Abstract
Receptor-activator of nuclear factor-κB (RANK), its ligand RANKL, and the soluble decoy receptor osteoprotegerin are the key regulators of osteoclast differentiation and bone remodeling. Here we show that RANK is also expressed in fully differentiated myotubes and skeletal muscle. Muscle RANK deletion has inotropic effects in denervated, but not in sham, extensor digitorum longus (EDL) muscles preventing the loss of maximum specific force while promoting muscle atrophy, fatigability, and increased proportion of fast-twitch fibers. In denervated EDL muscles, RANK deletion markedly increased stromal interaction molecule 1 content, a Ca2+ sensor, and altered activity of the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) modulating Ca2+ storage. Muscle RANK deletion had no significant effects on the sham or denervated slow-twitch soleus muscles. These data identify a novel role for RANK as a key regulator of Ca2+ storage and SERCA activity, ultimately affecting denervated skeletal muscle function.
Keywords: receptor-activator of nuclear factor-κB, sarco(endo)plasmic reticulum Ca2+-ATPase
receptor-activator of nuclear factor-κB ligand (RANKL), the membrane receptor RANK, and the soluble decoy receptor osteoprotegerin (OPG) are members of the tumor necrosis factor superfamily that regulate bone remodeling (26, 29). RANK/RANKL interaction activates Ca2+-dependent and NF-κB signaling pathways, which affect osteoclast differentiation, activation, and survival (29). The third protagonist, OPG, binds to RANKL and inhibits the RANK/RANKL interaction and subsequent osteoclastogenesis (46, 55). In addition to bone, RANK/RANKL has been detected in other tissues such as thymus, heart, kidney, liver, brain, blood vessels, and skeletal muscles (17, 32, 51). The RANK/RANKL pathway is known to be involved in a variety of physiological and pathological conditions such as lymph-node organogenesis, formation of lactating mammary gland, breast cancer, central thermoregulation, T cell/dendritic cell communication, vascular calcification, and bone metastasis (18, 36, 38, 41, 45).
Muscle hypertrophy/atrophy and gain/loss of bone mineral density occur in parallel in many physiological or pathological conditions, and endocrine, mechanical factors, inflammatory, and nutritional states affect simultaneously skeletal muscle and bone metabolism (3, 4, 16, 22, 39). These observations are consistent with the view that skeletal muscle and bone share common cell signaling pathways. For example, the Wnt/β-catenin signaling pathway is a major regulator of bone mass and muscle development and growth (24). Conditional deletion of Ctnnb1 gene in osteocytes, which encodes for β-catenin, leads to impaired bone maturation and mineralization with an increased RANKL-to-OPG ratio (27). In bone, RANKL/RANK interactions activate tumor necrosis factor receptor-associated factor 6 (TRAF-6), which subsequently induces the activation of downstream signaling molecules and intracellular Ca2+ concentration ([Ca2+]i) oscillations (53). The ATP-dependent Ca2+ pump, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), is essential for [Ca2+]i oscillations and plays a critical role in osteoclastogenesis (54). Moreover, it has been shown that TRAF-6 is required for functional bone resorption, and muscle-specific TRAF-6 deletion preserves function and reduces atrophy in a model of muscle wasting, indicating that TRAF-6 is an important regulator of both bone and muscle masses (19, 49, 50). Thus, common signaling pathways are emerging to explain the synchronicity between bone and skeletal muscle physiology and pathophysiology.
Muscle contraction involves the depolarization of the transverse-tubular system, which activates dihydropyridine receptors opening ryanodine receptor/Ca2+ release channels (RYR1) in the sarcoplasmic reticulum (SR) membrane. This results in the rapid influx of Ca2+ in the cytoplasm through RYR1 and binding of Ca2+ to troponin C causing the formation of actin-myosin crossbridges and force development (34). Ca2+ reuptake in the SR is a tightly control mechanism mediated almost exclusively by SERCA-1a in fast-twitch fibers and SERCA-2a in slow-twitch fibers. The regulation of Ca2+ is also implicated in other physiological processes such as the maintenance and adaptation of muscle phenotypes (7, 40), whereas chronic rise in [Ca2+]i is associated with different pathological states, including muscle dystrophy (1) and the triggering of apoptotic processes (12, 42). Therefore, the appropriate regulation of [Ca2+]i is a requirement for proper cell function, phenotype, and survival.
We recently reported that OPG treatments protect against muscular dystrophy, suggesting a potential role for the RANK/RANKL/OPG pathway in muscle disease. In addition, muscle-specific TRAF-6 deletion prevented muscle atrophy and decreased the expression of ubiquitin-proteasome components in models of denervation or starvation (28, 50). In the present study, we hypothesized that muscle RANK, a receptor upstream of TRAF-6, is an important regulator of denervated muscle function. We report that RANK/RANKL regulates Ca2+ storage, function, and phenotype, confirming a role for RANK in denervated skeletal muscles.
MATERIAL AND METHODS
Ethical approval.
All procedures were approved by the Université Laval Research Center Animal Care and Use Committee based on Canadian Council on Animal Care guidelines.
Animals.
Mice carrying the RANKfloxed or RANKdel alleles and muscle creatine kinase-Cre (mck-Cre) mice were backcrossed five times to a C57BL/10J background before generating a specific RANK skeletal muscle deletion, the mck-Cre RANKdel/floxed, hereafter named RANKmko mice (18). RANKmko mice are viable, healthy, and appeared indistinguishable from control RANKfloxed/floxed mice that do not carry the Cre recombinase, hereafter named RANKf/f mice. Male wild-type (C57BL/10J) mice were purchased from the Jackson Laboratory and bred at our animal facility. Mice were screened for the desired genotype by PCR analysis. Food and water were provided ad libitum. At the end of the different experimental procedures, the mice were killed by cervical dislocation under anesthesia.
Denervation.
Sciatic denervation was performed under anesthesia with isoflurane inhalation on adult mice aged between 12 and 18 wk. Briefly, the hindlimbs were shaved, and a small 0.5-cm incision was made proximal to the hip on the lateral side of the leg to expose and section a 3- to 5-mm piece of the sciatic nerve. The wounds were then closed with surgical sutures. The same surgery procedures were executed without sciatic denervation for sham mice. Mice were killed 14 days after sham or denervation procedures. Animals without chirurgical procedures were used as control mice.
Cell culture.
C2C12 myoblasts (ATCC) were cultured in high-glucose DMEM (HyClone) supplemented with 10% FBS (HyClone) and 1% antibiotic-antimycotic (Life Technologies) in 5% CO2 and at 37°C. When the myoblasts reached 90% confluence, the medium was replaced by high-glucose DMEM containing 1% FBS for 5 days to allow the myoblasts to differentiate into myotubes (11).
Genomic DNA for genotyping.
Genomic DNA from mouse tail tissue samples was isolated and amplified by PCR. RANK, cre, and dystrophin were identified by isolating genomic DNA from tail tissue and screening for the mutation or presence of the transgene by PCR. To detect delta, flox, and wild-type alleles primers used were p87, p88, and p105. Conditions were as follows: 94°C 2 min; 40 cycles of 94°C 30 s, 60°C 20 s, and 72°C 1 min; and 72°C 4 min. To detect the presence of the mck-cre primers used were ALP 130 and ALP 131. Conditions were as follows: 94°C 2 min; 40 cycles of 94°C 30 s, 58°C 10 s, and 72°C 1 min; and 72°C 4 min. To detect mdx allele primers used were p9427 and p259E. Conditions were as follows: 94°C 3 min; 45 cycles of 94°C 30 s, 57°C 30 s, and 72°C 20 s; and 72°C 10 min.
Isometric contractile properties.
Mice were injected with buprenorphine (0.1 mg/kg ip) and anesthetized with pentobarbital sodium (50 mg/kg ip) 15 min later. Mice were weighed, and the soleus (Sol) and extensor digitorum longus (EDL) muscles were carefully dissected and attached to an electrode and a force sensor (305B-LR; Aurora Scientific) to assess contractile properties as described previously (9, 44). Muscle fatigability was examined by stimulating muscle for 200 ms every second at 50 Hz until muscles lost 30% (Sol) or 50% (EDL) of their initial force. Last, muscle length was measured, tendons were removed, and muscles were weighed for calculation of muscle cross-sectional area and specific force (13). Functional measurements were analyzed with the Dynamic Muscle Data Analysis software (Aurora Scientific).
Immunohistochemistry.
Transversal Sol and EDL muscle sections (10 μm) were cut (CM1850; Leica Microsystems, Nussloch, Germany) in duplicate from the proximal and distal portions of the muscles. Sections were incubated overnight at 4°C with the following primary antibodies: anti-SERCA-1a (Abcam), anti-SERCA-2a (Abcam), anti-myosin heavy chain (MyHC) I (Novus Biological), anti-MyHC IIA (SC-71; DSHB), anti-MyHC IIB (BF-F3; DSHB), anti-MyHC IIX (6H1; DSHB), anti-dystrophin (NCL-Dys1; Vector Laboratories), and anti-RANK (R&D Systems). Pan-MyHC II was obtained by combining the following three antibodies: anti-MyHC IIA, anti-MyHC IIB, and anti-MyHC IIX (DSHB). Fiber-type differentiation by myosin immunohistochemistry was performed as described by Schiaffino et al. (43). Biotinylated secondary antibodies for immunohistochemistry were purchased from Vector Laboratories and Alexa Fluor secondary antibodies from Invitrogen. The localization of RANK and dystrophin in the sarcolemmal membrane of skeletal muscles was determined by confocal microscopy. Briefly, confocal series were acquired using a Quorum WaveFX spinning disc confocal system (Quorum Technologies, Guleph, Ontario) with 491- and 561-nm solid state laser lines for excitation of green and red (Alexa-488 and Alexa-594), combined with appropriate BrightLine single-bandpass emission filters (536/40 nm and 624/40 nm; Semrock). For DAPI visualization, wide-field z-series were acquired at the same time with a DAPI fluorescence filter cube (Chroma Technology). The CCD camera used to capture the images was a Hamamatsu ImagEM C-9100.
Western blotting.
Myotubes or skeletal muscles were homogenized in a lysis buffer containing 10 μl of protease inhibitor cocktail (Sigma-Aldrich). Protein homogenates were electrophoretically separated on SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad), blocked in 5% skim milk, and incubated overnight at 4°C. The following primary antibodies (all from Santa Cruz Biotechnology) were used: anti-SERCA-1a, anti-SERCA-2a, anti-stromal interaction molecule 1 (Stim1), anti-calsequestrin, and anti-RANK anti-GAPDH. The membranes were washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology). Protein bands were revealed using the ECL-Plus chemiluminescent detection system (Perkin-Elmer). Films (Denville Scientific) were used to detect a chemiluminescent signal, scanned, and analyzed using Quantity One software (version 4.6.6; Bio-Rad) (10).
Ca2+ measurements.
In another set of experiments, the concentration of total Ca2+ ([CaT]) in Sol and EDL muscles was determined using the Ca2+-dependent ultraviolet (UV) absorbance of 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), which is a good estimate of [CaT] in the SR (30). Briefly, whole Sol and EDL muscles from 12-wk-old mice were weighed and homogenized in a solution containing 0.3 mM BAPTA and SDS detergent to dissolve the surface and SR membranes. The mixture was then centrifuged removing proteins and other insoluble muscle components to prevent unwanted absorbance or light scattering. To determine the amount of [CaT], the supernatant was divided into four aliquots for separate absorbance measurements taken at 292 nm: 1) the supernatant alone; 2) the UV absorbance spectrum (240–390 nm) of the supernatant plus 1 mM EGTA added to give a zero Ca2+ BAPTA spectrum; 3) the supernatant with a known amount of Ca2+ standard added; and 4) the supernatant with excess Ca2+ added to complex essentially all of the binding sites on BAPTA with Ca2+. From the absorbance data and the equations given by Lamboley et al. (30), values of [CaT] concentration were estimated and reported here in units of millimoles per kilogram muscle weight. [CaT] measurements include the following components: extracellular, intracellular outside of the SR, and intracellular inside the SR ([CaT]SR). The Ca2+ chelator BAPTA should provide the best estimate of [CaT]SR under physiological conditions (30).
SERCA parameters.
Homogenates from the muscles of wild-type and knockout mice were used to determine Ca2+-dependent Ca2+-ATPase activity using a spectrophotometric assay (SPECTRAmax Plus; Molecular Devices) (47). Briefly, reaction buffer [in mM: 200 KCl, 20 HEPES (pH 7.0), 15 MgCl2, 1 EGTA, 10 NaN3, 5 ATP, and 10 phosphoenolpyruvate] containing 18 U/ml of both lactate dehydrogenase and pyruvate kinase, as well as the homogenate, was added to test tubes containing 15 different concentrations of Ca2+, ranging between 7.6 and 4.7 pCa units in the presence and absence of ionophore A-23187 (4.2 μM). In the absence of the ionophore, Ca2+ accumulates inside the SR vesicle and causes backinhibition of SERCA pumps, which is more relevant to the physiological system found in skeletal muscle. Aliquots (100 μl) were then transferred in duplicate to a clear-bottom 96-well plate (Costar, Corning), where 0.3 mM NADH was added to start the reaction. The plate was read at a wavelength of 340 nm for 30 min at 37°C. The different concentrations of Ca2+ in the wells were used to determine the maximal enzyme activity (Vmax) and pCa50, which is defined as the intracellular free Ca2+ concentration ([Ca2+]f) required to achieve 50% of Vmax. Last, cyclopiazonic acid (40 μM), a highly specific SERCA inhibitor, was used to determine background activity, which was subtracted from the [CaT]-ATPase activity measured in muscle homogenate. All data were then plotted against the negative logarithm of [Ca2+]f (pCa) using basic statistatical software (GraphPad Prism version 4) to determine Vmax and pCa50. pCa50 was determined by nonlinear regression curve fitting using the sigmoidal dose response.
Statistical analyses.
All values are expressed as means ± SE. The data were analyzed with Student's t-test, Chi square test, or one-way ANOVA followed by a Tukey's test (InStat). The levels of significance were set at P < 0.05, 0.01, and 0.001 for genotype (RANKf/f vs. RANKmko) or P < 0.05 for treatment (sham vs. denervated).
RESULTS
RANK is expressed in fully differentiated C2C12 myotubes and at the sarcolemmal membrane of skeletal muscle.
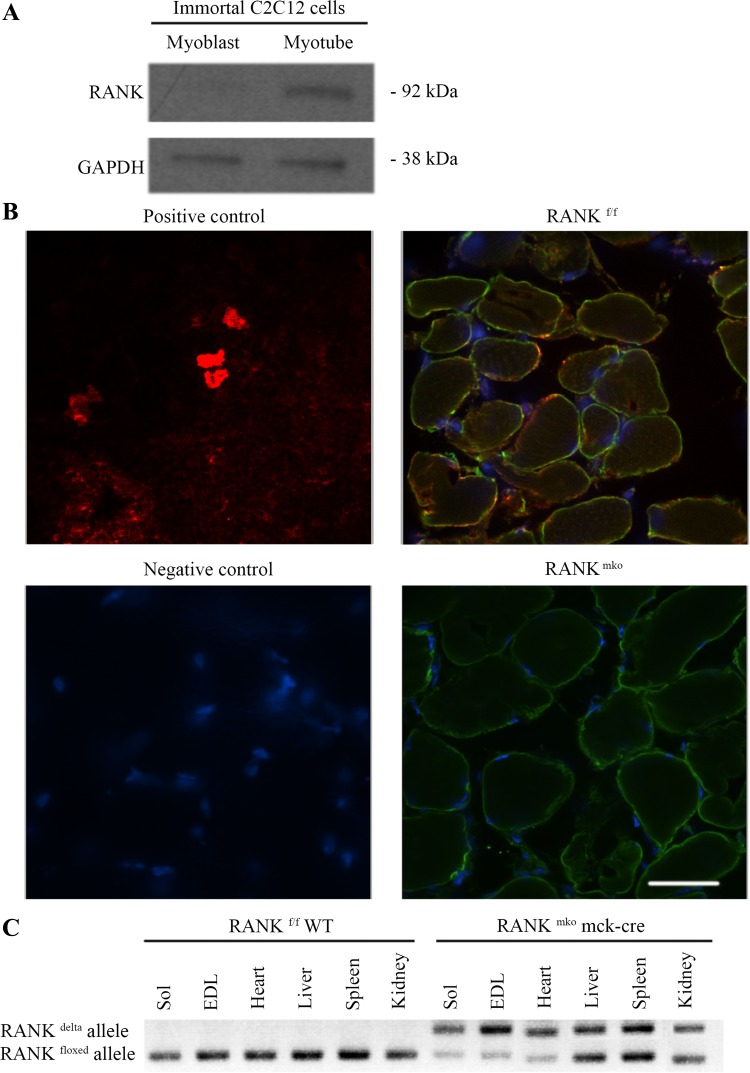
To assess RANK expression in muscle cells, we first analyzed its expression in C2C12 myoblasts and differentiated myotubes. RANK was found to be expressed in C2C12 myotubes but not in proliferating C2C12 myoblasts (Fig. 1A). We next analyzed RANK expression in skeletal muscle cells in situ using confocal microscopy. Confocal immunofluorescence confirmed the presence of RANK protein on the membrane of fast-twitch (EDL) (Fig. 1B) and slow-twitch (Sol; data not shown) skeletal muscle fibers. To confirm specific RANK expression in muscle, we crossed RANKf/f mice onto a muscle creatine kinase-Cre background to generate muscle-specific RANK knockout mice (RANKmko mice). PCR results validated the deletion of the RANK allele specifically in skeletal muscle tissue and partially in heart (Fig. 1C). Deletion efficiency was confirmed at the protein level in skeletal muscles where RANK immunostaining was not detectable (Fig. 1B).
Fig. 1.
Receptor-activator of nuclear factor-κB (RANK) expression in skeletal muscles and myotubes. A: Western blot showing that fully differentiated C2C12 myotubes but not myoblasts express RANK protein. GAPDH is shown as a loading control. B: confocal images showing colocalization of the intracellular face of the cytoplasmic membrane/sarcolemma dystrophin (green) and RANK (red) in RANKf/f and the absence of RANK in RANKmko extensor digitorum longus (EDL) muscles. Thymus sections were used as positive controls for RANK immunofluorescence. Omission of primary antibody was used as a negative control. Because nonmuscle cells in skeletal muscles can also express RANK, confocal images, rather than Western blots, were required to confirm the absence of muscle RANK in RANKmko mice. Bar = 100 μm. C: PCR analysis of genomic DNA isolated from soleus (Sol) muscle, EDL muscle, heart, liver, spleen, and kidney showing efficient Cre-mediated recombination of loxP sites in skeletal muscles from RANKmko mice.
RANK deletion affects denervated muscle mass and function.
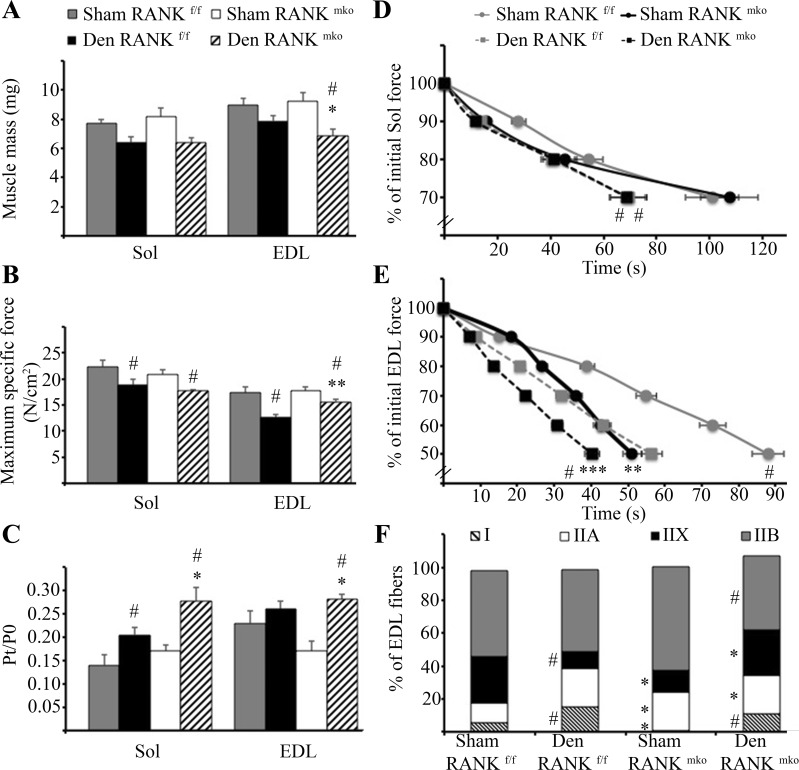
To examine the role of RANK/RANKL in muscle pathophysiology, muscle mass, contractile properties, and fiber type proportions were examined in sham and denervated Sol and EDL muscles from RANKf/f and RANKmko mice. Denervation and/or RANK deletion did not influence mouse body weight. Treatment, but not genotype, reduced EDL and Sol muscle masses when normalized to body weights (Table 1). Intriguingly, denervated EDL muscles from RANKmko mice exhibited inotropic effects as determined by the decrease in muscle mass (Fig. 2A), combined with the partial preservation of maximum specific force (N/cm2) compared with denervated RANKf/f muscles (Fig. 2B). The absolute force production (P0, g) was not different between RANKf/f and RANKmko EDL muscle, but RANK deletion prevented the loss of twitch tension (Pt) in denervated EDL muscles (Table 2). The contractile properties of control mice were similar between both genotypes (Table 2). Last, the Pt-to-P0 ratio was increased in both Sol and EDL muscles of denervated RANKmko mice (Fig. 2C), suggesting a change in myofilament Ca2+ sensitivity and/or SR Ca2+ release.
Table 1.
Body weights and muscle mass normalized to body weight of mice
| Mice |
||||
|---|---|---|---|---|
| Shamf/f | Denf/f | Shammko | Denmko | |
| Body weight, g | 24.7 ± 0.8 | 24.3 ± 0.7 | 23.8 ± 1.4 | 24.5 ± 1.9 |
| EDL muscle weight/body weight | 0.34 ± 0.05 | 0.28 ± 0.02# | 0.38 ± 0.04 | 0.25 ± 0.04# |
| Sol muscle weight/body weight | 0.29 ± 0.04 | 0.24 ± 0.03# | 0.26 ± 0.03 | 0.23 ± 0.04# |
Data are presented as means ± SE; n = 7 mice/group. Denervation (Den) and/or muscle receptor-activator of nuclear factor-κB (RANK) deletion did not influence body weight. Treatment, but not genotype, reduced extensor digitorum longus (EDL) and soleus (Sol) muscle mass normalized to body weight. The level of significance was set at #P < 0.05 for treatment (sham vs. Den).
Fig. 2.
Impact of RANK deletion on muscle contractility, fatigue, and phenotype. A: muscle atrophy was significantly more pronounced in denervated RANKmko relative to RANKf/f EDL muscles. B: however, ex vivo force measurements show that RANK ablation preserves the specific force tension of denervated (Den) EDL muscles but not in slow-twitch Sol muscles. C: RANK deletion increases the ratio of twitch tension (Pt) to absolute force production (P0) in Sol and EDL muscles following denervation. D: muscle glycolytic fatigue protocol was induced by a train of stimulations (200 ms on-800 ms off, 50 Hz) until Sol muscle force reaches 70% of its initial force. Fatigue time is decreased in denervated muscle but similar between RANKf/f and RANKmko muscles. E: muscle glycolytic fatigue protocol was induced by a train of stimulations (200 ms on-800 ms off, 50 Hz) until EDL muscle force reached 50% of its initial force. Sham and denervated RANKmko EDL muscles exhibit increased fatigability compared with sham and denervated RANKf/f EDL muscles. F: fiber typing of EDL muscle analysis showed that the slow-twitch fibers were nearly absent in sham RANKmko EDL muscles, whereas the proportion of fast-twitch fibers (IIA + IIB + IIX) was significantly increased in denervated RANKmko EDL muscles compared with denervated RANKf/f EDL muscles. The levels of significance were set at *P < 0.05, **P < 0.01, and ***P < 0.001 for genotype (RANKf/f vs. RANKmko) or #P < 0.05 for treatment (Sham vs. Den). Data are presented as means ± SE, n = 3–7 experiments.
Table 2.
Contractile properties of Sol and EDL muscles
| Sol |
EDL |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctrf/f | Shamf/f | Denf/f | Ctrmko | Shammko | Denmko | Ctrf/f | Shamf/f | Denf/f | Ctrmko | Shammko | Denmko | |
| P0, g | 26.6 ± 1.2 | 26.1 ± 0.6 | 19.6 ± 0.4# | 26.9 ± 1.5 | 25.6 ± 1.4 | 18.1 ± 1.8 | 33.5 ± 1.7 | 33.1 ± 1.6 | 21.3 ± 1.4 | 32.7 ± 1.8 | 33.5 ± 1.3 | 22.1 ± 1.4# |
| Pt, g | 3.8 ± 0.6 | 4.3 ± 0.2 | 4.2 ± 0.3 | 4.8 ± 0.5 | 4.3 ± 0.4 | 4.9 ± 0.4 | 7.1 ± 0.6 | 7.5 ± 0.8 | 5.6 ± 0.5 | 5.3 ± 0.6 | 5.8 ± 0.8 | 6.2 ± 0.4 |
Data are presented as means ± SE; n = 7 mice/group. Conrol (Ctr), sham, and denervated RANKf/f and RANKmko SOL and EDL muscles were incubated ex vivo and electrically stimulated to record maximal absolute force (P0) and twitch tension (Pt). P0 decreased independently of genotype following denervation. However, muscle RANK deletion prevented the loss of twitch force in denervated EDL muscles. No differences in P0 and Pt were observed in muscle RANK deletion of control mice. The level of significance was set at #P < 0.05 for treatment (sham vs. Den).
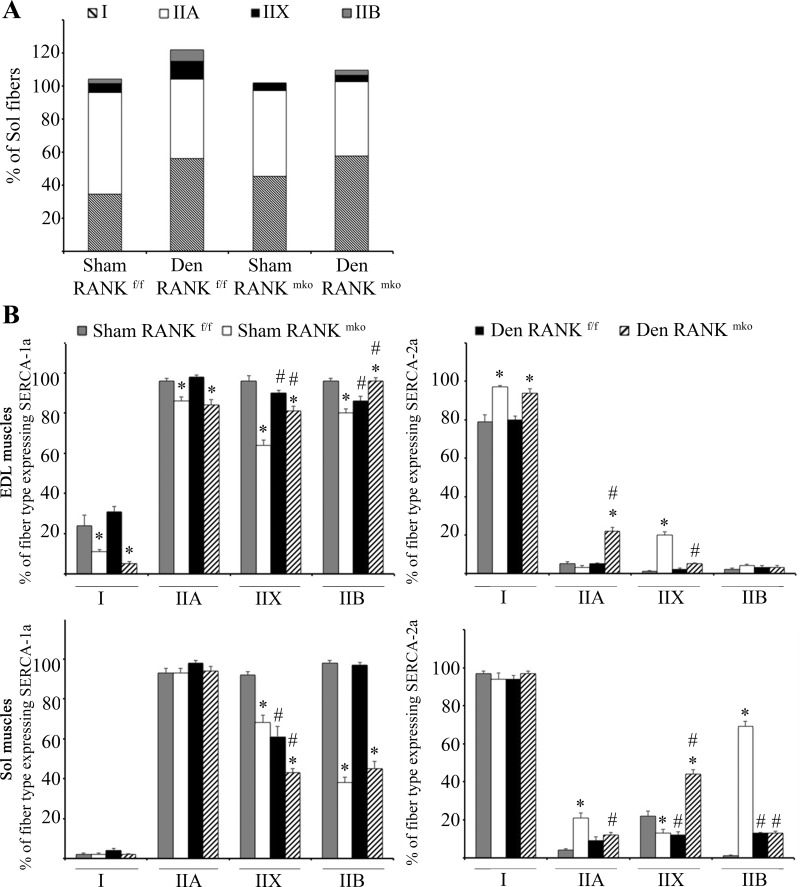
Denervation led to decreased fatigue resistance in both Sol and EDL muscles during a repetitive and glycolytic fatigue protocol (Fig. 2, D and E). Both sham and denervated RANKmko EDL muscles exhibited increased fatigability compared with sham and denervated RANKf/f muscles (Fig. 2, D and E). In accordance with the higher fatigability, immunohistochemical fiber typing showed that the slow-twitch fibers were nearly absent in sham RANKmko EDL muscles, whereas the proportion of fast-twitch fibers (IIA + IIX + IIB) was significantly increased in denervated RANKmko EDL muscles compared with denervated RANKf/f EDL muscles (Fig. 2F). The proportion of fiber type over 100% in denervated RANKmko EDL muscles indicated the presence of hybrid fibers expressing multiple isoforms of MyHC. No changes in muscle fiber type were observed in Sol muscles (Fig. 3A), indicating that the impact of muscle RANK deletion on muscle fatigue and phenotype is limited to fast-twitch EDL muscles. Noticeably, the proportion of fast-twitch fibers expressing SERCA-1a was reduced and the proportion of fast-twitch fibers expressing SERCA-2a was increased in RANKmko Sol and EDL muscles (Fig. 3B).
Fig. 3.
Percentage of type I and II fibers in Sol muscles and proportion of each fiber type expressing sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)-1a and SERCA-2a in Sol and EDL muscles. A: as opposed to fast-twitch EDL muscles, no changes in phenotype were observed in sham or denervated RANKf/f and RANKmko in Sol muscles. B: sham and denervated RANKmko muscles exhibited a lower proportion of fast-twitch fibers expressing SERCA-1a and a higher proportion of fast-twitch fibers expressing SERCA-2a. The levels of significance were set at *P < 0.05 for genotype (RANKf/f vs. RANKmko) or #P < 0.05 for treatment (Sham vs. Den). Data are presented as means ± SEM, n = 6–8.
[CaT] and SERCA activity and expression in RANKf/f and RANKmko muscles.
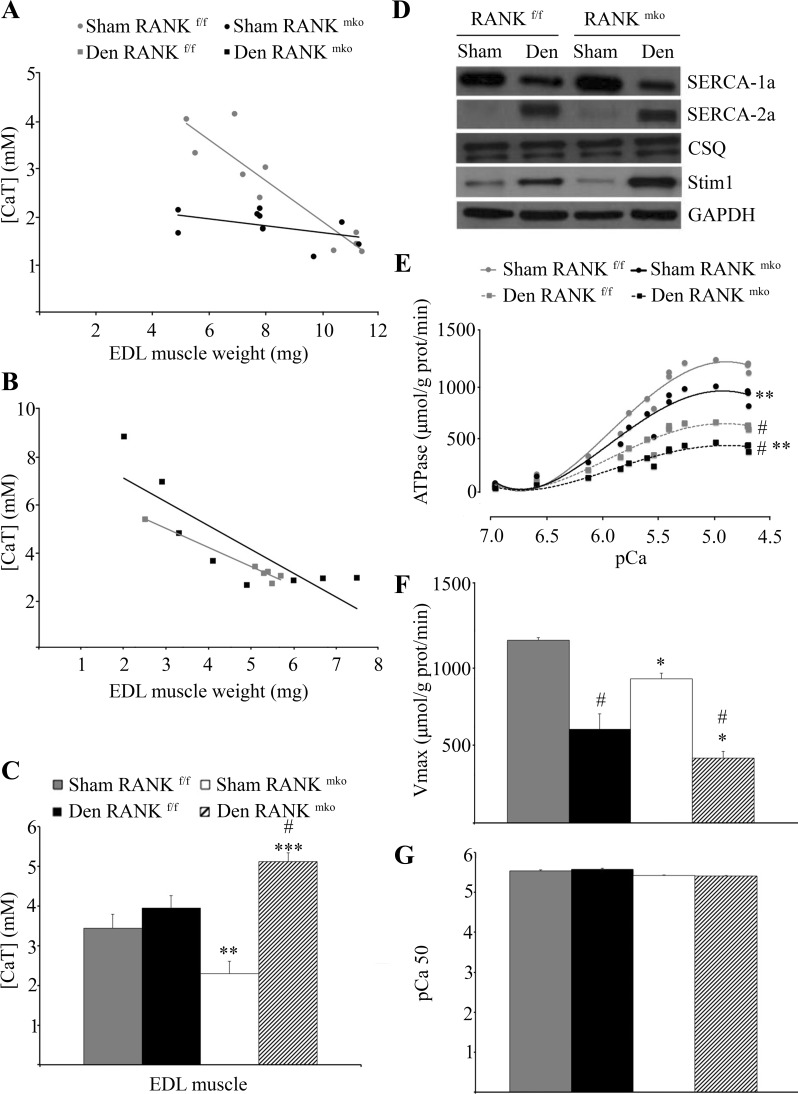
Our recent published data show an inverse relationship between [CaT] and muscle mass where the highest values of [CaT] are seen with the lowest muscle weights (30). Because RANK deletion promotes muscle atrophy and prevents force deficits in denervated muscles, we next measured [CaT] in EDL muscles (Fig. 4, A–C). Despite similar muscle masses, the average values of [CaT] were 42% lower in sham RANKmko relative to sham RANKf/f EDL muscles (Fig. 4C), indicating that, under nonpathological conditions, muscle RANK is important in Ca2+ storage of skeletal muscles. Consistent with the observed inverted relationship between [CaT] and muscle weights, we found that muscle weights below <4.5 mg had much greater [CaT] than the bigger muscles, but data were nonlinear because of a floor effect in the assay (Fig. 4B). Consistent with the reduced muscle mass in denervated RANKmko EDL muscles (Fig. 2A), [CaT] increased by 93% in denervated RANKmko EDL muscles, whereas it did not significantly increase in denervated RANKf/f EDL muscles (Fig. 4C).
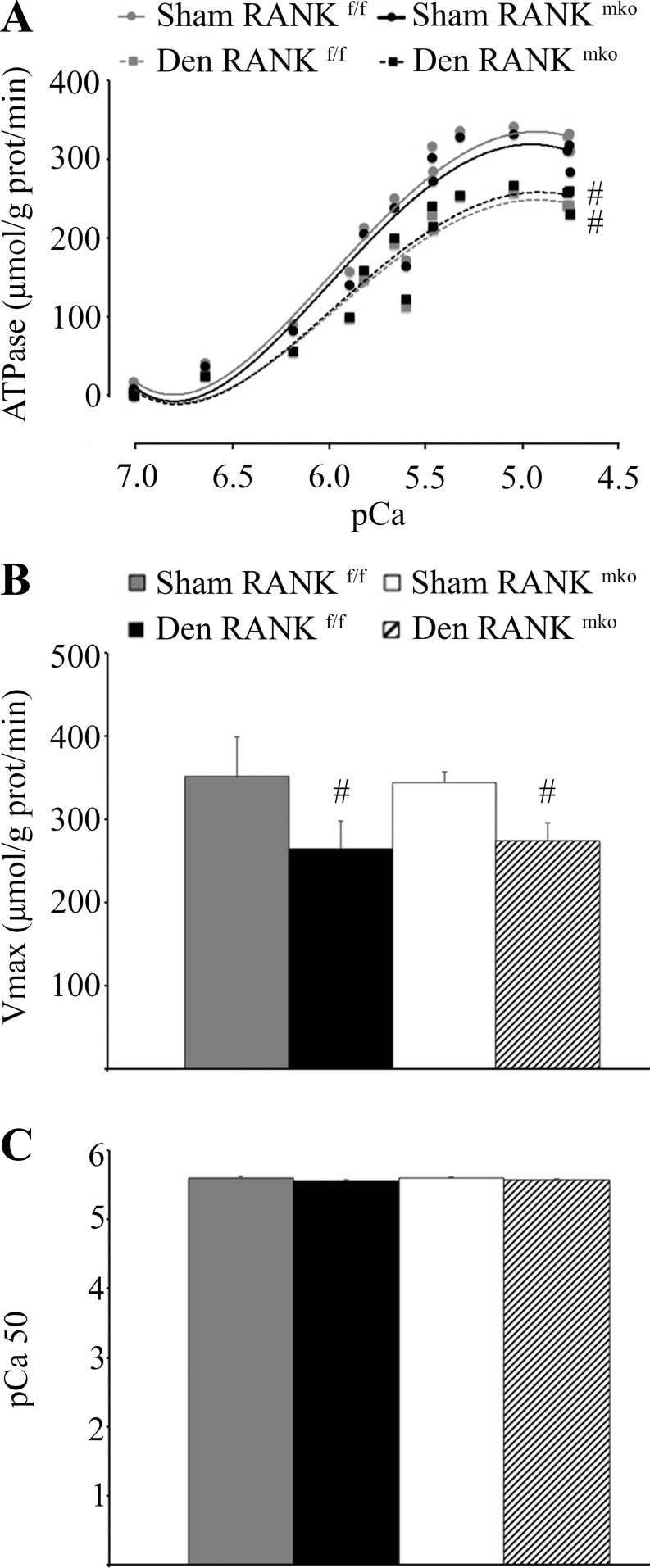
Fig. 4.
Total Ca2+ ([CaT]) content, SERCA activity, and Ca2+ protein contents in RANKf/f and RANKmko EDL muscles. A–C: [CaT] was decreased in sham RANKmko EDL muscles relative to sham RANKf/f EDL muscles but increased sharply in denervated RANKmko EDL muscles. D: Western blots show that SERCA-1a content is reduced, whereas SERCA-2a content is increased, following denervation. No changes in calsequestrin content were observed, but stromal interaction molecule 1 (Stim1) content increased markedly in denervated RANKmko EDL muscles. E: SERCA Ca2+-dependent ATPase activity was assessed in muscle homogenates from sham and denervated RANKf/f and RANKmko EDL muscles over Ca2+ concentrations ranging from pCa 7.4-5.0. F and G: maximal ATPase activity (Vmax, F) and pCa value required to elicit 50% of maximal activity (pCa50, G). The levels of significance were set at *P < 0.05, **P < 0.01, and ***P < 0.001 for genotype (RANKf/f vs. RANKmko) or #P < 0.05 for treatment (Sham vs. Den). Data are presented as means ± SE, n = 3–7.
Because SERCA is critical for Ca2+ cycling in the SR, we analyzed SERCA-1a and SERCA-2a expression and SERCA activity in Sol and EDL muscle extracts. Western blotting analyses show that SERCA-1a content is significantly reduced in denervated muscles, whereas SERCA-2a content is significantly increased following denervation, independent of the genotype (Fig. 4D). No difference in calsequestrin content, a Ca2+-binding protein located in SR, was observed between RANKf/f and RANKmko muscles (Fig. 4D). However, the content of the Stim1, which functions as a Ca2+ sensor in the SR, increased by 77 and 411% in denervated RANKf/f and RANKmko EDL muscles, relative to their respective sham (Fig. 4D). The concentration of Stim1 increased significantly by 184% in denervated RANKmko compared with denervated RANKmko EDL muscles. Next, we assessed SERCA Ca2+-dependent ATPase activity in muscle homogenates from sham and denervated RANKf/f and RANKmko Sol and EDL muscles over Ca2+ concentrations ranging from pCa 7.0 to 4.5. Interestingly, upon denervation, maximal SERCA activity was reduced in both the Sol and EDL muscles (Figs. 4, E-G, and 5). Moreover, maximal SERCA activity was significantly reduced in both the sham and denervated RANKmko EDL compared with the sham and denervated RANKf/f EDL (Fig. 4, C–F). This inhibitory effect of RANK deletion on SERCA activity was limited to EDL muscles and did not affect Sol muscles. Overall, these findings demonstrate a direct role for muscle RANK in the regulation of SERCA activity and Stim1 content in fast-twitch EDL muscles.
Fig. 5.
SERCA Ca2+-dependent ATPase parameters from sham and denervated RANKf/f and RANKmko in Sol muscles. Denervation induced a significant reduction of SERCA Ca2+-dependent ATPase activity (A), Vmax (B), and pCa50 (C) that was similar between control and experimental Sol muscles. The level of significance treatment (Sham/Den) was set at #P < 0.05. Data are presented as means ± SE, n = 3–7.
DISCUSSION
We previously demonstrated that systemic injection of OPG, the decoy receptor of RANKL, restores muscle force and improves muscle histology in dystrophic mdx mice (9). However, whether the RANK/RANKL/OPG system directly or indirectly affects skeletal muscle was unknown. In this paper, using muscle-specific RANK mutant mice, we demonstrate that RANK is expressed in skeletal muscle cells where it directly regulates muscle function. Importantly, muscle-specific RANK deletion protects from denervation-induced loss of muscle force and modulates [CaT] storage and SERCA activity.
RANK is well characterized in osteoclasts where it leads to the activation of different signaling pathways, especially NF-κB and Ca2+-dependent pathways (5). In skeletal muscle, Ca2+ is a master regulator of multiple intracellular processes, such as myosin-actin crossbridging, protein synthesis and degradation, mitochondrial adaptation, and fiber-type shifting, through the control of Ca2+-sensitive proteases and transcriptional factors such as nuclear factor of activated T cells sytoplasmic 1 (NFATc1) (2). Consistent with a role of RANK in the regulation of Ca2+ handling, conditional deletion of RANK in skeletal muscle affected muscle contraction postdenervation, atrophy, and fiber type, all of which are regulated by Ca2+ (2). Intriguingly, muscle-specific RANK deletion prevents the loss of specific force production but not the loss of muscle mass following denervation. Without significant changes in muscle mass, muscle strength can be altered by 1) the amplitude or duration of the Ca2+ transient or 2) the sensitivity of the myofilaments to Ca2+. For instance, reduced force production in aged muscle is partially caused by reduced Ca2+ content and myofilament sensitivity (31). Our findings show that RANKmko denervated muscles have higher [CaT], which originates from increased intracellular Ca2+ stocks (30, 33). Therefore, higher Ca2+ content potentially results in greater rates of Ca2+ release, increased myoplasmic Ca2+ transients, and, ultimately, preserved muscle force production. These results are supported by the increased specific force and Pt-to-P0 ratio in denervated RANKmko EDL muscle, indicating that these muscles are able to generate a stronger contraction following submaximal stimulation.
The higher releasable Ca2+ content is not explained by greater SERCA activity since we found that denervated RANKmko muscles had low SERCA activity rate compared with the denervated RANKf/f. Moreover, we observed no change in calsequestin (CSQ) content, making it unlikely to explain the greater releasable Ca2+ content; however, in the normal EDL muscles, it was shown that the resting SR Ca2+ content is only a relatively small proportion of its maximal content and that Ca2+ bound to CSQ is only one-third of its saturated level (14, 35, 52). Thus, it is likely that the increase in [CaT] observed in the denervated RANKmko EDL reflects an increased binding of Ca2+ in the SR, thereby increasing releasable SR Ca2+ and ultimately specific force production. In this respect, the relatively greater inhibition of SERCA activity may serve to prolong the Ca2+ transient and increase force production, while decreasing the fatigue resistance. Accordingly, we observed higher muscle strength following a single contraction (Pt) in denervated RANKmko EDL muscle combined with increased fatigability following repeated maximal contractions. The exact mechanisms lowering SERCA activity in response to RANK deletion are unknown and require further investigation, and analysis of sarcolipin and phospholamban, two well-known regulators of the SERCA pump (15), indicate increased expression of both proteins in response to denervation; however, no significant differences were found between genotype (data not shown).
Enhanced Ca2+ entry could be responsible for any increase in SR Ca2+ content in muscle. In osteoclasts, RANKL-mediated Ca2+ entry could arise from intracellular or extracellular origin (6, 25). Members of the transient receptor potential vanilloid channels (TRPV) family, TRPV2 and TRPV5, were demonstrated to mediate RANKL-induced Ca2+ entry (6, 23). Store-operated Ca2+ entry (SOCE) channels, which sense declining Ca2+ concentration in the SR, were also associated with RANKL-induced Ca2+ entry (23). For instance, silencing of the SOCE channel Orai1 with specific short-hairpin RNA inhibits RANKL-induced osteoclastogenesis by suppressing NFATc1 induction (20). Mice lacking SOCE specifically in skeletal muscle exhibit reduced muscle mass and increased susceptibility to fatigue, whereas Stim1−/− myotubes failed to refill their stores and altered expression of key SR proteins (48). Furthermore, the use of SERCA inhibitor suggests that under some conditions the role of SERCA in replenishing Ca2+ stores is limited (37). Our current findings that denervation treatment and RANK deletion markedly increased Stim1 content (184%) may explain, in part, the discrepancy between the rise in [CaT] and the depression of SERCA activity in denervated RANKmko EDL muscles. It is thus tempting to speculate that SOCE would compensate for the lack of SERCA activity in denervated RANKmko EDL muscles. The mechanisms by which RANKL/RANK controls Ca2+ storage in skeletal muscle cells requires further experiments; nevertheless, our findings provide the first evidence that RANK is a novel regulator of Ca2+ storage in skeletal muscle cells.
With respect to EDL muscle fatigability, we found that both denervation and RANK deletion led to higher fatigability than their wild-type and sham-operated counterparts. Consistent with this observation, we found that RANKmko muscles increased the number of fast-twitch type II fibers; however, the disproportionate increase of fatigability in RANKmko muscle compared with the relatively modest changes in fiber type and that no changes on muscle contractility, fatigue, and phenotype were observed in Sol muscles suggest that the muscle fiber type phenotype may not be the main reason to explain the fatigue in RANKmko muscle. Indeed, the decreased fatigue resistance observed following denervation was associated with an increased proportion of type I fibers in both RANKf/f and RANKmko Sol and EDL muscles. It could be speculated that the altered Ca2+ signaling following muscle-specific RANK deletion impairs mitochondrial function given the well-established role of the Ca2+-dependent effectors calcineurin and NFATc1 in enhancing muscle endurance and mitochondrial respiratory capacity (21). Nevertheless, our findings indicate that RANK deletion enhances force production and promotes a fast-twitch phenotype and fatigability while increasing Stim1 content and decreasing SERCA activity.
Perspective and Conclusion
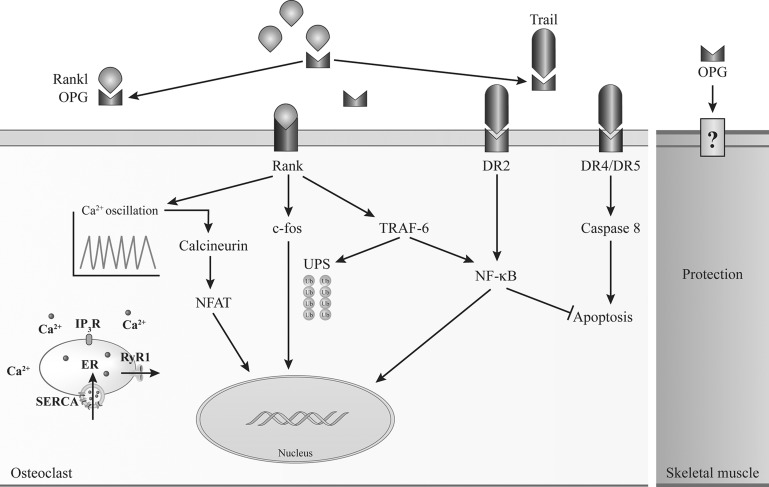
Osteoporosis and muscle wasting occur simultaneously in a variety of pathologies, although common signaling pathways between these two processes were not identified (Fig. 6). Here, we show that, in addition to its role in bone homeostasis, RANK signaling also regulates Ca2+ storage, muscle mass, and muscle performance. Muscle-specific deletion of RANK has an inotropic effect on denervated fast-twitch EDL muscles, largely composed of type IIA, IIX, and IIB fibers. Fast-twitch fibers are usually the first to be affected in several forms of muscular and neuromuscular diseases and aging conditions, leading to premature loss of function and important incapacities (8). Our findings show for the first time that fast-twitch fibers may be specifically targeted to enhance their force production. Although these results are preliminary and the long-term effects remain to be determined, this discovery opens a whole new field of research and new therapeutic avenues for conditions affecting in synchrony bone and skeletal muscle and potentially heart diseases.
Fig. 6.
Schematic representation of the RANK/RANKL/OPG pathway as a common regulator of bone and muscle cells. In osteoclast, RANK/RANKL interaction regulates osteoclastogenesis and/or cell apoptosis through modulation of SERCA activity, Ca2+ oscillation, and Ca2+-calcineurin-nuclear factor of activated T cells (NFAT) and NF-κB pathways. Muscle cells also express RANK, and RANK/RANKL interaction is an important regulator of SERCA activity and Ca2+ storage in fast-twitch EDL muscles. These results highlight the importance of a common signaling pathway opening potentially new treatment for both skeletal muscle and bone.
GRANTS
J. Frenette is supported by CIHR and NSERC and J. M. Penninger by an advanced ERC grant and the Austrian Academy of Sciences.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.D., N.A.D., J.M.P., P.C.P., and J.F. conception and design of research; S.S.D., N.A.D., A.B.-P., V.A.F., D.G., S.A.K.-G., R.O.D., P.B., and x.L. performed experiments; S.S.D., N.A.D., A.B.-P., V.A.F., D.G., S.A.K.-G., R.O.D., P.B., x.L., P.C.P., A.R.T., and J.F. analyzed data; S.S.D., N.A.D., V.A.F., D.G., S.A.K.-G., R.O.D., P.B., x.L., J.M.P., P.C.P., A.R.T., and J.F. interpreted results of experiments; S.S.D. and N.A.D. prepared figures; S.S.D., N.A.D., and J.F. drafted manuscript; S.S.D., N.A.D., A.B.-P., V.A.F., D.G., J.M.P., P.C.P., A.R.T., and J.F. edited and revised manuscript; S.S.D., N.A.D., A.B.-P., V.A.F., D.G., S.A.K.-G., R.O.D., P.B., x.L., J.M.P., P.C.P., A.R.T., and J.F. approved final version of manuscript.
REFERENCES
- 1.Allen DG, Gervasio OL, Yeung EW, Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88: 83–91, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Berchtold MW, Brinkmeier H, Müntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80: 1215–1265, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bloomfield SA. Changes in musculoskeletal structure and function with prolonged bed rest. Med Sci Sports Exerc 29: 197–206, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Boss GR, Seegmiller JE. Age-related physiological changes and their clinical significance. West J Med 135: 434–440, 1981. [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 423: 337–342, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Chamoux E, Bisson M, Payet MD, Roux S. TRPV-5 mediates a receptor activator of NF-kappaB (RANK) ligand-induced increase in cytosolic Ca2+ in human osteoclasts and down-regulates bone resorption. J Biol Chem 285: 25354–25362, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin ER. Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol 99: 414–423, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45: 2191–2199, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Dufresne SS, Dumont NA, Bouchard P, Lavergne É, Penninger JM, Frenette J. Osteoprotegerin protects against muscular dystrophy. Am J Pathol 185: 920–926, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Dufresne SS, Frenette J. Investigation of wild-type and mycolactone-negative mutant Mycobacterium ulcerans on skeletal muscle: IGF-I protects against mycolactone-induced muscle catabolism. Am J Physiol Regul Integr Comp Physiol 304: R753–R762, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Dumont N, Frenette J. Macrophages protect against muscle atrophy and promote muscle recovery in vivo and in vitro: a mechanism partly dependent on the insulin-like growth factor-1 signaling molecule. Am J Pathol 176: 2228–2235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francini F, Formigli L, Meacci E, Vassalli M, Nosi D, Quercioli F, Tiribilli B, Bencini C, Squecco R, Bruni P, Orlandini SZ. Ca+2 homeostasis and cytoskeletal rearrangement operated by sphingosine 1-phosphate in C2C12 myoblastic cells. J Gravitational Physiol J Int Soc Gravitational Physiol 9: P281–P282, 2002. [PubMed] [Google Scholar]
- 13.Frenette J, St-Pierre M, Côté CH, Mylona E, Pizza FX. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am J Physiol Regul Integr Comp Physiol 282: R351–R357, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol 493: 357–370, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamu D, Bombardier E, Smith IC, Fajardo VA, Tupling AR. Sarcolipin provides a novel muscle-based mechanism for adaptive thermogenesis. Exerc Sport Sci Rev 42: 136–142, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Goldspink DF. The effects of denervation on protein turnover of the soleus and extensor digitorum longus muscles of adult mice. Comp Biochem Physiol B 61: 37–41, 1978. [DOI] [PubMed] [Google Scholar]
- 17.Hanada R, Hanada T, Sigl V, Schramek D, Penninger JM. RANKL/RANK-beyond bones. J Mol Med Berl Ger 89: 647–656, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Hanada R, Leibbrandt A, Hanada T, Kitaoka S, Furuyashiki T, Fujihara H, Trichereau J, Paolino M, Qadri F, Plehm R, Klaere S, Komnenovic V, Mimata H, Yoshimatsu H, Takahashi N, von Haeseler A, Bader M, Kilic SS, Ueta Y, Pifl C, Narumiya S, Penninger JM. Central control of fever and female body temperature by RANKL/RANK. Nature 462: 505–509, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Hindi SM, Sato S, Choi Y, Kumar A. Distinct roles of TRAF6 at early and late stages of muscle pathology in the mdx model of Duchenne muscular dystrophy. Hum Mol Genet 23: 1492–1505, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang SY, Putney JW. Orai1-mediated calcium entry plays a critical role in osteoclast differentiation and function by regulating activation of the transcription factor NFATc1. FASEB J 26: 1484–1492, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang LQ, Garcia-Roves PM, de Castro Barbosa T, Zierath JR. Constitutively active calcineurin in skeletal muscle increases endurance performance and mitochondrial respiratory capacity. Am J Physiol Endocrinol Metab 298: E8–E16, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Jost PD. Simulating human space physiology with bed rest. Hippokratia 12, Suppl 1: 37–40, 2008. [PMC free article] [PubMed] [Google Scholar]
- 23.Kajiya H, Okamoto F, Nemoto T, Kimachi K, Toh-Goto K, Nakayana S, Okabe K. RANKL-induced TRPV2 expression regulates osteoclastogenesis via calcium oscillations. Cell Calcium 48: 260–269, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium 38: 439–446, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Komarova SV, Pilkington MF, Weidema AF, Dixon SJ, Sims SM. RANK ligand-induced elevation of cytosolic Ca2+ accelerates nuclear translocation of nuclear factor kappa B in osteoclasts. J Biol Chem 278: 8286–8293, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–309, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30: 3071–3085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Bhatnagar S, Paul PK. TWEAK and TRAF6 regulate skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 15: 233–239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacey D, Timms E, Tan HL, Kelley M, Dunstan C, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle W. Osteoprotegerin Ligand Is a Cytokine that Regulates Osteoclast Differentiation and Activation. Cell 93: 165–176, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Lamboley CRH, Kake Guena SA, Touré F, Hébert C, Yaddaden L, Nadeau S, Bouchard P, Wei-LaPierre L, Lainé J, Rousseau EC, Frenette J, Protasi F, Dirksen RT, Pape PC. New method for determining total calcium content in tissue applied to skeletal muscle with and without calsequestrin. J Gen Physiol 145: 127–153, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamboley CR, Wyckelsma VL, Dutka TL, McKenna MJ, Murphy RM, Lamb GD. Contractile properties and sarcoplasmic reticulum calcium content in type I and type II skeletal muscle fibres in active aged humans. J Physiol 593: 2499–2514, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leibbrandt A, Penninger JM. RANK/RANKL: regulators of immune responses and bone physiology. Ann NY Acad Sci 1143: 123–150, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Manno C, Ríos E. A better method to measure total calcium in biological samples yields immediate payoffs. J Gen Physiol 145: 167–171, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta 1241: 59–116, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol 587: 443–460, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy V, Penninger JM. The RANKL-RANK story. Gerontology 61: 534–542, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Okubo Y, Suzuki J, Kanemaru K, Nakamura N, Shibata T, Iino M. Visualization of Ca2+ Filling Mechanisms upon Synaptic Inputs in the Endoplasmic Reticulum of Cerebellar Purkinje Cells. J Neurosci 35: 15837–15846, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page G, Miossec P. RANK and RANKL expression as markers of dendritic cell-T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum 52: 2307–2312, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Pang MYC, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 16: 1769–1779, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson MF. Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am J Physiol Cell Physiol 291: C518–C528, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Perlot T, Penninger JM. Development and function of murine B cells lacking RANK. J Immunol 188: 1201–1205, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Sandri M. Apoptotic signaling in skeletal muscle fibers during atrophy. Curr Opin Clin Nutr Metab Care 5: 249–253, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10: 197–205, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol 248: C265–C270, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Sigl V, Penninger JM. RANKL/RANK:- from bone physiology to breast cancer. Cytokine Growth Factor Rev 25: 205–214, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Simonet W, Lacey D, Dunstan C, Kelley M, Chang MS, Lüthy R, Nguyen H, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes T, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle W. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PloS One 8: e68924, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, Williams RS, Eu JP, Rosenberg P. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol 10: 688–697, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun H, Gong Y, Qiu J, Chen Y, Ding F, Zhao Q. TRAF6 inhibition rescues dexamethasone-induced muscle atrophy. Int J Mol Sci 15: 11126–11141, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun H, Qiu J, Chen Y, Yu M, Ding F, Gu X. Proteomic and bioinformatic analysis of differentially expressed proteins in denervated skeletal muscle. Int J Mol Med 33: 1586–1596, 2014. [DOI] [PubMed] [Google Scholar]
- 51.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 20: 795–823, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Trinh HH, Lamb GD. Matching of sarcoplasmic reticulum and contractile properties in rat fast- and slow-twitch muscle fibres. Clin Exp Pharmacol Physiol 33: 591–600, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol 5: 511, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang YM, Kim MS, Son A, Hong JH, Kim KH, Seo JT, Lee SI, Shin DM. Alteration of RANKL-induced osteoclastogenesis in primary cultured osteoclasts From SERCA2+/− Mice. J Bone Miner Res 24: 1763–1769, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95: 3597–3602, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]