Abstract
Diuretics acting on specific nephron segments to inhibit Na+ reabsorption have been used clinically for decades; however, drug interactions, tolerance, and derangements in serum K+ complicate their use to achieve target blood pressure. ROMK is an attractive diuretic target, in part, because its inhibition is postulated to indirectly inhibit the bumetanide-sensitive Na+-K+-2Cl− cotransporter (NKCC2) and the amiloride- and benzamil-sensitive epithelial Na+ channel (ENaC). The development of small-molecule ROMK inhibitors has created opportunities for exploring the physiological responses to ROMK inhibition. The present study evaluated how inhibition of ROMK alone or in combination with NKCC2, ENaC, or the hydrochlorothiazide (HCTZ) target NCC alter fluid and electrolyte transport in the nephron. The ROMK inhibitor VU591 failed to induce diuresis when administered orally to rats. However, another ROMK inhibitor, termed compound A, induced a robust natriuretic diuresis without kaliuresis. Compound A produced additive effects on urine output and Na+ excretion when combined with HCTZ, amiloride, or benzamil, but not when coadministered with bumetanide, suggesting that the major diuretic target site is the thick ascending limb (TAL). Interestingly, compound A inhibited the kaliuretic response induced by bumetanide and HCTZ, an effect we attribute to inhibition of ROMK-mediated K+ secretion in the TAL and CD. Compound A had no effect on heterologously expressed flow-sensitive large-conductance Ca2+-activated K+ channels (Slo1/β1). In conclusion, compound A represents an important new pharmacological tool for investigating the renal consequences of ROMK inhibition and therapeutic potential of ROMK as a diuretic target.
Keywords: hypertension; mechanism; pharmacology; potassium, sodium
approximately one in three american adults has hypertension, an insidious disorder that increases the risk of stroke, heart failure, and kidney disease. Diuretics are prescribed to help patients reach a target blood pressure of <140/90 mmHg. Loop, thiazide, and K+-sparing diuretics work by inhibiting net Na+ and/or Cl− reabsorption in specific nephron segments, thereby reducing the osmotic driving force for water reabsorption in the collecting duct (CD). Loop diuretics (e.g., bumetanide) inhibit the Na+-K+-2Cl− cotransporter (NKCC2) expressed in the thick ascending limb (TAL) of Henle, whereas thiazide diuretics (e.g., HCTZ) inhibit the NaCl cotransporter (NCC) which is expressed in the distal convoluted tubule (DCT) and connecting tubule (CNT). The K+-sparing diuretics amiloride and benzamil inhibit the epithelial Na+ channel, ENaC, which is expressed in the distal nephron, including the CD. Benzamil is a more specific blocker of ENaC than amiloride, since amiloride at higher doses also inhibits the type 3 Na/H exchanger (NHE3) in the proximal tubule (PT). Inhibition of NKCC2 or NCC impairs NaCl reabsorption in the TAL or DCT, respectively, leading to increased delivery of Na+ and fluid to the distal nephron. The ensuing increase in Na+ reabsorption in the CD depolarizes the luminal membrane potential and thus increases the electrochemical driving force for K+ secretion into the urine. Increased fluid delivery specifically stimulates K+ secretion through flow-sensitive large-conductance Ca2+-activated K+ (BK) channels. Thus loop or thiazide diuretic use leads to urinary K+ excretion and hypokalemia (2).
ROMK is broadly expressed in the nephron and plays critical roles in the regulation of fluid and electrolyte transport. In the TAL, ROMK recycles K+ into the tubule fluid so that the concentration of K+ does not limit the activity of NKCC2. In principal cells of the CD, ROMK constitutes the major pathway for regulated K+ excretion and contributes to the driving force for Na+ reabsorption by ENaC. A complete loss of ROMK function causes antenatal Bartter's syndrome, a life-threatening renal tubulopathy characterized by polyuria, hypokalemic metabolic alkalosis, and low blood pressure (8). However, a partial loss of ROMK function lowers blood pressure without causing Bartter's syndrome (5). These observations have raised the possibility that ROMK represents a new target for diuretics. Pharmacological inhibition of ROMK in the TAL is expected to starve NKCC2 of luminal K+ and indirectly inhibit Na+ reabsorption in that segment. ROMK inhibition in principal cells of the CD is postulated to depolarize luminal membrane potential and reduce the electrochemical driving force for Na+ reabsorption via ENaC. Thus, in theory, a ROMK inhibitor is expected to impair Na+ reabsorption in two different nephron segments, which should induce a more robust natriuretic response than that observed with conventional diuretics acting on single-nephron segments. ROMK inhibition is also expected to limit urinary K+ excretion in the CD.
In the present study, a newly developed ROMK inhibitor, termed compound A, was administered alone or in combination with bumetanide, HCTZ, amiloride, or benzamil to evaluate how acute ROMK inhibition alters fluid and electrolyte transport in the kidney. The results suggest that the major diuretic target site of ROMK inhibition is the TAL, whereas the K+-sparing effects are likely due to inhibition of ROMK in the TAL and CD.
METHODS
Chemical synthesis.
6-Nitro-2-[(6-nitro-1H-benzimidazol-2-yl)methoxymethyl]-1H-benzimidazole hydrochloride (VU591) was purchased from Sigma-Aldrich or Tocris. 5-(2-(4-(2-(4-(1H-tetrazol-1-yl) phenyl) acetyl) piperazin-1-yl) ethyl)isobenzofuran-1(3H)-one (compound A) was synthesized locally.
Animal dosing and urine collection.
All studies involving animals were approved by Institutional Animal Care and Use Committee. Rats (250–300 g) were allowed to acclimatize to single housing in metabolic cages for 2 h before an experiment. Access to food and water was restricted during experiments. Rats were given drug or vehicle (10% ethanol+40% PEG400+50% saline) by oral gavage (PO). Dose-escalation experiments were performed for individual inhibitors to establish maximally effective doses, as determined by urine volume measurement. After 30 min, voiding was induced by giving each animal a saline load (18 ml/kg by oral gavage), and urine was collected at 2- and 4-h time points. Urine samples were measured, centrifuged, aliquoted, and frozen at −80°C until analyzed.
Urinalysis.
Urine Na+ and K+ concentrations were measured by flame photometry (model 943, Instrumentation Laboratories, Lexington, MA). Samples were diluted 1:1 with distilled water to readings within the working range of 1–200 mM for both cations.
Whole-cell patch-clamp analysis of BK channel activity in HEK293 cells.
BK channel activity was measured essentially as described previously (1).
Statistical analysis.
The results are presented as means ± SE, and the statistical analyses were performed using an unpaired two-tailed Student's t-test.
RESULTS
VU591 does not induce diuresis in rats.
VU591 was the first publically disclosed small-molecule inhibitor of ROMK that is sufficiently potent (IC50 = 240 nM) and selective (over >70 potential off-targets) to enable in vivo experiments (1). We administered VU591 orally at a dose of 100 mg/kg, which is near the solubility limit of VU591. Urine output over a 4-h period increased from 2.23 ± 0.13 ml/100 g body wt (BW) in control animals treated with vehicle alone to 4.00 ± 0.41 ml/100 g BW in animals treated with bumetanide (25 mg/kg; positive control). However, VU591 had no significant (P > 0.05) effect on urine output (2.59 ± 0.28 ml/100 g BW). We therefore employed an in vivo active ROMK inhibitor, termed compound A (3) for the remainder of the studies.
Effects of compound A and diuretics on urine output.
Compound A inhibits ROMK with an IC50 of ∼90 nM and is selective for ROMK over Kir2.1, Kir2.3, Kir4.1, Kir7.1, and hERG K+ channels (3). Garcia et al. (3) reported that compound A increases urine output and Na+ excretion in rats and dogs and does so without increasing urinary K+ excretion. The mechanisms by which compound A augments renal Na+ and K+ excretion have not been reported.
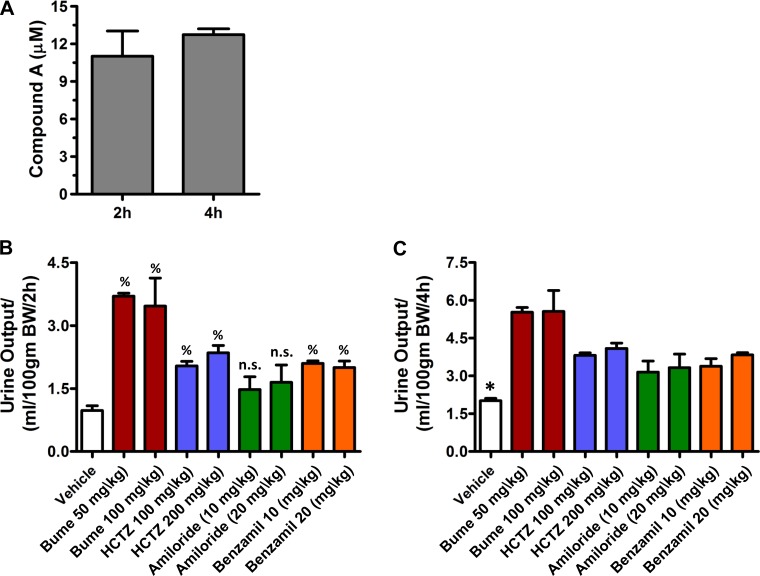
To evaluate the mechanisms of action, volume-loaded rats (see methods) were administered compound A (30 mg/kg) alone or together with bumetanide, HCTZ, amiloride, or benzamil. The maximally effective dose of compound A administered PO in rats is reported to be 10 mg/kg (3). We therefore used a dose three times higher than that to maximize ROMK inhibition. Mass spectrometric analysis revealed that the concentration of compound A in the urine at 2 and 4 h is ∼10 μM (Fig. 1A), indicating that compound A reaches the lumen of the nephron and fully inhibits ROMK. Dose-escalation experiments were performed to define the maximally effective doses of bumetanide (50 mg/kg), HCTZ (100 mg/kg), amiloride (20 mg/kg), and benzamil (10 mg/kg) used in this study (Fig. 1). Urine was collected after 2 and 4 h for the measurement of urine volume and electrolyte (i.e., Na+ and K+) concentrations. In principle, additive effects of compound A should reflect inhibition of ROMK in nephron segments that are not inhibited by the other diuretics, whereas nonadditivity is expected if two drugs are acting on the same segment.
Fig. 1.
Establishment of maximally effective drug doses used in this study. A: concentration of compound A excreted in the urine over 4-h period (n = 3) as measured by mass spectrometry. Total urine was collected and measured at 2-h (B) or 4-h (C) time points following oral administration of bumetanide (50 and 100 mg/kg, n = 3 each), hydrochlorothiazide (HCTZ; 100 and 200 mg/kg, n = 3 each), amiloride (10 and 20 mg/kg, n = 4 each), benzamil (10 and 20 mg/kg, n = 3 each), or the vehicle (n = 6) alone. n.s., Not significant. %P < 0.05, significantly different from vehicle alone. *P < 0.05, significantly different from all other drug treatments.
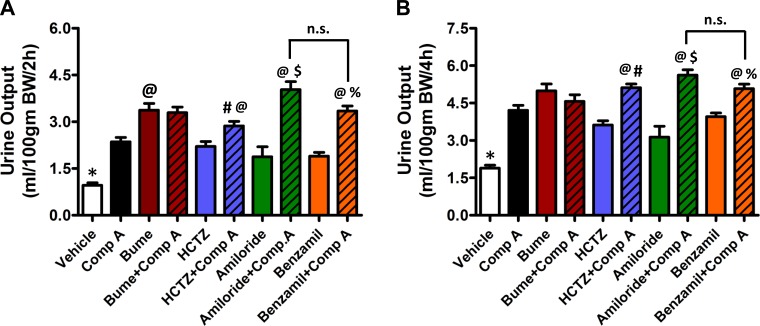
The effects of inhibitors on urine output are summarized in Fig. 2. Consistent with the report by Garcia et al. (3), oral administration of compound A led to a significant increase in urine output. The diuretics used at their maximally effective dose increased urine output with a rank-order potency of bumetanide > HCTZ ∼ amiloride ∼ benzamil. Significant (P < 0.05) additive effects on urine output were observed when compound A was coadministered with HCTZ, amiloride, and benzamil, but not when coadministered with bumetanide.
Fig. 2.
Effect on urine output of compound A alone or in combination with other diuretics. Total urine was collected and measured over 2-h (A) and 4-h (B) period following oral administration of compound A (30 mg/kg, n = 5), bumetanide (50 mg/kg, n = 8), HCTZ (100 mg/kg, n = 9), amiloride (20 mg/kg, n = 6), benzamil (10 mg/kg, n = 6), or vehicle (n = 7) alone or in combination with compound A (bumetanide, n = 4; HCTZ, n = 10; amiloride, n = 5; benzamil, n = 5). *P < 0.05, significantly different from all other drug treatments. @P < 0.05, significantly different from compound A alone. #P < 0.05, significantly different from HCTZ alone. $P < 0.05, significantly different from amiloride alone. %P < 0.05, significantly different than benzamil alone.
Effects of compound A and diuretics on urine Na+ and K+.
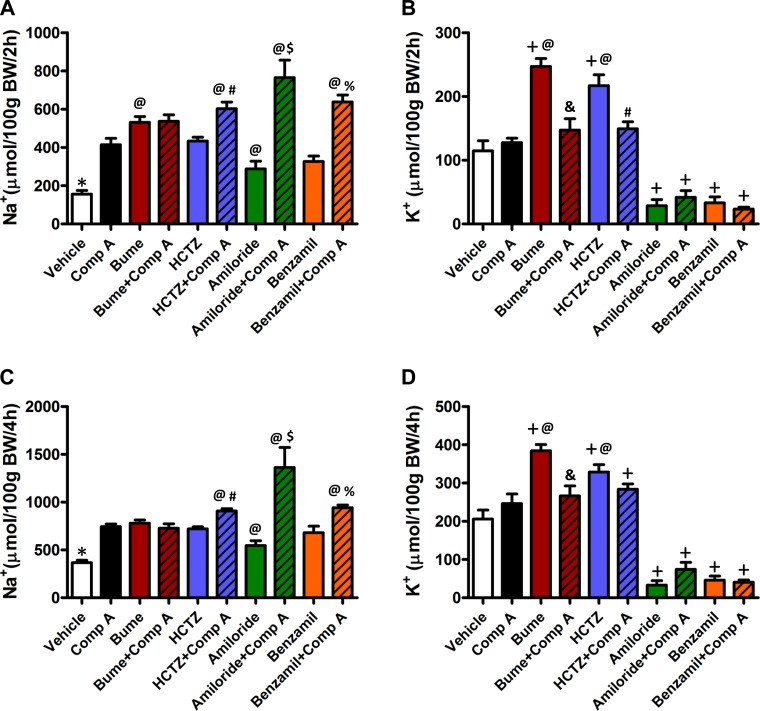
Urine Na+ and K+ concentrations were measured to determine how compound A alters electrolyte transport along the nephron. As shown in Fig. 3, A and C, compound A and all four diuretics significantly (P < 0.05) increased urinary Na+ excretion above that observed in vehicle-treated control animals at the 2- and 4-h time points. There was no significant additive effect of compound A and bumetanide on Na+ excretion; however, significant (P < 0.05) additivity was observed with HCTZ, amiloride, and benzamil.
Fig. 3.
Effect on Na+ and K+ excretion of compound A alone or in combination with other diuretics. Total Na+ excreted over a period of 2 h (A) or 4 h (C) and total K+ excreted over a period of 2 h (B) or 4 h (D) after drug administration were measured using flame photometry. Vehicle (n = 8), compound A (n = 5), bumetanide (n = 8), bumetanide+compound A (n = 4), HCTZ (n = 11), HCTZ+compound A (n = 10), amiloride (n = 4), amiloride+compound A (n = 5), benzamil (n = 7), benzamil+compound A (n = 7). *P < 0.05, significantly different from all other drug treatments. @P < 0.05, significantly different from compound A alone. #P < 0.05, significantly different from HCTZ alone. %P < 0.05, significantly different from benzamil alone. $P < 0.05, significantly different from all other treatments &P < 0.05, significantly different from bumetanide alone. +P < 0.05, significantly different from vehicle alone.
Bumetanide and HCTZ significantly (P < 0.05) increased, whereas amiloride and benzamil decreased urinary K+ excretion, compared with vehicle controls (Fig. 3, B and D). In contrast, compound A did not induce K+ excretion at either time point. No additive effects on K+ excretion were observed when compound A was coadministered with either amiloride or benzamil. Interestingly, however, coadministration of compound A inhibited the K+ excretion induced by HCTZ at the 2-h time point and by bumetanide at both time points (Fig. 3B).
Compound A does not inhibit BK channel activity.
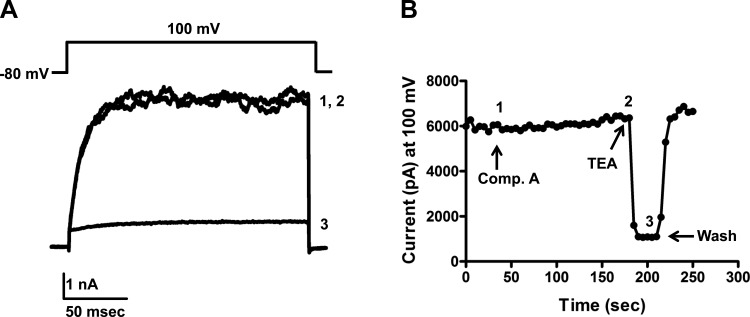
To rule out the possibility that compound A inhibits urinary K+ excretion by inhibiting BK channel activity, we tested whether compound A inhibits Slo1/β1 BK channels expressed in HEK293 cells. Compound A did not inhibit BK-mediated currents measured at 100 mV; indeed, a small but significant (P < 0.05) 10.7 ± 1.7% increase over basal current was observed during bath application of compound A (Fig. 4). The control blocker tetraethylammonium (2 mM) inhibited BK currents by 82.5 ± 3.9% (n = 8).
Fig. 4.
Compound A does not inhibit large-conductance Ca2+-activated K channel (BK) activity. A: representative BK (Slo1/β1) currents evoked from HEK293 cells at 100 mV. Current traces 1 (control), 2 (10 μM compound A), and 3 [2 mM tetraethylammonium (TEA)] were taken from the time course experiment shown in B where indicated with corresponding numbers.
DISCUSSION
The two major findings of this study are 1) that the primary site of diuretic action of compound A is the TAL, and 2) that compound A inhibits diuretic-induced K+ excretion in the TAL and CD. The evidence for these conclusions is as follows. Compound A induces additive effects on Na+ excretion when coadministered with maximally effective doses of HCTZ, amiloride, or benzamil, but not when administered with bumetanide. The simplest interpretation of these data, and the one we favor, is that the observed additivity with compound A reflects ROMK inhibition in nephron segments not targeted by the other three diuretics, namely, the TAL. We previously postulated (2) that ROMK inhibition might also inhibit Na+ reabsorption in the CD by depolarizing the principal cell membrane potential and thereby reducing the electrochemical driving force for Na+ reabsorption via ENaC. Although we cannot rule out a contribution of principal cell ROMK inhibition to the natriuretic response to compound A, the amiloride and benzamil coadministration data indicate that compound A does not completely inhibit ENaC-mediated Na+ reabsorption. For example, if ROMK inhibition completely inhibits Na+ reabsorption by ENaC, then coadministration of amiloride or benzamil would not be expected to produce an additive effect on Na+ excretion, as we observed. A caveat of using amiloride for these studies is that it can also inhibit NHE3-mediated Na+ reabsorption in the PT, which could contribute to the synergistic effect of compound A+amiloride compared with compound A alone. To address this possibility, we performed a separate set of experiments using a maximally effective dose of benzamil, a more specific blocker of ENaC. At both time points, Na+ excretion was higher in animals treated with benzamil+compound A than with compound A alone, suggesting that ROMK inhibition does not abolish ENaC-mediated Na+ transport in the CD. We speculate that the higher Na+ excretion observed with amiloride+compound A, compared with benzamil+compound A, reflects a combination of 1) amiloride-dependent inhibition of PT Na+ reabsorption by NHE3, and 2) compound A-dependent inhibition of Na+ reabsorption by ROMK/NKCC2 in the TAL secondary to inhibition of PT NHE3.
Perhaps one of the most interesting findings of this study is that compound A inhibits K+ excretion induced by bumetanide. This observation could reflect inhibition of ROMK-mediated K+ recycling/secretion in the TAL and/or ROMK-mediated K+ secretion in principal cells of the CD. In support of the first mechanism, Hropot and colleagues (4) demonstrated using free-flow micropuncture techniques that inhibition of NKCC2 with furosemide induces a marked increase in the delivery of K+ (and Na+) to the distal nephron, suggesting K+ recycling/secretion by ROMK in the TAL is a significant source of urinary K+ loss during furosemide treatment (7). If ROMK recycling/secretion in the TAL contributes to K+ wasting during bumetanide treatment, as we suspect it does, then inhibition of ROMK in the TAL by compound A is expected to limit the kaliuresis induced by this drug. The observation that compound A inhibits K+ excretion induced by HCTZ at the 2-h time point suggests that compound A also inhibits ROMK activity in the CD. Increased Na+ delivery to the distal nephron following NCC inhibition by HCTZ will depolarize the principal cell membrane potential and thus increase the driving force for K+ secretion through ROMK. It is therefore plausible that ROMK inhibition in the CD will limit K+ secretion stimulated by HCTZ treatment and Na+ delivery to the distal nephron.
In conclusion, our data suggest for the first time that the major site of diuretic action of compound A is likely the TAL and that the K+-sparing effect of compound A is likely mediated through inhibition of ROMK in the TAL and CD. However, we wish to emphasize the following limitations of this study. First, our interpretations of the data are based on the assumption that different diuretics act on well-defined, discrete nephron segments. Second, these data do not demonstrate directly that compound A inhibits Na+ transport in the TAL. Future studies in isolated, perfused TAL tubules will be used to directly measure the effects of compound A on Na+ transport in the TAL and CD. Third, our data do not exclude the possibility that compound A has effects on vascular tone and renal perfusion pressure. This will also be addressed in future experiments. Nevertheless, this study raises important mechanistic questions that remain to be addressed. First, does compound A inhibit Na+ transport in the CD? Based on the results of the present study, we hypothesize that a robust driving force for Na+ transport still exists in the CD during ROMK inhibition, perhaps as a consequence of the large basolateral K+ conductance (6, 9). Second, does inhibition of ROMK-mediated K+ secretion in the TAL contribute to the K+-sparing property of compound A, or does this reflect inhibition of flow- and/or Na+ load-induced K+ secretion in the CD? The lack of effect of compound A on heterologously expressed BK channel activity suggests that inhibition of flow-stimulated K+ secretion does not play a role. Third, does the establishment of hypertension or changes in dietary Na+ and/or K+ alter the efficacy of compound A on kidney function? Fourth, does long-term administration of compound A lead to remodeling of the nephron in a manner analogous to that observed in ROMK−/− mice? Finally, and importantly, is there a therapeutically effective dose of compound A that can be administered chronically without causing a Bartter's syndrome-like phenotype (i.e., hypokalemic metabolic alkalosis)? The development of compound A creates unprecedented opportunities for answering these and other important questions related to the integrative physiology of ROMK function in the kidney.
GRANTS
This work was supported in part by DK082884-03 (J. S. Denton). Compound A was provided by the Vanderbilt Institute of Chemical Biology Synthesis Core, Vanderbilt University (Nashville, TN).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.V.K., C.W.L., L.M.S., and J.S.D. provided conception and design of research; S.V.K. performed experiments; S.V.K. and D.F., analyzed data; S.V.K., D.F., L.M.S., and J.S.D. interpreted results of experiments; S.V.K. and J.S.D. prepared figures; S.V.K., D.F., C.W.L., L.M.S., and J.S.D. drafted manuscript; S.V.K., D.F., C.W.L., L.M.S., and J.S.D. edited and revised manuscript; S.V.K., D.F., C.W.L., L.M.S., and J.S.D. approved final version of manuscript.
REFERENCES
- 1.Bhave G, Chauder BA, Liu W, Dawson ES, Kadakia R, Nguyen TT, Lewis LM, Meiler J, Weaver CD, Satlin LM, Lindsley CW, Denton JS. Development of a selective small-molecule inhibitor of Kir1.1, the renal outer medullary potassium channel. Mol Pharmacol 79: 42–50, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denton JS, Pao AC, Maduke M. Novel diuretic targets. Am J Physiol Renal Physiol 305: F931–F942, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia ML, Priest BT, Alonso-Galicia M, Zhou X, Felix JP, Brochu RM, Bailey T, Thomas-Fowlkes B, Liu J, Swensen A, Pai LY, Xiao J, Hernandez M, Hoagland K, Owens K, Tang H, de Jesus RK, Roy S, Kaczorowski GJ, Pasternak A. Pharmacologic inhibition of the renal outer medullary potassium channel causes diuresis and natriuresis in the absence of kaliuresis. J Pharmacol Exp Ther 348: 153–164, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Hropot M, Fowler N, Karlmark B, Giebisch G. Tubular action of diuretics: distal effects on electrolyte transport and acidification. Kidney Int 28: 477–489, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Ji W, Foo JN, O'Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP. Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koeppen BM. Conductive properties of the rabbit outer medullary collecting duct: outer stripe. Am J Physiol Renal Fluid Electrolyte Physiol 250: F70–F76, 1986. [DOI] [PubMed] [Google Scholar]
- 7.Morgan T, Tadokoro M, Martin D, Berliner RW. Effect of furosemide on Na+ and K+ transport studied by microperfusion of the rat nephron. Am J Physiol 218: 292–297, 1970. [DOI] [PubMed] [Google Scholar]
- 8.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Stanton BA. Characterization of apical and basolateral membrane conductances of rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 256: F862–F868, 1989. [DOI] [PubMed] [Google Scholar]