Abstract
Activation of small GTPase Rac1 in podocytes is associated with rodent models of kidney injury and familial nephrotic syndrome. Induced Rac1 activation in podocytes in transgenic mice results in rapid transient proteinuria and foot process effacement, but not glomerular sclerosis. Thus it remains an open question whether abnormal activation of Rac1 in podocytes is sufficient to cause permanent podocyte damage. Using a number of transgenic zebrafish models, we showed that moderate elevation of Rac1 activity in podocytes did not impair the glomerular filtration barrier but aggravated metronidazole-induced podocyte injury, while inhibition of Rac1 activity ameliorated metronidazole-induced podocyte injury. Furthermore, a further increase in Rac1 activity in podocytes was sufficient to cause proteinuria and foot process effacement, which resulted in edema and lethality in juvenile zebrafish. We also found that activation of Rac1 in podocytes significantly downregulated the expression of nephrin and podocin, suggesting an adverse effect of Rac1 on slit diaphragm protein expression. Taken together, our data have demonstrated a causal link between excessive Rac1 activity and podocyte injury in a dosage-dependent manner, and transgenic zebrafish of variable Rac1 activities in podocytes may serve as useful animal models for the study of Rac1-related podocytopathy.
Keywords: Rac1, podocyte injury, zebrafish, periorbital edema
podocytes are highly specialized epithelial cells that play a pivotal role in maintaining the integrity of the glomerular filtration barrier (GFB). Located on the outer surface of the glomerular capillaries, podocytes extend protrusions with numerous branches called foot processes. The foot processes of neighboring podocytes interdigitate to form slit diaphragms, which are critical for the GFB. Mutations of slit diaphragm proteins such as nephrin and podocin disrupt the GFB and cause congenital nephrotic syndrome. Podocytes typically undergo foot process effacement in response to stress and injury. This subsequently leads to the loss of slit diaphragms and podocyte detachment, which underlie a variety of human glomerular diseases (7).
The Rho family small GTPases and their regulators have been implicated in podocyte development and pathogenesis (1, 11, 14, 18). Among the three major Rho family GTPases, Cdc42 is essential for podocyte development whereas Rac1 and RhoA are more important in podocyte maintenance (23). They antagonistically and cooperatively maintain the actin cytoskeleton and cell motility (5, 13, 20). Recent studies indicated the involvement of Rac1 and RhoA in podocyte injury in chronic kidney disease (3). Activation of either constitutively active or dominant negative RhoA in podocytes induces podocyte injury, proteinuria, and glomerular sclerosis via distinct mechanisms (30). Although activation of Rho family GTPases causes rapid actin cytoskeleton rearrangement in vitro, the consequent proteinuric phenotype by RhoA activation requires a long period of 2 wk or more (30, 34), implying the complexity of the role of RhoA in podocytes in vivo. A growing collection of literature has shown that elevated Rac1 activity in glomeruli is associated with disrupted glomerular function in puromycin aminonucleoside-mediated nephrosis (2), high salt- and angiotensin II-induced hypertension (24), genetic models of nephrosis (1, 25), as well as human patients with nephrotic syndrome (11). Intriguingly, podocyte-specific overexpression of constitutively active Rac1 in mice induced a rapid onset of proteinuria with altered foot process morphology, a transient but not sustained event that failed to result in glomerular sclerosis (31). Therefore, the exact consequence and pathomechanism of sustained Rac1 activation in podocytes are still unclear. In this study, we aimed to investigate the role of Rac1 in podocyte pathology by constantly expressing constitutively active or dominant negative Rac1 in zebrafish podocytes using the Gal4-UAS system.
MATERIALS AND METHODS
Zebrafish maintenance.
Zebrafish (AB* strain) were used for making transgenic fish. All zebrafish were raised and maintained in a facility in compliance with the University Committee on Use and Care of Animals (UCUCA) standards, and all animal procedures were approved by the UCUCA at the University of Michigan. Fish eggs were collected in petri dishes and raised in incubators at a constant temperature of 28.5°C.
DNA constructs and transgenic zebrafish.
The podocyte-specific Gal4 driver lines Tg(pod:Gal4) were generated as previously reported (29). RAC1Q61L and RAC1T17N, the constitutively active form (CA-RAC1) and the dominant negative form (DN-RAC1) of the human RAC1 gene, were amplified from plasmids obtained from Addgene (12981, 12982) and cloned into pME-MCS. Enhanced green fluorescent protein (EGFP)-tagged rac1G12V (CA-rac1) and rac1T17N (DN-rac1), the constitutively active form and the dominant negative form of the zebrafish rac1, were obtained by subcloning the mutated zebrafish orthologs of RAC1 (6) into pEGFP-C1 and then into pME-MCS. The transgenic constructs Tol2-UAS:CA-RAC1/DN-RAC1/EGFP-CA-rac1/EGFP-DN-rac1 were assembled using the multisite Gateway method (17). Tol2-pod:CreERT2 was created by replacing Gal4 with CreERT2 in the Tol2-pod:Gal4 construct (29). Each transgenic construct was coinjected with the in vitro synthesized capped RNA of the Tol2 transposase into one-cell-stage embryos. Transgenic zebrafish Tg(UAS:CA/DN-RAC1,cmlc2:EGFP) were screened based on EGFP expression in the myocardium, and Tg(UAS:EGFP-CA/DN-rac1) were screened by pronephric EGFP expression in the presence of Tg(pod:Gal4). Tg(ubi:loxP-mCherry-loxP-GFP) were obtained from Dr. Daniel Goldman (22). Tg(pod:CreERT2) fish were confirmed by constant GFP expression in the pronephric glomeruli after 4-hydroxytamoxifen (4-OHT) treatment at a concentration of 2 μM for 24 h. Each transgenic line was maintained from three independent transgenic founders to maximize the heterogeneity of the genetic background and minimize the nonspecific effect of the genetic background. Zebrafish containing multiple transgenes were obtained by several rounds of intercrosses between different transgenic zebrafish lines sequentially. All of the primers are listed in Table 1.
Table 1.
List of primers for DNA constructs and real-time PCR
| Genes | Application | Forward | Reverse |
|---|---|---|---|
| RAC1 | cDNA | ATCGAATTCACCATGCAGGCCATCAAGTGTG | ATAGACGTCTTACAACAGCAGGCATTTTCTC |
| rac1 | cDNA | ATCGTCGACCAGGCCATAAAGTGTGTGGT | ACTGGATCCTCACAGAAGGAGACATCTTCTC |
| Gal4 | Real-time PCR | GCTACTCTCCCAAAACCAAAAG | TGTTCCAGTCTTTCTAGCCTTG |
| RAC1 | Real-time PCR | GGTGAATCTGGGCTTATGGG | TCAGGATACCACTTTGCACG |
| nephrin | Real-time PCR | GCCTCGATGTGCTCTTTCCT | TCGTCTGGGTTTGCAGAGAC |
| podocin | Real-time PCR | CAGTGTGAGGGAACGGATAAA | CAGCTCACAAACTCCAAGGTA |
Metronidazole treatment and phenotype characterization.
Tg(pod:Gal4;UAS:NfsB-mCherry;UAS:RAC1,cmlc2:EGFP) were sorted out at 3 days postfertilization (dpf) based on fluorescence in the pronephros (red) and the heart (green). Thirty larvae were pooled as a cohort and subjected to metronidazole (MTZ) treatment for 48 h. Periorbital edema (POE) was characterized by the ratio of pupil spacing distance vs. body length as described previously (29). All experiments were repeated independently three times, and statistical analyses were performed based on Student's t-test.
Proteinuria assay.
Transgenic fish were crossed to Tg(lfabp:vdbp-gfp) (33), and progenies were sorted out at 3 dpf based on GFP expression. Ten embryos were pooled together in 500 μl E3 embryonic medium at designated time points. The GFP concentration in the embryonic medium was measured by ELISA as reported previously (33). lrp2a-MO (16) was injected at the one-cell stage. The measurements were repeated for three or four times. Statistical analyses were performed based on Student's t-test.
Real-time PCR.
Pronephric glomeruli were isolated from transgenic larvae at 5 dpf based on GFP fluorescence in the podocytes. Total RNA was extracted and followed by first-strand cDNA synthesis (Quanta). Quantitative real-time PCR was performed using a SYBR Green Kit (Bio-Rad). The primers used are listed in Table 1.
Whole-mount in situ hybridization.
Human RAC1 was amplified and cloned into pCMV-Script, and the riboprobe was synthesized using a DIG RNA Labeling Kit (Roche) following the manufacturer's protocol. Embryos were fixed in 4% paraformaldehyde in PBS and processed for whole-mount in situ hybridization according to a standard procedure (27).
Immunostaining and confocal microscopy.
Dissected zebrafish adult kidneys were fixed in 4% paraformaldehyde at 4°C overnight. After several washes with PBS and Tween 20 (PBST), kidneys were dehydrated with methanol, rehydrated, and washed with PBST. Kidneys were permeabilized in acetone for 8 min and washed with PBST. Blocking was carried out by incubating with 2% sheep serum in PBST for 1 h at room temperature. An anti-Rac1 antibody (1:1,000, NewEast Biosciences) (19) was diluted in blocking solution and incubated at 4°C overnight. After washing with PBST, kidneys were incubated at 4°C overnight with Alexa 594-conjugated secondary antibody (1:500, Invitrogen). Kidneys were washed with PBST and mounted in mounting medium for confocal imaging. Anti-cleaved caspase 3 (1:1,000, BD Pharmingen) staining was performed following a previously published procedure (33). Images were taken under a Leica SP5X confocal microscopy (Leica).
Histology.
Embryos were fixed in 4% paraformaldehyde in PBS and embedded in JB4 resin (Electron Microscope Science) according to the manufacturer's protocol (26). Thin sections were obtained using a Leica RM2265 microtome, followed by methylene blue staining.
Transmission electron microscopy.
Embryos at 5 dpf were fixed and processed by following the standard protocol (15). Transmission electron microscopy images were captured using a Philips CM-100 transmission electronic microscope.
RESULTS
Overexpression of constitutively active Rac1 exacerbates podocyte injury while dominant negative Rac1 protects podocytes against injury.
We first verified using immunofluorescence the expression of rac1 in podocytes in zebrafish renal glomeruli. Staining of Tg(pod:GFP) zebrafish adult kidneys, in which podocytes are labeled with GFP expression (32), with an anti-Rac1 antibody (19) indicated the evident endogenous rac1 expression in normal podocytes (Fig. 1). To test whether Rac1 activation could lead to podocyte injury in zebrafish, we generated transgenic zebrafish that express either constitutively active (CA-RAC1) or dominant negative (DN-RAC1) human RAC1 (11) under the control of the UAS promoter. When combined with a podocyte-specific Gal4-driver transgenic line (29), these transgenic fish expressed either CA-RAC1 or DN-RAC1 specifically in the podocytes of pronephric glomeruli (Fig. 2, A and B). However, neither the Tg(pod:Gal4;UAS:CA-RAC1,cmlc2:EGFP) nor the Tg(pod:Gal4;UAS:DN-RAC1,cmlc2:EGFP) double transgenic fish developed any obvious abnormality by 5 dpf (Fig. 1C), and both were viable and fertile in adulthood.
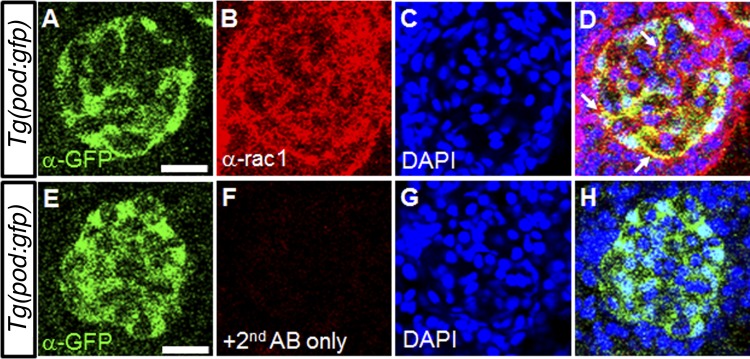
Fig. 1.
The endogenous expression of rac1 in zebrafish podocytes. A–D: coimmunostaining of green fluorescent protein (GFP; A) and rac1 (B) in a mesonephric glomerulus in Tg(pod:GFP) zebrafish. GFP expression demarcates podocytes, and 4,6-diamidino-2-phenylindole (DAPI; C) is used to label nuclei. The arrows indicate that endogenous rac1 colocalizes with GFP in podocytes. E–H: coimmunostaining of a mesonephric glomerulus of in Tg(pod:GFP). No signal is detected when the primary anti-rac1 antibody is not used. Scale bar = 20 µm.
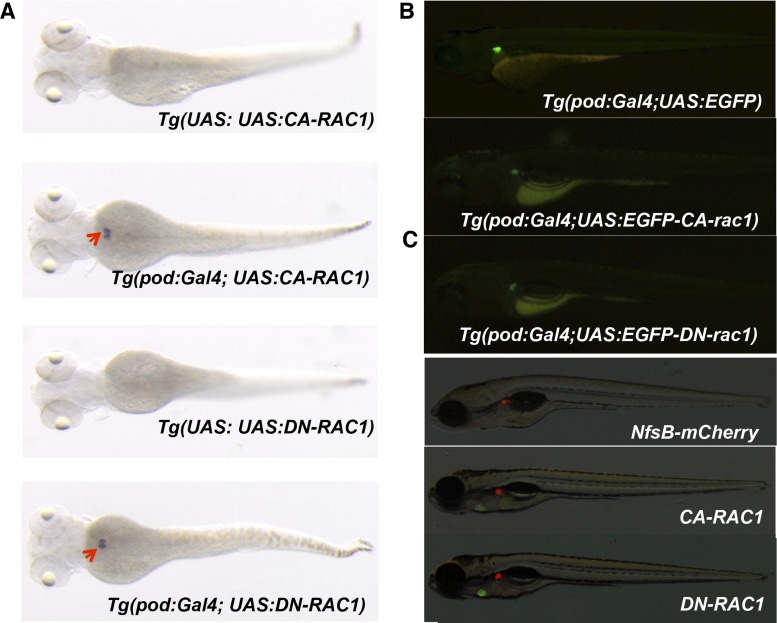
Fig. 2.
Generation of transgenic zebrafish expressing RAC1 in podocytes. A: whole-mount in situ hybridization for RAC1 in transgenic embryos at 3 days postfertilization (dpf). Red arrows show pronephric glomeruli with RAC1 overexpression. B: lateral views of double transgenic zebrafish Tg(pod:Gal4; UAS:EGFP), Tg(pod:Gal4; UAS:EGFP-rac1G12V) and Tg(pod:Gal4; UAS:EGFP-rac1T17N) at 4 dpf. Note that rac1G12V and rac1T17N are constitutively active and dominant negative forms of zebrafish rac1, respectively. C: lateral views of transgenic larvae Tg(pod:Gal4; UAS:NfsB-mCherry), Tg(pod:Gal4; UAS:NfsB-mCherry; UAS:CA-RAC1,cmlc2:EGFP) and Tg(pod:Gal4;UAS:NfsB-mCherry;UAS:DN-RAC1,cmlc2:EGFP) at 5 dpf.
Since Rac1 activation in podocytes has been reported in several animal models of podocyte injury, we sought to test whether modulation of Rac1 activity in podocytes affects the severity of podocyte injury. Previously we have generated an inducible podocyte injury model in zebrafish by applying MTZ to transgenic fish expressing a bacterial nitroreductase (NfsB) only in podocytes. In the transgenic fish Tg(pod:gal4;UAS:NfsB-mCherry), MTZ is converted to a cytotoxin solely in podocytes and causes podocyte apoptosis (33). We have also established POE as a phenotypic consequence of podocyte injury (Figs. 3 and 4A) (29). Using this new method, we found that overexpression of CA-RAC1 resulted in a significant increase in the POE phenotype in a MTZ-dosage dependent manner, indicating that forced Rac1 activation in podocytes aggravated podocyte injury (Fig. 4B). To confirm this result, we employed the proteinuria assay for zebrafish previously established by us (33). Vitamin D binding protein (VDBP) is a plasma protein and homolog of albumin, so we tagged the zebrafish ortholog of VDBP with GFP, rendering it a molecular tracer of GFB selectivity for zebrafish. In the transgenic fish Tg(lfabp:vdbp-gfp), GFP-tagged VDBP is expressed by the hepatocytes and secreted into plasma. Upon podocyte injury, VDBP-GFP leaks out of the glomeruli and can be visualized by fluorescence in the proximal tubules as well as detected in fish water by ELISA. Therefore, we showed by proteinuria assessment that CA-RAC1 expression predisposed zebrafish to more severe proteinuria following the MTZ treatment (Fig. 3C). Mice with podocyte-specific knockout of Rac1 were reported to have no podocyte defects and resist the protamine sulfate-induced foot process effacement (4). Consistently, transgenic fish expressing DN-RAC1 in podocytes develop normally without any proteinuria or POE (Fig. 2, B and C). In addition, these fish had less manifestation of POE and reduced proteinuria when subjected to MTZ-induced podocyte injury (Fig. 4, B and D), suggesting that inhibition of Rac1 activity protects podocytes from injury.
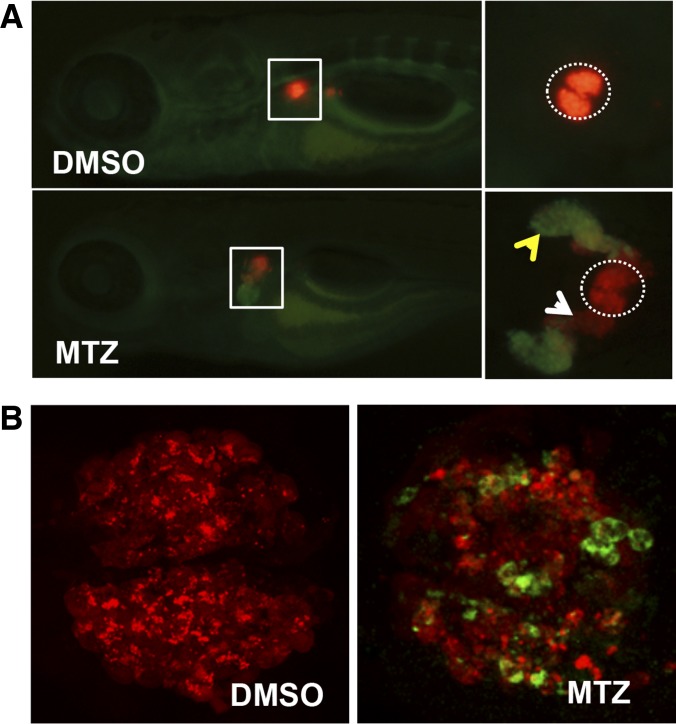
Fig. 3.
Metronidazole (MTZ) treatment induces podocyte injury and proteinuria in transgenic zebrafish. A: Tg(pod:Gal4;UAS:NfsB-mCherry;lfabp:vdbp-gfp) larvae exhibit podocyte loss [along with vitamin D binding protein-GFP leaking out and being reabsorbed in convoluted tubules (yellow arrowhead)] when subjected to MTZ treatment for 24 h. Dotted circles represent the pronephric glomeruli. Note reduction in mCherry fluorescence in the glomeruli and appearance in proximal tubules (white arrowhead), presumably due to tubular uptake of mCherry proteins from the disintegrated podocytes. B: podocytes undergo apoptosis after MTZ treatment for 24 h as revealed by anti-cleaved caspase 3 staining (green) of pronephros in Tg(pod:Gal4;UAS:NfsB-mCherry).
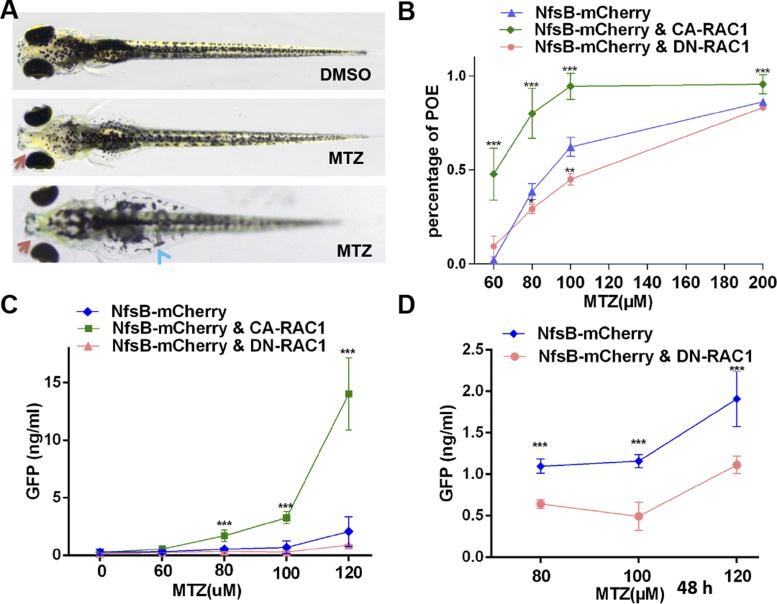
Fig. 4.
Overexpression of CA-RAC1 aggravates MTZ-induced podocyte injury and proteinuria in zebrafish. A: Tg(pod:Gal4;UAS:NfsB-mCherry) larvae develop periorbital edema and abdominal edema at 5 dpf following MTZ treatment for 48 h. Red arrows, periorbital edema; blue arrowheads, abdominal edema. B: overexpression of CA-RAC1 (green) increases and DN-RAC1 (red) decreases the penetrance of periorbital edema phenotype after MTZ treatment. C: larvae with podocyte-specific overexpression of CA-RAC1 exhibit more severe proteinuria after MTZ treatment for 24 h than the controls, while DN-RAC1 overexpression in podocytes does not show any obvious reduction of proteinuria, presumably due to the low level of proteinuria over 24 h following induction of podocyte injury with MTZ. D: over 48 h after MTZ treatment, DN-RAC1 overexpression (red) in the podocytes significantly attenuates proteinuria compared with the controls (blue). NfsB-mCherry: Tg(pod:Gal4;UAS:NfsB-mCherry); NfsB-mCherry and CA-RAC1: Tg(pod:Gal4;UAS:NfsB-mCherry;UAS:CA-RAC1); NfsB-mCherry and DN-RAC1: Tg(pod:Gal4;UAS:NfsB-mCherry;UAS:DN-RAC1). All larvae for proteinuria assay carry the lfabp:vdbp-gfp transgene. ***P < 0.001
A high level of Rac1 activity induces POE and proteinuria.
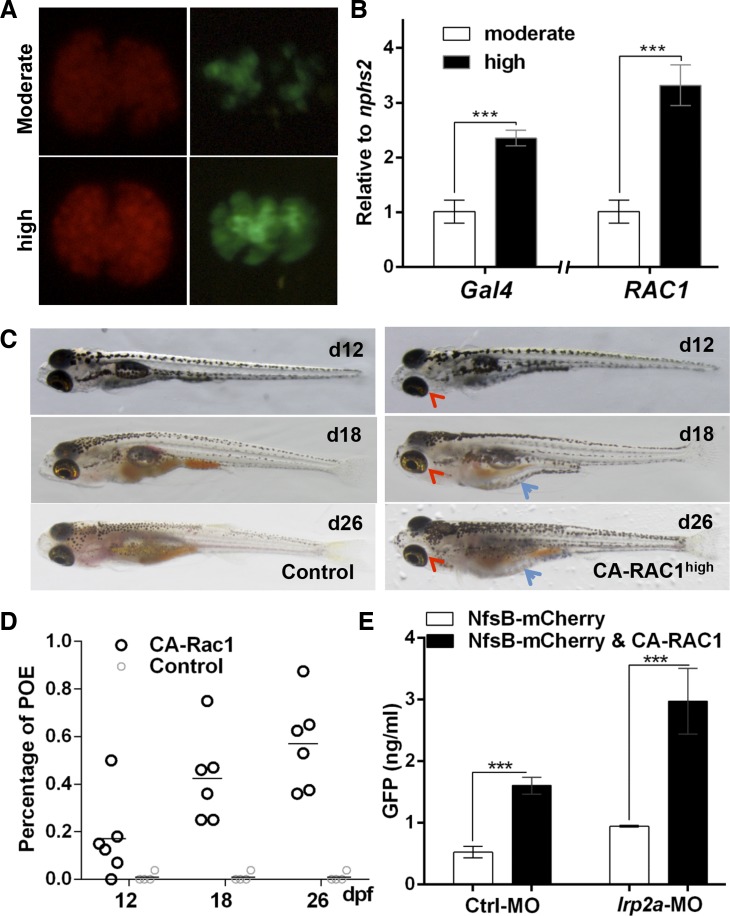
The GFB defect seen in CA-RAC1 transgenic zebrafish appears rather milder than in transgenic mice with podocyte-specific expression of Rac1 (31), possibly due to a relatively low expression level of CA-RAC1 in zebrafish podocytes. To facilitate the analyses of the Rac1 expression level, we generated transgenic fish Tg(pod:Gal4;UAS:EGFP-rac1G12V) and Tg(pod:Gal4;UAS:EGFP-rac1T17N), in which constitutively active and dominant negative forms of zebrafish Rac1 were tagged with GFP at the N terminus (8). In fact, Rac1 expression was mosaic in Tg(pod:Gal4;UAS:EGFP-rac1G12V) (Fig. 5A). To achieve a higher level of CA-RAC1 expression, we utilized a stronger Gal4 driver line Tg(pod:Gal4high) that was confirmed via both mCherry reporter and EGFP-rac1G12V expression in the glomeruli (Fig. 5A). The transcript levels of Gal4 and CA-RAC1 were nearly doubled and tripled, respectively, compared with those driven by the moderate Gal4 driver Tg(pod:Gal4) (Fig. 5B). Starting at 12 dpf, Tg(pod:Gal4high;UAS:CA-RAC1,cmlc2:EGFP) developed POE without induction of podocyte injury. The edematous phenotype became progressively severe and eventually led to whole-body edema and lethality between 12 days and 1 mo of age (Fig. 5, C and D). Seven of 10 clutches of double transgenic larvae from different pairs of parents developed the edematous and lethal phenotype (Fig. 5D and Table 2), presumably due to variable expression levels of the transgene. Furthermore, significant proteinuria was detected in transgenic larvae Tg(pod:Gal4high;UAS:CA-RAC1,cmlc2:EGFP) (Fig. 5E). Since the renal tubular epithelia are shown to modulate proteinuria through tubular reabsorption of proteins in urine (28), knockdown of lrp2a, encoding the megalin transporter in renal epithelial cells, revealed a significant increase in proteinuria in Tg(pod:Gal4high;UAS:CA-RAC1,cmlc2:EGFP) transgenic fish, confirming that the GFB in these fish was defective even without induction of podocyte injury (Fig. 5E). This further supported the conclusion that activation of Rac1 in podocytes imposes a detrimental effect on the GFB.
Fig. 5.
Increase in Rac1 activity induces severe podocyte injury and proteinuria. A: comparison of NfsB-mCherry (red) and EGFP-rac1G12V (green) expression in glomeruli driven by [Tg(pod:Gal4); moderate] and [Tg(pod:Gal4high); high], respectively. Tg(pod:Gal4high) delivers a higher expression level of the transgenes under the control of UAS. B: comparison of transcriptional levels of Gal4 and active RAC1 in glomerulus when driven by either Tg(pod:Gal4) or Tg(pod:Gal4high). C: lateral views of Tg(pod:Gal4high; UAS:CA-RAC1) larvae with obvious edematous phenotype at various ages. Red arrowheads point to periorbital edema (POE), and blue arrows show abdominal edema. D: quantitation of the POE phenotype in Tg(pod:Gal4high; UAS:EGFP-rac1G12V) larvae and juveniles at day 12, day 18, and day 26, respectively. E: measurement of proteinuria of Tg(pod:Gal4high;UAS:NfsB-mCherry;lfabp:vdbp-egfp) and Tg(pod:Gal4high;UAS:NfsB-mCherry;UAS:CA-RAC1;lfabp:vdbp-gfp). lrp2a-MO was used to inhibit the expression of megalin and hence the renal tubular protein uptake. ***P < 0.001
Table 2.
Quantitative record of the POE phenotype for multiple crosses of transgenic lines
| Embryos with POE |
||||
|---|---|---|---|---|
| Genotype | D12 | D18 | D26 | Total |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #1 | 0 | 0 | 0 | 9 |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #2 | 0 | 0 | 0 | 15 |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #3 | 0 | 0 | 0 | 12 |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #4 | 2 | 4 | 1 | 13 |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #5 | 1 | 4 | 0 | 14 |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #6 | 3 | 5 | 3 | 17 |
| Tg(pod:Gal4 s; UAS:CA-RAC1) #7 | 1 | 1 | 0 | 3 |
| Tg(pod:Gal4 s; UAS:EGFP-CA-Rac1) #1 | 0 | 2 | 3 | 8 |
| Tg(pod:Gal4 s; UAS:EGFP-CA-Rac1) #2 | 2 | 4 | 1 | 8 |
| Tg(pod:Gal4 s; UAS:EGFP-CA-Rac1) #3 | 1 | 2 | 1 | 8 |
| Tg(pod:Gal4 s) #1 | 0 | 0 | 0 | 12 |
| Tg(pod:Gal4 s) #2 | 1 | 0 | 0 | 26 |
| Tg(pod:Gal4 s) #3 | 0 | 0 | 0 | 17 |
| Tg(pod:Gal4 s) #4 | 0 | 0 | 0 | 8 |
| Tg(pod:Gal4 s) #5 | 0 | 0 | 0 | 8 |
| Tg(pod:Gal4 s) #6 | 0 | 0 | 0 | 8 |
| Tg(UAS: UAS:CA-RAC1) #1 | 0 | 0 | 0 | 32 |
| Tg(UAS: UAS:CA-RAC1) #2 | 0 | 0 | 0 | 25 |
| Tg(UAS: UAS:CA-RAC1) #3 | 0 | 0 | 0 | 64 |
| Tg(UAS: EGFP-CA-Rac1) #1 | 0 | 0 | 0 | 122 |
| Tg(UAS: EGFP-CA-Rac1) #2 | 0 | 0 | 0 | 36 |
| Tg(UAS: EGFP-CA-Rac1) #3 | 0 | 0 | 0 | 46 |
POE, periorbital edema; D, day. Bold crosses produced POE phenotypes.
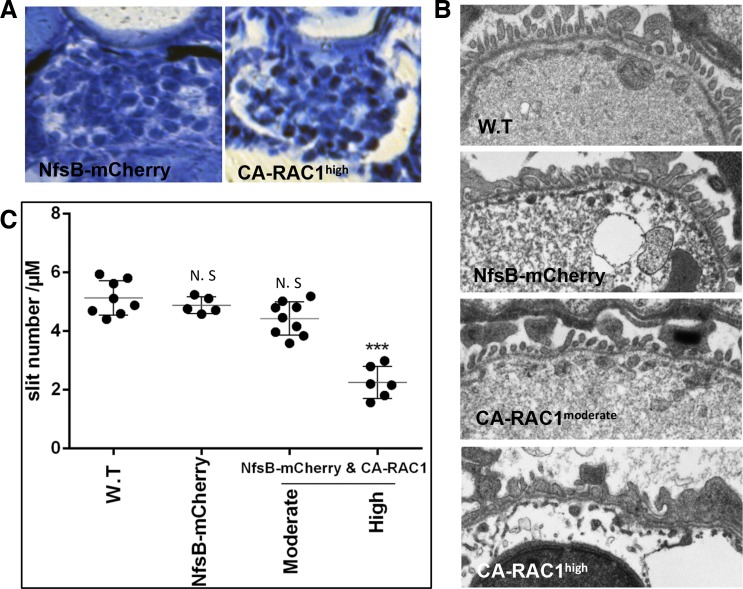
The ultrastructural change in foot processes is an early event of podocyte injury associated with altered glomerular permeability and proteinuria (9). As expected, histological sections of Tg(pod:Gal4high;UAS:CA-RAC1,cmlc2:EGFP) larvae at as early as 5 dpf revealed a dilated Bowman's capsule, which was not seen in larvae with moderate CA-RAC1 expression (Fig. 6A). Consistently, reduction of slit diaphragm density and widened foot processes were observed only in larvae with high CA-RAC1 expression but not in the normal controls or those with moderate CA-RAC1 expression (Fig. 6, B and C).
Fig. 6.
A high level of Rac1 activity in podocytes damages pronephric glomeruli in zebrafish. A: cross sections of pronephros expressing either NsfB-mCherry (left) or constitutively active RAC1 (right) in podocytes when driven by pod:Gal4high. B: representative micrographs of podocyte foot processes in wild-type (W.T) and transgenic larvae. NfsB-mCherry: Tg(pod:Gal4;UAS:NfsB-mCherry); CA-RAC1moderate: Tg(pod:Gal4moderate;UAS:CA-RAC1,cmlc2:EGFP); CA-RAC1high: Tg(pod:Gal4high;UAS:CA-RAC1,cmlc2:EGFP). C: measurement of slit density of podocytes in wild-type and transgenic larvae. NS, not significant. ***P < 0.001.
Rac1 activation in podocytes downregulates the expression of podocin and nephrin.
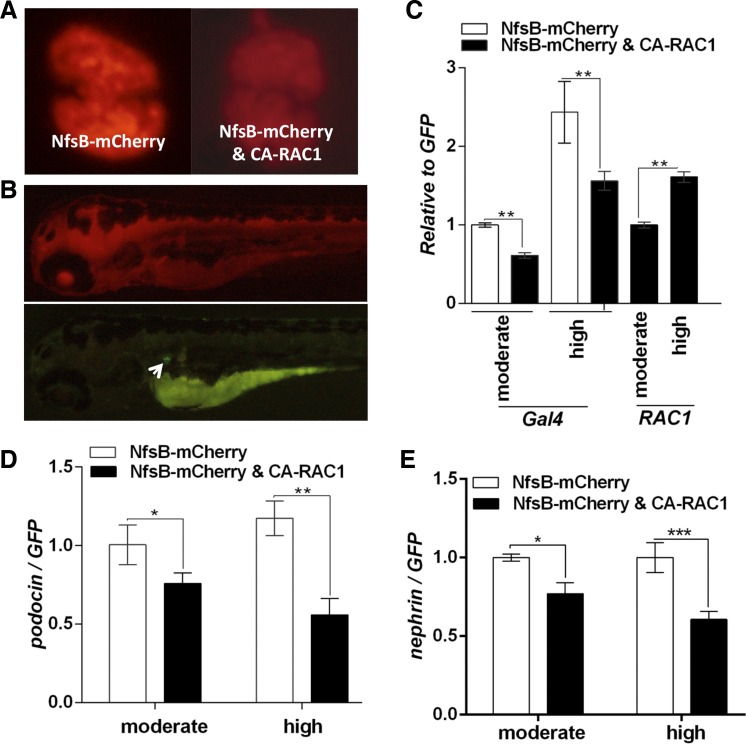
In the pronephric glomeruli of Tg(pod:Gal4;UAS:NfsB-mCherry;UAS:CA-RAC1,cmlc2:EGFP), we noticed a significant decrease in mCherry expression compared with Tg(pod:Gal4;UAS:NfsB-mCherry) (Fig. 7A). This suggested that overexpression of CA-RAC1 may have inhibited podocin promoter activity. Conventionally, podocin is used as a podocyte-specific reference gene for gene expression analyses in podocytes given its exclusive expression in podocytes. However, in this scenario, the expression of podocin itself may have been affected by Rac1 activation, so it is not suitable to use the transcript level of podocin as a reference. To precisely compare the gene expression specifically in podocytes, we genetically tagged podocytes with GFP using a new transgenic line Tg(pod:Cre-ERT2; ubi:loxP-mCherry-loxP-GFP). In this transgenic fish, mCherry is expressed in all the cells under the control of the ubiquitin promoter while GFP expression is blocked. We could induce GFP expression using tamoxifen (4-OHT) induction of Cre recombinase activity specifically in podocytes, which cleaved the loxP-mCherry-loxP blockage of GFP expression (Fig. 7B). The consequential GFP expression is under the control of the ubiquitin promoter, independently of the regulation by the podocin promoter but still restricted in podocytes. We thus quantitated the podocyte-specific gene expression by normalization to GFP and confirmed that the mRNA level of Gal4 was decreased in podocytes with CA-RAC1 expression (Fig. 7C), and so were those of nephrin and podocin (Fig. 7, D and E). Particularly, a higher expression of CA-RAC1 with the Tg(pod:Gal4high) driver further downregulated nephrin and podocin expression. Using this new method, we also excluded the possible reduction of podocyte-specific gene expression due to podocyte loss, because we used GFP as our reference gene, whose expression level reflected the quantity of remaining podocytes regardless of podocyte loss. Taken together, these results provided the first evidence that Rac1 activation in podocytes is causal to the decreased expression of nephrin and podocin, which are major components of the slit diaphragm and essential for maintaining normal GFB.
Fig. 7.
A high level of Rac1 activity decreases nephrin and podocin expression in podocytes. A: comparison of mCherry expression in podocytes between Tg(pod:Gal4high;UAS:Nfsb-mCherry) and Tg(pod:Gal4high;UAS:Nfsb-mCherry;UAS:CA-RAC1,cmlc2:EGFP) at 5 dpf. B: ubiquitous mCherry expression in Tg(pod:CreERT2;ubi:loxP-mCherry-loxP-GFP). GFP-tagged podocytes are induced (white arrow) after 4-hydroxytamoxifen (4-OHT) treatment. The yolk sac is visible due to autofluorescence. C: comparison of Gal4 and RAC1 expression in pronephric glomeruli in transgenic larvae after 4-OHT induction. NfsB-mCherry: Tg(pod:Gal4;UAS:NfsB-mCherry;pod:CreERT2;ubi:loxP-mCherry-loxP-GFP); NfsB-mCherry and CA-RAC1: Tg(pod:Gal4;UAS:NfsB-mCherry;UAS:CA-RAC1,cmlc2:EGFP;pod:CreERT2;ubi:loxP-mCherry-loxP-GFP). D and E: comparison of podocin and nephrin expression levels in isolated glomeruli. [Tg(pod:Gal4); moderate] or [Tg(pod:Gal4high); high] is used, respectively, to drive the transgene expression in podocytes. *P < 0.05. **P < 0.01.***P < 0.001.
DISCUSSION
In this study, we have generated and analyzed a number of zebrafish models of Rac1-associated podocytopathy and found that different levels of Rac1 activity had distinct impacts on podocyte health in zebrafish. In addition, we revealed that Rac1 activation in podocytes decreased the expression of podocin promoter as well as slit diaphragm proteins nephrin and podocin.
The significant Rac1 activation in podocytes in rodent models of kidney disease and human nephrotic syndrome implicates that sustained Rac1 activation may induce podocyte injury (1, 11, 14). However, no kidney failure was seen in transgenic rodents with Rac1 overexpression in podocytes although rapid transient proteinuria and foot process effacement were observed (31). This was attributed to the mosaic expression of the Nphs1:rtTA driver in podocytes of transgenic mice and the permanent loss of these cells after long-term induction of Rac1 expression. We have also observed the mosaic expression of the Rac1 transgene in our transgenic zebrafish model; however, it is not due to the mosaic expression of the pod:Gal4 driver because NfsB-mCherry expression appears quite uniform in podocytes when the same Gal4-driver line is used. As a matter of fact, using a genetic-tagging approach in podocytes, we revealed that overexpression of CA-RAC1 downregulated podocin promoter activity, which restricted the expression of the CA-RAC1 transgene in podocytes.
Besides actin cytoskeleton reorganization in cultured podocytes (2), Rac1 activation was associated with multiple cellular process including inflammation, fibrosis, and apoptosis in kidney disease (21), oxidative stress and mitochondrial apoptosis in acute kidney disease (10), and mineralocorticoid receptor activation in a salt-induced hypertension and kidney disease model (24). The activity of the podocin promoter has been proposed to be an indicator of podocyte health (12), so it is unclear whether the decline in podocin and nephrin expression in the CA-RAC1-expressing glomerulus is a direct or indirect regulatory effect of Rac1 or even a comprehensive consequence of podocyte injury.
Several studies have suggested that a balanced activity of small GTPases is crucial for normal podocyte function (1, 4, 23, 25). Consistent with this notion, in zebrafish excessive Rac1 activity, detrimental to podocytes while lowering Rac1 activity is protective against podocyte injury. Using two pod:Gal4 driver lines with distinct expression levels, we showed that a moderate increase in Rac1 activity only slightly impaired the GFB without podocyte loss but a further increase in Rac1 activity led to severe damage to the GFB and podocytes. Compared with the rapid onset of a glomerular phenotype in transgenic mice reported earlier, our zebrafish model of moderate Rac1 activation may represent a more relevant model for chronic glomerular diseases in the light of the progressive nature of these diseases.
In conclusion, our study highlighted that Rac1 affects podocyte health in a dosage-dependent manner in association with altered expression of slit diaphragm components podocin and nephrin.
GRANTS
This research was supported by Grants DK091405 and DK081943 from the National Institutes of Health to W. Zhou, the Janette Ferrantino Investigator Award (W. Zhou), and the American Society of Nephrology Carl W. Gottschalk Scholar Research Grant (W. Zhou). The TEM technical service was supported by the George O'Brien Kidney Translational Core Center at the University of Michigan (DK081943).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.W., M.-S.L., and W.Z. performed experiments; X.W., M.-S.L., and W.Z. analyzed data; X.W., M.-S.L., and W.Z. interpreted results of experiments; X.W., M.-S.L., and W.Z. prepared figures; X.W., M.-S.L., and W.Z. drafted manuscript; M.-S.L. and W.Z. edited and revised manuscript; W.Z. provided conception and design of research; W.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors appreciate Dr. Kristen Kwan for sharing the Tol2 Gateway Kit, Dr. Gage Crump for sharing the zebrafish Rac1 constructs, and Dr. Daniel Goldman for sharing the zebrafish reporter line.
REFERENCES
- 1.Akilesh S, Suleiman H, Yu H, Stander MC, Lavin P, Gbadegesin R, Antignac C, Pollak M, Kopp JB, Winn MP, Shaw AS. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J Clin Invest 121: 4127–4137, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attias O, Jiang R, Aoudjit L, Kawachi H, Takano T. Rac1 contributes to actin organization in glomerular podocytes. Nephron Exp Nephrol 114: e93–e106, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Babelova A, Jansen F, Sander K, Lohn M, Schafer L, Fork C, Ruetten H, Plettenburg O, Stark H, Daniel C, Amann K, Pavenstadt H, Jung O, Brandes RP. Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS One 8: e80328, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, Vining C, Randolph A, Herbach N, Wanke R, Atkins KB, Gyung Kang H, Henger A, Brakebusch C, Holzman LB, Kretzler M. Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 84: 920–930, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chauhan BK, Lou M, Zheng Y, Lang RA. Balanced Rac1 and RhoA activities regulate cell shape and drive invagination morphogenesis in epithelia. Proc Natl Acad Sci USA 108: 18289–18294, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe CP, Collazo A, Trinh le A, Pan L, Moens CB, Crump JG. Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev Cell 24: 296–309, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Daugaard M, Nitsch R, Razaghi B, McDonald L, Jarrar A, Torrino S, Castillo-Lluva S, Rotblat B, Li L, Malliri A, Lemichez E, Mettouchi A, Berman JN, Penninger JM, Sorensen PH. Hace1 controls ROS generation of vertebrate Rac1-dependent NADPH oxidase complexes. Nat Commun 4: 2180, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duner F, Lindstrom K, Hultenby K, Hulkko J, Patrakka J, Tryggvason K, Haraldsson B, Wernerson A, Pettersson E. Permeability, ultrastructural changes, and distribution of novel proteins in the glomerular barrier in early puromycin aminonucleoside nephrosis. Nephron Exp Nephrol 116: e42–e52, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Invest 124: 4989–5001, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, Beck BB, Gribouval O, Zhou W, Diaz KA, Natarajan S, Wiggins RC, Lovric S, Chernin G, Schoeb DS, Ovunc B, Frishberg Y, Soliman NA, Fathy HM, Goebel H, Hoefele J, Weber LT, Innis JW, Faul C, Han Z, Washburn J, Antignac C, Levy S, Otto EA, Hildebrandt F. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest 123: 3243–3253, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He N, Zahirieh A, Mei Y, Lee B, Senthilnathan S, Wong B, Mucha B, Hildebrandt F, Cole DE, Cattran D, Pei Y. Recessive NPHS2 (Podocin) mutations are rare in adult-onset idiopathic focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2: 31–37, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Kawarazaki W, Nagase M, Yoshida S, Takeuchi M, Ishizawa K, Ayuzawa N, Ueda K, Fujita T. Angiotensin II- and salt-induced kidney injury through Rac1-mediated mineralocorticoid receptor activation. J Am Soc Nephrol 23: 997–1007, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires nephrin, podocin and the FERM domain protein Mosaic eyes. Dev Biol 285: 316–329, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kur E, Christa A, Veth KN, Gajera CR, Andrade-Navarro MA, Zhang JJ, Willer JR, Gregg RG, Abdelilah-Seyfried S, Bachmann S, Link BA, Hammes A, Willnow TE. Loss of Lrp2 in zebrafish disrupts pronephric tubular clearance but not forebrain development. Dev Dyn 240: 1567–1577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Rao J, Zha XL, Xu H. Angiopoietin-like 3 induces podocyte F-actin rearrangement through integrin alpha(V)beta(3)/FAK/PI3K pathway-mediated Rac1 activation. Biomed Res Int 2013: 135608, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiuthed A, Chanvorachote P. Cisplatin at sub-toxic levels mediates integrin switch in lung cancer cells. Anticancer Res 34: 7111–7117, 2014. [PubMed] [Google Scholar]
- 20.Moorman JP, Luu D, Wickham J, Bobak DA, Hahn CS. A balance of signaling by Rho family small GTPases RhoA, Rac1 and Cdc42 coordinates cytoskeletal morphology but not cell survival. Oncogene 18: 47–57, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86–98, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol 518: 4196–4212, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott RP, Hawley SP, Ruston J, Du J, Brakebusch C, Jones N, Pawson T. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J Am Soc Nephrol 23: 1149–1154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, Takai Y, Ishikawa A, Shimosawa T, Ando K, Nagase M, Fujita T. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest 121: 3233–3243, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan-Brown J, Bisher ME, Burdine RD. Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nat Protoc 6: 46–55, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Wagner MC, Campos-Bilderback SB, Chowdhury M, Flores B, Lai X, Myslinski J, Pandit S, Sandoval RM, Wean SE, Wei Y, Satlin LM, Wiggins RC, Witzmann FA, Molitoris BA. Proximal tubules have the capacity to regulate uptake of albumin. J Am Soc Nephrol 27: 482–494, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan X, Chen Z, Choi WI, Gee HY, Hildebrandt F, Zhou W. Loss of epithelial membrane protein 2 aggravates podocyte injury via upregulation of caveolin-1. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Ellis MJ, Gomez JA, Eisner W, Fennell W, Howell DN, Ruiz P, Fields TA, Spurney RF. Mechanisms of the proteinuria induced by Rho GTPases. Kidney Int 81: 1075–1085, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Suleiman H, Kim AH, Miner JH, Dani A, Shaw AS, Akilesh S. Rac1 activation in podocytes induces rapid foot process effacement and proteinuria. Mol Cell Biol 33: 4755–4764, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol 299: F1040–F1047, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol 23: 1039–1047, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Jiang R, Aoudjit L, Jones N, Takano T. Activation of RhoA in podocytes induces focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1621–1630, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]