Abstract
Resistance exercise training (RT) is the most effective method for increasing skeletal muscle mass in older adults; however, the amount of RT-induced muscle growth is highly variable between individuals. Recent evidence from our laboratory and others suggests ribosome biogenesis may be an important factor regulating RT-induced hypertrophy, and we hypothesized that the extent of hypertrophy is at least partly regulated by the amount of RT-induced ribosome biogenesis. To examine this, 42 older adults underwent 4 wk of RT aimed at inducing hypertrophy of the knee extensors (e.g., 2 sets of squat, leg press, and knee extension, 10–12 repetition maximums, 3 days/wk), and vastus lateralis muscle biopsies were performed pre- and post-RT. Post hoc K-means cluster analysis revealed distinct differences in type II myofiber hypertrophy among subjects. The percent change in type II myofiber size in nonresponders (Non; n = 17) was −7%, moderate responders (Mod; n = 19) +22%, and extreme responders (Xtr; n = 6) +83%. Total muscle RNA increased only in Mod (+9%, P < 0.08) and Xtr (+26%, P < 0.01), and only Xtr increased rRNA content (+40%, P < 0.05) and myonuclei/type II fiber (+32%, P < 0.01). Additionally, Mod and Xtr had a greater increase in c-Myc protein levels compared with Non (e.g., approximately +350 and +250% vs. +50%, respectively, P < 0.05). In vitro studies showed that growth factor-induced human myotube hypertrophy is abolished when rRNA synthesis is knocked down using the Pol I-specific inhibitor CX-5461. Overall, these data implicate ribosome biogenesis as a key process regulating the extent of RT-induced myofiber hypertrophy in older adults.
Keywords: skeletal muscle, ribosome, rRNA, c-Myc, Pol I
loss of skeletal muscle mass with aging (i.e., sarcopenia) and disease (e.g., cachexia) is associated with poor clinical outcomes (23, 24) and higher all-cause mortality (44); thus, maximizing mechanisms of muscle regrowth is a high priority. Restoration of muscle mass and contractile function is dependent on an accumulation of various protein pools (myofibrillar, mitochondrial, etc.) within myofibers that leads to overall myofiber hypertrophy. To achieve this, the primary focus to date has been on mechanisms regulating translation initiation. Many have reported acute induction of the relevant signaling pathways in response to various hypertrophy stimuli (protein nutrition, anabolic drugs, exercise) (3, 10, 14, 17, 27, 41), and we have shown that some of these induced signals regulate myofiber hypertrophy (e.g., eIF2Bε) (29). On the other hand, little attention has been given to other potentially rate-limiting processes, including overall ribosomal capacity, which is dependent on efficiency and the number of available ribosomes. Here we tested the hypothesis that induction of ribosome biogenesis plays a central role in muscle regrowth using two human models.
First, we treated atrophied older adults with a stimulus known to induce widely divergent myofiber hypertrophy adaptations across individuals [resistance exercise training (RT)] as we (7) and others (13, 22, 31, 38) have shown. This offers the unique ability to probe potential mechanisms in humans by studying clusters of individuals based on responsiveness (e.g., nonresponders to extreme responders) via K-means cluster analysis. Interrogating ribosome biogenesis via cluster analysis in the current human trial was grounded on several lines of evidence we have published previously with this model, which suggest ribosome biogenesis may be a key regulator of myofiber regrowth: 1) only individuals with an extreme hypertrophic response to RT (Xtr) increased total muscle RNA content within the first 24 h following the initial bout of resistance exercise [while modest (Mod) and nonresponders (Non) did not] (25). Because ∼85% of total RNA in muscle is rRNA (43), this suggests a rapid increase in ribosome biogenesis may be an important factor for maximizing RT-induced muscle growth. 2) Induction (phosphorylation) of p70S6 kinase, which modulates both translation initiation and ribosome biogenesis, was noted after the first exercise stimulus only in the Xtr cluster (29). 3) Via microarray analysis of resting skeletal muscle, we found that the Mod and Xtr clusters had higher baseline levels (vs. Non) of several ribosomal protein mRNAs and higher mRNA levels of two major upstream regulators of ribosome biogenesis, c-Myc and n-Myc (46). Furthermore, we recently reported that older adults, who, on average, realize less RT-induced hypertrophy than gender-matched young (8), display a blunted increase in ribosome biogenesis following a bout of resistance exercise compared with young (42). In addition to our work, a recent study (16) showed the extent of change in an individual's total muscle RNA content was positively correlated with the extent of change in muscle cross-sectional area (CSA) following 8 wk of RT, further suggesting ribosome biogenesis may be an important regulator of human myofiber hypertrophy.
Next, we tested whether ribosome biogenesis is necessary for cellular hypertrophy in human primary myotubes in vitro. Previous studies have used in vitro models to suggest that ribosome biogenesis may be an important factor contributing to muscle cell growth. For example, Nader et al. (34) have shown that FBS-induced myotube hypertrophy is associated with increased total RNA content, suggesting augmented ribosome biogenesis. Inhibition of mammalian target of rapamycin (mTOR) via rapamycin treatment blunted the increase in total RNA and ultimately blunted myotube hypertrophy. These data indicate that growth factor-induced ribosome biogenesis is at least partly controlled by mTOR-dependent increases in rRNA; however, it is not known to what extent this contributes to hypertrophy, since rapamycin treatment also blocks mTOR-mediated increases in translational efficiency. In separate experiments, Armstrong et al. showed that activation of the β-catenin/c-Myc pathway is increased during muscle hypertrophy (5), and is in fact required (6). Given the importance of c-Myc in regulating ribosome biogenesis in all cells, these data strongly suggest ribosome biogenesis is necessary for successful muscle cell hypertrophy. Here, we directly tested this in human myotubes during FBS-induced hypertrophy in the absence or presence of a chemical inhibitor that prevents ribosome biogenesis by specifically blocking de novo Pol I-mediated transcription of rDNA to pre-rRNA. Based on our findings, we suggest the induction of ribosome biogenesis does indeed play a central role in myofiber hypertrophy, which has important implications for the development of treatments to induce muscle regrowth following aging- or disease-related atrophy.
METHODS
Subjects.
Forty-two older adults (age 60–75 yr) were recruited from the Birmingham, Alabama, metropolitan area. Each subject completed a comprehensive physical examination and a diagnostic graded exercise stress test with a 12-lead electrocardiogram before participation in the study. Individuals were excluded for: lidocaine allergy, prescription anticoagulants, acute illness or active infection, chronic end-stage disease, uncontrolled hypertension, unstable or exercise-induced angina pectoris or myocardial ischemia, diabetes mellitus, or any known contraindication to exercise training or testing. Additionally, subjects who were currently adherent to a weight reduction diet, had a body mass index of >30, or had performed regular resistance training during the previous three years were excluded from the study. The study was approved by the Institutional Review Board of the University of Alabama at Birmingham, and all subjects provided written informed consent before participation.
Resistance training protocol.
Subjects underwent 4 wk of RT (3 days/wk) with an emphasis on knee extensor training. The RT program consisted of two sets of 8–12 repetitions for 10 movements, including: machine squat, knee extension, leg press, heel raise, seated overhead press, incline chest press, seated cable row, arm curl, triceps push down, and abdominal flexion. The exercise intensity for each set was 60–75% of the subject's one-repetition maximum strength [1RM; determined before training using our established methods (37)], except for abdominal flexion, in which each set was performed to fatigue. This RT protocol resulted in neuromuscular strength and power adaptations, and also induced modest myofiber hypertrophy, allowing us to study the mechanisms regulating RT-induced muscle growth as the muscle was actively undergoing hypertrophy.
Dietary assessment.
Before beginning RT, subjects met with a dietician to learn how to complete accurate 4-day diet records. Subjects were informed to consume ad libitum and to maintain a fairly consistent intake throughout the 4-wk training program. To supplement dietary protein, all subjects ingested 0.6 g/kg of whey protein isolate (provided by the U.S. Dairy Export Council) on exercise training days. One-half of the whey protein supplement (0.3 g/kg) was ingested before exercise, and the other one-half (0.3 g/kg) was ingested immediately postexercise. Four-day diet records were collected at the beginning of the study across two weekdays and two weekend days in succession to account for any weekend changes in dietary habits. Total energy, macronutrient, and amino acid intakes were determined using Nutrition Data Systems for Research (NDSR software version 5.0, Minneapolis, MN).
Muscle biopsies.
Muscle samples were obtained before (week 0) and after (week 4) the RT protocol. Biopsies were performed under local anesthetic (1% lidocaine) from the vastus lateralis by percutaneous needle biopsy. All visible connective and adipose tissue was removed from the biopsy samples, and muscle samples were snap-frozen (≈30 mg) in liquid nitrogen and stored at −80°C for future protein and RNA analysis. A separate portion for immunohistochemistry was mounted cross sectionally on cork in optimum cutting temperature mounting medium mixed with tragacanth gum and frozen in liquid nitrogen-cooled isopentane.
Immunohistochemistry.
Myofiber type distribution (I, IIa, IIx) and type-specific myofiber size were assessed as previously described (7) via myosin heavy chain (MHC) isoform immunofluorescence microscopy. Briefly, 6-μm muscle serial cross sections were fixed in 3% neutral-buffered formalin at room temperature for 45 min, washed in PBS, and blocked with 5% goat serum for 20 min. Anti-MHC type I (NCL-MHCs, 1:100; NovoCastra Laboratories), anti-MHC type IIa (1:80; University of Iowa Hybridoma Bank), and anti-laminin (VP-L551, 1:80; NovoCastra Laboratories) primary mouse monoclonal antibodies were used to detect type I myofibers, type IIa myofibers, and basal lamina, respectively (type IIx fibers are the remaining unstained fibers). Images used for fiber size analysis were captured at ×10, and images used for myonuclear number analysis were captured at ×20 using an Olympus BX51 fluorescent microscope with an Olympus MagnaFire SP camera (S99810). Image analysis for myofiber type distribution, CSA, and the number of myonuclei/fiber was performed by a technician blinded to age, sex, and time point using Image-Pro Plus 6.0 software as previously described (7, 35).
RNA isolation and analysis.
Muscle samples were pulverized, and total muscle RNA was isolated using Tri-Reagent (Molecular Research Center, Cincinnati, OH) in accordance with the manufacturer's instructions. RNA quantity was determined using a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Rockford, IL), and total RNA content/tissue weight was used as a surrogate marker of rRNA abundance. To more accurately assess rRNA abundance, a subset of RNA samples (n = 19; 5 Non, 10 Mod, and 4 Xtr) with RNA integrity numbers (RIN) that were >5 (average RIN among all samples was ≈6, with no differences between clusters) were analyzed via electrophoretic separation using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). The bioanalyzer software generates an electropherogram with peaks corresponding to the 18S, 28S, and 5S rRNAs. The areas under these peaks were quantified, summed, and divided by tissue weight to obtain measures of rRNA abundance.
Protein isolation and analysis.
Muscle samples were pulverized and homogenized in 6 μl/mg of ice-cold lysis buffer [150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 0.5% Nonidet P-40, 1% deoxycholate, 0.1% SDS, 1% Triton X-100, and 5 mM EDTA] with protease and phosphatase inhibitors (P2714 and P0044; Sigma) and centrifuged at 15,000 g for 40 min at 4°C. The supernatant was assayed for protein content using the bicinchonic acid (BCA) technique with BSA as a standard. Mixed muscle protein lysate (35 μg) was resolved on 4–12% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Membranes were blotted with antibodies from Cell Signaling Technologies (CS; Danvers, MA), Santa Cruz Biotechnology (SC; Dallas, TX), Sigma-Aldrich (St. Louis, MO), or Abcam (AB; Cambridge, MA) against phospho (p)-UBF (upstream binding factor, Ser388) (SC-21637), total UBF (SC-13125), p-retinoblastoma (Rb, Ser780) (CS-3590), total Rb (CS-9309), p-β-catenin (Ser33/37/Thr41) (CS-9561), total β-catenin (CS-8480), Frizzled-1 (Fzd1, AB-187122), and c-Myc (SC-788) to examine differences in upstream regulators of ribosome biogenesis. Additionally, to determine levels of select ribosomal proteins (rp), membranes were probed with antibodies against rpS3 (CS-9538), rpS6 (CS-2217), rpL3 (SC-86828), and rpL7a (CS-2403). Antibodies were used at a 1:1,000 dilution (except for c-Myc and UBF, which were 1:500, and rpS6, which was 1:2,000) in 5% goat serum (monoclonal antibodies) or 2% milk + 2% BSA (polyclonal antibodies). Horseradish peroxidase-conjugated secondary antibody (Thermo Scientific) was used at 1:50,000 (wt/vol) dilution, followed by chemiluminescent detection in a Bio-Rad (Hercules, CA) ChemiDoc imaging system with band densitometry performed using Bio-Rad Quantity One software (version 4.5.1).
Satellite cell isolation and in vitro experimental protocol.
Skeletal muscle satellite cells were isolated from an untrained young adult male (28 yr) according to previously established procedures (30). Briefly, ≈50 mg of muscle tissue were minced, subjected to pronase digestion, preplated to remove fibroblasts, and maintained on collagen-coated tissue culture plates at 37°C humid atmosphere with 5% CO2. The myoblasts obtained from this protocol were grown in DMEM containing 20% FBS, 5 ng/ml fibroblast growth factor, 100 μl/ml streptomycin, and 100 U/ml penicillin until they reached ≈80% confluence. Myoblasts were then switched to differentiation media (DMEM containing 2% horse serum, 100 μl/ml streptomycin, and 100 U/ml penicillin) for 4 days to induce formation of multinucleated myotubes. To examine the role ribosome biogenesis plays in regulating growth factor-stimulated myotube hypertrophy, myotubes were treated for 24 h with either 20% FBS or 20% FBS + CX-5461 (Millipore, Billerica, MA), a chemical inhibitor of Pol I-mediated pre-rRNA transcription. Importantly, CX-5461 does not directly inhibit DNA, mRNA, or protein synthesis, and is not cytotoxic in normal cells, up to a concentration of at least 10 μM (15, 19). Our preliminary experiments showed that a CX-5461 concentration of 1 μM can reduce growth factor-induced increases in rRNA by ∼60% after 24 h, and that a concentration of 5 μM can completely abolish the increase in rRNA. Thus, CX-5461 was reconstituted at a 5 μM concentration in acetic acid, and the myotubes that were treated with only 20% FBS were treated with an equivalent amount of acetic acid (vehicle control).
Following treatment, protein and RNA were isolated from the myotubes (see protocols above). Additionally, myotubes were stained using a myosin heavy chain protein antibody (25 μg/ml, MF-20; Developmental Studies Hybridoma Bank) and Alexa 488 secondary antibody. Cover slips were mounted with Prolong Gold (Life Technologies, Carlsbad, CA) containing 4′,6-diamidino-2-phenylindole for nuclei labeling, and images were captured at ×4 magnification. Myotube size was assessed using Adobe Photoshop CS6 according to previously established procedures (12). A detailed protocol for this analysis is described (2). Myotube total RNA and rRNA abundance were assessed similar to the protocol used for whole tissue (see above). For protein analysis, myotubes were lysed in 150 μl of ice-cold RIPA buffer containing protease and phosphatase inhibitors. Cell lysate was centrifuged at 5,000 g for 5 min at 4°C, and supernatant was assayed using the BCA technique. Before being lysed, myotubes were given a 10-min pulse of puromycin (10 μg/ml, P9620; Sigma) to assess protein synthesis rates using the surface sensing of translation technique (40). Cell protein lysate (15 μg) was resolved on 4–12% SDS-PAGE gels, transferred to polyvinylidene difluoride membranes, and subsequently blotted with an anti-puromycin antibody (1:1,000; Millipore, Billercia, MA) to assess the amount of puromycin incorporation in newly synthesized proteins. Membranes were imaged using a Bio-Rad ChemiDoc imaging system (see above). All in vitro assays were performed in triplicate.
Statistical analysis.
K-means cluster analysis was performed to identify three responder clusters based on the percent change in type II myofiber CSA from week 0 to week 4 (Statistica 12; StatSoft, Tulsa, OK). We defined the clusters as Non, Mod, and Xtr, corresponding to the individual subject's change in CSA (e.g., Non = no change; Mod = modest increase; Xtr = extreme increase). Three by two repeated-measures ANOVA (cluster × time) was used to examine differences between clusters for total RNA, rRNA, signaling proteins, ribosomal proteins, and number of myonuclei/type II fiber from pre- to post-RT. Fisher's least-significant difference post hoc analysis was used to examine any interaction effects. For in vitro experiments, unpaired t-tests were used to assess differences in RNA abundance, protein synthesis rates, and myotube volume between FBS (± CX-5461)-treated and control myotubes. Data are reported as means ± SE, and statistical significance was set at P ≤ 0.05.
RESULTS
K-means cluster.
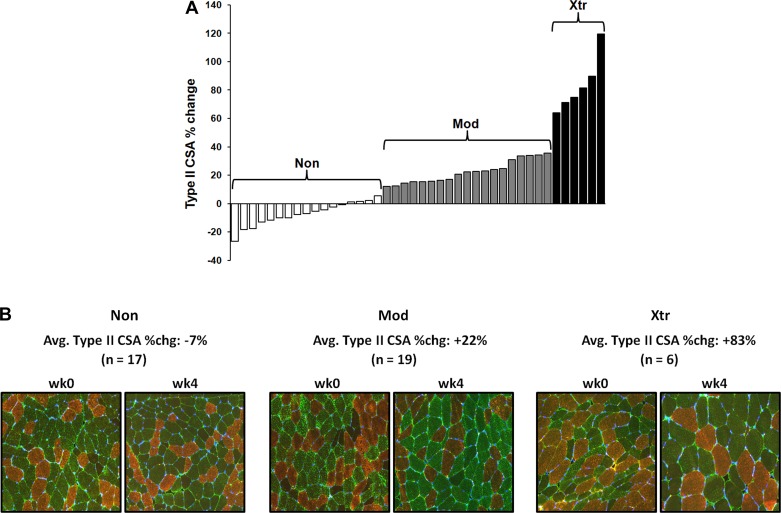
Overall, the 4-wk training program resulted in a significant increase in type II myofiber CSA (+19%, P < 0.001), and a modest type IIx-to-IIa fiber type shift (≈50% of type IIx fibers shifted to IIa) in the entire cohort of subjects. Post hoc K-means cluster analysis identified three distinct clusters based on the percent change in type II myofiber CSA from week 0 to week 4. The average percent change in type II myofiber CSA for Non was −7.4 ± 8.5% (n = 17), Mod was +22.3 ± 8.0% (n = 19), and Xtr was +83.4 ± 19.7 (n = 6) (Fig. 1). There was no difference in baseline type I or type II myofiber size, or myofiber type distribution between clusters (Table 1). Importantly, training intensity did not differ between clusters throughout the course of the 4-wk training period (e.g., all groups maintained ≈75% 1RM leg press training intensity). Additionally, when normalized to body weight, there were no differences in total energy, macronutrient, branched-chain amino acid, or leucine intakes between clusters (Table 1).
Fig. 1.
K-means cluster analysis defined three distinct groups [nonresponders (Non), moderate responders (Mod), extreme responders (Xtr)] based on the percent change in type II myofiber cross-sectional area (CSA) from pre- to post-resistance exercise training (RT) (A). Each histogram bar represents an individual subject's type II CSA %change. B: representative ×10 immunohistochemical images. Type I myofibers are stained red, type IIa are stained green, and type IIx are black (negative).
Table 1.
Myofiber size/type distribution and dietary intake of each cluster
| Non | Mod | Xtr | |
|---|---|---|---|
| Sex | 9F, 8M | 9F, 10M | 1F, 5M |
| Age, yr | 65.9 ± 0.8 | 64.4 ± 0.8 | 67.3 ± 1.7 |
| Myofiber size and type distribution | |||
| Type I CSA, μm2 | |||
| Week 0 | 4,836 ± 512 | 4,010 ± 252 | 4,132 ± 300 |
| Week 4* | 4,672 ± 473 | 4,336 ± 250 | 5,953 ± 659† |
| Type II CSA, μm2 | |||
| Week 0 | 4,344 ± 595 | 3,483 ± 304 | 4,015 ± 720 |
| Week 4* | 3,979 ± 509 | 4,235 ± 358† | 7,371 ± 1,384† |
| Type I distribution, % | |||
| Week 0 | 38.8 ± 2.9 | 38.2 ± 2.7 | 39.7 ± 3.5 |
| Week 4 | 34.1 ± 3.3 | 37.6 ± 2.4 | 36.5 ± 4.8 |
| Type IIa distribution, % | |||
| Week 0 | 49.1 ± 2.6 | 48.5 ± 2.3 | 41.8 ± 5.8 |
| Week 4* | 57.2 ± 3.3† | 55.3 ± 2.6† | 58.8 ± 4.2† |
| Type IIx distribution, % | |||
| Week 0 | 12.0 ± 2.9 | 13.3 ± 2.2 | 18.5 ± 3.4 |
| Week 4* | 8.7 ± 1.8 | 7.1 ± 1.8† | 4.7 ± 1.5† |
| Dietary intake | |||
| Energy, kcal·kg−1·day−1 | 22.5 ± 3.3 | 22.7 ± 1.4 | 24.5 ± 2.2 |
| Carbohydrate, g·kg−1·day−1 | 2.6 ± 0.3 | 2.5 ± 0.2 | 2.8 ± 0.2 |
| Protein, g·kg−1·day−1 | 0.9 ± 0.2 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| Fat, g·kg−1·day−1 | 0.8 ± 0.2 | 0.9 ± 0.1 | 1.1 ± 0.1 |
| BCAA, g·kg−1·day−1 | 0.16 ± 0.03 | 0.18 ± 0.01 | 0.16 ± 0.02 |
| Leucine, g·kg−1·day−1 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 |
Values are means ± SE. Non, nonresponders; Mod, moderate responders; Xtr, extreme responders; M, male; F, female; CSA, cross-sectional area; BCAA, branched-chain amino acids.
Significant change from pre- to post-resistance exercise training, P < 0.01 (main effect of time).
Significantly different from week 0 within the same group, P < 0.01.
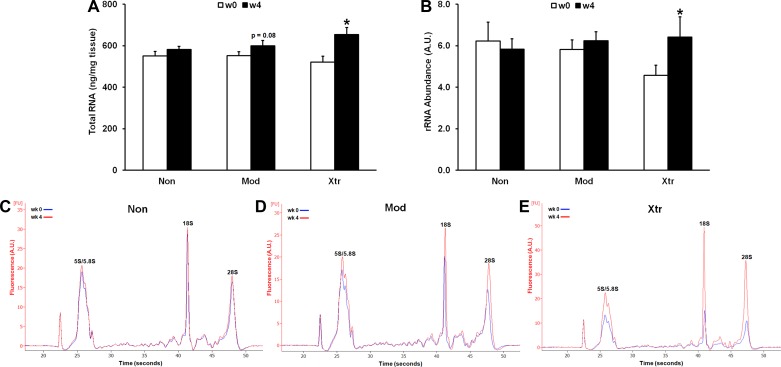
Total RNA and rRNA abundance.
Total RNA and rRNA abundance did not differ between clusters at baseline. Following 4 wk of RT, Non did not increase total RNA content, whereas Mod tended to increase (+9%, P < 0.08) and Xtr significantly increased (+26%, P < 0.01) RNA content, indicating augmented rRNA production in Mod and Xtr. Only Xtr significantly increased rRNA abundance as assessed by the Agilent 2100 Bioanalyzer following 4 wk RT (+40%, P < 0.05) (Fig. 2). While the Mod cluster did not appear to significantly increase rRNA, it is possible that a lower sample size for this assay did not allow us to detect a significant change in rRNA despite Mod tending to increase total RNA content.
Fig. 2.
Cluster differences in whole tissue total RNA content (A) and rRNA content (B) normalized to tissue weight. C–E: representative electropherogram plots with labeled rRNA peaks. *Significantly different from previous time point (P < 0.05).
Ribosomal protein content and markers of ribosome biogenesis signaling.
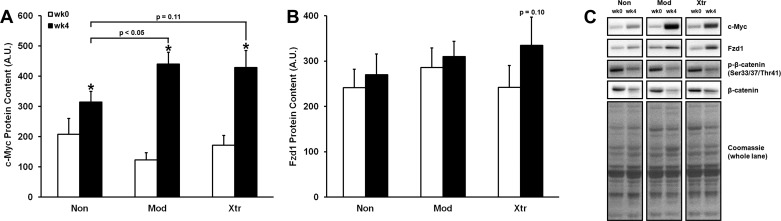
We examined levels of several ribosomal proteins of the 40S and 60S subunits, and, contrary to the changes observed in rRNA, we did not find that levels of any of the ribosomal proteins increased following 4 wk of RT in any of the clusters. In fact, levels of rpS6 and rpL7a actually decreased from week 0 to week 4 (−22 and −27%, respectively, P < 0.05 for both). Interestingly, we found that levels of rpL3, a ribosomal protein recently found to be sensitive to mechanical loading (11), had the highest levels in the Xtr group at baseline (+21% vs. Non, +19% vs. Mod, P < 0.05 and 0.07, respectively), although levels did not significantly change following RT (data not shown).
Overall, several markers of ribosome biogenesis changed from week 0 to week 4. Levels of c-Myc significantly increased among all groups, and follow-up of a cluster × time interaction effect revealed that Mod and Xtr increased c-Myc levels to a greater extent than Non (+357 and +250% vs. +51%, respectively; all P < 0.05) (Fig. 3). Interestingly, both p-β-catenin (Ser33/37/Thr41) and total β-catenin levels decreased from week 0 to week 4 among all clusters (−33 and −28%, respectively, P < 0.001 for both). Upstream of β-catenin, a significant time effect revealed that Fzd1 levels increased from week 0 to week 4, and post hoc analysis showed that Xtr tended to increase the most when compared with the other groups (+38%, P < 0.10).
Fig. 3.
Cluster differences in the abundance of signaling proteins that regulate ribosome biogenesis. Representative immunoblots are shown in C. *Significantly different from previous time point (P < 0.05).
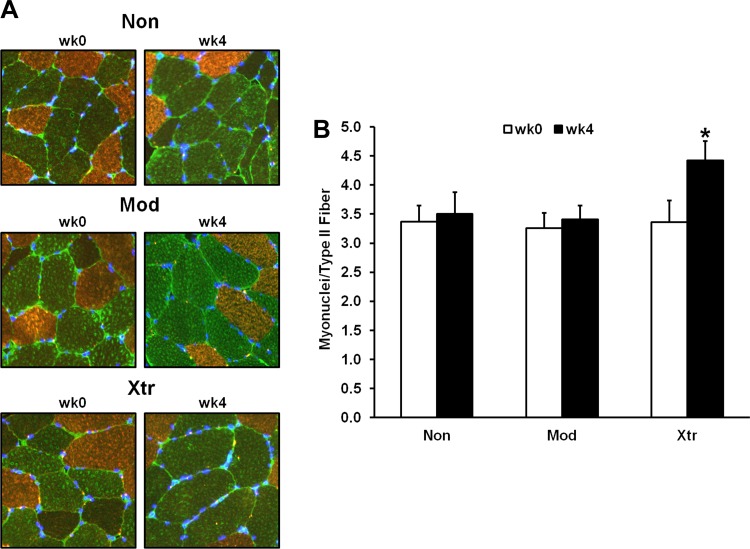
Robust hypertrophy and rRNA accumulation are associated with myonuclear addition.
There were no cluster differences in the baseline number of myonuclei per type II fiber, nor the baseline myonuclear domain (type II fiber CSA divided by myonuclei per fiber). Both Mod and Xtr significantly increased myonuclear domain from week 0 to week 4 (+19 and +40%, respectively, both P < 0.01; data not shown). Post hoc analysis of a cluster × time interaction effect revealed that only Xtr significantly increased the number of myonuclei per type II fiber (+32%, P < 0.01; Fig. 4).
Fig. 4.
Cluster differences in type II myofiber myonuclear addition from pre- to post-RT. A: representative ×20 immunohistochemical images [nuclei labeled with 4′,6-diamidino-2-phenylindole (DAPI)]. B: average no. of myonuclei per type II myofiber within each responder cluser before resistance training (wk0) and posttraining (wk4). *Significantly different from previous time point (P < 0.05). Non, nonresponder; Mod, modest responder; Xtr, extreme responder.
Pol I inhibition blunts growth factor-induced myotube hypertrophy.
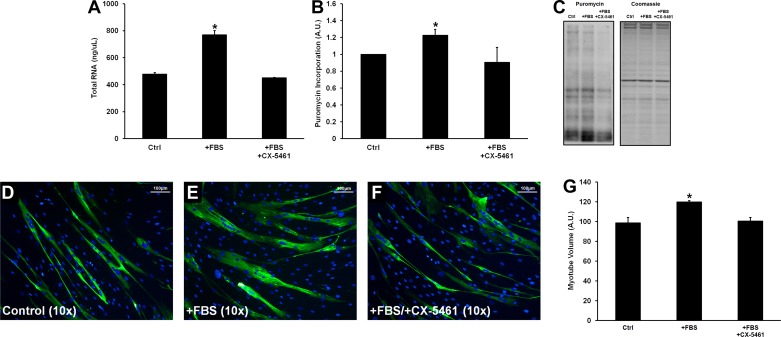
Following terminal differentiation, myotubes treated with 20% FBS for 24 h significantly increased total RNA content (+61%, P < 0.01), protein synthetic rates (+22%, P < 0.05), and myotube volume (+21%, P < 0.05) compared with control myotubes (Fig. 5). FBS treatment did not induce cell proliferation, as assessed by nuclear counting (control group: 989 ± 26 nuclei/image; +FBS group: 1,045 ± 91 nuclei/image; +FBS/+CX-5461 group: 1,052 ± 59 nuclei/image; data not shown). Pretreatment with CX-5461 completely inhibited the FBS-induced increase in total RNA content (−5% compared with vehicle control), protein synthetic rate (−9% compared with vehicle control), and myotube volume (+2% compared with vehicle control) (all P > 0.05 vs. control). CX-5461 had no effect on cell viability relative to vehicle-treated control cells as determined by MTS assay (P > 0.05 between treatment conditions; data not shown) (Promega, Madison, WI).
Fig. 5.
Effects of 24 h of FBS treatment or FBS + CX-5461 on myotube total RNA content (A), protein synthesis measured via the surface sensing of translation (SUnSET) method (B), and myotube volume (G). C–F: representative puromycin immunoblots (C) and ×10 immunocytochemical images (myosin heavy chain stained green; D–F). *Significantly different from control (P < 0.05).
DISCUSSION
Here, using K-means cluster analysis on a cohort of 42 older adults, we show that there is a widely divergent hypertrophic response to short-term RT. This interindividual response heterogeneity to a standardized RT program has been reported in numerous studies (7, 13, 22, 31, 38), but the molecular mechanisms governing this phenomenon have yet to be fully understood. Based on previous studies, it appears that the magnitude of RT-induced skeletal muscle hypertrophy is not determined by the magnitude of change in circulating hormones or acute increases in myofibrillar protein synthesis shortly after a single resistance exercise bout (31, 32). Instead, the magnitude of RT-induced hypertrophy seems to be at least partly associated with processes such as the extent of mTOR activation following a bout of resistance exercise (29, 31, 45), as well as the extent of myonuclear addition following long-term RT (36). In the current study, we contribute to the understanding of potential mechanisms regulating RT-induced hypertrophic response heterogeneity by providing data that implicate ribosome biogenesis as a likely key regulator. The evidence provided here suggests that augmented RT-induced ribosome biogenesis may support increased muscle protein synthesis over time and thus help maximize the hypertrophic adaptation to RT.
Recently, it has been found that skeletal muscle ribosome biogenesis is impaired in older mice that are subjected to synergist ablation (26), and we have also shown that markers of ribosome biogenesis appear to be blunted in older humans following an acute bout of resistance exercise (42). We suggest that this blunted ribosome biogenesis response in the elderly is a possible cause of their impaired hypertrophic response to long-term RT compared with young. The findings of the current study show that a subset of older adults who have a remarkable hypertrophic response (i.e., Xtr, +83% type II CSA) also significantly increase rRNA content (+40%) following short-term RT. These findings suggest increased ribosome biogenesis facilitates extreme hypertrophy in these older adults. Furthermore, previous data from our laboratory and others suggest the magnitude of ribosome biogenesis may be predictive of the hypertrophic response irrespective of age. For example, using a K-means cluster analysis on a cohort of 66 younger and older adults, we have previously shown that the individuals clustered as Xtr after 16 wk of training have a significant increase in total muscle RNA content 24 h after the first full exercise bout (+26%) (25). Similarly, in a small group of 14 young adults, Figueiredo et al. (16) showed that there was a significant positive correlation between the fold change in total muscle RNA content from pre- to post-RT and the percent increase in muscle CSA after 8 wk of training. These data support the concept that enhanced ribosome biogenesis following mechanical loading may help facilitate a maximal hypertrophic response, irrespective of age.
The process of ribosome biogenesis is extremely complicated. Synthesis of a single functional ribosome requires transcription to produce four rRNAs (28S, 18S, 5S, and 5.8S rRNAs) and production of ∼80 ribosomal proteins. Hundreds of molecules are involved in the production, assembly, and transport of the ribosome, and these processes are tightly regulated at many levels. A major rate-limiting step in ribosome biogenesis is the production of rRNA (28). In the current study, we show that only the Xtr cluster increased muscle rRNA content after 4 wk of RT (+40%). Because ribosome biogenesis requires the production of both rRNA and ribosomal proteins, we examined whether levels of select ribosomal proteins were also increased in Xtr following RT. Previous microarray data from our laboratory (46) and others (38) show that individuals with the greatest hypertrophic gains following RT seem to have higher baseline levels of ribosomal protein mRNAs, which might be expected to be translated into higher amounts of ribosomal proteins following a loading stimulus. However, none of the clusters significantly increased the abundance of any ribosomal proteins that we assessed following RT. It is possible that there is a sufficient basal pool of ribosomal proteins to support RT-induced ribosome biogenesis and subsequent hypertrophy, but that production of rRNA may be rate limiting. On the other hand, because we only measured levels of 4 of the ≈80 known ribosomal proteins, we may have missed potential RT-induced changes in the abundance of several other ribosomal proteins that were not measured. Nevertheless, the robust increase in rRNA in the Xtr cluster is likely a major contributor to augmented RT-induced ribosome biogenesis in this group, since rRNA constitutes ≈60% of the molecular weight of the entire ribosome (33).
In an effort to better understand the mechanisms regulating the RT-induced increase in ribosome biogenesis, we examined upstream cell signaling pathways. Activation of the mTOR pathway is required for load-induced skeletal muscle hypertrophy (9), and the extent of phosphorylation of its downstream target, p70S6 kinase, appears to be predictive of the magnitude of muscle hypertrophy with long-term RT (29, 31, 45). Activation of mTOR can induce muscle hypertrophy via increases in both translational efficiency and translational capacity. In vitro, it has been found that mTOR activation can regulate myotube hypertrophy by phosphorylating Rb, thus releasing UBF and allowing it to be available for Pol I holoenzyme-mediated rDNA transcription (34). In the current study, we did not find any cluster differences in Rb phosphorylation or UBF content from pre- to post-RT, although total levels of UBF tended to modestly increase in the entire cohort of subjects following RT. Thus, it does not appear that changes in Rb phosphorylation or UBF content were important in regulating the cluster differences in RT-induced rRNA production. Another aspect of mTOR signaling that regulates ribosome biogenesis is its ability to drive selective translation of c-Myc mRNA (49), which is a major transcription factor that directly enhances Pol I-mediated transcription of rDNA (18). Interestingly, in the current study, we found that the Mod and Xtr clusters increased total c-Myc protein levels to a greater extent than Non following 4 wk of RT. These data are in support of our previous microarray findings, which show that, in a different cohort of subjects, individuals clustered as Mod and Xtr have higher basal levels of n-Myc and c-Myc transcripts (46). This elevation in c-Myc protein content in the Mod and Xtr responder clusters following RT is a novel finding that leads us to suggest c-Myc-driven increases in ribosome biogenesis may facilitate RT-induced myofiber hypertrophy. It is important to note that recent evidence suggests that the resistance exercise-induced upregulation of c-Myc (and several other regulators of ribosome biogenesis) is not entirely dependent on mTOR signaling (48). Thus, we cannot be sure whether increased c-Myc protein levels in the Mod and Xtr clusters following RT were due to heightened mTOR signaling in these subjects. Regardless of the mechanism(s) regulating this augmented c-Myc response to RT, our data suggest that c-Myc may be a critical regulator of RT-induced ribosome biogenesis and myofiber growth.
Similar to mTOR, activation of the Wnt/β-catenin pathway occurs in response to mechanical loading, and is required for overload-induced hypertrophy (5, 6). Interestingly, β-catenin also regulates c-Myc expression, but at the level of transcription (21). Thus, given the differential magnitude of change in c-Myc protein accumulation among clusters, we sought to examine if upstream Wnt/β-catenin signaling was altered. Surprisingly, both phosphorylated (Ser33/37/Thr41) and total β-catenin levels were significantly decreased from week 0 to week 4 (both approximately −30%) in the entire cohort of subjects. We did not collect acute response samples following the initial exercise bout and thus do not know whether β-catenin levels were altered (up or down) acutely. However, we did find that protein content of the Wnt receptor Fzd1 tended to increase only in the Xtr cluster (approximately +40%) from pre- to post-RT. Despite there being no cluster differences in β-catenin levels, increased Fzd1 receptor abundance in the Xtr cluster may have allowed for an augmented downstream Wnt/β-catenin signaling response to any subsequent mechanical loading event, and perhaps enhanced β-catenin-mediated c-Myc transcription. Overall, because c-Myc is required for activating rDNA transcription in response to mitogenic stimuli (4), it is likely that the observed increase in RT-induced c-Myc production contributed to a heightened ribosome biogenesis response in the Mod and Xtr clusters.
An interesting observation in the current study is that only the Xtr cluster experienced significant myonuclear addition to type II myofibers (≈+30%) following just 4 wk of RT. This is consistent with our previous report showing that individuals with the greatest magnitude of myofiber hypertrophy following 16 wk of training also had the greatest extent of myonuclear addition (36). Whether myonuclear addition is required for load-induced muscle hypertrophy is debatable; however, some suggest a myonuclear domain threshold that may demand myonuclear addition in order to hypertrophy any further (1). The myonuclear domain concept has been discussed for decades (20), suggesting that, within a multinucleated myofiber, each nucleus services a specific domain of the myofiber. Based on the data from the current study, we hypothesize that a major purpose of RT-induced myonuclear addition is to provide more rDNA template to facilitate ribosome biogenesis, which may be required to support the increased cytoplasmic volume of the growing myofiber. Because rRNA is required for ribosome biogenesis, a critical size limit of the myonuclear domain makes sense because eventually, with no nuclear addition, rRNA transcription and diffusion throughout the myofiber would inevitably be impaired, halting hypertrophy due to an insufficient amount of translational machinery. While increased translational efficiency may help compensate for the increased myofiber size, it may not be enough to allow further myofiber growth without an increase in ribosome number. In the current study, the increases in rRNA in the Xtr cluster are coupled with significant myonuclear addition, suggesting that myonuclear addition may have played some part in augmenting ribosome biogenesis in these subjects.
While our in vivo data support the hypothesis that ribosome biogenesis likely plays an important role in regulating the magnitude of RT-induced myofiber hypertrophy, it is difficult to determine whether increased ribosome biogenesis is completely necessary. Thus, we used an in vitro model of myotube hypertrophy (FBS stimulation) to explore this question. Here, we show that treatment with a Pol I-specific inhibitor (CX-5461) effectively knocks down de novo human myotube rRNA production, and abolishes the FBS-induced hypertrophic response. These data are in agreement with those from Nader et al. (34), which show that rapamycin treatment blocks FBS-induced increases in myotube Rb phosphorylation and UBF availability, as well as total RNA content and hypertrophy. It cannot be determined from the study by Nader et al. whether the rapamycin effects were due primarily to reduced mTOR-mediated changes in translational efficiency or capacity. The present findings indicate translational capacity is central to the myotube hypertrophic response. In support of our findings, West et al. (48) have recently shown that inhibiting c-Myc in C2C12 myotubes significantly blunts ribosome biogenesis and protein synthesis (but not acute protein synthesis following an anabolic stimulus), demonstrating that c-Myc regulates muscle ribosome biogenesis, and that the process of ribosome biogenesis is crucial for maintaining myotube protein synthesis. To complement our current findings, future studies should examine the effects of the Pol I inhibitor CX-5461 during a more physiologically relevant scenario, such as overload-induced hypertrophy, and whether blocking Pol I differentially affects hypertrophic responses in young and aged muscle.
While the data presented here are novel, they are not without limitation. First, as with most human muscle biopsy trials, the timing of the biopsies is a limitation to the findings. We chose to examine biopsies obtained after just 4 wk of RT in an effort to examine the mechanisms by which muscle grows early on in response to a hypertrophic stimulus. It would have been optimal to also obtain and analyze biopsies after the first single resistance exercise bout, along with later time points following long-term training. These biopsies would have enabled us to examine acute cell signaling events that may play a role in regulating the disparate RT-induced hypertrophic response, and allow us to track whether individuals in the Non group could hypertrophy with longer-term training, or if Mod and Xtr could continue to hypertrophy even further. Another limitation of the current study is that we only assessed specific markers of ribosome biogenesis, not the entire process. Undoubtedly, it would be extremely difficult to comprehensively assess the entire process of ribosome biogenesis, since synthesis of a single ribosome requires 4 rRNAs, ≈80 ribosomal proteins, and hundreds of accessory molecules. Although we did find cluster differences in RT-mediated changes in rRNA content, we did not observe any cluster differences in RT-induced changes in the few ribosomal proteins assayed (only 4 out of ≈80 total). Interestingly, we did find that basal levels of rpL3 tended to be ≈20% higher in the Xtr group compared with Mod and Non. Recently, it has been shown that transcript levels of rpL3 are expressed at very low levels in skeletal muscle compared with other tissues (47), but that its expression is highly upregulated in response to mechanical overload (11). The importance of this specific ribosomal protein in skeletal muscle is not yet known, and it is a prime example of “ribosome heterogeneity,” demonstrating that not all ribosomes in all tissues/cells are compromised of the same molecules (reviewed in Ref. 39). Future research should attempt to examine if there are RT-induced changes in any of the ≈80 ribosomal proteins that were not measured in the current study, and examine if the ribosomes produced during RT are functionally different from ribosomes in untrained muscle.
In conclusion, we show here that older adults who have a robust hypertrophic response to short-term RT significantly increase rRNA production, a major rate-limiting step in ribosome biogenesis. The increased rRNA production in this cohort was accompanied by remarkable c-Myc accumulation during RT (possibly via enhanced mTOR and/or Wnt/β-catenin activation), as well as substantial myonuclear addition. These data suggest that augmented ribosome biogenesis may help facilitate maximal RT-induced muscle hypertrophy in older adults, a population we have recently shown to have a blunted ribosome biogenesis response to a single bout of resistance exercise (42). Finally, we show that inhibiting de novo ribosome biogenesis with a Pol I-specific inhibitor blunts growth factor-induced increases in myotube size in vitro. It would be of great value to further explore the upstream regulators of load-induced skeletal muscle ribosome biogenesis, and to determine to what extent ribosome biogenesis is required for human muscle hypertrophy.
GRANTS
This work was supported by National Institutes of Health Grants 5R01-AG-017896 (M. M. Bamman), F31-AG-044109 (M. J. Stec), and T32-HD-071866 (N. A. Kelly and G. M. Many) and the UAB Center for Clinical and Translational Science (UL1-TR-000165).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.S., N.A.K., G.M.M., and M.M.B. conception and design of research; M.J.S., N.A.K., G.M.M., S.T.W., and S.C.T. performed experiments; M.J.S. and N.A.K. analyzed data; M.J.S., N.A.K., G.M.M., and M.M.B. interpreted results of experiments; M.J.S. prepared figures; M.J.S., G.M.M., and M.M.B. drafted manuscript; M.J.S., N.A.K., G.M.M., S.T.W., S.C.T., and M.M.B. edited and revised manuscript; M.J.S., N.A.K., S.T.W., S.C.T., and M.M.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to the participants for their efforts and dedication.
REFERENCES
- 1.Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol 2: 2829–2870, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Agley CC, Velloso CP, Lazarus NR, Harridge SD. An image analysis method for the precise selection and quantitation of fluorescently labeled cellular constituents: application to the measurement of human muscle cells in culture. J Histochem Cytochem 60: 428–438, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131: 856S–860S, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Arabi A, Wu S, Ridderstrale K, Bierhoff H, Shiue C, Fatyol K, Fahlen S, Hydbring P, Soderberg O, Grummt I, Larsson LG, Wright AP. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat Cell Biol 7: 303–310, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DD, Esser KA. Wnt/beta-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol 289: C853–C859, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong DD, Wong VL, Esser KA. Expression of beta-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol 291: C185–C188, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol 102: 2232–2239, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Science Sports Exercise 43: 1177–1187, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaillou T, Kirby TJ, McCarthy JJ. Ribosome biogenesis: emerging evidence for a central role in the regulation of skeletal muscle mass. J Cell Physiol 229: 1584–1594, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrick KL, Stec MJ, Merritt EK, Windham ST, Thomas SJ, Cross JM, Bamman MM. Serum from human burn victims impairs myogenesis and protein synthesis in primary myoblasts. Frontiers Physiol 6: 184, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol 110: 309–317, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drygin D, Lin A, Bliesath J, Ho CB, O'Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A, Streiner N, Quin JE, Sanij E, Bywater MJ, Hannan RD, Ryckman D, Anderes K, Rice WG. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res 71: 1418–1430, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab 309: E72–E83, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle 1: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandori C, Gomez-Roman N, Felton-Edkins ZA, Ngouenet C, Galloway DA, Eisenman RN, White RJ. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I Nat Cell Biol 7: 311–318, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Haddach M, Schwaebe MK, Michaux J, Nagasawa J, O'Brien SE, Whitten JP, Pierre F, Kerdoncuff P, Darjania L, Stansfield R, Drygin D, Anderes K, Proffitt C, Bliesath J, Siddiqui-Jain A, Omori M, Huser N, Rice WG, Ryckman DM. Discovery of CX-5461, the first direct and selective inhibitor of RNA polymerase I, for cancer therapeutics. Med Chem Lett 3: 602–606, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell 59: 771–772, 1989. [DOI] [PubMed] [Google Scholar]
- 21.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Science Sports Exercise 37: 964–972, 2005. [PubMed] [Google Scholar]
- 23.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 159: 413–421, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol 103: 1488–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol 119: 321–327, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 25: 6384–6391, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol 589: 3023–3037, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol 115: 937–948, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLos One 8: e78636, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell CJ, Churchward-Venne TA, Parise G, Bellamy L, Baker SK, Smith K, Atherton PJ, Phillips SM. Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men. PLos One 9: e89431, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore PB. The three-dimensional structure of the ribosome and its components. Ann Rev Biophys Biomol Struct 27: 35–58, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol 289: C1457–C1465, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291: E937–E946, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol 104: 1736–1742, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol 98: 211–220, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet 9: e1003389, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sauert M, Temmel H, Moll I. Heterogeneity of the translational machinery: Variations on a common theme. Biochimie 114: 39–47, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6: 275–277, 2009. [DOI] [PubMed] [Google Scholar]
- 41.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279: E715–E729, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol 119: 851–857, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugden PH, Fuller SJ. Regulation of protein turnover in skeletal and cardiac muscle. Biochem J 273: 21–37, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr 91: 1227–1236, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, Mascher H, Blomstrand E. Resistance exercise-induced increase in muscle mass correlates with p70S6 kinase phosphorylation in human subjects. Eur J Appl Physiol 102: 145–152, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Thalacker-Mercer A, Stec M, Cui X, Cross J, Windham S, Bamman M. Cluster analysis reveals differential transcript profiles associated with resistance training-induced human skeletal muscle hypertrophy. Physiol Genomics 45: 499–507, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorrez L, Van Deun K, Tranchevent LC, Van Lommel L, Engelen K, Marchal K, Moreau Y, Van Mechelen I, Schuit F. Using ribosomal protein genes as reference: a tale of caution. PLos One 3: e1854, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West DW, Baehr LM, Marcotte GR, Chason CM, Tolento L, Gomes AV, Bodine SC, Baar K. Acute resistance exercise activates rapamycin-sensitive and -insensitive mechanisms that control translational activity and capacity in skeletal muscle. J Physiol 594: 453–468, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West MJ, Stoneley M, Willis AE. Translational induction of the c-myc oncogene via activation of the FRAP/TOR signalling pathway. Oncogene 17: 769–780, 1998. [DOI] [PubMed] [Google Scholar]