Abstract
No therapies have been shown to accelerate recovery or prevent fibrosis after acute kidney injury (AKI). In part, this is because most therapeutic candidates have to be given at the time of injury and the diagnosis of AKI is usually made too late for drugs to be efficacious. Strategies to enhance post-AKI repair represent an attractive approach to address this. Using a phenotypic screen in zebrafish, we identified 4-(phenylthio)butanoic acid (PTBA), which promotes proliferation of embryonic kidney progenitor cells (EKPCs), and the PTBA methyl ester UPHD25, which also increases postinjury repair in ischemia-reperfusion and aristolochic acid-induced AKI in mice. In these studies, a new panel of PTBA analogs was evaluated. Initial screening was performed in zebrafish EKPC assays followed by survival assays in a gentamicin-induced AKI larvae zebrafish model. Using this approach, we identified UPHD186, which in contrast to UPHD25, accelerates recovery and reduces fibrosis when administered several days after ischemia-reperfusion AKI and reduces fibrosis after unilateral ureteric obstruction in mice. UPHD25 and 186 are efficiently metabolized to the active analog PTBA in liver and kidney microsome assays, indicating both compounds may act as PTBA prodrugs in vivo. UPHD186 persists longer in the circulation than UPHD25, suggesting that sustained levels of UPHD186 may increase efficacy by acting as a reservoir for renal metabolism to PTBA. These findings validate use of zebrafish EKPC and AKI assays as a drug discovery strategy for molecules that reduce fibrosis in multiple AKI models and can be administered days after initiation of injury.
Keywords: acute kidney injury, drug discovery, phenotypic screening, PTBA analogs
severe acute kidney injury (AKI) affects >90,000 patients in the United States each year, while milder, non-dialysis-dependent AKI affects >1.5 million/yr (1, 15, 22). AKI is most often precipitated by major surgery, particularly cardiopulmonary bypass surgery, prolonged hypotension from any cause, sepsis, drug and systemic toxicities, and urinary obstruction (1, 22). AKI is a precursor to chronic kidney disease (CKD), and there is increased risk of end-stage renal disease in patients with severe AKI (2–4, 8, 28, 29). Despite this, to date, no therapeutic interventions have been shown to either prevent injury, accelerate recovery, reduce postinjury fibrosis, or prevent progressive CKD in patients after an episode of AKI (19). In part, this is because drug candidates usually have to be administered within hours of the initiating injury and the diagnosis of AKI is usually made late, often days after the injury has occurred (1). Therapeutic strategies designed to enhance post-AKI repair mechanisms represent an attractive approach, as repair occurs largely by proliferation of surviving, dedifferentiated, tubular epithelial cells a number of days after the initiating injury (12). To address this, we developed a high-content phenotypic screen using zebrafish embryos to identify compounds that promote proliferation of embryonic kidney progenitor cells (EKPCs) (9, 23). Since proliferating tubular epithelial cells express markers of kidney progenitor cells after injury (5), we reasoned that compounds promoting expansion of zebrafish EKPCs might also increase proliferation of surviving epithelial cells after AKI. Using this approach, we identified the small molecule 4-(phenylthio)butanoic acid (PTBA), a histone deacetylase inhibitor (HDACi) that expands EKPCs in zebrafish embryos (9, 23), and the methyl ester of PTBA methyl-4-(phenylthio)butanoate (M4PTB/UPHD25), which increases tubular cell proliferation, accelerates recovery, and reduces renal fibrosis when administered after injury in two different mouse models of AKI: ischemia-reperfusion-induced AKI (IR-AKI) and aristolochic acid-induced AKI (AA-AKI) (7, 20).
In these studies, a new series of PTBA analogs were evaluated for efficacy in accelerating recovery and reducing postinjury fibrosis after AKI. Primary screening was performed using two complementary zebrafish EKPC assays. This was followed by secondary and tertiary screens in larval zebrafish and mouse models of AKI, respectively, to evaluate the most promising PTBA analogs for their ability to enhance postinjury repair. Using this functional phenotypic screening approach, we found that activity in zebrafish and mice is dependent on exposure to PTBA itself and developed the PTBA prodrug UPHD186, which accelerates recovery and reduces postinjury fibrosis when administered several days after the initiating injury. We also show that UPHD186 is effective in reducing postinjury renal fibrosis after unilateral ureteric obstruction (UUO) in mice, extending the spectrum of PTBA analog activity from ischemia and toxin-induced injury to a model of post-renal AKI. These findings establish the sequential use of zebrafish EKPC and AKI models as a drug discovery strategy to identify molecules that accelerate recovery and reduce postinjury fibrosis after AKI. The discovery of UPHD186 represents an important preclinical advance since no established treatments have been shown to accelerate recovery or reduce progressive CKD when administered late after the diagnosis of AKI has been made.
METHODS
Zebrafish EKPC Assays
Embryos were processed for kidney expansion by in situ hybridization (ISH) or cognitive network technology (CNT) analysis.
ISH assays.
Wild-type PITT-AB embryos were treated with compounds at 3.0, 1.5, 0.8, 0.4, and 0.2 μM at the 512 cell stage by serial dilution in E3 medium containing 0.5% dimethyl sulfoxide (DMSO, Sigma), as described (9). Embryos were incubated at 22°C overnight and fixed in 4% paraformaldehyde at the 10-somite stage. In situ hybridization was performed using lhx1a riboprobes, as described (14). At least 20 embryos/condition were visually scored for increased lhx1a mRNA expression. Data are presented as the percentage of embryos with expanded lhx1a.
CNT assays.
CNT assays were used to quantify the half maximum effective concentrations (EC50) for compounds that increased Lhx1 mRNA expression. For this we utilized transgenic zebrafish, Tg(cdh17:EGFP)pt305, as described (23). At the 512 cell stage, 10–15 embryos in 12-well plates were treated with vehicle or increasing concentrations of compounds (3.0-0.2 μM) in E3 medium containing 0.5% DMSO. Embryos were allowed to develop for 48 h, dechorionated, arrayed in flat-bottom 96-well plates, and anesthetized with 40 μg/ml MS222 (tricaine methanesulfonate; Sigma) to restrict movement during imaging. Images were acquired at a single wavelength (GFP). Because the elongated morphology of 48-h postfertilization (hpf) embryos necessitated capture of the entire well, a MetaXpress journal was generated that acquired two sites that were stitched together into a single montage. Archived fluorescence micrographs were uploaded into Developer (Definiens, Munich, Germany) using the Cellenger module. CNT rule sets were used that quantified Cdh17-EGFP expression within the developing embryo, as described (23).
Zebrafish Larval Maximum Tolerated Dose and AKI Survival Assays
Maximum tolerated dose (MTD) assays were performed with minor modifications, as described (7). Cohorts of 15 zebrafish larvae were arrayed in 6-well plates and treated with 1% DMSO vehicle, or increasing concentrations of compounds in E3 medium plus 1% DMSO. Treatment was initiated at 5 days postfertilization (dpf) and continued for 24 h. For zebrafish larval AKI, compounds were used at one-quarter the MTD. Three-day dpf larvae were treated with 8 ng gentamicin (Sigma) diluted in 1 nl normal saline injected into the common cardinal vein. Lucifer yellow dextran (10 kDa; 1 mg/ml; Invitrogen) was included with the gentamicin to verify successful microinjection, as described (6). After injection, larvae were incubated at 28°C for 2 days. At 2 days postinjection (dpi), larvae showing signs of pericardial edema were transferred to E3 medium containing 1% DMSO with or without compounds. Treatment was continued for 24 h at 28°C when treatment solution was replaced with E3 medium without compounds. Larvae were assayed for survival at 4, 5, 6, and 7 dpi. The Pittsburgh University Institutional Animal Care and Use Committee approved experimental protocols in zebrafish.
Mouse IR-Induced AKI, Renal Biopsy, and UUO
Surgeries were performed on a water bath-heated platform at 38°C on 10- to 12-wk-old male BALB/c mice. To induce IR-AKI, mice underwent left renal pedicle clamping for 31 min, and a delayed contralateral nephrectomy was performed after 8 days, as described (24). None of the mice died in either the vehicle or treatment groups over the course of these studies. Serum creatinine was evaluated using enzymatic cascade assays (Pointe Scientific, Canton, MI). Mice were dosed with 1 mg/kg, 10 mg/kg or 50 mg/kg UPHD25, or 50 mg/kg PTBA, UPHD29, 36, 186, 263, or vehicle control (20% 2-hydroxypropyl-β-cyclodextrin in PBS) by daily intraperitoneal (ip) injection, starting 24 h after injury for 7 days, or 3 days after injury for 4 days, as indicated. To study the effect of UPHD186's effect on regression of fibrosis, mice underwent left pedicle clamping for 35 min and an open-kidney biopsy on day 30 to assess fibrosis, as described (17). Mice were then randomized into vehicle or 50 mg/kg UPHD186 IP daily for 10 days, and kidneys were harvested on day 50. For UUO surgery, male BALB/c mice underwent left-sided ureteral ligation and treated with either vehicle or 50 mg/kg UPHD25 or 186 ip daily for 7 days starting at the time of surgery. Obstructed kidneys were harvested 8 days after surgery for analysis of renal fibrosis. Experimental protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee.
Analysis of Renal Fibrosis and Histone Acetylation
To assess fibrosis, kidneys were harvested, fixed, and mounted in paraffin, and Sirius red staining and quantification were performed to evaluate renal fibrosis/collagen accumulation in kidney sections, as described (7). Image capture and semiquantitative evaluation was performed by observed blinded to treatment (N. I. Skrypnyk and K. Patel). RNA isolation and quantitative RT-PCR were performed to evaluate expression of renal fibrosis markers in snap-frozen kidneys using primer sequences and RNA extraction, as previously described (7). Histone acetylation was determined in snap-frozen kidneys by Western blotting using rabbit monoclonal anti-histone H4 (clone 62) and rabbit anti-acetylated H4 lysine 5/8/12 and 16 antibodies (pan H4 acetylation, Millipore), and infrared 680LT-conjugated donkey anti-rabbit IgG secondary antibodies (LI-COR), and fluorescence images were captured using the Odyssey Laser Fluorescence Detection System, as previously described (7).
Pharmacokinetic Studies
Eight- to twelve-week male BALB/c mice were given 50 mg/ml PTBA, UPHD25, 36, 186, or 263 in 20% 2-hydroxypropyl-β-cyclodextrin in water ip, and 60 μl of blood collected in three time sets: 0.08, 0.25, and 0.5 h; 1, 2, and 4 h; and 6, 8, and 24 h postinjection (3 mice/compound in 3 time sets). Plasma samples were separated, stored below −70°C, and processed for analysis after precipitation using acetonitrile followed by liquid chromatography and tandem mass spectrometry (LC-MS/MS) to identify compounds and PTBA (Supplemental Methods 1; all supplemental material is available on the journal web site). A noncompartmental analysis module in Phoenix WinNonlin [version (8) 6.3] was used to assess pharmacokinetic (PK) parameters. This experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) at SAI Life Sciences.
Synthetic chemistry for all of the PTBA analogs is described in supplemental data (Supplemental Methods 2).
In Vitro Stability and Activity Assays
We used a solution containing 1 mg/ml mouse liver and kidney microsomes (CD-1 male, Xenotech), 10 μM test compound ±1 mM NADPH (Sigma-Aldrich) in 50 mM potassium phosphate buffer, pH 7.4. Time-zero samples were immediately quenched with 1.0 ml acetonitrile containing 0.1% formic acid. Test samples were incubated at 37°C for 50 min before quenching. Standard curves were generated by spiking time-zero microsomal preparations with compounds. Supernatants were dried under nitrogen and reconstituted in water for analysis by liquid chromatography and mass spectrometry (LC-MS) in the Vanderbilt Mass Spectrometry Core Laboratory, as described in the supplemental data (Supplemental Methods 3).
Statistical Analyses
Statistical analyses performed included by Student's two-tailed t-test for paired group comparisons, one-way ANOVA for multiple between-group comparisons using Tukey's correction for post hoc, pairwise between-group comparisons, as indicated in the figure legends. The minimal level of significance was set at P ≤ 0.05, and statistical analyses were performed using GraphPad Prism V6.
RESULTS
Screening of PTBA Analogs in EKPC Assays
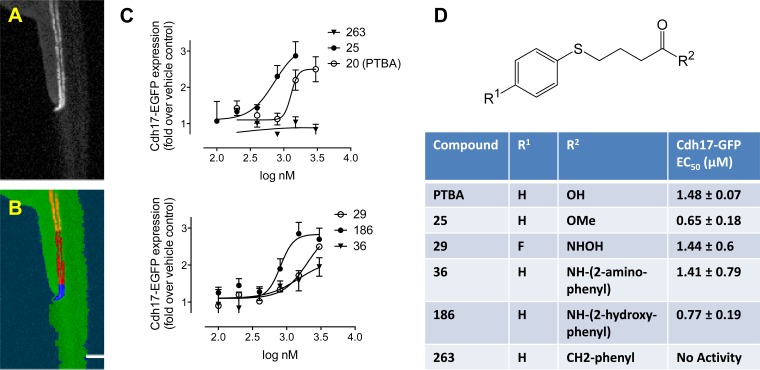
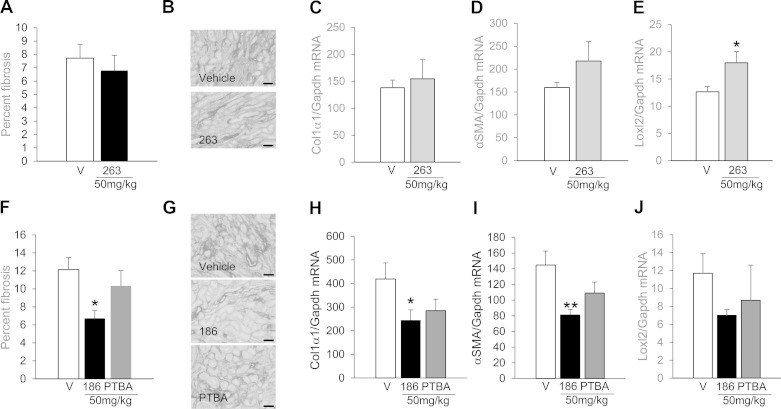
To identify PTBA analogs with more favorable activities than M4PTB/UPHD25 in accelerating recovery and reducing postinjury fibrosis after AKI, we synthesized and tested a new series of PTBA analogs in zebrafish EKPC assays. Based on our finding that PTBA and the PTBA methyl ester UPHD25 expand zebrafish EKPCs by ISH (9, 23), we screened a panel of PTBA analogs for effects on expansion of the lhx1a mRNA-positive embryonic kidney fields (9). Sixty-four PTBA analogs were screened and 20 were found to be active (estimated EC50 >3 μM, Supplemental Table). These compounds contained structural modifications in the benzene ring (R1, predicted to enhance stability) and modifications to the acid functionality with different zinc-chelating moieties (R2, predicted to increase HDAC inhibition) (13) (see Fig. 1D). Representative active analogs that contained an ester (UPHD25), a hydroxamic acid (UPHD29), two benzamides (UPHD36 and 186), as well as an inactive ketone lacking a zinc-chelating moiety as a negative control (UPHD263), were further evaluated further using the CNT EKPC assay. The CNT assay uses the transgenic zebrafish line Tg(cdh17:EGFP)pt305 and complements the ISH assay by providing an independent, quantitative measure of changes to the size of the kidney field in larvae exposed to compounds for 48 hpf (Fig. 1, A and B) (23). Using this assay, we measured the EC50 for expansion of the kidney field for each compound (Fig. 1, C and D). In addition to PTBA (EC50 = 1.48 μM), we evaluated: 1) UPHD25 (EC50 = 0.65 μM), the PTBA methyl ester (9); 2) UPHD29 (EC50 = 1.44 μM), a fluoro-substituted hydroxamic acid analog of PTBA; 3) UPHD36 (EC50 = 1.41 μM), an analog in which the acid was replaced with a 2-aminoanilide moiety; and 4) UPHD186 (EC50 = 0.77 μM), an analog in which the acid was replaced with a 2-hydroxyanilide moiety (Supplemental Table, Fig. 1, C and D). In addition, as anticipated based on the ISH lhx1a assay, the inactive ketone, UPHD263, had no effect on expansion of the cdh17:EGFP domain in the CNT assay.
Fig. 1.
Quantitation of kidney organ expansion by automated imaging. Tg(chd17:EGFP) transgenic zebrafish (A) were treated for 48 h at the 256–512 cell stage with vehicle (0.5% DMSO) or specified compounds, followed by washout. At 72-h postfertilization (hpf), embryos were dechorionated and imaged in 96-well plates (8 embryos/condition). Images were acquired in the green fluorescent protein (GFP) channel and analyzed by a Definiens Cognition Network Technology (CNT) rule set. Starting from the original image (A), the rule set successively detected the entire embryo and the fluorescently labeled kidney (B, orange). Further subsegmentation identified the cloaca based on brightness and proximity to the zebrafish edge (B, blue), enabling final measurements to be made in a defined length segment relative to the cloaca (B, red). C: quantitation of kidney expansion by UPHD20, 25, 29, 36, 186, and 263. Images were analyzed by CNT, and total transgene expression in the tubular segment was calculated. Each data point represents the average ± SE of 3 independent experiments. D: structure of 4-(phenylthio)butanoic acid (PTBA) analogs with sites of modification (R1 and R2) and EC50 for compounds UPHD20 (PTBA), 25, 29, 36, 186, and 263.
PTBA Analog Efficacy in Zebrafish AKI Assays
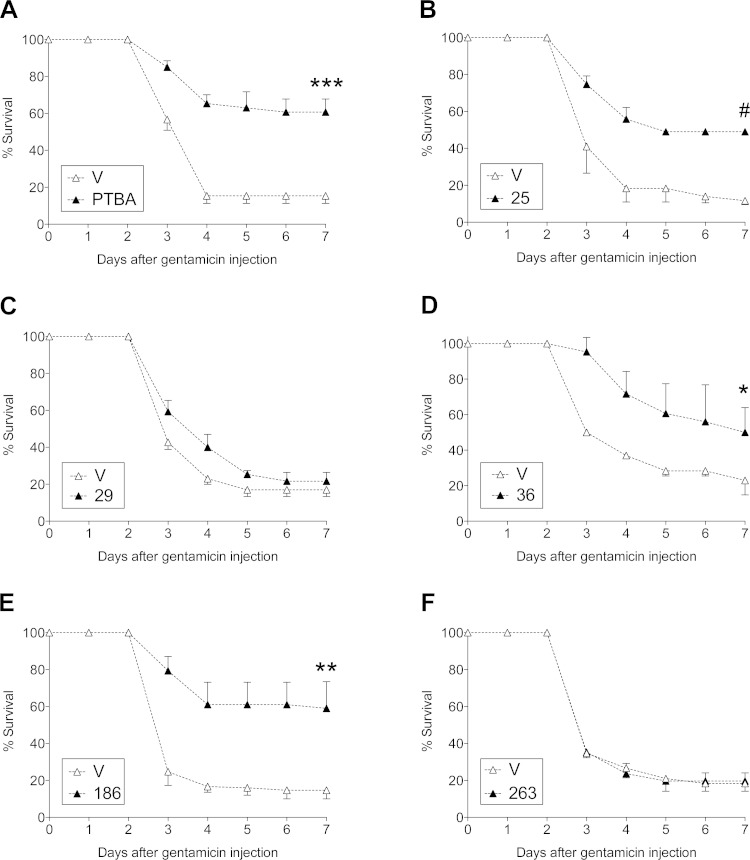
Previous studies using our zebrafish ISH assay indicate that it predicts efficacy in mouse models of AKI (7, 20). However, it was impractical to screen the large number of positive compounds identified in the EKPC assay in mice. To address this, we utilized a model of gentamicin-induced AKI in zebrafish larvae and scored for larval survival. Since gentamicin toxicity is specific to renal epithelial cells and the inner ear due to selective uptake in these organs (16), and edema is a sign of renal dysfunction (10), we selected edematous larvae for treatment and scored for organism survival as an initial indicator of renal injury and repair following compound treatment, as previously described (6, 7). We evaluated PTBA, UPHD25, 29, 36, 186, and 263 for efficacy in improving survival of zebrafish larvae after gentamicin-induced AKI at one-quarter their MTD (Supplemental Figure) (7). In this model, we treated zebrafish larvae with a single dose of compound 2 days after gentamicin injection and assayed for survival over the next 4 days. While PTBA, UPHD25, 36, and 186 significantly improved postinjury survival, UPHD29 and 263 showed no effect (Fig. 2).
Fig. 2.
Augmented survival after treatment with PTBA analogs in zebrafish larval acute kidney injury (AKI). A: survival after gentamicin-induced AKI. Gentamicin-injected larvae were treated with either 1% DMSO vehicle (V; △) or at one-quarter maximum tolerated dose (MTD) for compounds (▲): PTBA (1 μM; A), UPHD25 (2 μM; B), UPHD29 (2 μM; C), UPHD36 (8 μM; D), UPHD186 (4 μM; E), and UPHD263 (4 μM; F) at 2 days post-gentamicin injection (dpi). Key indicates PTBA and UPHD identification numbers. The survival rate was scored at 4, 5, 6, and 7 dpi. Values are pooled from 3 survival assays and expressed as means ± SE by 2-tailed t-test on 7 dpi. *P < 0.05. **P < 0.01. ***P < 0.005. #P < 0.0001.
PTBA Analog Efficacy in Accelerating Recovery and Reducing Renal Fibrosis After AKI in Mice
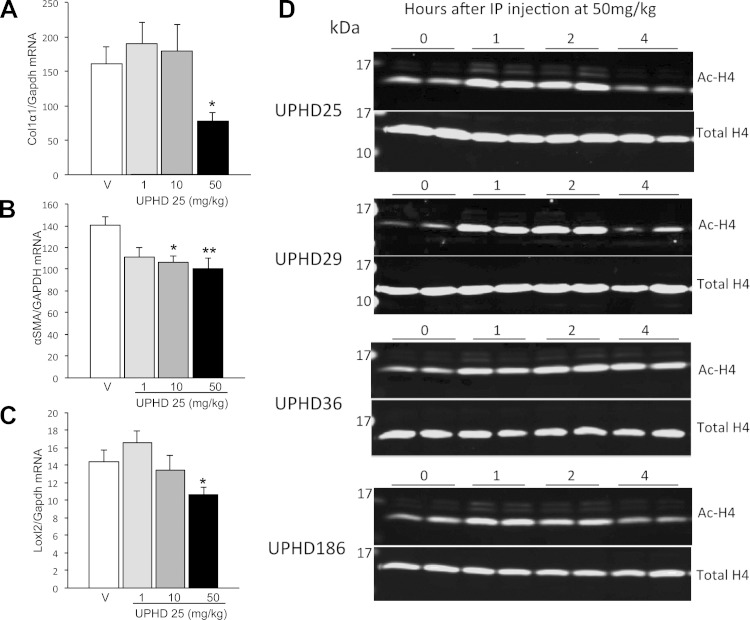
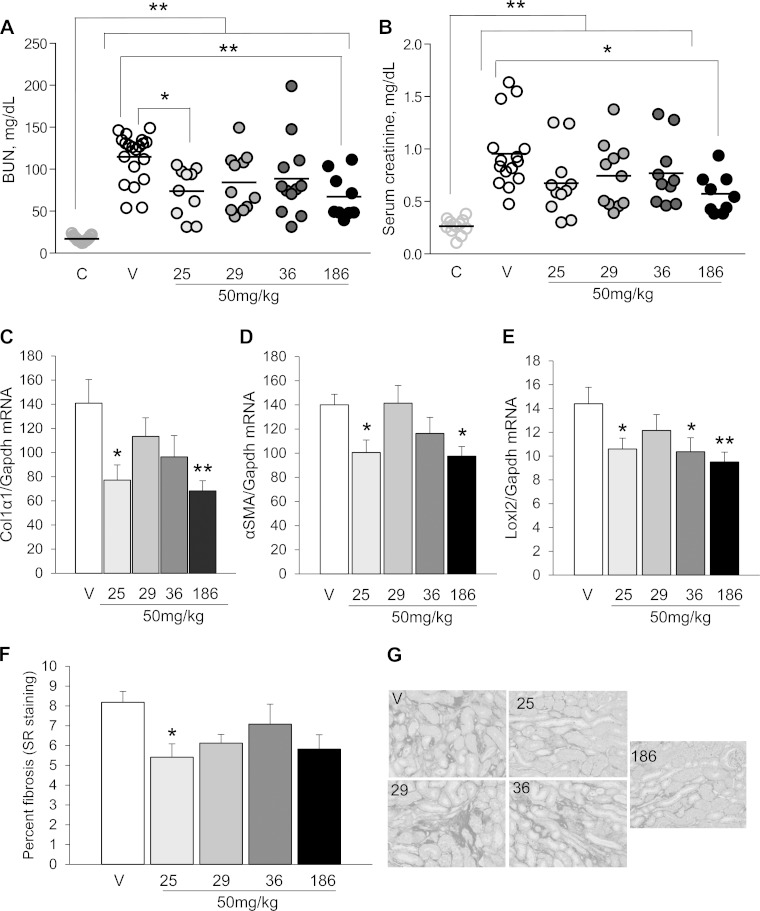
To determine whether results from the larval AKI screen were predictive of efficacy in mice, we first evaluated the efficacy of UPHD25, 36, and 186, which showed activity in the zebrafish EKPC and AKI assays, and of UPHD29, which showed activity in zebrafish EKPC but not the zebrafish AKI assays, in a mouse model of IR-AKI. To determine optimal dosing, we initially evaluated the effects of UPHD25 at 1, 10, and 50 mg/kg administered via ip injection on days 1–7 on expression of fibrosis markers in the injured kidney 28 days after unilateral IR-AKI. Dose ranges were based on previous experience treating mice with UPHD25 (7, 20). There was a dose-dependent reduction in expression of the fibrosis markers Col1α1, α-SMA, and LoxL2 mRNAs, with maximal effects at 50 mg·kg−1·day−1 of UPHD25 (Fig. 3, A–C). Since we have previously shown that PTBA analogs are histone deacetylase inhibitors (HDACi) that increase levels of hyperacetylated histones in the kidney in mice (7), we compared the effects of a single 50 mg/kg ip dose of the PTBA analogs UPHD25, 29, 36, and 186 on renal histone acetylation (Fig. 3D). All of the PTBA analogs increased renal histone H4 acetylation within an hour of treatment. Histone H4 acetylation returned to baseline 4 h after treatment with UPHD25, 29, and 186, but persisted up to 4 h with UPHD36. Based on these results, we evaluated the effects of PTBA analogs at a dose of 50 mg·kg−1·day−1 on days 1–7 after injury for all studies, unless otherwise indicated. We performed unilateral IR-AKI followed by a contralateral nephrectomy 8 days after injury to evaluate functional recovery and postinjury fibrosis, as described (7, 24). UPHD25- and 186-treated mice have reduced blood urea nitrogen (BUN) compared with vehicle 24 h after the contralateral nephrectomy (Fig. 4A). UPHD186 also reduced serum creatinine 24 h after contralateral nephrectomy (Fig. 4B). The relatively high BUN levels that we observed in some of the mice relative to changes in serum creatinine may reflect a degree of dehydration that occurs at the time these assays were performed, 24 h after contralateral nephrectomy surgery. It is well recognized that dehydration preferentially increases BUN vs. serum creatinine values after AKI (25). In contrast, UPHD36 had no effect on BUN or serum creatinine after AKI. Renal fibrosis markers showed a reduction in Col1α1, α-SMA, and LoxL2 mRNA in UPHD25- and 186-treated mice 28 days after injury (Fig. 4, C–E). There was also a reduction in renal fibrosis assessed by Sirius red staining in UPHD25-treated mice (Fig. 4, F and G). Consistent with effects on BUN and serum creatinine, UPHD29 had no effect on postinjury renal fibrosis, while UPHD36 reduced expression of one of three fibrosis markers but had no effect on Sirius red staining.
Fig. 3.
Dosing with PTBA analogs in mice. A–C: dose-dependent effects of UPHD25 on postinjury fibrosis after ischemia-reperfusion (IR)-induced AKI (IR-AKI). Male BALB/c mice underwent left-sided renal pedicle clamping for 31 min to induce IR-AKI, were treated for 7 days with vehicle or 1, 10, or 50 mg/kg UPHD25 intraperitoneally (ip) daily for 7 days starting 24 h after injury, as indicated. Injured kidneys were harvested 28 days after injury and evaluated for expression of renal fibrosis markers Col1α1, α-SMA, and LoxL2 mRNA relative to Gapdh mRNA control. Values are means ± SE (injured vehicle controls, n = 17; 1 mg/kg, n = 7; 10 mg/kg, n = 8; 50 mg/kg, n = 11 mice) by 1-way ANOVA (P < 0.05) with Dunnett's correction for multiple between-group testing. *P ≤ 0.05, **P < 0.01 vs. injured vehicle control, as indicated. D: kinetics of renal histone hyperacetylation after ip injection of PTBA analogs in mice. CD1 mice were injected once with 50 mg/kg of PTBA analogs UPHD25, 29, 36, and 186 ip, as indicated. Kidneys were isolated at different time points for histone extraction and analysis of histone H4 acetylation. Fluorescence immunoblots for total renal histone H4 and acetylated histone H4 K5/8/1/16 (Acetyl H4) were captured using the Odyssey Infrared Imaging System. Replicates for each of the time points obtained from different mice are shown.
Fig. 4.
UPHD25 and 186 accelerate functional recovery and postinjury fibrosis after IR-AKI. Male BALB/c mice underwent left-sided renal pedicle clamping for 31 min to induce IR-AKI and were treated with vehicle or 50 mg/kg UPHD25, 29, 36, or 186 ip daily for 7 days starting 24 h after injury. A and B: functional recovery. Blood urea nitrogen (BUN; A) and serum creatinine (B) were measured at day 9, 1 day later after right nephrectomy to assess functional recovery (nos. of mice for A and B: uninjured mice, n = 12/15, day 9 after injury; vehicle controls, n = 20/15; UPHD25, n = 10/11; UPHD29, n = 11 each; UPHD36, n = 12/10; UPHD186, n = 9 each). Individual data points and means for each group are shown. C–G: renal fibrosis 28 days after IR-AKI. C–E: expression of renal fibrosis markers Col1α1, α-SMA, and LoxL2 mRNA relative to Gapdh mRNA control. F and G: Sirius red staining for interstitial collagen. F: quantification of Sirius red staining (% total area). G: representative images for Sirius red-stained tissues (outer medulla; scale bars = 50 μm). C–G: nos. of mice: vehicle controls, n = 11; UPHD25, n = 11; UPHD29, n = 11; UPHD36, n = 10; UPHD186, n = 10. Values are means ± SE. We performed 1-way ANOVA (P < 0.05) with Dunnett's correction for multiple between-group testing. *P < 0.05, **P < 0.01 vs. uninjured control or vs. injured vehicle control, as indicated; C–F vs. vehicle control only.
We next evaluated effects of UPHD263 and PTBA treatment in reducing postinjury fibrosis after IR-AKI. Treatment with UPHD263 on days 1–7 after IR-AKI had no effect on renal fibrosis 28 days after injury (Fig. 5, A and B) and increased expression of only one of three renal fibrosis markers (Fig. 5, C–E). Since UPHD263 was inactive in zebrafish EKPC assays, this suggests that lack of activity in the EKPC assay is predictive of a lack of efficacy in mouse AKI. Since PTBA was active in both the EKPC and larval AKI models, we also compared the efficacy of PTBA with that of UPHD186 after IR-AKI in mice. Unlike UPHD186, PTBA had no effect on postinjury fibrosis and no significant effects on the expression of fibrosis markers 28 days after injury (Fig. 5, F–J). Taken together, these data indicate that lack of activity in zebrafish EKPC and/or larval AKI assays can be used to eliminate PTBA analogs that are ineffective in reducing postinjury fibrosis after mouse IR-AKI. However, these data also indicate that not all of the compounds that show activity in both of the zebrafish assays are effective in reducing renal fibrosis in mouse IR-AKI.
Fig. 5.
UPHD263 and PTBA do not reduce postinjury fibrosis after IR-AKI. Male BALB/c mice underwent left renal pedicle clamping for 31 min and were treated for 7 days with either vehicle or 50 mg/kg compound ip daily for 7 days starting 24 h after injury. Injured kidneys were harvested 28 days after injury and evaluated for markers of renal fibrosis. A–E: UPHD263 (injured vehicle controls, n = 12; UPHD263, n = 10). F–J: UPHD186 and PTBA (injured vehicle controls, n = 7; UPHD186, n = 9; PTBA, n = 9). A–C, F–H: expression of renal fibrosis markers Col1α1, α-SMA, and LoxL2 mRNA relative to Gapdh mRNA control. A and F: percentage of fibrosis in the outer medulla (OM). B and G: representative images of Sirius red-stained tissue section in the OM (scale bars = 50 μm). Values are means ± SE (A–D) by 2-tailed t-test. *P < 0.05, **P < 0.01 and (F–I) 1-way ANOVA (P < 0.05) with Dunnett's correction for multiple between-group testing. *P < 0.05, **P < 0.01 vs. injured vehicle control, as indicated.
PK of PTBA Analogs in Mice
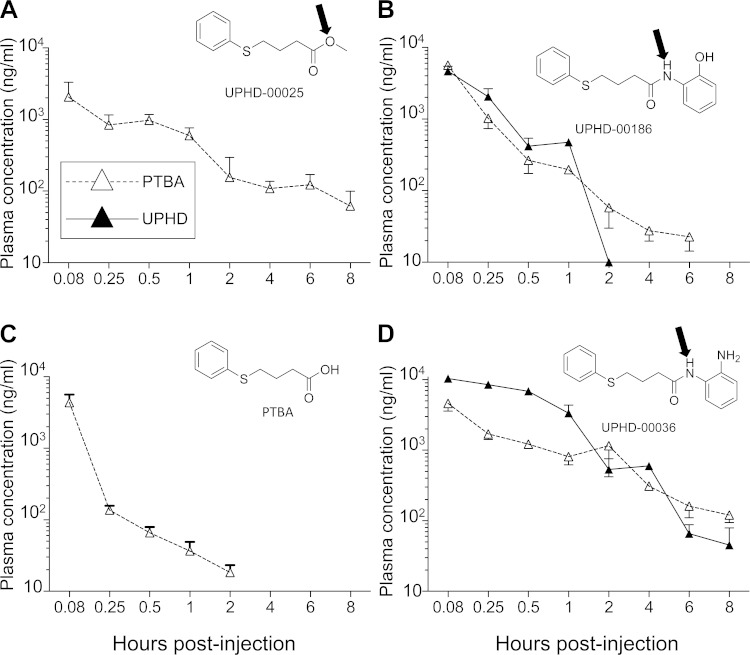
Since biological activity in mice is dependent on absorption, distribution, metabolism, and excretion (ADME) characteristics, we performed a comparative plasma PK study in mice after ip injection of PTBA analogs (Fig. 6, Table 1). PTBA itself was rapidly absorbed with the peak plasma level obtained within 5 min (Table 1). UPHD25 was undetectable after ip injection in mice. However, peak plasma levels of PTBA were detected 5 min after ip injection of UPHD25 (Fig. 6A, Table 1), suggesting that UPHD25 is rapidly bioconverted to PTBA. Also evident was the rapid absorption and metabolic conversion of UPHD186 to PTBA within 5 min (Fig. 6B, Table 1). This suggests UPHD25 and 186 may be acting as PTBA prodrugs, most likely hydrolyzed to the acid, PTBA, by ubiquitous and promiscuous tissue and serum esterase and amidases (Fig. 6, A and B; arrows indicate potential cleavage sites) (21). This is consistent with our finding that UPHD25 was only detectable in spiked mouse plasma samples if the samples were pretreated with an esterase inhibitor (Supplemental Methods 1). In addition, our observation that PTBA increases survival in zebrafish AKI (Fig. 2A) is consistent with the hypothesis that PTBA is the active metabolite of UPHD25 and 186. However, PTBA itself was ineffective in reducing post-AKI fibrosis in mice (Fig. 5, F–J). It is notable that the area under the curve (AUC) for PTBA was markedly reduced compared with PTBA levels after injecting UPHD25 and 186 (Table 1). Since the peak plasma levels of PTBA after ip injection of UPHD25, 186, and PTBA were similar (Cmax, Table 1), this suggests that the efficacy of UPHD25 and 186 is driven by the AUC, while decreased efficacy of PTBA in mice may result from decreased tissue exposure to PTBA associated with a reduced AUC. Since zebrafish are soaked continuously in PTBA, it is likely that efficacy in zebrafish models reflects greater tissue exposure in this model compared with mice.
Fig. 6.
Pharmacokinetics of PTBA analogs in mice. Male BALB/c mice were injected with a single dose of PTBA analog at 50 mg/kg ip, and plasma samples were drawn at the indicated intervals over the next 8 h. Parent compound (▲) and PTBA metabolite (△) concentrations were determined in ng/ml plasma by liquid chromatography and tandem mass spectrometry (LC-MS/MS). A: UPHD25. No detectable parent compound was detected at any time point up to 8 h after ip injection of UPHD25. Data are shown for the UPHD25 metabolite PTBA only. B: UPHD186. C: PTBA. D: UPHD36. Chemical structures are shown. Potential esterase (UPHD25) and amidase (UPHD36 and 186) cleavage sites are indicated with arrows. Results from 3 mice per compound and time point are shown. Values are expressed graphically as means ± SE of 3 measurements per time point (3 mice).
Table 1.
Pharmacokinetics of plasma PTBA, UPHD25, 36, 186, and 263 after intraperitoneal (ip) injection in mice
| Compound | Matrix | Route | Dose, mg/kg | Tmax, h | Cmax, ng/ml | AUC, h·g−1·ml−1 | T1/2, h |
|---|---|---|---|---|---|---|---|
| PTBA | Plasma | ip | 50 | 0.08 | 4,380.71 | 690.9 | 4.03 |
| UPHD25 | Plasma | ip | 50 | ND | ND | ND | ND |
| PTBA | 0.08 | 2,063.38 | 2,010.89 | 4.51 | |||
| UPHD36 | Plasma | ip | 50 | 0.08 | 10,380.57 | 10,753.71 | 3.82 |
| PTBA | 0.08 | 4,618.54 | 4,811.3 | 2.94 | |||
| UPHD186 | Plasma | ip | 50 | 0.08 | 4,659.77 | 15,32.21 | 0.23 |
| PTBA | 0.08 | 5,630.29 | 1,380.91 | 1.13 | |||
| UPHD263 | Plasma | ip | 50 | 0.08 | 1,031.42 | 366.9 | 1.13 |
| PTBA | ND | ND | ND | ND |
Male BALB/c mice were injected with PTBA analog at 50 mg/kg ip, and plasma samples were drawn at intervals over the next 8 h. ND, not determined. Parent compound and PTBA concentrations were determined in the plasma by LC-MS/MS. Cmax was determined at 0.08-h postinjection. The noncompartmental analysis module in Phoenix WinNonlin was used to derive AUC and T1/2 for the parent and PTBA metabolite. See text for other definitions.
UPHD36 was active in zebrafish EKPC and AKI assays but had only minor effects on fibrosis in mice after AKI. Similar Cmax and AUC levels of PTBA were detected after ip dosing of UPHD36, 25, and 186 (Fig. 6D, Table 1). Like UPHD186, UPHD36 contains an amide group that could be hydrolyzed to PTBA (Fig. 6D). However, the Cmax for the parent UPHD36 was higher than that for UPHD186 (Table 1). This suggests that hydrolysis of UPHD36 to PTBA is less efficient than UPHD186. As expected, we were unable to detect PTBA in the plasma up to 8 h after injection of UPHD263 due to the absence of a hydrolyzable amide (Table 1). Since UPHD263 is inactive in mouse and zebrafish AKI models, these findings suggest that conversion to PTBA is necessary but not sufficient for biological activity in zebrafish and mouse AKI.
Metabolic Stability and Activity of PTBA Analogs In Vitro
To explore the metabolism of PTBA analogs further, we performed in vitro stability and activity (appearance of PTBA) assays using mouse liver and kidney microsome preparations. PTBA was stable in liver and kidney microsome preparations, while UPHD25 and 186 were efficiently metabolized and converted to PTBA when incubated with both liver and kidney microsomes for 50 min (Table 2). Metabolism of parent compounds and the appearance of PTBA were NADPH independent, indicating that UPHD25 and 186 metabolism is not a cytochrome P-450 reaction (30). This contrasts with UPHD36 metabolism, as this is NADPH dependent and occurs at a slower rate than UPHD186, particularly in kidney microsomes (Table 2). These findings lend further support to our hypothesis that UPHD25, 36 and 186 are prodrugs for the active species, PTBA, and differences in efficacy in mice are likely to result from differences in the rates of metabolism and release of PTBA at the site of injury.
Table 2.
Liver and kidney microsome stability and activity
| Remaining After 50 min, μM |
PTBA Measured After 50 min, μM |
|||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Liver + | Liver − | Kidney + | Kidney − | Liver + | Liver − | Kidney + | Kidney − |
| PTBA | 8.0 | 8.1 | 8.6 | 8.9 | N/A | N/A | N/A | N/A |
| UPHD25 | ND | ND | ND | ND | 5.1 | 7.2 | 3.2 | 5.1 |
| UPHD36 | 0.8 | 6.8 | 5.9 | 13* | 0.4 | 0.5 | 0.2 | 0.3 |
| UPHD186 | 0.37 | 0.37 | 0.18 | 0.18 | 2.4 | 6.8 | 2.1 | 5.4 |
Compounds (10 μM) were incubated with liver or kidney microsomes ± NADPH, as indicated. Concentrations of the parent compound and PTBA were determined after 50-min incubation by LC-MS using standard curves. Intact UPHD25 could not be detected using electrospray ionization in positive or negative modes. Experiment was repeated 3 times with similar results.
Concentration within expected error for the calculation.
Delayed Treatment with UPHD186 Reduces Postinjury Fibrosis After IR-AKI
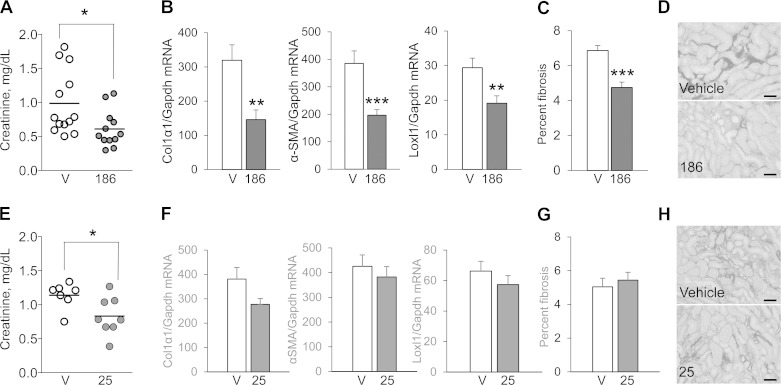
Having established the efficacy of UPHD25 and 186 in accelerating recovery and reducing postinjury fibrosis when administered 24 h after the initiating injury, we evaluated the effects of delayed treatment on IR-AKI outcomes in mice. For these studies, we again performed unilateral IR-AKI followed by a delayed contralateral nephrectomy 8 days after the initial injury, but now PTBA analog treatment was delayed until 3 days after injury and continued for only 4 days. Treatment with UPHD186 3 days after injury reduced serum creatinine, decreased postinjury fibrosis, and reduced expression of all three renal fibrosis markers 28 days after injury (Fig. 7, A–D). In contrast, while UPHD25 reduced serum creatinine values 9 days after injury, delayed treatment with UPHD25 had no effect on renal fibrosis (Fig. 7, E–H).
Fig. 7.
Delayed treatment with UPHD186 accelerates recovery and reduces postinjury fibrosis after IR-AKI. Male BALB/c mice underwent left-sided renal pedicle clamping for 31 min to induce IR-AKI and then were treated with either vehicle or 50 mg/kg UPHD25 or 186 ip daily for 4 days, starting 3 days after injury. Mice underwent a right nephrectomy on day 8, and injured kidneys were harvested 28 days after injury and evaluated for markers of renal fibrosis. A–D: UPHD186 (injured vehicle controls, n = 13; UPHD186 treatment, n = 12). E–H: UPHD25 (injured vehicle controls, n = 7; UPHD25 treatment, n = 8). A and E: serum creatinine values 9 days after injury, 1 day after right nephrectomy to assess functional recovery. Individual data points and means for each group are shown. B and F: expression of renal fibrosis markers Col1α1, α-SMA, and LoxL2 mRNA relative to Gapdh mRNA control. C and D, G and H: Sirius red staining for interstitial collagen. C and G: quantification of Sirius red staining (% total area). D and H: representative images for Sirius red-stained tissues (outer medulla; scale bars = 50 μm). Values are means ± SE by 2-tailed t-test vs. vehicle controls. *P < 0.05. **P < 0.01. ***P < 0.001.
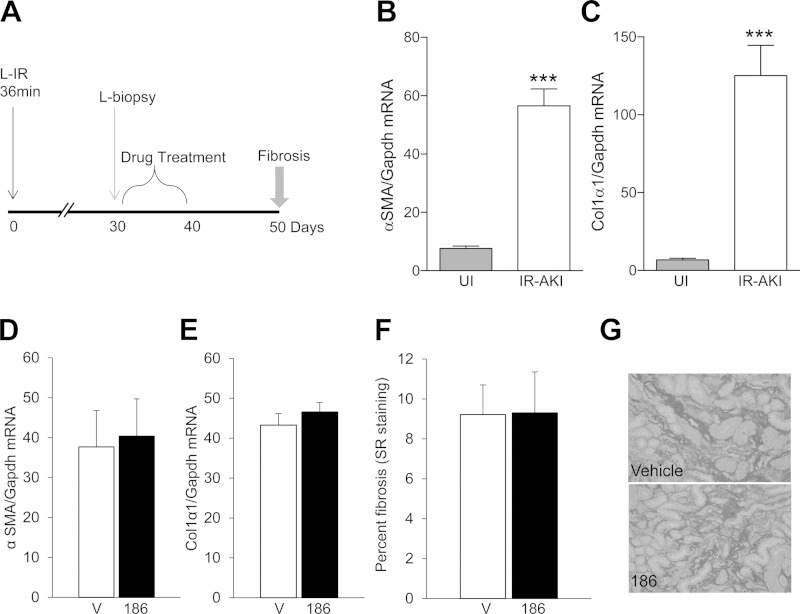
Since delayed treatment with UPHD186 reduces postinjury fibrosis when administered late after the initiating injury, we asked whether UPHD186 could reverse established postinjury fibrosis. For this, we developed a new model of IR-AKI that allowed us to evaluate effects of UPHD186 on established postinjury renal fibrosis. Mice underwent prolonged unilateral renal pedicle clamping (35 min vs. 31 min used in previous studies), followed by an open renal biopsy 30 days after injury to assess baseline postinjury fibrosis. After the renal biopsy, mice were treated with 100 mg/kg UPHD186 ip for 10 days, and injured kidneys were harvested 50 days after initial injury (Fig. 8A). Kidneys harvested from mice 30 days after injury were visibly atrophic, with markedly increased expression of fibrosis markers' Col1α1 and αSMA mRNAs in renal biopsies (Fig. 8, B and C). Renal Col1α1 and αSMA mRNAs were still increased 50 days after injury, but there was no change in expression of either marker in UPHD186-treated mice, and the extent of renal fibrosis as determined by Sirius red staining was no different in UPHD186- and vehicle-treated mice (Fig. 8, D–G). Taken together, these data suggest that UPHD186 is only effective in reducing postinjury fibrosis after IR-AKI when administered during the active regenerative phase after IR-AKI but not once the postinjury fibrosis has been established.
Fig. 8.
UPHD186 does not reverse established fibrosis after severe IR-AKI. A: male BALB/c mice underwent left-sided renal pedicle clamping for 36 min to induce severe IR-AKI and were treated with vehicle or 50 mg/kg UPHD186 ip daily for 10 days starting 30 days after injury, as indicated in the model. Before starting UPHD186, mice underwent an open renal biopsy to assess fibrosis, and kidneys were harvested 50 days after the initial injury to evaluate the effect of delayed treatment with UPHD186 on postinjury fibrosis. B and C: expression of renal fibrosis markers α-SMA and Col1α1 mRNA relative to Gapdh mRNA control in uninjured mouse kidneys (UI), and injured mouse kidneys 30 days after injury before treating with UPHD186 (uninjured, n = 5; IR-AKI, n = 14). D–G: renal fibrosis 50 days after IR-AKI in mice treated with UPHD186 (injured vehicle controls, n = 7; UPHD186 treatment, n = 7). D and E: expression of renal fibrosis markers α-SMA and Col1α1 mRNA relative to Gapdh mRNA control. F: quantification of Sirius red staining. G: representative images for Sirius red-stained tissues (outer medulla; scale bars = 50 μm). Values are means ± SE by 2-tailed t-test vs. controls. ***P < 0.001.
UPHD186 Reduces Renal Fibrosis After UUO in Mice
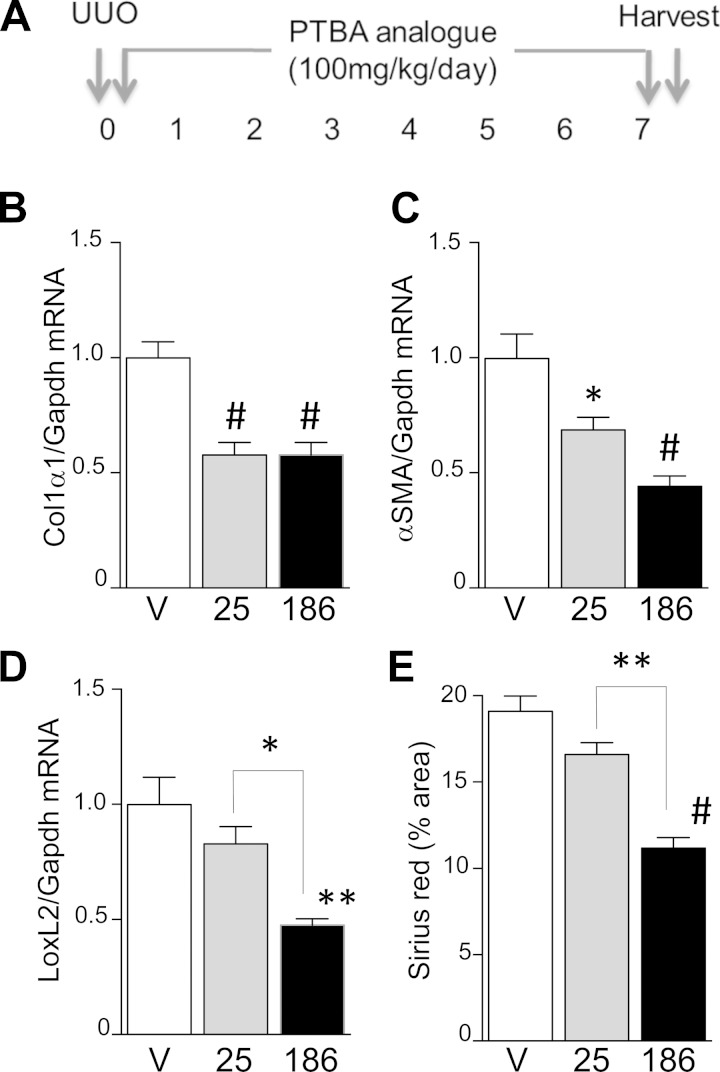
Finally, to determine whether UPHD186 is also effective in other models of AKI, we compared the efficacy of UPHD25 and 186 in reducing renal fibrosis after UUO. For these studies, UPHD25 and 186 were administered at a dose of 100 mg·kg−1·day−1 starting at the time of surgery and continuing for 7 days (Fig. 9A). There was a significant reduction in two of three fibrosis mRNA markers in mice treated with UPHD25 8 days after surgery compared with vehicle control, but no significant reduction in renal fibrosis assessed by Sirius red staining for renal collagen deposition (Fig. 9, B–E). In contrast, UPHD186-treated mice showed a significant reduction in expression of all three fibrosis markers, and there was less renal fibrosis by Sirius red staining in UPHD186- compared with UPHD25-treated mice. These data indicate that UPHD186 is more effective than UPHD25 in reducing renal fibrosis after UUO.
Fig. 9.
UPHD186 prevents renal fibrosis after unilateral ureteric obstruction (UUO). A: male BALB/c mice underwent left-sided ureteric ligation and were treated with vehicle, 50 mg/kg UPHD25, or 186 ip daily for 7 days starting at the time of surgery. Obstructed kidneys were harvested 8 days after surgery and evaluated for markers of fibrosis. B–D: expression of renal fibrosis markers Col1α1, α-SMA, and LoxL2 mRNA relative to Gapdh mRNA control (vehicle controls, n = 6; UPHD25, n = 5–6; UPHD186, n = 5). D: quantification of Sirius red staining (injured controls, n = 5; UPHD25, n = 5; UPHD186, n = 4). Values are means ± SE by 1-way ANOVA (P < 0.05) with Dunnett's correction for multiple testing. *P < 0.05, **P < 0.01, #P < 0.0001 vs. injured vehicle, or vs. UPHD25, as indicated by brackets.
DISCUSSION
Prior studies from our laboratories used phenotypic screens in zebrafish embryos to identify the PTBA analog UPHD25, which accelerates recovery from AKI in zebrafish and mice and reduces postinjury fibrosis in IR and aristolochic acid-induced models of AKI in mice (7, 20). In the current study, we have refined this approach to characterize a phenotypic drug discovery paradigm in which screening was performed using ISH for expression of the EKPC marker lhx1a and an automated fluorescence assay to quantify expansion of kidney structures in zebrafish embryos. This was followed by zebrafish larval AKI survival assays to evaluate the efficacy in reducing injury and accelerating renal repair after AKI. Both models are largely unaffected by drug delivery and absorption problems that often occur in early drug discovery in mice. Using this approach, we identified the PTBA prodrug UPHD186, which accelerates recovery and reduces postinjury fibrosis after IR-AKI and is effective when administered several days after the initiating injury. UPHD186 also reduces postinjury fibrosis after UUO. In contrast, UPHD25 has no effect on renal fibrosis when administered late after injury in IR-AKI and has limited effects on fibrosis after UUO. These findings establish the sequential use of zebrafish EKPC and larval AKI models as an effective drug discovery platform to identify molecules that reduce renal fibrosis after AKI in mice. The ability of UPHD186 to reduce fibrosis when administered late after injury and its efficacy in an obstruction model of AKI represent important new preclinical advances. Agents that can be administered late after the initiation of renal injury are more likely to be effective in human AKI as patients often present late in the course of their illness (1). In addition, agents that are active in multiple models of AKI are more likely to be effective in human AKI, in which pathogenesis is often complex and multifactorial.
A retrospective analysis of FDA-approved drugs over the last decade showed that the majority of first-in-class agents were discovered in phenotypic, not target-based, screens (27). The discovery of UPHD186 validates use of our phenotypic screen in zebrafish to identify compounds that are effective in accelerating recovery and reducing fibrosis when administered several days after the initiating injury. The discovery of UPHD186 was only possible through screening in a living organism since the molecular events mediating recovery are poorly understood. These studies also provide insight into efficacy, since they suggest that differences in activity of PTBA analogs in mice do not depend on the nature of zinc-chelating moieties predicted to increase HDAC inhibition (13), but rather on the efficiency with which they are metabolized to PTBA.
Our studies show that PTBA is active in zebrafish AKI assays and is present as the dominant metabolite in mice treated with UPHD25 and 186. This suggests that PTBA is the active species for the prodrugs UPHD25 and 186. However, when delivered ip, PTBA is not effective in preventing fibrosis in mice with AKI. Since plasma PTBA decreases rapidly after ip injection, it is likely its lack of efficacy results from rapid elimination from the circulation and that UPHD25 and 186 are active because there is increased total exposure to PTBA. However, UPHD36 is also metabolized to PTBA in mice. Unlike UPHD186, UPHD36 undergoes cytochrome P-450-dependent metabolism, and conversion of UPHD36 to PTBA is less efficient than for UPHD25 and 186. This suggests that while UPHD36 is effective in zebrafish AKI assays because metabolite production is not limited by decreasing levels of the parent compound in these assays, UPHD36 is ineffective in mice because there is inefficient conversion to PTBA. On this basis, we propose that differences in the efficacy of UPHD25, 36, and 186 in mouse AKI result from differences in the rate of metabolism and release of PTBA. Since UPHD186 is efficiently converted to PTBA in kidney microsomes, it is likely that increased efficacy of delayed treatment with UPHD186 compared with UPHD25 results from prolonged local release of PTBA in the kidney from UPHD186.
In summary, our studies establish a novel, sequential phenotypic screening approach using zebrafish to identify molecules that accelerate recovery and reduce postinjury fibrosis in mice. Using this approach, we have identified a novel prodrug, UPHD186, which is particularly effective when administered days after the initiating injury in mouse AKI. PK and metabolism studies suggest that the efficacy of UPHD186 in mouse AKI results from its efficient conversion to the active metabolite PTBA in the kidney.
GRANTS
National Institutes of Health (NIH) Grants 1R01 HL093057, 1RC4DK090770, VUMC44339-R/P30DK079307-07, VUMC43321/U24 DK076169, 2R01 DK069403, and the Vanderbilt Center for Kidney Disease (VCKD) supported M. P. de Caestecker. N. A. Hukriede was supported by NIH Grants 2R01 DK069403, 1RC4 DK090770, and 1P30DK079307, and Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant 2R01 HD053287.
DISCLOSURES
M. P. de Caestecker performed consultancy work for NephroGenex.
AUTHOR CONTRIBUTIONS
Author contributions: N.I.S., S.S., L.B.-S., T.N., C.W., T.C., K.P., N.D.G., L.M., P.N.V., M.W.C., and A.V. performed experiments; N.I.S., S.S., L.B.-S., T.N., C.W., T.C., N.D.G., P.N.V., M.W.C., A.V., N.H., and M.P.d.C. analyzed data; N.I.S., S.S., L.B.-S., T.N., T.C., N.D.G., L.M., P.N.V., D.M.H., L.A.V., A.V., N.H., and M.P.d.C. interpreted results of experiments; N.I.S., S.S., L.B.-S., T.N., A.V., N.H., and M.P.d.C. prepared figures; N.I.S., S.S., T.C., L.M., P.N.V., D.M.H., L.A.V., A.V., N.H., and M.P.d.C. edited and revised manuscript; N.I.S., S.S., L.B.-S., T.N., C.W., T.C., K.P., N.D.G., L.M., P.N.V., M.W.C., D.M.H., A.V., N.H., and M.P.d.C. approved final version of manuscript; L.M., P.N.V., M.W.C., D.M.H., L.A.V., N.H., and M.P.d.C. provided conception and design of research; M.P.d.C. drafted manuscript.
Supplementary Material
REFERENCES
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet 380: 756–766, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE 2nd, Perkins RM. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82: 516–524, 2012. [DOI] [PubMed] [Google Scholar]
- 5.Chiba T, Hukriede N, de Caestecker MP. Kidney regeneration: lessons from development. Curr Pathobiol Rep 3: 67–79, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cianciolo Cosentino C, Roman BL, Drummond IA, Hukriede NA. Intravenous microinjections of zebrafish larvae to study acute kidney injury. J Vis Exp: 2079, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C, West J, Korotchenko VN, McDermott L, Day BW, Davidson AJ, Harris RC, de Caestecker MP, Hukriede NA. Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24: 943–953, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groh ED, Swanhart LM, Cosentino CC, Jackson RL, Dai W, Kitchens CA, Day BW, Smithgall TE, Hukriede NA. Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol 21: 794–802, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol 293: F1746–F1750, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288: F923–F929, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen FE, Lewis JA, Cohen SM. The design of inhibitors for medicinally relevant metalloproteins. ChemMedChem 2: 152–171, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kudoh T, Tsang M, Hukriede NA, Chen X, Dedekian M, Clarke CJ, Kiang A, Schultz S, Epstein JA, Toyama R, Dawid IB. A gene expression screen in zebrafish embryogenesis. Genome Res 11: 1979–1987, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet 382: 170–179, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 79: 33–45, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Ma LJ, Nakamura S, Aldigier JC, Rossini M, Yang H, Liang X, Nakamura I, Marcantoni C, Fogo AB. Regression of glomerulosclerosis with high-dose angiotensin inhibition is linked to decreased plasminogen activator inhibitor-1. J Am Soc Nephrol 16: 966–976, 2005. [DOI] [PubMed] [Google Scholar]
- 18.McKee RA, Wingert RA. Zebrafish renal pathology: emerging models of acute kidney injury. Curr Pathobiol Rep 3: 171–181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molitoris BA, Okusa MD, Palevsky PM, Kimmel PL, Star RA. Designing clinical trials in acute kidney injury. Clin J Am Soc Nephrol 7: 842–843, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Novitskaya T, McDermott L, Zhang KX, Chiba T, Paueksakon P, Hukriede NA, de Caestecker MP. A PTBA small molecule enhances recovery and reduces postinjury fibrosis after aristolochic acid-induced kidney injury. Am J Physiol Renal Physiol 306: F496–F504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oda S, Fukami T, Yokoi T, Nakajima M. A comprehensive review of UDP-glucuronosyltransferase and esterases for drug development. Drug Metab Pharmacokinet 30: 30–51, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Sanker S, Cirio MC, Vollmer LL, Goldberg ND, McDermott LA, Hukriede NA, Vogt A. Development of high-content assays for kidney progenitor cell expansion in transgenic zebrafish. J Biomol Screen 18: 1193–1202, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skrypnyk NI, Harris RC, de Caestecker MP. Ischemia-reperfusion model of acute kidney injury and post injury fibrosis in mice. J Vis Exp: 50495, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens LA, Lafayette RA, Perrone RD. Laboratory evaluation of kidney function. In: Diseases of the Kidney and Urinary Tract, edited by Schrier RW. Philadelphia, PA: Lippincott, Williams and Wilkins, 2007. [Google Scholar]
- 26.Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, Hukriede NA. Zebrafish kidney development: basic science to translational research. Birth Defects Res C Embryo Today 93: 141–156, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov 10: 507–519, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Wald R, Quinn RR, Adhikari NK, Burns KE, Friedrich JO, Garg AX, Harel Z, Hladunewich MA, Luo J, Mamdani M, Perl J, Ray JG, University of Toronto Acute Kidney Injury Research Group. Risk of chronic dialysis and death following acute kidney injury. Am J Med 125: 585–593, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, Chu TS, Wu PC, Chao CT, Wang JY, Kao TW, Young GH, Tsai PR, Tsai HB, Wang CL, Wu MS, Chiang WC, Tsai IJ, Hu FC, Lin SL, Chen YM, Tsai TJ, Ko WJ, Wu KD. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80: 1222–1230, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Luo G, Ding X, Lu C. Preclinical experimental models of drug development and disposition in drug discovery and development. Acta Pharma Sinica B 2: 549–561, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.