Abstract
The phosphorylation of the myosin regulatory light chain (RLC) is an important modulator of skeletal muscle performance and plays a key role in posttetanic potentiation and staircase potentiation of twitch contractions. The structural basis for these phenomena within the filament lattice has not been thoroughly investigated. Using a synchrotron radiation source at SPring8, we obtained X-ray diffraction patterns from skinned rabbit psoas muscle fibers before and after phosphorylation of myosin RLC in the presence of myosin light chain kinase, calmodulin, and calcium at a concentration below the threshold for tension development ([Ca2+] = 10−6.8 M). After phosphorylation, the first myosin layer line slightly decreased in intensity at ∼0.05 nm−1 along the equatorial axis, indicating a partial loss of the helical order of myosin heads along the thick filament. Concomitantly, the (1,1/1,0) intensity ratio of the equatorial reflections increased. These results provide a firm structural basis for the hypothesis that phosphorylation of myosin RLC caused the myosin heads to move away from the thick filaments towards the thin filaments, thereby enhancing the probability of interaction with actin. In contrast, 2,3-butanedione monoxime (BDM), known to inhibit contraction by impeding phosphate release from myosin, had exactly the opposite effects on meridional and equatorial reflections to those of phosphorylation. We hypothesize that these antagonistic effects are due to the acceleration of phosphate release from myosin by phosphorylation and its inhibition by BDM, the consequent shifts in crossbridge equilibria leading to opposite changes in abundance of the myosin-ADP-inorganic phosphate complex state associated with helical order of thick filaments.
Keywords: myosin regulatory light chain, phosphorylation, X-ray diffraction, skinned fiber, twitch potentiation
the phosphorylation of myosin regulatory light chain (RLC) is one of the important regulators of muscle contraction. In vertebrate skeletal muscle, although the main site of the activation resides in the thin filament, muscle performance depends on the history of contractile events. Following a brief tetanic stimulation, the isometric twitch tension of a fast skeletal muscle is enhanced and the speed of the initial rise of tension is accelerated, a phenomenon called posttetanic potentiation (PTP) (7). Staircase potentiation is a related phenomenon in which successive isometric twitches are potentiated during low-frequency stimulation (31). Perry and coworkers first discovered that myosin RLC can be phosphorylated by a specific enzyme, myosin light chain kinase (MLCK) (45, 49). Subsequent work showed that a single tetanus could lead to substantial phosphorylation of RLC (5), and that the ensuing decline of RLC phosphorylation was quantitatively correlated with the time course of decay of PTP (36). It is now established that skeletal MLCK is a calmodulin-sensing kinase that becomes activated by the elevated Ca2+ level during the tetanus, and that knocking out the skeletal muscle MLCK gene virtually abolishes PTP (79). The phosphorylation of RLC in cardiac muscle is also considered to underlie the basis of twitch potentiation by α-adrenergic agents (22, 50), endothelin (54), and prostenoids (3, 53, 66), though the kinase responsible differs from that in skeletal muscle (79).
The physiological mechanism underlying twitch potentiation by RLC phosphorylation has been extensively studied (58, 60, 65). In skinned striated fast muscle fibers, phosphorylation of RLC enhances force generation at a submaximal Ca2+ concentration, owing to an increase in Ca2+ sensitivity, but has no effect on maximally activated tension (62). This property would enable intact fibers with phosphorylated RLC to increase in twitch force without increasing the magnitude of the Ca2+ transient. Mechanical analysis revealed that phosphorylation of the RLC is associated with an increase in the rate of tension redevelopment after a quick release at low levels of activation, which is due to the enhancement of the rate of Ca2+-activated crossbridge attachment to binding sites on actin (38, 61), while the rate of crossbridge detachment is reduced (43).
Insight into how RLC phosphorylation influences the rate of crossbridge attachment came from structural analysis of thick filament regulated invertebrate striated muscles that are activated by phosphorylation of RLC (10). Electron microscopy of tarantula thick filaments revealed that, under relaxing conditions, they are highly ordered structures in which the myosin heads are arranged in a helical manner such that the heads from axially neighboring myosin molecules appear to interact with one another. This helix order is disrupted by RLC phosphorylation (10). Similar order-to-disorder transitions upon RLC phosphorylation have been observed in thick filaments of dually regulated Limulus muscle (33), as well as rabbit skeletal muscle (34). These observations led to the hypothesis that thick filaments in relaxed striated muscles of a wide range of animal species have a common structure in which the myosin heads are held down onto the thick filament core, and that RLC phosphorylation releases the myosin heads, allowing them to swing out towards thin filaments, thereby enhancing their probability of interacting with thin filaments (34, 38, 61).
X-ray diffraction studies on tarantula skeletal and mammalian cardiac muscle fibers added structural support for the view that RLC phosphorylation shifts myosin heads towards thin filaments in the context of an intact myofilament array. In tarantula striated muscle, the (1,1/1,0) intensity ratio of equatorial reflections showed a significant increase upon thiophosphorylation of RLC (42). In the mouse cardiac muscle, a similar increase in the (1,1/1,0) intensity ratio occurred following RLC phosphorylation (8). However, these earlier X-ray diffraction studies were limited to analysis of equatorial reflections, but changes in myosin layer lines in these muscles expected on the basis of the observed order-to-disorder transitions of isolated thick filaments following RLC phosphorylation referred to above have not been reported. Furthermore, X-ray diffraction experiments on the effects of RLC phosphorylation on mammalian skeletal muscle have not been studied.
In this paper, we analyzed two-dimensional (2-D) X-ray diffraction patterns of resting skinned rabbit fast skeletal muscle fibers to test the hypothesis that RLC phosphorylation would induce correlated changes in layer lines and equatorial reflections within a physiological myofilament lattice consistent with the notion that myosin heads move from their ordered arrangement on thick filaments towards thin filaments. We also examined the effects of BDM on the diffraction patterns. This reagent has been shown to dephosphorylate RLC in intact cardiac muscle fibers (8, 41, 64, 66), and we hoped to use it as a method of enhancing the diffraction signal-to-noise ratio due to RLC phosphorylation. The results showed that RLC phosphorylation caused a distinct order-to-disorder transition of thick filaments associated with a movement of myosin heads towards thin filaments. BDM, a known inhibitor of muscle contraction and myosin ATPase activity (19), induced the reverse of these effects of phosphorylation apparently without dephosphorylating the skeletal RLC. These observations are discussed in the context of a unifying hypothesis for the apparent multiple effects of RLC phosphorylation and BDM on myosin structure and function.
METHODS
Solutions.
The relaxing solution used in this study was composed of (in mM) 4.7 Na2ATP, 6.5 Mg-(methanesulfonate)2 (MgMs2), 10 EGTA, 55 K-(methanesulfonate) (KMs), and 20 PIPES (adjusted to pH 7). The phosphorylation solution was prepared by adding 2 μM calmodulin (CaM), 0.15 μM skeletal MLCK, and 10 μM phosphatase inhibitor (tautomycin) to a solution with a subthreshold concentration of calcium for tension development (pCa 6.8) composed of (in mM) 4.9 Na2ATP, 6.8 MgMs2, 10 EGTA, 5 KMs, 7.5 Ca-(methanesulfonate)2 (CaMs2), and 20 PIPES (adjusted to pH 7). The enzyme solution used for testing nonspecific binding of enzymes to myofibrillar proteins in muscle fibers was prepared by adding CaM and MLCK to relaxing solution. The BDM solution was prepared from the relaxing solution by adding BDM to a concentration of 10 mM.
Preparation and treatment of muscle fibers.
Eight male rabbits (Japan White, slc:JW/CSK, Sankyo Lab. Industry) with age ranging 15–17 wk were used with the approval of the Institutional Animal Care and Use Committee of The Jikei University. The rabbits were killed by an intravenous injection of pentobarbiturates (120 mg/kg). Thin fiber bundles dissected from rabbit psoas muscle were chemically skinned for 3 h in a skinning solution prepared by adding 0.2% Triton X-100 to the relaxing solution. After the skinning, the bundles were transferred to the relaxing solution diluted to half-strength with glycerin and stored at −20°C. To detect the effect of phosphorylation on the myofibrillar structure without the complication of force-generating crossbridges, phosphorylation was achieved by incubating the fibers in the phosphorylation solution for 20 min at a pCa of 6.8, which is effective for activating CaM/MLCK but remains subthreshold for tension development. All the fibers used for studying the effect of phosphorylation on diffraction patterns were pretreated in 10 mM BDM solution for 10 min because BDM has been reported to decrease endogenous RLC phosphorylation level in cardiac muscle cells (8, 41, 60, 64), although it emerged that our own results failed to demonstrate dephosphorylation of skeletal RLC with BDM (see below). X-ray diffraction analyses of fiber bundles were also performed before and after the treatment in the BDM solution to evaluate the effect of BDM. X-ray diffraction patterns were obtained from the fibers in the relaxing solution after CaM/MLCK or BDM was washed out by rinsing the fibers in the relaxing solution for more than 2 min.
X-ray diffraction experiment.
X-ray diffraction experiments were carried out at BL45XU in SPring8 (Hyogo, Japan) using a setup summarized in Fig. 1. Experiments were carried out at 20°C. At this temperature, PTP is reduced compared with that at body temperature but is still prominent (32). Diffraction patterns were obtained from 4–5 pairs of parallel fibers set in tandem along the X-ray beam with a mean center-to-center separation of 120 μm between fiber pairs. This arrangement allows the X-ray beam to pass through many fibers to enhance the diffraction signal without compromising the fast diffusion of solutes into and out of the fibers (23). Images were recorded using an imaging plate system (BAS 2500, Fuji Xerox). The X-ray wavelength was 0.09 nm, beam size was 250 μm wide and 150 μm in height, and the specimen-to-detector distance was 1.8 m. The sarcomere length of each fiber bundle was adjusted to the desired value in the range from 1.9 to 2.7 μm, guided by laser diffraction. Dithiothreitol (5 mM) and catalase (1,000 U/ml) were added to the solutions to minimize tissue damage from free radicals produced by X-rays (30).
Fig. 1.
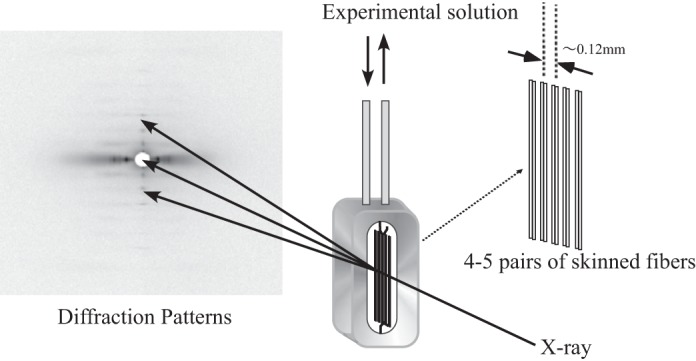
Experimental setup and the arrangement of skinned muscle fibers used in this study. For details, see text.
Two-dimensional electrophoresis of myosin light chains.
The phosphorylation level of the myosin RLC was evaluated by 2-D electrophoresis as described by Gonzalez et al. (14) with slight modifications. Briefly, skinned fibers were put in a sample buffer consisting of 7 M urea, 2 M thiourea, 1% aminosulfobetaine-14 (ASB-14), 0.5% Triton X-100, 10 mM dithiothreitol, and 0.5% ampholyte (pH range 3–10). The pH of the first-dimension gel was in the range from 3.9 to 5.1. The second-dimension gel consisted of 4% stacking gel and 8–16% gradient gel. After electrophoresis, myosin RLC was stained using antibody MF5 (55) after it was transblotted onto a nitrocellulose membrane.
RESULTS
Effectiveness of the phosphorylation protocol.
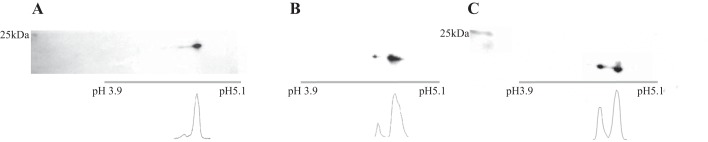
The protocol used here to phosphorylate RLC has been extensively used and its effectiveness verified in previous studies (47, 62, 76). To determine the effectiveness of our phosphorylation protocol, 2-D polyacrylamide gel electrophoresis of RLC was carried out to compare the results for untreated native fibers (Fig. 2A) with fibers treated with BDM (Fig. 2B) and fibers after phosphorylation (Fig. 2C). The results showed that the phosphorylated RLC content after BDM treatment was unchanged relative to that of native fibers, while phosphorylation enhanced the ratio of phosphorylated RLC to unphosphorylated RLC by approximately fivefold. This suggests that BDM is unable to dephosphorylate mammalian skeletal RLC, and that the phosphorylating protocol does work in our hands.
Fig. 2.
Western blots and corresponding densitograms of myosin regulatory light chain (RLC) from skinned fiber bundles following two-dimensional (2-D) gel electrophoresis. A: untreated fibers showing phosphorylated RLC (RLC-P, weak spot on the left) and unphosphorylated RLC (RLC, strong spot on the right). The ratio of RLC-P to RLC as determined by the areas under the curves is 0.10. B: fibers treated with 2,3-butanedione monoxime (BDM) but not phosphorylated; ratio of RLC-P to RLC is 0.11. C: fibers after phosphorylation; ratio of RLC-P to RLC is 0.56.
Changes in layer lines in response to RLC phosphorylation.
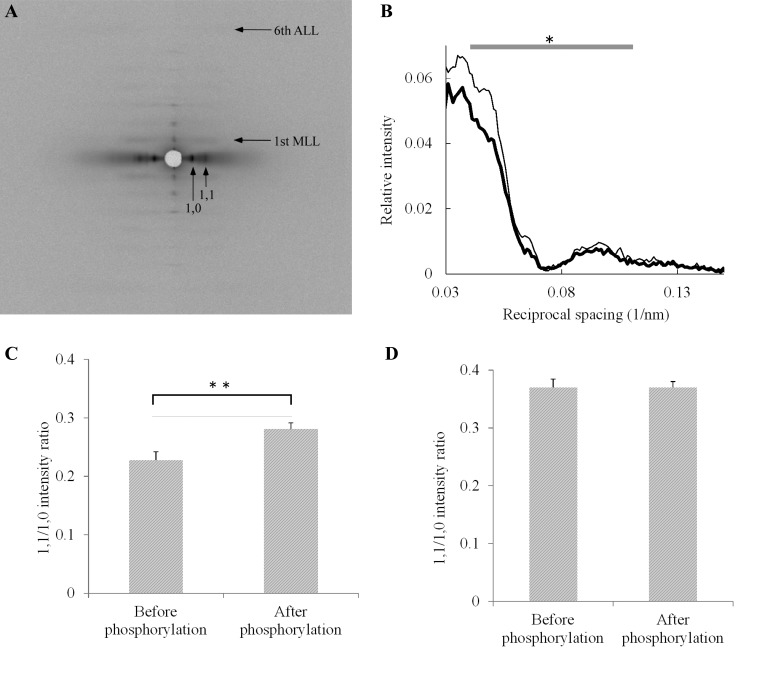
Figure 3A shows a representative composite image of 2-D X-ray diffraction patterns obtained before (left half) and after (right half) incubating fibers in the phosphorylation solution for 20 min. Figure 3B shows averaged profiles of the first myosin layer line scanned along the equatorial axes obtained before and after phosphorylation. This layer line arises from the helical arrangement of myosin heads around the thick filament backbone. The intensity of the first myosin layer line was lower after phosphorylation, and the decrease was particularly marked at around 0.05 nm−1 of reciprocal spacing along the equatorial axis. These effects of phosphorylation were not influenced by changes in sarcomere length (data not shown), showing that the changes did not involve interactions with thin filaments.
Fig. 3.
Changes in diffraction patterns of fiber bundles in response to RLC phosphorylation. A: representative composite image of 2-D X-ray diffraction patterns obtained before (left half) and after (right half) phosphorylation. Horizontal arrows point to first myosin layer line (MLL) and the sixth actin layer line (ALL). Vertical arrows point to the equatorial (1,0) and (1,1) reflections. B: averaged profiles of the first MLL before (thin line, n = 9) and after (thick line, n = 9) phosphorylation. MLL intensity is presented relative to the integrated intensity of the sixth ALL (at 5.9 nm−1). The integral of the averaged intensity from 0.040 to 0.11 nm−1 in reciprocal equatorial spacing was significantly lower after phosphorylation (*P < 0.05, t-test). Fiber bundles were obtained from the same rabbit, and the sarcomere lengths of the fibers were within the range of 1.9 to 2.6 μm. C: mean equatorial (1,1/1,0) intensity ratios at short sarcomere lengths before (n = 12) and after (n = 11) phosphorylation. Bars represent standard errors of the mean (SE). The increase in mean (1,1/1,0) intensity ratio after phosphorylation is statistically significant (**P < 0.01, t-test). Sarcomere lengths were within the range of 1.9 to 2.2 μm. The (1,0) lattice spacing (means ± SE) was not significantly different before (41.6 ± 0.26 nm) and after (42.0 ± 0.39 nm) phosphorylation. Fiber bundles were obtained from the same rabbit. D: mean equatorial (1,1/1,0) intensity ratios at long sarcomere lengths before (n = 5) and after (n = 5) phosphorylation. Bars represent SE. The ratios were not significantly different (P > 0.05, t-test). Sarcomere lengths were within the range of 2.35 to 2.6 μm. The (1,0) lattice spacing (means ± SE) was not significantly different before (40.8 ± 0.19 nm) and after (40.6 ± 0.33 nm) phosphorylation. Fiber bundles were obtained from the same rabbit.
Changes in equatorial reflections in response to RLC phosphorylation.
The equatorial reflections (1,1) and (1,0) are due to longitudinally oriented planes in the muscle filament lattice that pass through thick and thin filaments (1,1), and thick filaments only (1,0), respectively. Figure 3C shows the average (1,1/1,0) intensity ratios of equatorial reflections before and after the phosphorylation treatment, at short sarcomere length. The (1,1/1,0) intensity ratio increased significantly after phosphorylation and remained unchanged after washing out the enzyme. The (1,0) lattice spacing did not change significantly with phosphorylation (see legend, Fig. 3C).
When unphosphorylated fiber bundles were stretched from short sarcomere lengths (range: 1.9–2.2 μm) to longer lengths (range: 2.35–2.6 μm), the equatorial (1,1/1,0) intensity ratio increased from 0.23 ± 0.015 (SE) to 0.37 ± 0.038 (SE) as the lattice spacing is reduced and myosin heads are brought closer to the thin filaments. However, at the longer sarcomere lengths, the averaged equatorial (1,1/1,0) intensity ratio after RLC phosphorylation (0.37 ± 0.025 SE) did not increase further (Fig. 3D).
Effects of BDM on diffraction patterns.
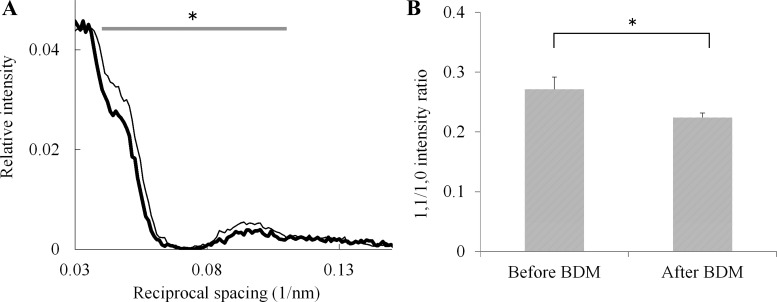
BDM has been shown to dephosphorylate proteins, including the RLC in intact cardiac muscle fibers (8, 41, 64, 66), but it also reversibly inhibits muscle contraction (19, 20, 75). Because glycerinated fibers prepared using a protocol similar to ours contain some endogenously phosphorylated RLC (47), we initially planned to enhance the diffraction signal-to-noise ratio in response to phosphorylation by attempting to use BDM to dephosphorylate the endogenously phosphorylated RLC. We found that BDM treatment significantly increased the intensity of the first myosin layer line at around 0.05 nm−1 of reciprocal equatorial spacing (Fig. 4A), and this was associated with a significant decrease in the (1,1/1,0) intensity ratio of equatorial reflections (Fig. 4B).
Fig. 4.
Effects of BDM treatment on diffraction patterns of skinned fibers. A: averaged profiles of the first MLL before (thick line, n = 21) and after (thin line, n = 21) treatment with BDM. MLL intensity is presented relative to the integrated intensity of the sixth actin layer line (ALL; at 5.9 nm−1). The integral of the averaged intensity from 0.040 to 0.11 nm−1 in reciprocal equatorial spacing was significantly higher after BDM treatment (*P < 0.025, t-test). The sarcomere lengths of the fibers, which were from the same rabbit, were within the range 1.9 to 2.2 μm. B: mean equatorial (1,1/1,0) intensity ratios before (n = 16) and after (n = 16) treatment with BDM. Bars represent SE. The decrease in mean intensity ratio after BDM treatment is statistically significant (*P < 0.025, t-test). Sarcomere lengths of the fibers, which were from the same rabbit, were within the range of 1.9 to 2.2 μm. The (1,0) lattice spacing of the fibers (means ± SE) was not significantly different before (42.6 ± 0.20 nm) and after (41.6 ± 0.18 nm) BDM treatment.
The observed effects of BDM on the diffraction pattern are nearly exactly opposite to those of phosphorylation described above, and thus appeared to be consistent with the intended dephosphorylation of endogenously phosphorylated RLC. However, unexpectedly, electrophoretic analyses of RLC from fibers before (Fig. 2A) and after (Fig. 2B) BDM treatment showed no difference in the level of RLC phosphorylation.
DISCUSSION
Significance of the changes in diffraction pattern due to RLC phosphorylation.
Analyses of the X-ray diffraction patterns of the muscle fibers show the RLC phosphorylation leads to significant changes in both equatorial reflections and layer lines: an increase in the intensity ratio of the equatorial (1,1/1,0) reflections associated with a marked decrease in the intensity of the first myosin layer line at 0.05 nm−1 reciprocal spacing. BDM showed the reverse of these effects.
The observed increase in the (1,1/1,0) intensity ratio of equatorial reflections after RLC phosphorylation may be caused by the movement of myosin heads on the thick filaments towards the thin filament due to the phosphorylation of RLC. However, it may also be caused by the binding to thin filaments of residual MLCK after the phosphorylation solution washout. Although the skeletal isoform of MLCK lacks the amino terminal of the smooth muscle MLCK that has a high-affinity binding site for thin filaments (35, 40) which is responsible for its binding to thin filaments in smooth muscle cells, some workers (12, 17) reported that skeletal MLCK binds to thin filaments in the presence of CaM. Thus, binding of MLCK to thin filaments could increase the (1,1/1,0) intensity ratio, similarly to the effect of aldolase binding to thin filaments on the equatorial reflections (56). However, we observed that the (1,1/1,0) intensity ratio did not increase significantly in the absence of Ca2+, reportedly the optimal condition for MLCK binding to thin filaments (12), suggesting that it was not likely that MLCK was significantly bound to thin filaments under our experimental conditions. Moreover, the phosphorylation-induced increase in (1,1/1,0) intensity ratio showed sarcomere length dependence, being absent at long sarcomere length, and this behavior cannot be attributed to MLCK binding to thin filaments. Therefore, the observed increase in the equatorial (1,1/1,0) intensity ratio can be attributed to the radial shift of myosin heads towards actin. These changes in equatorial reflections associated with RLC phosphorylation observed here in skeletal muscle are consistent with earlier X-ray diffraction results in other types of muscle: tarantula muscle (42), the contraction of which is modulated by the phosphorylation of RLC, and in vertebrate cardiac muscle (8), which is thin filament regulated.
The intensity peak of the first myosin layer line at around 0.05 nm−1 of reciprocal spacing in mammalian muscle can be explained by the sampling effect due to the orderly arrangement of myosin heads in the equatorial plane within the hexagonal myofilament superlattice (21, 24). The observed decrease in intensity of this peak could be caused either by the disorder in the helix structure formed by myosin heads around thick filaments, or by the disorder in the alignment of the thick filaments in the equatorial plane following phosphorylation. However, the latter is not likely to occur because our phosphorylation procedure produced no active tension, nor a change in passive tension that could bring about a change in thick filament alignment. Also, the peak width of equatorial reflections did not increase noticeably, suggesting that lattice disorder did not take place. Therefore, the observed decrease in the intensity of the first myosin layer line can be attributed to the disturbance of the regular helical order of myosin heads on thick filaments. The fact that the effect of phosphorylation on myosin layer line was mainly observed on the intensity peak attributed to the lattice sampling, which is sensitive to many factors, including thick filament alignment and lattice disorder, may help to explain why this effect has not been reported in previous studies. Activation of MLCK with calcium below tension threshold, technical improvements in the experimental setup, and the use of synchrotron radiation probably contributed to our success in detecting these changes.
A phosphorylation-induced radial shift of myosin heads towards actin filaments inferred from the increased equatorial (1,1/1,0) intensity ratio shown earlier in tarantula and mammalian cardiac muscles (8, 42), and confirmed here in mammalian skeletal muscle, does not necessarily imply that this movement originates from the helically arranged myosin heads on the thick filament. X-ray diffraction analyses have suggested that, in relaxed mammalian skeletal muscle, there are at least two populations of disordered myosin heads in dynamic equilibrium with an ordered third population helically arranged around thick filaments (72). The radial shift could involve only these disordered myosin heads. Our observation that the phosphorylation-induced increase in equatorial (1,1/1,0) intensity ratio is concomitantly associated with a decrease in intensity of the first myosin layer line at 0.05 nm−1 reciprocal spacing strongly supports the hypothesis that this movement involves the detachment of phosphorylated myosin heads from their helically arranged positions in the thick filaments and their subsequent movement towards the thin filaments, as proposed earlier (34, 38, 42, 61) and refined by more recent work (26). By linking order-to-disorder transition in the thick filaments to crossbridge movement towards the thin filaments within a physiological myofilament lattice, the present work makes a significant contribution to our understanding of the structural basis for tension modulation by RLC phosphorylation in striated muscles.
At long sarcomere lengths, the equatorial (1,1/1,0) intensity ratio failed to increase with phosphorylation, while the order-to-disorder transition of myosin heads on thick filaments remained unaffected. This suggests that phosphorylation can still detach myosin heads from thick filaments at long sarcomere lengths, but when myosin heads are brought closer to thin filaments as lattice spacing is reduced by the stretch, further movement of the phosphorylated myosin heads towards the thin filaments could no longer take place. These observations correlate well with mechanical analysis showing that the phosphorylation-induced increase in Ca2+ sensitivity is reduced by an increased sarcomere length as well as by osmotic compression of the lattice spacing (76), and that PTP is reduced at long fiber (51) or sarcomere (52) lengths.
Mechanism of action of BDM.
In this study, we failed to dephosphorylate skeletal RLC with BDM even though many authors have reported on its effectiveness in dephosphorylating cardiac RLC in intact myocytes (8, 41, 64, 66). The residual phosphorylation level in fibers treated for 10 min in 10 mM BDM (Fig. 2B) was no different from that in skinned fibers not treated with BDM (Fig. 2A). This suggests that BDM is unable to dephosphorylate RLC in the skinned skeletal muscle fibers, which differs in structure from cardiac RLC (67). This possibility is further supported by the finding that skinned skeletal fibers can be fully phosphorylated even in the presence of 7.5 mM BDM (11). Therefore, the observed effects of BDM on diffraction patterns are likely due to its inhibitory action on the contractile machinery (75) rather than to any action on RLC dephosphorylation.
In a previous X-ray diffraction study on the inhibitory action on muscle contraction of 10 mM BDM, a concentration sufficient to virtually abolish tetanic contraction, a decrease in the equatorial (1,1/1,0) intensity ratio similar to that reported here was observed in resting intact frog sartorius muscle using a weak X-ray source, but changes in myosin layer lines were not reported (75). With the more intense synchrotron radiation used in the present study, we showed that BDM not only significantly decreased the equatorial (1,1/1,0) intensity ratio, but also significantly increase the intensity of the first myosin layer line at around 0.05 nm−1 of reciprocal equatorial spacing.
The site of action of BDM in inhibiting muscle contraction has been localized to the myosin molecule (19). BDM inhibits myosin ATPase as well as myofibrillar ATPase by acting as an uncompetitive inhibitor. It accelerates ATP hydrolysis and inhibits Pi release by stabilizing the “weak” actin binding state myosin-ADP-inorganic phosphate complex (M.ADP.Pi), thereby increasing the steady-state concentration of this intermediate at the expense of the “strong” actin binding state myosin-ADP complex (M.ADP) (18). Mechanically, BDM decreases the rate constant for crossbridge attachment (4), precisely contrary to the effect of RLC phosphorylation. In the presence of actin and in intact fibers, inhibition of Pi release by BDM stabilizes the low-force actin-myosin- ADP.Pi (A.M.ADP.Pi) state at the expense of the high-force states (A.M*.ADP.Pi or A.M.ADP) during activation, thereby weakening the interaction of actin and myosin and reducing tetanic force (37).
Helical order of thick filaments requires myosin heads in the weakly actin binding M.ADP.Pi state (71), in which the switch 2 element of the myosin motor domain is in the closed conformation (73). This M.ADP.Pi is the state stabilized by BDM (18). Thus, BDM acting on a resting muscle would be expected to shift the equilibrium of myosin heads towards the M.ADP.Pi state, thus promoting helical order of thick filaments. This shift in equilibrium would be at the expense of reducing the two populations of disordered myosin heads (72). These changes in myosin head distribution would explain the observed increase in intensity of the first myosin layer line at 0.05 nm−1 reciprocal spacing, as well as the decrease in equatorial (1,1/1,0) intensity ratio induced by BDM. Blebbistatin is another uncompetitive inhibitor of myosin ATPase with similar properties to BDM. It also inhibits Pi release from myosin by stabilizing the closed conformation of the switch 2 element of the myosin motor domain (29) and promotes helical order of thick filaments (74, 78) and the super relaxed state (68).
Structural basis for the helical order of thick filaments.
Recent cryoelectron microscopy in combination with 3-D reconstitution and atomic fitting have shown a common structural motif on the surfaces of thick filaments in striated muscles of animals ranging from invertebrates to mammals which resembles the structure of the smooth muscle myosin in the off state (1, 48, 69, 70, 77, 80). In this motif, the two heads of each myosin interact with one another and with its S2 segment, the actin binding region of the “blocked” head interacts with converter region and the essential light chain of the “free” head, thereby preventing actin binding in the blocked head, while the ATPase activity in the free head is inhibited by preventing the movement of the converter needed for Pi release (70). These heads also interact with their S2 segments and those of neighboring myosins (2), helping to hold the myosin heads to the thick filament core (25). These head-head and head-tail interactions have also been observed in vertebrate striated and cardiac myosins in the relaxed state (25). For vertebrate striated muscle thick filaments, this motif is the structural basis for the helical order and the super relaxed state with very low ATPase activity (57).
A functional consequence of the interacting heads in tarantula thick filaments which may in part be applicable to vertebrate striated muscles is that the RLCs of the two heads of each myosin have different susceptibilities to phosphorylation, and thus are sequentially phosphorylated and mobilized, depending on the activation level of the kinases (2, 6, 59). In view of these findings, the structural changes observed in this study, which was performed under a relatively low concentration of Ca2+ (pCa = 6.8) and thus a low level of activation of MLCK, may result from a change in one of the two myosin heads that is more susceptible to phosphorylation than the other, thus making it more flexible and more likely to interact with actin than the other myosin head. This explanation is consistent with the relatively low level of phosphorylation observed in this study (Fig. 2).
Mechanism of action of RLC phosphorylation at the molecular level.
Experiments using RLC mutants suggest that RLC phosphorylation causes myosin heads to swing out towards thin filaments by destabilizing the electrostatic interactions between the RLC and the thick filament which hold the myosin heads close to the thick filament, and that reducing net charge in the N-terminal region of RLC adequately explains the enhanced force at low activating Ca2+ in skinned fibers (63). However, recent work on the conformation of the RLC in cardiac muscle using polarized fluorescence techniques showed that the orientation of the unphosphorylated RLC did not change during activation (27), suggesting that the interaction between unphosphorylated RLC and the thick filament is maintained during crossbridge cycling. Further, the effect of phosphorylation of RLC allows movement of the crossbridge towards actin without completely detaching the RLC from the thick filament (26).
There exists biochemical and biophysical evidence pointing to the existence of direct effects of RLC phosphorylation on the interaction between actin and myosin even when extracted from the filament lattice. The affinity of phosphorylated myosin for actin is about twofold higher than that for dephosphorylated myosin (39, 44, 46). This is in sharp contrast to the weakening of this affinity by BDM (37). Phosphorylated myosin also has a twofold higher affinity for ADP (15), which most probably explains its increase in the myosin duty cycle and the consequent slowing of the maximal sliding velocity under unloaded conditions in motility assays (16) and in skinned fibers (28). Further, phosphorylation ameliorates the slowing of velocity in motility assays under fatigue-like conditions (increased ADP, Pi, and lowered pH) (15) as well as enhances maximal activated tension in skinned fibers under these conditions (13).
Davis et al. (11) made the important observation that phosphorylation of RLC partially reverses the inhibitory effect of BDM on tension in a fully activated skinned fiber, even though phosphorylation has no effect on maximally activated tension in the absence of BDM. Since BDM is known to inhibit the release of Pi from A.M.ADP.Pi (37), this observation led them to suggest that RLC phosphorylation accelerates the release of Pi from A.M.ADP.Pi. In other words, BDM and RLC phosphorylation operate on the same step in the crossbridge cycle, namely, Pi release, but in opposing directions. This notion is consistent with their opposite effects on the rate of crossbridge attachment (4, 38, 61), the affinity of myosin for actin (37, 39) and the diffraction changes reported here. Since blebbistatin blocks Pi release in the same way as BDM does, it is likely that RLC phosphorylation would also oppose the inhibitory action of blebbistatin on tetanic tension as BDM does (11). Conversely, blebbistatin is likely to reverse significantly the phosphorylation-induced helical disorder of myosin heads.
Phosphorylation-induced enhancement of Pi release in a relaxed fiber would lead to a shift in the equilibrium of myosin heads away from the M.ADP.Pi, the state associated with helical order (71), towards the two disordered states which include a population of myosin heads weakly attached to actin (72), thereby explaining the observed reduced thick filament helical order and increased equatorial (1,1/1,0) intensity ratio. Since myosin heads associated with the helical order are in a super relaxed state with very low myosin ATPase activity (57), moving myosin heads from this state to the relaxed state with higher ATPase activity would enhance the ATPase activity of resting muscle fibers. In an intact animal, this would contribute significantly to thermogenesis (9). Phosphorylation-induced enhancement of Pi release may also explain the amelioration of the slowing of velocity in motility assays (15) and the enhancement of maximal activated tension in skinned fibers under fatigue conditions (13) when crossbridge cycling is severely inhibited by the elevated ADP and Pi.
RLC phosphorylation enhances the affinity of myosin for ADP by twofold (15), reflecting the stabilization of the M.ADP complex. This action may allow inorganic Pi to dissociate from M.ADP.Pi complex more readily, which may contribute to the enhancement of actin-activated myosin ATPase activity (39, 44, 46). However, the maximal actin-activated ATPase activity (Vmax) remains unchanged (39, 44, 46), possibly because the increased affinity of myosin for ADP slows the dissociation of ADP and so the detachment rate of attached crossbridges (43), thereby limiting the enhancement of Vmax.
In summary, we show that in skinned mammalian fast muscle fibers, RLC phosphorylation is associated with X-ray diffraction changes in both layer lines and equatorial reflections, which provide strong structural evidence for the hypothesis that phosphorylation causes myosin heads to move from their regular helically arranged positions in thick filaments towards thin filaments, thereby facilitating actin myosin interaction. These findings provide new insights into the structural basis for the mechanism of the tension modification in the striated muscles. We also show that BDM has opposite effects on these diffraction changes, which can be accounted for by the hypothesis that BDM inhibits Pi release from M.ADP.Pi complex, while RLC phosphorylation accelerates it.
GRANTS
This work was supported by the Australian Academy of Science with a Travel Grant to J. F. Y. Hoh and the Jikei University Research Fund to M. Yamaguchi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.Y. and J.F.Y.H. conception and design of research; M.Y., M.K., Z.B.L., T.O., S.T., J.F.Y.H., and N.Y. performed experiments; M.Y., Z.B.L., S.T., and J.F.Y.H. analyzed data; M.Y., M.K., Z.B.L., T.O., S.T., J.F.Y.H., and N.Y. interpreted results of experiments; M.Y. prepared figures; M.Y. drafted manuscript; M.Y., M.K., T.O., J.F.Y.H., and N.Y. edited and revised manuscript; M.Y., M.K., Z.B.L., T.O., S.T., J.F.Y.H., and N.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
The X-ray diffraction experiments were performed at BL45XU in SPring8 with the approval of the Program Review Committee of the Japan Synchrotron Radiation Research Institute (JASRI; 2003A0416, 2004A0267). We are grateful to Dr. James Stull for supplying MLCK and CaM. We are also grateful to Dr. Tetsuro Fujisawa for help in the X-ray diffraction experiment and to Shizuko Syoji for preparing the experimental solutions.
REFERENCES
- 1.Al-Khayat HA, Kensler RW, Squire JM, Marston SB, Morris EP. Atomic model of the human cardiac muscle myosin filament. Proc Natl Acad Sci USA 110: 318–323, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamo L, Wriggers W, Pinto A, Bartoli F, Salazar L, Zhao FQ, Craig R, Padron R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol 384: 780–797, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen GO, Qvigstad E, Schiander I, Aass H, Osnes JB, Skomedal T. Alpha(1)-AR-induced positive inotropic response in heart is dependent on myosin light chain phosphorylation. Am J Physiol Heart Circ Physiol 283: H1471–H1480, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bagni MA, Cecchi G, Colomo F, Garzella P. Effects of 2,3-butanedione monoxime on the crossbridge kinetics in frog single muscle fibres. J Muscle Res Cell Motil 13: 516–522, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Barany K, Barany M. Phosphorylation of the 18,000-dalton light chain of myosin during a single tetanus of frog muscle. J Biol Chem 252: 4752–4754, 1977. [PubMed] [Google Scholar]
- 6.Brito R, Alamo L, Lundberg U, Guerrero JR, Pinto A, Sulbaran G, Gawinowicz MA, Craig R, Padron R. A molecular model of phosphorylation-based activation and potentiation of tarantula muscle thick filaments. J Mol Biol 414: 44–61, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Close R, Hoh JFY. The after-effects of repetitive stimulation on the isometric twitch contraction of rat fast skeletal muscle. J Physiol 197: 461–477, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 588: 981–993, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys Rev 3: 33–45, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig R, Padron R, Kendrick-Jones J. Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J Cell Biol 105: 1319–1327, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis JS, Satorius CL, Epstein ND. Kinetic effects of myosin regulatory light chain phosphorylation on skeletal muscle contraction. Biophys J 83: 359–370, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita K, Ye LH, Sato M, Okagaki T, Nagamachi Y, Kohama K. Myosin light chain kinase from skeletal muscle regulates an ATP-dependent interaction between actin and myosin by binding to actin. Mol Cell Biochem 190: 85–90, 1999. [PubMed] [Google Scholar]
- 13.Godt RE, Nosek TM. Changes of intracellular milieu with fatigue or hypoxia depress contraction of skinned rabbit skeletal and cardiac muscle. J Physiol 412: 155–180, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez B, Negredo P, Hernando R, Manso R. Protein variants of skeletal muscle regulatory myosin light chain isoforms: prevalence in mammals, generation and transitions during muscle remodelling. Pflügers Arch 443: 377–386, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg MJ, Mealy TR, Jones M, Szczesna-Cordary D, Moore JR. The direct molecular effects of fatigue and myosin regulatory light chain phosphorylation on the actomyosin contractile apparatus. Am J Physiol Regul Integr Comp Physiol 298: R989–R996, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR. The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol 297: R265–R274, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatch V, Zhi G, Smith L, Stull JT, Craig R, Lehman W. Myosin light chain kinase binding to a unique site on F-actin revealed by three-dimensional image reconstruction. J Cell Biol 154: 611–617, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann C, Wray J, Travers F, Barman T. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry 31: 12227–12232, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi H, Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem 105: 638–643, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Horiuti K, Higuchi H, Umazume Y, Konishi M, Okazaki O, Kurihara S. Mechanism of action of 2,3-butanedione 2-monoxime on contraction of frog skeletal muscle fibres. J Muscle Res Cell Motil 9: 156–164, 1988. [DOI] [PubMed] [Google Scholar]
- 21.Huxley HE, Brown W. The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol 30: 383–434, 1967. [DOI] [PubMed] [Google Scholar]
- 22.Iwakura K, Hori M, Watanabe Y, Kitabatake A, Cragoe EJ, Yoshida H, Kamada T. Alpha 1-adrenoceptor stimulation increases intracellular pH and Ca2+ in cardiomyocytes through Na+/H+ and Na+/Ca2+ exchange. Eur J Pharmacol 186: 29–40, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto H, Oiwa K, Suzuki T, Fujisawa T. X-ray diffraction evidence for the lack of stereospecific protein interactions in highly activated actomyosin complex. J Mol Biol 305: 863–874, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto H, Wakayama J, Fujisawa T, Yagi N. Static and dynamic X-ray diffraction recordings from living mammalian and amphibian skeletal muscles. Biophys J 85: 2492–2506, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung HS, Komatsu S, Ikebe M, Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol Biol Cell 19: 3234–3242, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kampourakis T, Irving M. Phosphorylation of myosin regulatory light chain controls myosin head conformation in cardiac muscle. J Mol Cell Cardiol 85: 199–206, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kampourakis T, Sun YB, Irving M. Orientation of the N- and C-terminal lobes of the myosin regulatory light chain in cardiac muscle. Biophys J 108: 304–314, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol 294: R948–R955, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279: 35557–35563, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Kraft T, Xu S, Brenner B, Yu LC. The effect of thin filament activation on the attachment of weak binding cross-bridges: a two-dimensional X-ray diffraction study on single muscle fibers. Biophys J 76: 1494–1513, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krarup C. Enhancement and diminution of mechanical tension evoked by staircase and by tetanus in rat muscle. J Physiol 311: 355–372, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krarup C. Temperature dependence of enhancement and diminution of tension evoked by staircase and by tetanus in rat muscle. J Physiol 311: 373–387, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine RJ, Chantler PD, Kensler RW, Woodhead JL. Effects of phosphorylation by myosin light chain kinase on the structure of Limulus thick filaments. J Cell Biol 113: 563–572, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine RJ, Kensler RW, Yang Z, Stull JT, Sweeney HL. Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71: 898–907, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin P, Luby-Phelps K, Stull JT. Binding of myosin light chain kinase to cellular actin-myosin filaments. J Biol Chem 272: 7412–7420, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Manning DR, Stull JT. Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun 90: 164–170, 1979. [DOI] [PubMed] [Google Scholar]
- 37.McKillop DF, Fortune NS, Ranatunga KW, Geeves MA. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibres. J Muscle Res Cell Motil 15: 309–318, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol 93: 855–883, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michnicka M, Kasman K, Kakol I. The binding of actin to phosphorylated and dephosphorylated myosin. Biochim Biophys Acta 704: 470–475, 1982. [DOI] [PubMed] [Google Scholar]
- 40.Nunnally MH, Stull JT. Mammalian skeletal muscle myosin light chain kinases. A comparison by antiserum cross-reactivity. J Biol Chem 259: 1776–1780, 1984. [PubMed] [Google Scholar]
- 41.Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol 287: H2712–H2718, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Padron R, Pante N, Sosa H, Kendrick-Jones J. X-ray diffraction study of the structural changes accompanying phosphorylation of tarantula muscle. J Muscle Res Cell Motil 12: 235–241, 1991. [DOI] [PubMed] [Google Scholar]
- 43.Patel JR, Diffee GM, Huang XP, Moss RL. Phosphorylation of myosin regulatory light chain eliminates force-dependent changes in relaxation rates in skeletal muscle. Biophys J 74: 360–368, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pemrick SM. The phosphorylated L2 light chain of skeletal myosin is a modifier of the actomyosin ATPase. J Biol Chem 255: 8836–8841, 1980. [PubMed] [Google Scholar]
- 45.Perrie WT, Smillie LB, Perry SV. A phosphorylatable light chain component of myosin from skeletal muscle. Biochem J 135: 151–164, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persechini A, Stull JT. Phosphorylation kinetics of skeletal muscle myosin and the effect of phosphorylation on actomyosin adenosinetriphosphatase activity. Biochemistry 23: 4144–4150, 1984. [DOI] [PubMed] [Google Scholar]
- 47.Persechini A, Stull JT, Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal muscle fibers. J Biol Chem 260: 7951–7954, 1985. [PubMed] [Google Scholar]
- 48.Pinto A, Sanchez F, Alamo L, Padron R. The myosin interacting-heads motif is present in the relaxed thick filament of the striated muscle of scorpion. J Struct Biol 180: 469–478, 2012. [DOI] [PubMed] [Google Scholar]
- 49.Pires E, Perry SV, Thomas MA. Myosin light-chain kinase, a new enzyme from striated muscle. FEBS Lett 41: 292–296, 1974. [DOI] [PubMed] [Google Scholar]
- 50.Puceat M, Clement O, Pelosin JM, Ventura-Clapier R, Vassort G. Neurohormonal control of calcium sensitivity in rat single heart cells. Circ Res 67: 517–524, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Rassier DE. The effects of length on fatigue and twitch potentiation in human skeletal muscle. Clin Physiol 20: 474–482, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Rassier DE, MacIntosh BR. Sarcomere length-dependence of activity-dependent twitch potentiation in mouse skeletal muscle. BMC Physiol 2: 19, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riise J, Nguyen CH, Qvigstad E, Sandnes DL, Osnes JB, Skomedal T, Levy FO, Krobert KA. Prostanoid F receptors elicit an inotropic effect in rat left ventricle by enhancing myosin light chain phosphorylation. Cardiovasc Res 80: 407–415, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Rossmanith GH, Hoh JF, Turnbull L, Ludowyke RI. Mechanism of action of endothelin in rat cardiac muscle: cross-bridge kinetics and myosin light chain phosphorylation. J Physiol 505: 217–227, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shimizu T, Reinach FC, Masaki T, Fischman DA. Analysis of the metal-induced conformational change in myosin with a monoclonal antibody to light chain two. J Mol Biol 183: 271–282, 1985. [DOI] [PubMed] [Google Scholar]
- 56.Stewart M, Morton DJ, Clarke FM. Changes associated with glycolytic-enzyme binding in the equatorial X-ray-diffraction pattern of glycerinated rabbit psoas muscle. Biochem J 183: 663–667, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA 107: 430–435, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys 510: 120–128, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sulbaran G, Biasutto A, Alamo L, Riggs C, Pinto A, Mendez F, Craig R, Padron R. Different head environments in tarantula thick filaments support a cooperative activation process. Biophys J 105: 2114–2122, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol Cell Physiol 264: C1085–C1095, 1993. [DOI] [PubMed] [Google Scholar]
- 61.Sweeney HL, Stull JT. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: implications for regulation of actin-myosin interaction. Proc Natl Acad Sci USA 87: 414–418, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sweeney HL, Stull JT. Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol Cell Physiol 250: C657–C660, 1986. [DOI] [PubMed] [Google Scholar]
- 63.Sweeney HL, Yang Z, Zhi G, Stull JT, Trybus KM. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci USA 91: 1490–1494, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turnbull L, Hoh JFY, Ludowyke RI, Rossmanith GH. Troponin I phosphorylation enhances crossbridge kinetics during β-adrenergic stimulation in rat cardiac tissue. J Physiol 542: 911–920, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vandenboom R, Gittings W, Smith IC, Grange RW, Stull JT. Myosin phosphorylation and force potentiation in skeletal muscle: evidence from animal models. J Muscle Res Cell Motil 34: 317–332, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Venema RC, Raynor RL, Noland TAJ, Kuo JF. Role of protein kinase C in the phosphorylation of cardiac myosin light chain 2. Biochem J 294 401–406, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westwood SA, Perry SV. Two forms of the p light chain of myosin in rabbit and bovine hearts. FEBS Lett 142: 31–34, 1982. [DOI] [PubMed] [Google Scholar]
- 68.Wilson C, Naber N, Pate E, Cooke R. The myosin inhibitor blebbistatin stabilizes the super-relaxed state in skeletal muscle. Biophys J 107: 1637–1646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodhead JL, Zhao FQ, Craig R. Structural basis of the relaxed state of a Ca2+-regulated myosin filament and its evolutionary implications. Proc Natl Acad Sci USA 110: 8561–8566, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padron R. Atomic model of a myosin filament in the relaxed state. Nature 436: 1195–1199, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Xu S, Gu J, Rhodes T, Belknap B, Rosenbaum G, Offer G, White H, Yu LC. The M.ADP.Pi state is required for helical order in the thick filaments of skeletal muscle. Biophys J 77: 2665–2676, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu S, Malinchik S, Gilroy D, Kraft T, Brenner B, Yu LC. X-ray diffraction studies of cross-bridges weakly bound to actin in relaxed skinned fibers of rabbit psoas muscle. Biophys J 73: 2292–2303, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu S, Offer G, Gu J, White HD, Yu LC. Temperature and ligand dependence of conformation and helical order in myosin filaments. Biochemistry 42: 390–401, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Xu S, White HD, Offer GW, Yu LC. Stabilization of helical order in the thick filaments by blebbistatin: further evidence of coexisting multiple conformations of myosin. Biophys J 96: 3673–3681, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yagi N, Takemori S, Watanabe M, Horiuti K, Amemiya Y. Effects of 2,3-butanedione monoxime on contraction of frog skeletal muscles: an X-ray diffraction study. J Muscle Res Cell Motil 13: 153–160, 1992. [DOI] [PubMed] [Google Scholar]
- 76.Yang Z, Stull JT, Levine RJ, Sweeney HL. Changes in interfilament spacing mimic the effects of myosin regulatory light chain phosphorylation in rabbit psoas fibers. J Struct Biol 122: 139–148, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Zhao FQ, Craig R, Woodhead JL. Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J Mol Biol 385: 423–431, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao FQ, Padron R, Craig R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch 2 closed state. Biophys J 95: 3322–3329, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci USA 102: 17519–17524, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci USA 105: 2386–2390, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]