondary antibodies: goat anti-mouse IgG2b AF647 (no. A21242, type 1), IgG1 AF488 (no. A21121, type 2a), and IgM AF555 (no. A21426, type 2x), all from Invitrogen (Grand Island, NY). Slides were postfixed in methanol before being mounted with fluorescent mounting media (Vectashield, no. H-1000; Vector Laboratories, Burlingame, CA).
For capillary and arteriole staining, slides were fixed for 10 min in ice-cold acetone and then blocked for 1 h in 2.5% normal horse serum. Slides were incubated overnight in antibody against α-smooth muscle actin (α-SMA, no. sc-130616; Santa Cruz Biotechnology, Santa Cruz, CA) and rhodamine-labeled Ulex Europaeus agglutinin I (no. RL-1062; Vector Laboratories), a human endothelial cell marker (32). The next day, slides were incubated in secondary antibody: goat anti-mouse IgG2a AF488 (no. A-21131; Invitrogen, α-SMA) before being mounted with fluorescent mounting media.
For Pax7/MyHC type 1/laminin staining, slides were fixed for 10 min in ice-cold acetone, endogenous peroxidases were blocked with 3% H2O2, and then slides were incubated overnight in anti-laminin (no. L9393; Sigma Aldrich, St. Louis, MO) and MyHC type 1 antibodies at 4°C. The next day, slides were incubated in goat anti-rabbit AF488 (no. A11034; Invitrogen, laminin) and goat anti-mouse IgG2b, AF647 (no. A21242; Invitrogen, type 1) for 1 h and then blocked for 1 h in 2.5% normal horse serum (no. S-2012; Vector Laboratories). Slides were incubated overnight at 4°C in anti-Pax7 antibody (DSHB), followed by goat anti-mouse biotin secondary antibody (no. 115-065-205; Jackson ImmunoResearch, West Grove, PA) for 1 h and reacted with streptavidin-horseradish peroxidase and AF555 tyramide included with the TSA kit (no. T20935; Invitrogen). Slides were costained with 4′,6-diamidino-2-phenylindole (DAPI, no. D35471; Invitrogen) before being mounted with fluorescent mounting media.
For CD56 (neural cell adhesion molecule, NCAM)/MyHC type 1/laminin staining, slides were fixed for 10 min in ice-cold acetone, and then slides were incubated overnight in anti-laminin (no. L9393; Sigma Aldrich) and anti-MyHC type 1 antibodies (no. BA.D5; IgG2b, DSHB) at 4°C. The next day, slides were incubated in goat anti-rabbit AF488 (no. A11034; Invitrogen, laminin) and goat anti-mouse IgG2b, AF647 (no. A21242; Invitrogen, type 1) for 1 h and then blocked for 1 h in 2.5% normal horse serum (no. S-2012; Vector Laboratories). Slides were incubated overnight at 4°C in anti-CD56/NCAM antibody (no. 555514; BD Biosciences, San Jose, CA), followed by goat anti-mouse IgG1, AF555 (no. A21127; Invitrogen, CD56/NCAM). Slides were costained with DAPI before being mounted with fluorescent mounting media.
For MyoD/MyHC type 1/laminin staining, slides were fixed for 10 min in ice-cold acetone, endogenous peroxidases were blocked with 3% H2O2, and then slides were incubated overnight in anti-laminin (no. L9393; Sigma Aldrich) and anti- MyHC type 1 antibodies (no. BA.D5; IgG2b, DSHB) at 4°C. The next day, slides were incubated in goat anti-rabbit AF488 (no. A11034; Invitrogen, laminin) and goat anti-mouse IgG2b, AF647 (no. A21242; Invitrogen, type 1) for 1 h and then blocked for 1 h in 2.5% normal horse serum (no. S-2012; Vector Laboratories). Slides were incubated overnight at 4°C in anti-MyoD antibody (no. 554130; BD Biosciences), followed by goat anti-mouse biotin secondary antibody (no. 115-065-205; Jackson ImmunoResearch) for 1 h, and reacted with streptavidin-horseradish peroxidase and AF555 tyramide included with the TSA kit (no. T20935; Invitrogen). Slides were costained with DAPI before being mounted with fluorescent mounting media.
For dystrophin/MyHC type 1/MyHC type 2/DAPI staining, unfixed slides were washed with PBS and then incubated overnight in anti-dystrophin (no. sc-15376; Santa Cruz Biotechnology), MyHC type 1 (BA.D5) and type 2a (SC.71). The next day, slides were incubated in goat anti-rabbit AF488 (no. A11034, dystrophin), goat anti-mouse IgG2b AF647 (no. A21242, type 1), goat anti-mouse IgG1 AF555 (no. A21127, type 2a; all from Invitrogen). Slides were costained with DAPI before being mounted with fluorescent mounting media.
Image acquisition and analysis.
Images were captured at ×100 and ×200 total magnification at room temperature with a Zeiss upright microscope (AxioImager M1; Zeiss, Oberkochen, Germany), and analysis was carried out using the AxioVision Rel software (v. 4.9). Average number of fibers analyzed for each immunohistochemical protocol was as follows: fiber typing and CSA: 347 ± 44 fibers Pre-BR, 337 ± 41 fibers Post-BR; Pax7: 330 ± 46 fibers Pre-BR, 395 ± 53 fibers Post-BR; CD56: 453 ± 57 fibers Pre-BR, 494 ± 76 fibers Post-BR; MyoD: 382 ± 127 fibers Pre-BR, 314 ± 63 fibers Post-BR; myonuclear counts: 419 ± 144 fibers Pre-BR, 382 ± 76 fibers Post-BR; capillary/arteriole: 390 ± 26 fibers Pre-BR, 433 ± 64 fibers Post-BR.
Fiber type-specific satellite cell abundance was assessed using Pax7 staining in conjunction with MyHC type I/laminin, and only those loci that were scored as Pax7 positive and DAPI positive within the laminin border were counted. Fiber types were scored as MyHC type 1 positive (type 1) or MyHC type 1 negative (type 2). Fiber type-specific satellite cell abundance was also assessed using CD56 staining in conjunction with MyHC type I/laminin, and only those loci that were scored as CD56 positive and DAPI positive within the laminin border were counted. Fiber type-specific satellite cell activation was assessed using MyoD staining in conjunction with MyHC type I/laminin, and only those loci that were scored as MyoD positive and DAPI positive within the laminin border were counted. DAPI-positive nuclei residing within the dystrophin border were counted as myonuclei associated with either MyHC type 1 or 2 fibers. A nucleus was identified as a myonucleus if it met one of the following criteria: 1) it was clearly located within the dystrophin boundary; 2) it was on the boundary facing inside the fiber; or 3) ∼50% of the area fell inside the dystrophin boundary. Myonuclear domain, the muscle fiber cytosolic volume per myonucleus, was estimated in two dimensions by expressing fiber CSA relative to myonuclear content (μm2/myonucleus). For fiber typing, fibers were sequentially scored as positive/negative in the Alexa Fluor 488 (type 2a), Alexa Fluor 555 (type 2x), and Alexa Fluor 647 (type 1) channels. All fibers that were scored as positive in the AF555 channel (type 2x) also scored positive in the AF488 channel (type 2a), as has been recently reported (19). For the sake of clarity in the current manuscript, these fibers are denoted as type 2x, emphasizing their status as 2x-positive fibers, regardless of their expression of the 2a isoform. Fibers that were scored as positive on type 1 and 2a channels are denoted as 1/2a hybrid fibers. Arterioles were scored as Ulex Europaeus agglutinin positive and α-SMA positive, whereas capillaries were Ulex Europaeus agglutinin positive and α-SMA negative. Capillary and arteriole counts are normalized to both fiber number and muscle area (mm2). NCAM-positive denervated fibers were quantified by ubiquitous expression of NCAM (CD56) throughout the fiber.
All samples for a given immunohistochemical protocol (i.e., myonuclear counts, Pax7, etc.) were performed and analyzed together. Muscle biopsies were cut on the cryostat, stained immunohistochemically, and then imaged in an unblinded manner. Images from each biopsy were then blinded for bed rest (Pre-BR vs. Post-BR) before image analysis by assignment of a random number. Once image analysis was complete, images and associated data were unblinded for statistical analysis.
Statistical analysis.
Pre-BR/Post-BR-dependent variables were analyzed using a mixed model with factors including bed rest (Pre-BR and Post-BR) and fiber type (MyHC type 1 and 2 or type 1, 1/2a, 2a, and 2x) with P ≤ 0.05. Post hoc testing was performed with Holm-Sidak pairwise comparisons when significant main effects or interactions were found. When fiber type was not an appropriate factor, differences between Pre-BR/Post-BR-dependent variables were analyzed with a paired Student's t-test. Where appropriate, simple correlations were tested by assessing the existence of a linear fit between the appropriate outcome measures with significance set at P ≤ 0.05. All analyses were performed with SPSS 20 (IBM SPSS Statistics, Armonk, NY). Data are presented as means ± SE.
RESULTS
Fourteen days of bed rest decreases MyHC type 2a fiber frequency and fiber CSA.
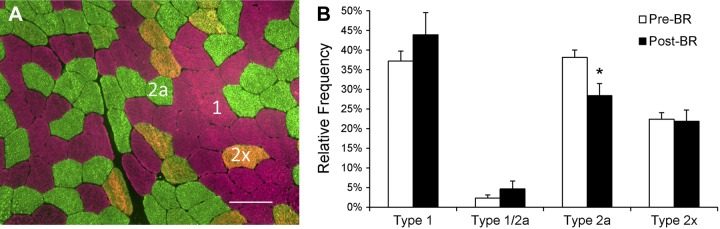
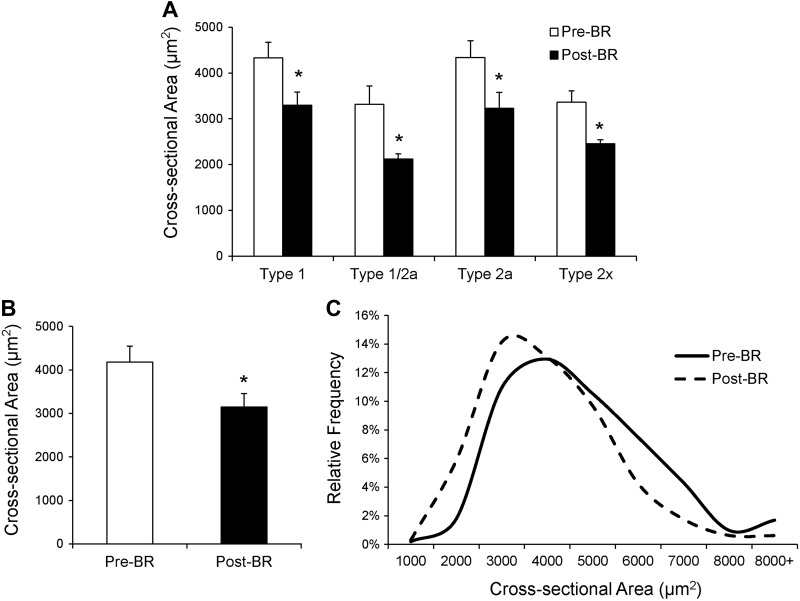
Following 14 days of bed rest, a shift in relative fiber type distribution was observed. There was a main effect of bed rest on fiber type distribution (P < 0.05), and post hoc analyses revealed a significant reduction in relative MyHC type 2a fiber frequency with no change in type 1, 1/2a, or 2x fiber frequency (Fig. 1, A and B). Analysis of mean fiber CSA showed robust atrophy of muscle fibers with MyHC types 1, 1/2a, 2a, and 2x fibers all demonstrating significant (P < 0.05) declines in mean fiber CSA (Fig. 2A). Mean MyHC type 1 fiber CSA declined by 23.4 ± 4.4%, MyHC type 1/2a fiber CSA declined by 24.2 ± 8.0%, MyHC type 2a fiber CSA declined by 23.8 ± 7.5%, and MyHC type 2x fiber CSA declined by 25.0 ± 6.0%. Pooled mean fiber CSA declined by 23.8 ± 5.2% (Fig. 2B). When presented as a binned histogram, a clear leftward shift in fiber size distribution was observed (Fig. 2C). Individual changes in mean fiber CSA can be seen in Fig. 9G. The reduction in mean fiber CSA was accompanied by significant atrophy at the whole muscle level [leg lean mass: −875 ± 125 g (−6.0 ± 0.4%); P < 0.05]. Knee extensor strength also declined by 14 ± 3 Nm (−13.5 ± 2.3%; P < 0.05), and knee extensor total work decreased by 221 ± 56 J (−13.1 ± 2.6%; P < 0.05) during the bed rest period.
Fig. 1.
Myosin heavy chain (MyHC) type 2a fiber frequency is reduced after 14 days of bed rest. A: representative immunohistochemical image of MyHC type 1 (pink), type 2a (green), and type 2x (orange) fiber identification. Scale bar = 100 μm. B: quantification of MyHC fiber type from immunohistochemical analysis, presented as mean fiber type frequency ± SE. *Significantly different from pre-bed rest (Pre-BR) value (P < 0.05).
Fig. 2.
Mean fiber cross-sectional area (CSA) is decreased after 14 days of bed rest. A: fiber CSA (μm2) of MyHC type 1, 1/2a, 2a, and 2x fibers presented as means ± SE. B: pooled fiber CSA presented as means ± SE. C: histogram distribution of pooled fiber CSA. *Significantly different from Pre-BR value (P < 0.05).
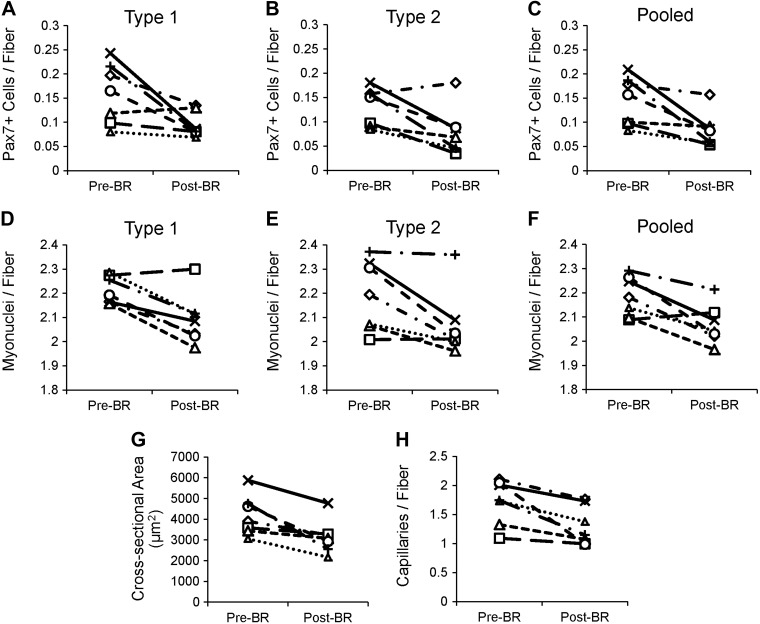
Fig. 9.
Individual data plots for fiber type-specific satellite cells, myonuclear content, mean fiber CSA, and capillary content. A: individual data plot for MyHC type 1 Pax7-positive satellite cells. B: individual data plot for MyHC type 2 Pax7-positive satellite cells. C: individual data plot for all Pax7-positive satellite cells. D: individual data plot for MyHC type 1 myonuclei. E: individual data plot for MyHC type 2 myonuclei. F: individual data plot for all myonuclei. G: individual data plot for mean fiber CSA. H: individual data plot for mean capillary content.
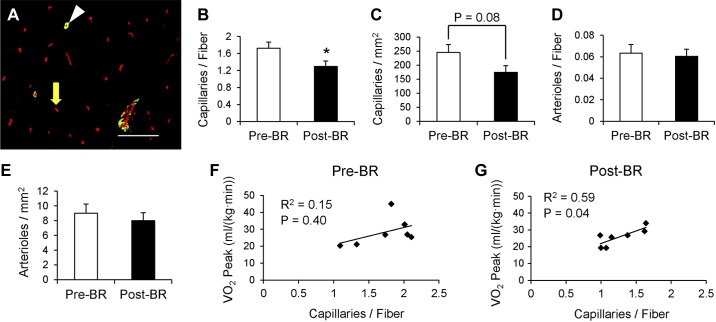
Bed rest leads to a reduction in capillaries but not arterioles.
The capillary content per fiber was significantly reduced following bed rest (−23.5 ± 5.6%, P < 0.05, Fig. 3, A and B), and there was a tendency for a reduced number of capillaries per fiber area (P = 0.08, Fig. 3C). Individual changes in capillary content per fiber are shown in Fig. 9H. Conversely, muscle arteriole content was unaffected by bed rest (Fig. 3, D and E). Subjects exhibited a 10% reduction in peak oxygen uptake (V̇o2 peak; Pre-BR: 29.6 ± 3.0 ml·kg−1·min−1; Post-BR: 26.6 ± 2.2 ml·kg−1·min−1; P < 0.05). Whereas Pre-BR capillary content was not significantly correlated with Pre-BR peak oxygen uptake (Fig. 3F), following bed rest, capillary content of the muscle was strongly correlated to peak oxygen uptake (Fig. 3G).
Fig. 3.
14 days of bed rest decreases muscle capillary content, and capillary content is associated with peak oxygen consumption following bed rest. A: representative immunohistochemical image of capillaries labeled with rhodamine-conjugated Ulex Europaeus agglutinin I (agglutinin, red) and arterioles labeled with α-smooth muscle actin (α-SMA, green). Arterioles are denoted as α-SMA positive and agglutinin positive (white arrowhead) and capillaries as α-SMA negative and agglutinin positive (yellow arrow). Scale bar = 100 μm. B: quantification of capillary content, presented as mean capillaries per fiber ± SE. C: quantification of capillary content, presented as mean capillaries per mm2 of muscle ± SE. D: quantification of arteriole content, presented as mean arterioles per fiber ± SE. E: quantification of arteriole content, presented as mean arterioles per mm2 of muscle ± SE. F: correlation of Pre-BR capillary content with Pre-BR peak oxygen consumption [V̇o2 peak; ml/(kg·min)]. G: correlation of Post-BR capillary content with Post-BR peak oxygen consumption. *Significantly different from Pre-BR value (P < 0.05).
Reduced satellite cell content and activity following 14 days of bed rest.
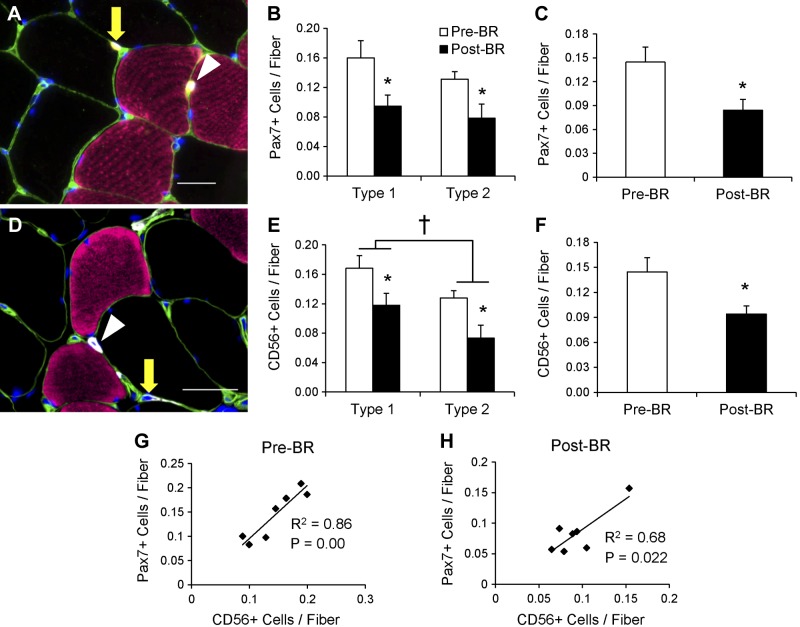
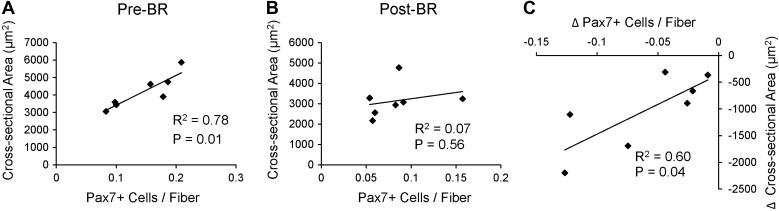
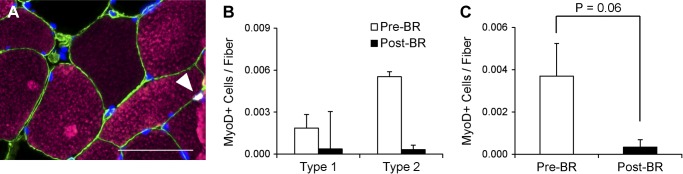
Pax7-positive satellite cells (Fig. 4A) associated with both type 1 (−33.3 ± 10.5%) and type 2 (−40.7 ± 10.9%) fibers declined, and total satellite cell content in the muscle was reduced by 38.7 ± 8.5% following the bed rest period (Fig. 4, B and C). Individual changes in type 1- and type 2-associated Pax7-positive satellite cells and pooled Pax7-positive satellite cells can be seen in Fig. 9, A–C. CD56-positive satellite cell content was also assessed (Fig. 4D) and demonstrated a similar decline in cells associated with both type 1 (−26.6 ± 8.7%) and type 2 (−39.9 ± 11.7%) fibers (Fig. 4E). A main effect for fiber type-specific CD56-positive satellite cells also existed, with greater CD56-positive satellite cell content associated with type 1 fibers compared with type 2 fibers Pre-BR and Post-BR. Fourteen days of bed rest also induced a 33.2 ± 6.2% decline in total CD56-positive satellite cell content (Fig. 4F). Pax7-positive satellite cell content and CD56-positive satellite cell content were significantly correlated both Pre-BR and Post-BR (Fig. 4, G and H). As has been previously reported in older adults (70), Pre-BR Pax7-positive satellite cell content was positively associated with Pre-BR mean fiber CSA (Fig. 5A). This relationship was not maintained Post-BR (Fig. 5B); however, the bed-rest induced-change in Pax7-positive satellite cells was significantly correlated with the change in mean fiber CSA (Fig. 5C). Satellite cell activation was assessed by the expression of MyoD (Fig. 6A), and there were no differences in MyoD-positive satellite cells associated with type 1 or type 2 fibers following bed rest (Fig. 6B). Fourteen days of bed rest did induce a trend for decline in total MyoD-positive satellite cells (P = 0.06; Fig. 6C).
Fig. 4.
Satellite cells associated with MyHC type 1 and 2 fibers decrease following 14 days of bed rest. A: representative immunohistochemical image demonstrating laminin (green), MyHC type 1 (pink), Pax7 (yellow), and DAPI (blue). Pax7-positive satellite cells associated with MyHC type 1 fiber (white arrowhead) and MyHC type 2 fiber (yellow arrow) are identified. Scale bar = 25 μm. B: quantification of fiber type-specific Pax7-positive satellite cell content, expressed as mean satellite cells per fiber ± SE. C: quantification of Pax7-positive satellite cell content, expressed as mean satellite cells per fiber ± SE. D: representative immunohistochemical image demonstrating laminin (green), MyHC type 1 (pink), CD56 (white), and DAPI (blue). CD56-positive satellite cells associated with MyHC type 1 fiber (white arrowhead) and MyHC type 2 fiber (yellow arrow) are identified. Scale bar = 50 μm. E: quantification of fiber type-specific CD56-positive satellite cell content, expressed as mean satellite cells per fiber ± SE. F: quantification of CD56-positive satellite cell content, expressed as mean satellite cells per fiber ± SE. G: correlation of Pre-BR CD56-positive satellite cell content with Pre-BR Pax7-positive satellite cell content. H: correlation of Post-BR CD56-positive satellite cell content with Post-BR Pax7-positive satellite cell content. *Significantly different from Pre-BR value (P < 0.05). †Main effect for fiber type (P < 0.05).
Fig. 5.
The change in satellite cell content is associated with the change in mean fiber CSA following 14 days of bed rest. A: correlation of Pre-BR Pax7-positive satellite cell content with Pre-BR mean fiber CSA. B: correlation of Post-BR Pax7-positive satellite cell content with Post-BR mean fiber CSA. C: correlation of the change in Pax7-positive satellite cell content with the change in mean fiber CSA.
Fig. 6.
14 days of bed rest induces a nonsignificant reduction in MyoD-positive satellite cells. A: representative immunohistochemical image demonstrating laminin (green), MyHC type 1 (pink), MyoD (white), and DAPI (blue). A MyoD-positive satellite cell associated with a MyHC type 1 fiber (white arrowhead) is identified. Scale bar = 50 μm. B: quantification of fiber type-specific MyoD-positive satellite cell content, expressed as mean satellite cells per fiber ± SE. C: quantification of MyoD-positive satellite cell content, expressed as mean satellite cells per fiber ± SE.
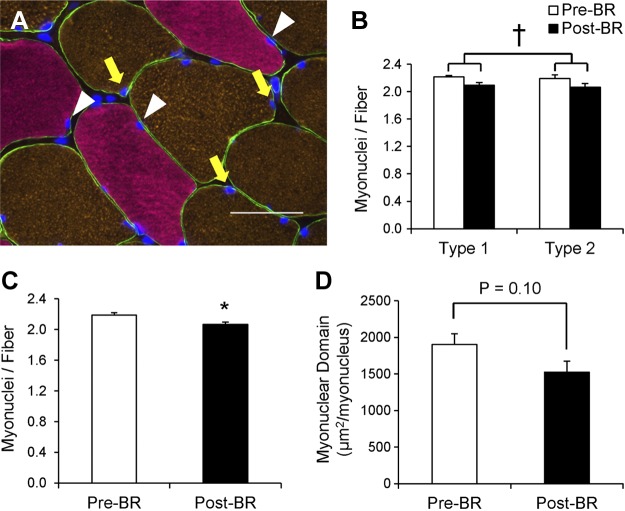
Fourteen days of bed rest induces a subtle decline in myonuclear content and myonuclear domain.
Following 14 days of bed rest, a main effect for a decline in fiber type-specific myonuclear content (Fig. 7, A and B) was observed (P < 0.05). Total myonuclear content significantly declined following bed rest (−5.3 ± 1.4%, P < 0.05; Fig. 7C). Individual changes in type 1- and type 2-associated myonuclei and pooled myonuclei can be seen in Fig. 9, D–F. The myonuclear domain decreased by 19.6 ± 5.3% rest (P = 0.10; Fig. 7D) following 14 days of bed rest.
Fig. 7.
Myonuclear content is decreased after 14 days of bed rest. A: representative image demonstrating dystrophin (green), MyHC type 1 (pink), MyHC type 2 (orange), and DAPI (blue). Myonuclei associated with type 1 fibers are denoted with white arrowheads, and myonuclei associated with type 2 fibers are denoted with yellow arrows. Scale bar = 50 μm. B: quantification of fiber type-specific myonuclear content, expressed as mean myonuclei per fiber ± SE. C: quantification of myonuclear content, expressed as mean myonuclei per fiber ± SE. D: quantification of myonuclear domain, calculated as the mean fiber CSA (μm2) expressed relative to the number of myonuclei per fiber ± SE. *Significantly different from Pre-BR value (P < 0.05). †Main effect for bed rest (P < 0.05).
Increase in percentage of NCAM-positive denervated fibers following bed rest.
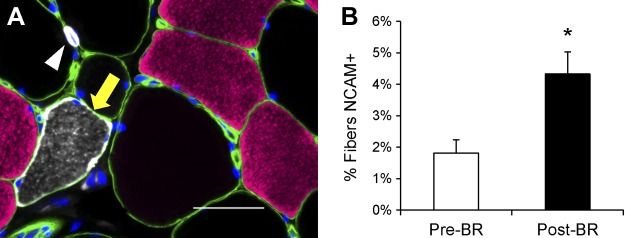
In addition to serving as a marker for satellite cells, CD56 or NCAM has also been used to identify denervated muscle fibers in rodents (3) and humans (28, 45). NCAM-positive muscle fiber frequency (Fig. 8A) increased significantly following 14 days of bed rest (P < 0.05; Fig. 8B).
Fig. 8.
14 days of bed rest increases the frequency of NCAM-positive muscle fibers. A: representative image demonstrating laminin (green), MyHC type 1 (pink), NCAM/CD56 (white), and DAPI (blue). An NCAM/CD56-positive muscle fiber is denoted with a yellow arrow, and a NCAM/CD56-positive satellite cell is denoted with a white arrowhead. Scale bar = 50 μm. B: quantification of NCAM-positive fibers, expressed as mean percentage of fibers positive for NCAM ± SE. *Significantly different from Pre-BR value (P < 0.05).
DISCUSSION
Fourteen days of bed rest unloading induced rapid lower limb skeletal muscle loss and deleterious alterations in lower limb skeletal muscle architecture of middle-aged adults, emphasizing the need for appropriate countermeasures to preserve muscle function in astronauts during spaceflight. In addition to a significant decrease in mean fiber CSA, bed rest elicited a shift in relative fiber type distribution with a reduction in MyHC type 2a frequency. A subtle but significant decline in myonuclear content occurred following 14 days of bed rest (Fig. 9). Bed rest also decreased satellite cell number, with a similar reduction occurring in satellite cells associated with MyHC type 1 and 2 fibers. Furthermore, the change in satellite cells that occurred during bed rest was associated with the change in mean fiber CSA during 14 days of bed rest. Fourteen days of bed rest also led to reduced capillary density, and post-BR capillary content was significantly associated with post-BR peak aerobic capacity.
Satellite cells contribute to fiber hypertrophy (24) and are necessary for skeletal muscle regeneration following injury (42, 48). They are key mediators in the maintenance of muscle mass (7, 52, 61). Satellite cell content and activity decline with age accompany sarcopenia (44, 68, 69). Chronic muscle disuse as seen in spinal cord injury results in significant fiber atrophy with a concomitant reduction in satellite cell content (68). Conversely, a recent study of young to middle-aged men following 28 days of bed rest showed no decrement in satellite cell content (8). However, in the study by Brooks and colleagues (8), satellite cell content was normalized to nuclear content, and, following bed rest, subjects experienced a trend for decreased myonuclear content, which could confound the interpretation of satellite cell data. In another study of healthy young subjects (24 yr), satellite cell content was unaltered despite muscle fiber atrophy over a 2-wk period of leg immobilization (64). The modest fiber atrophy experienced in the immobilized leg of young subjects (7–15%) (64) was notably less than the marked fiber atrophy observed in the middle-aged bed rest participants of the present study (∼24%). Differences in subject age and disuse model (cast immobilization vs. bed rest) may have contributed to the discrepancy in satellite cell content observed in the present study compared with the study by Snijders and colleagues (64). Complete removal of gravitational loading may also play a key role in mediating changes to satellite cell activity during disuse. Support for this is seen during hind limb-unloading studies in rodents, in which reductions in satellite cell content similar to those observed in humans during bed rest have been observed. Several studies have demonstrated that hind limb unloading in mice and rats results in a decline in satellite cell content and proliferation (16, 46, 73). In the present study, we assessed satellite cell content through two well-established markers, Pax7 (8, 25) and CD56 (64, 68), and demonstrate with both methods a similar decline in satellite cell content. Importantly, Pre-BR and Post-BR CD56 and Pax7 data demonstrate significant correlation, providing confidence in our findings that satellite cell content declines in middle-aged adults following bed rest. The change in satellite cell content that occurred following bed rest was significantly correlated with the change in mean fiber CSA, providing evidence for a relationship between muscle fiber remodeling/atrophy and satellite cell alterations. We cannot conclude from our data whether the loss of satellite cells affects fiber size or whether the reduction in fiber size affects the need for satellite cells, therefore reducing the satellite cell content. Similar relationships have been observed during the regrowth period following disuse-induced muscle atrophy; the change in satellite cell content was associated with improvements in muscle fiber CSA (67).
Satellite cell activity during skeletal muscle atrophy is a biologically complex issue. Studies in rodent models of disuse atrophy have shown both proliferation/fusion of satellite cells (23) and reductions in satellite cell activity (39) during mechanical unloading. In human studies of disuse atrophy, mRNA expression of various myogenic regulatory factors (MyoD, myogenin) is increased (8, 64, 67), providing evidence that satellite cells may not be quiescent during myofiber atrophy. In the present study, we found that 14 days of bed rest induced a nonsignificant reduction in the number of MyoD-positive satellite cells. The frequency of MyoD-positive satellite cells was far less than Pax7/CD56-positive satellite cells, making interpretation of this data somewhat difficult. It is likely that the decrease in active satellite cells was influenced by the overall reduction in satellite cell content observed in the present study.
Mechanical unloading induces myofiber atrophy by modulating skeletal muscle protein turnover. Extended periods (14–30 days) of bed rest causes myofiber atrophy in the range of 15–19% in healthy, young male subjects (4, 13, 31); longer-term bed rest (60 days) induces an even greater muscle fiber atrophy (30–40%) in younger adults (57, 58). Marked myofiber atrophy ranging from 11–26% occurs following 14 days of cast immobilization in young and older adults (66, 67). Intriguingly, younger adults experienced a greater relative decline in mean fiber CSA compared with older subjects (66, 67). Alterations in protein metabolism, specifically a decline in rates of protein synthesis at the whole body and skeletal muscle level, contribute to skeletal muscle atrophy during moderate periods of bed rest (14 days) (22). Accelerated muscle fiber atrophy can occur during bed rest during periods of critical illness; comatose patients experienced significant fiber atrophy (16–24%) in a hospital stay of only 3–10 days (17). Middle-aged subjects in the present study demonstrated myofiber atrophy of ∼24% following a moderate (14 days) period of bed rest. The severe myofiber atrophy experienced by subjects in the present study provides evidence that middle-aged adults may be more susceptible to the deleterious effects of bed rest than their younger counterparts.
Additionally, subjects in the present study experienced a decrease in the relative abundance of MyHC type 2a fibers. Nonsignificant reductions in MyHC type 2a frequency have previously been noted following 14 days of bed rest using both histochemical and electrophoretic methodologies (4). The shift in fiber type distribution following bed rest was accompanied by a small but significant increase in the frequency of NCAM-positive muscle fibers. NCAM expression has been used as a proxy for fiber denervation in both rodents (3) and humans (28, 45). The changes in fiber type distribution and fiber innervation status illustrate profound tissue plasticity in response to 14 days of bed rest. Changes in knee extensor strength have also been correlated to changes in NCAM fiber expression in the absence of fiber CSA alterations (45). In the present study, both fiber atrophy and an increase in fiber denervation likely contributed to the reduced knee extensor peak torque and total work following bed rest.
Disuse and physical inactivity reduce capillary density and diameter in skeletal muscle of both rodents (26, 37, 56) and humans (31, 57, 58). In the present study, middle-aged adults experienced an ∼23% reduction in capillary density during 14 days of bed rest. This is similar to the 20–35% decline in capillary content observed in younger subjects following longer periods of bed rest (30–60 days) (31, 57). The reduced capillary content seen in the present study was accompanied by a 10% reduction in peak oxygen consumption. Several studies have reported strong associations between muscle capillarity and aerobic capacity in sedentary and trained subjects (72, 76). Although there was no correlation between capillary density and oxygen consumption before the bed rest period, Post-BR capillary content and peak oxygen consumption were strongly correlated. Following a period of inactivity, reduced capillary content and, by proxy, muscle perfusion may contribute to deleterious changes in functional capacity (59). Several additional adaptations to bed rest, including decreased circulating blood volume (15), reduced maximal cardiac output (11), and reduced oxidative enzyme activity (5), may have also contributed to the decrease in aerobic capacity observed in the present study. In moderate duration bed rest studies (<30 days), the decline in maximal cardiac output closely mirrors the reduction in aerobic capacity (33).
In reports of various animal models of mechanical unloading, the effect of inactivity on myonuclear content remains unclear with data supporting both decreases (1, 41, 62) and no changes (9, 10). In the present study, we found a subtle but statistically significant 5% decrease in the number of myonuclei per fiber. These data are supported by a recent study demonstrating a nonsignificant ∼11% decline in myonuclear content in young and middle-aged adults following 28 days of bed rest (8). The myonuclear domain theory posits that each myonucleus within a muscle fiber is responsible for the regulation of its surrounding cytosolic volume (30). During periods of fiber atrophy and hypertrophy, the myonuclear domain remains relatively constant through changes in myonuclear number, consistent with fiber diameter changes (2, 36, 54). Although we were unable to assess fiber volume in the present study, normalizing fiber CSA to myonuclear content provides an estimation of the myonuclear domain in two dimensions. We found a nonsignificant 19% reduction in the myonuclear domain (μm2/myonucleus; P = 0.10). The myonuclear domain did not decrease to a similar extent as fiber CSA because of the reduction in myonuclear content. A recent report shows that myonuclear addition occurs concomitantly with increased fiber CSA, demonstrating a coordinated regulation of the myonuclear domain during fiber hypertrophy (63). The addition of myonuclei that accompanies resistance training (53, 54) may facilitate recovery of atrophied skeletal muscles following disuse, offering a proactive approach to facilitate faster skeletal muscle recovery after periods of mechanical unloading (e.g., upon reaching a terrestrial destination during exploration spaceflight). This effect has been observed in mice, in which myonuclear addition before fiber atrophy promoted subsequent recovery of fiber CSA (18). Additionally, overload-induced myonuclear accretion has been shown to be protective against skeletal muscle atrophy during unloading in mice (10). It is likely that myonuclear domain size is flexible (25, 35), especially during the early phases of adaptation; however, more evidence is needed to determine the magnitude of myonuclear changes that occur during disuse atrophy.
In conclusion, 14 days of bed rest induced fiber atrophy and reduced capillary content, functional capacity, satellite cell, and myonuclear content in middle-aged adults. Although middle-aged adults do not display obvious functional signs of aging (55), during mechanical unloading, they experience deleterious changes in skeletal muscle that are comparable to those observed in older adults and much greater than those documented in young adults with disuse. Thus middle-aged adults should be considered an at-risk population when exposed to disuse and stand to benefit from aggressive countermeasures (e.g., high-intensity exercise and/or nutritional supplementation) to combat skeletal muscle maladaptations that occur during spaceflight or clinical bed rest.
GRANTS
This work was funded by NSBRI grant NNJ08ZSA002N, NIH R01NR012973 (D. Paddon-Jones), a Texas Space Grant Consortium Fellowship (K. English), NIH grant T32HD007539, and in part by 1UL1RR029876-01 from the National Center for Research Resources. C. Fry is a KL2 scholar supported by the UTMB Claude D. Pepper Older Americans Independence Center NIH/NIA grant P30 AG024832. The study was conducted with the support of UTMB Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences (NIH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.A.-L., K.L.E., and C.S.F. performed experiments; E.A.-L. and C.S.F. analyzed data; E.A.-L. and C.S.F. interpreted results of experiments; E.A.-L., K.L.E., D.P.-J., and C.S.F. edited and revised manuscript; E.A.-L., K.L.E., D.P.-J., and C.S.F. approved final version of manuscript; K.L.E., D.P.-J., and C.S.F. conception and design of research; C.S.F. prepared figures; C.S.F. drafted manuscript.
ACKNOWLEDGMENTS
The authors sincerely thank Dr. Elena Volpi, Dr. Randall Urban, and Dr. Charles Mathers for medical oversight, the nurses and staff of the ITS-CRC, and our volunteers for their time and effort.
REFERENCES
- 1.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol 273: C579–C587, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol 78: 1969–1976, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Andersson AM, Olsen M, Zhernosekov D, Gaardsvoll H, Krog L, Linnemann D, Bock E. Age-related changes in expression of the neural cell adhesion molecule in skeletal muscle: a comparative study of newborn, adult and aged rats. Biochem J 290: 641–648, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen MC. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol 84: 157–163, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Berg HE, Dudley GA, Hather B, Tesch PA. Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading. Clin Physiol 13: 337–347, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Bergstrom J. Clinical studies on cell electrolytes. Scand J Clin Lab Invest 15, Suppl 76: 16–78, 1963. [PubMed] [Google Scholar]
- 7.Blau HM, Cosgrove BD, Ho AT. The central role of muscle stem cells in regenerative failure with aging. Nat Med 21: 854–862, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks NE, Cadena SM, Vannier E, Cloutier G, Carambula S, Myburgh KH, Roubenoff R, Castaneda-Sceppa C. Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve 42: 927–935, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest 118: 1450–1457, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci USA 107: 15111–15116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capelli C, Antonutto G, Kenfack MA, Cautero M, Lador F, Moia C, Tam E, Ferretti G. Factors determining the time course of VO2(max) decay during bedrest: implications for VO2(max) limitation. Eur J Appl Physiol 98: 152–160, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Cherin P, Voronska E, Fraoucene N, de Jaeger C. Prevalence of sarcopenia among healthy ambulatory subjects: the sarcopenia begins from 45 years. Aging Clin Exp Res 26: 137–146, 2014. [DOI] [PubMed] [Google Scholar]
- 13.Clarke MS, Bamman MM, Feeback DL. Bed rest decreases mechanically induced myofiber wounding and consequent wound-mediated FGF release. J Appl Physiol 85: 593–600, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Coker RH, Hays NP, Williams RH, Wolfe RR, Evans WJ. Bed rest promotes reductions in walking speed, functional parameters, and aerobic fitness in older, healthy adults. J Gerontol A Biol Sci Med Sci 70: 91–96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Convertino VA. Clinical aspects of the control of plasma volume at microgravity and during return to one gravity. Med Sci Sports Exerc 28: S45–S52, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol 67: 1827–1834, 1989. [DOI] [PubMed] [Google Scholar]
- 17.Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ. Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci 128: 357–365, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Egner IM, Bruusgaard JC, Eftestol E, Gundersen K. A cellular memory mechanism aids overload hypertrophy in muscle long after an episodic exposure to anabolic steroids. J Physiol 591: 6221–6230, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellefsen S, Vikmoen O, Zacharoff E, Rauk I, Slettalokken G, Hammarstrom D, Strand TA, Whist JE, Hanestadhaugen M, Vegge G, Fagernes CE, Nygaard H, Hollan I, Ronnestad BR. Reliable determination of training-induced alterations in muscle fiber composition in human skeletal muscle using quantitative polymerase chain reaction. Scand J Med Sci Sports 24: e332–e342, 2014. [DOI] [PubMed] [Google Scholar]
- 20.English KL, Mettler JA, Ellison JB, Mamerow MM, Arentson-Lantz E, Pattarini JM, Ploutz-Snyder R, Sheffield-Moore M, Paddon-Jones D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am J Clin Nutr 103: 465–473, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 13: 34–39, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira R, Neuparth MJ, Ascensao A, Magalhaes J, Vitorino R, Duarte JA, Amado F. Skeletal muscle atrophy increases cell proliferation in mice gastrocnemius during the first week of hindlimb suspension. Eur J Appl Physiol 97: 340–346, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu HL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J 28: 1654–1665, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fry CS, Noehren B, Mula J, Ubele MF, Westgate PM, Kern PA, Peterson CA. Fibre type-specific satellite cell response to aerobic training in sedentary adults. J Physiol 592: 2625–2635, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujino H, Kohzuki H, Takeda I, Kiyooka T, Miyasaka T, Mohri S, Shimizu J, Kajiya F. Regression of capillary network in atrophied soleus muscle induced by hindlimb unweighting. J Appl Physiol 98: 1407–1413, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci 72: 503–509, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Gosztonyi G, Naschold U, Grozdanovic Z, Stoltenburg-Didinger G, Gossrau R. Expression of Leu-19 (CD56, N-CAM) and nitric oxide synthase (NOS) I in denervated and reinnervated human skeletal muscle. Microsc Res Tech 55: 187–197, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Grounds MD, White JD, Rosenthal N, Bogoyevitch MA. The role of stem cells in skeletal and cardiac muscle repair. J Histochem Cytochem 50: 589–610, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell 59: 771–772, 1989. [DOI] [PubMed] [Google Scholar]
- 31.Hikida RS, Gollnick PD, Dudley GA, Convertino VA, Buchanan P. Structural and metabolic characteristics of human skeletal muscle following 30 days of simulated microgravity. Aviat Space Environ Med 60: 664–670, 1989. [PubMed] [Google Scholar]
- 32.Holthofer H, Virtanen I, Kariniemi AL, Hormia M, Linder E, Miettinen A. Ulex europaeus I lectin as a marker for vascular endothelium in human tissues. Lab Invest 47: 60–66, 1982. [PubMed] [Google Scholar]
- 33.Hung J, Goldwater D, Convertino VA, McKillop JH, Goris ML, DeBusk RF. Mechanisms for decreased exercise capacity after bed rest in normal middle-aged men. Am J Cardiol 51: 344–348, 1983. [DOI] [PubMed] [Google Scholar]
- 34.Ingemann-Hansen T, Halkjaer-Kristensen J. Computerized tomographic determination of human thigh components. The effects of immobilization in plaster and subsequent physical training. Scand J Rehabil Med 12: 27–31, 1980. [PubMed] [Google Scholar]
- 35.Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol 558: 1005–1012, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol 113: 99–103, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Kano Y, Shimegi S, Takahashi H, Masuda K, Katsuta S. Changes in capillary luminal diameter in rat soleus muscle after hind-limb suspension. Acta Physiol Scand 169: 271–276, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 297: 1772–1774, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Kujawa M, Baran W, Jankowska-Steifer E. Quantitative ultrastructural changes in satellite cells of rats immobilized after soleus muscle denervation. Exp Mol Pathol 78: 78–85, 2005. [DOI] [PubMed] [Google Scholar]
- 40.LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol 73: 2172–2178, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol 288: R1288–R1296, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 26: 2509–2521, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Messi ML, Li T, Wang ZM, Marsh AP, Nicklas B, Delbono O. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J Gerontol A Biol Sci Med Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell PO, Pavlath GK. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol 287: C1753–C1762, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec 170: 421–435, 1971. [DOI] [PubMed] [Google Scholar]
- 48.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab 91: 4836–4841, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Aarsland A, Wolfe RR, Ferrando AA. The catabolic effects of prolonged inactivity and acute hypercortisolemia are offset by dietary supplementation. J Clin Endocrinol Metab 90: 1453–1459, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab 89: 4351–4358, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Pallafacchina G, Blaauw B, Schiaffino S. Role of satellite cells in muscle growth and maintenance of muscle mass. Nutr Metab Cardiovasc Dis 23, Suppl 1: S12–S18, 2013. [DOI] [PubMed] [Google Scholar]
- 53.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Prado CM, Siervo M, Mire E, Heymsfield SB, Stephan BC, Broyles S, Smith SR, Wells JC, Katzmarzyk PT. A population-based approach to define body-composition phenotypes. Am J Clin Nutr 99: 1369–1377, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Roudier E, Gineste C, Wazna A, Dehghan K, Desplanches D, Birot O. Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J Physiol 588: 4579–4591, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rudnick J, Puttmann B, Tesch PA, Alkner B, Schoser BG, Salanova M, Kirsch K, Gunga HC, Schiffl G, Luck G, Blottner D. Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest. FASEB J 18: 1228–1230, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Salanova M, Schiffl G, Puttmann B, Schoser BG, Blottner D. Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J Anat 212: 306–318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saltin B, Rowell LB. Functional adaptations to physical activity and inactivity. Fed Proc 39: 1506–1513, 1980. [PubMed] [Google Scholar]
- 60.Schultz E, Lipton BH. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev 20: 377–383, 1982. [DOI] [PubMed] [Google Scholar]
- 61.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol 294: 50–66, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siu PM, Alway SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol 288: C1058–C1073, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Snijders T, Smeets JS, van Kranenburg J, Kies AK, van Loon LJ, Verdijk LB. Changes in myonuclear domain size do not precede muscle hypertrophy during prolonged resistance-type exercise training. Acta Physiol (Oxf) 216: 231–239, 2016. [DOI] [PubMed] [Google Scholar]
- 64.Snijders T, Wall BT, Dirks ML, Senden JM, Hartgens F, Dolmans J, Losen M, Verdijk LB, van Loon LJ. Muscle disuse atrophy is not accompanied by changes in skeletal muscle satellite cell content. Clin Sci 126: 557–566, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res 185: 399–408, 1977. [DOI] [PubMed] [Google Scholar]
- 66.Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Bayer M, Petersson SJ, Schroder HD, Andersen JL, Heinemeier KM, Aagaard P, Schjerling P, Kjaer M. Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One 7: e51238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suetta C, Frandsen U, Mackey AL, Jensen L, Hvid LG, Bayer ML, Petersson SJ, Schroder HD, Andersen JL, Aagaard P, Schjerling P, Kjaer M. Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 591: 3789–3804, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verdijk LB, Dirks ML, Snijders T, Prompers JJ, Beelen M, Jonkers RA, Thijssen DH, Hopman MT, Van Loon LJ. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med Sci Sports Exerc 44: 2322–2330, 2012. [DOI] [PubMed] [Google Scholar]
- 69.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg H, van Loon LJC. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292: E151–E157, 2007. [DOI] [PubMed] [Google Scholar]
- 70.Verdijk LB, Snijders T, Beelen M, Savelberg HH, Meijer K, Kuipers H, Van Loon LJ. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J Am Geriatr Soc 58: 2069–2075, 2010. [DOI] [PubMed] [Google Scholar]
- 71.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12: 898–906, 2013. [DOI] [PubMed] [Google Scholar]
- 72.Walton RG, Finlin BS, Mula J, Long DE, Zhu B, Fry CS, Westgate PM, Lee JD, Bennett T, Kern PA, Peterson CA. Insulin-resistant subjects have normal angiogenic response to aerobic exercise training in skeletal muscle, but not in adipose tissue. Physiol Rep 3: e12415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang XD, Kawano F, Matsuoka Y, Fukunaga K, Terada M, Sudoh M, Ishihara A, Ohira Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am J Physiol Cell Physiol 290: C981–C989, 2006. [DOI] [PubMed] [Google Scholar]
- 74.White MJ, Davies CT, Brooksby P. The effects of short-term voluntary immobilization on the contractile properties of the human triceps surae. Q J Exp Physiol 69: 685–691, 1984. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto N, Otsuka K, Kubo Y, Hayashi M, Mizuno K, Ohshima H, Mukai C. Effects of long-term microgravity exposure in space on circadian rhythms of heart rate variability. Chronobiol Int 32: 327–340, 2015. [DOI] [PubMed] [Google Scholar]
- 76.Zumstein A, Mathieu O, Howald H, Hoppeler H. Morphometric analysis of the capillary supply in skeletal muscles of trained and untrained subjects–its limitations in muscle biopsies. Pflügers Arch 397: 277–283, 1983. [DOI] [PubMed] [Google Scholar]