Abstract
Neonatal pigs are used as a model to study and optimize the clinical treatment of infants who are unable to maintain oral feeding. Using this model, we have shown previously that pulsatile administration of leucine during continuous feeding over 24 h via orogastric tube enhanced protein synthesis in skeletal muscle compared with continuous feeding alone. To determine the long-term effects of leucine pulses, neonatal piglets (n = 11–12/group) were continuously fed formula via orogastric tube for 21 days, with an additional parenteral infusion of either leucine (CON + LEU; 800 μmol·kg−1·h−1) or alanine (CON + ALA) for 1 h every 4 h. The results show that body and muscle weights and lean gain were ∼25% greater, and fat gain was 48% lower in CON + LEU than CON + ALA; weights of other tissues were unaffected by treatment. Fractional protein synthesis rates in longissimus dorsi, gastrocnemius, and soleus muscles were ∼30% higher in CON + LEU compared with CON + ALA and were associated with decreased Deptor abundance and increased mTORC1, mTORC2, 4E-BP1, and S6K1 phosphorylation, SNAT2 abundance, and association of eIF4E with eIF4G and RagC with mTOR. There were no treatment effects on PKB, eIF2α, eEF2, or PRAS40 phosphorylation, Rheb, SLC38A9, v-ATPase, LAMTOR1, LAMTOR2, RagA, RagC, and LAT1 abundance, the proportion of polysomes to nonpolysomes, or the proportion of mRNAs encoding rpS4 or rpS8 associated with polysomes. Our results demonstrate that pulsatile delivery of a leucine supplement during 21 days of continuous enteral feeding enhances lean growth by stimulating the mTORC1-dependent translation initiation pathway, leading to protein synthesis in skeletal muscle of neonates.
Keywords: leucine, growth, protein metabolism, orogastric feeding, infant
growth and development during childhood is the traditional measure of overall nutritional status. Poor growth in early infancy is associated with increased risk for adverse long-term health outcomes, including programming of obesity (63), risk of cardiovascular disease (4), cognitive impairment (56), and reduced muscle mass and strength (52, 69). Feeding by orogastric tube, using either continuous or intermittent bolus delivery of formula or human milk, is commonly used in infants with impaired suckling, swallowing, or breathing (26). Pediatric patients who are meal feeding intolerant are fed continuously (26, 64). Recently, we demonstrated, using the neonatal pig as a model for the human neonate, that continuous feeding blunts protein synthesis and reduces protein deposition compared with intermittent bolus feeding, even though the same nutrient load is consumed (20, 31, 40). Thus, strategies are needed to improve the growth of neonates who require continuous feeding.
Feeding increases protein synthesis in all tissues of the neonate, but the greatest response occurs in skeletal muscle (16, 17, 39, 84). This effect is due largely to the postprandial rise in circulating concentrations of insulin and amino acids (18) that independently activate the master protein kinase, mechanistic (a.k.a. mammalian) target of rapamycin (mTOR), which regulates translation initiation (21, 62, 76). We have shown that intermittent bolus feeding enhances muscle protein synthesis by inducing a pulsatile pattern of amino acid- and insulin-stimulated translation initiation (20, 40). However, the low and constant hormone and substrate patterns elicited by continuous feeding attenuate translation initiation signaling.
The protein kinase mTOR interacts with signaling components to form two complexes: mTOR complex 1 (mTORC1), which regulates translation initiation, and mTORC2, a regulator of protein kinase B (PKB) (48, 53, 92). Upstream regulators of mTOR are not well characterized but include Deptor, an inhibitor of mTORC1 and mTORC2 (65), and proline-rich Akt substrate of 40 kDa (PRAS40), which inhibits mTORC1 by binding to Raptor, a core mTORC1 member (82). Studies suggest that amino acids regulate recruitment of mTORC1 to the lysosomal surface, where it becomes activated (23). In the current model, amino acids enter the lysosome and activate v-ATPase and Ragulator (LAMTOR1-5) which promotes the formation of RagA/BGTP-RagC/DGDP complexes that bind to mTORC1 and recruit the inactive mTORC1 to the lysosome surface. Subsequently, activated Rheb binds and activates mTORC1. Recent findings implicate the lysosomal amino acid transporter SLC38A9 in mTORC1 activation (67, 83). Transporters that are critical for amino acid transport into the cell include system L and system A transporters, L-type amino acid transporter 1 (LAT1), and small neutral amino acid transporter 2 (SNAT2) (25, 73). Activation of mTORC1 results in the phosphorylation of the 70-kDa ribosomal protein S6 kinase 1 (p70S6K1) and the eukaryotic initiation factor (eIF)4E-binding protein 1 (4E-BP1), which promotes eIF4E association with eIF4G and translation initiation activation (47). Translation initiation is also controlled by phosphorylation of eIF2α (28), and peptide chain elongation regulation involves phosphorylation of eukaryotic elongation factor 2 (eEF2) (9, 43).
The essential amino acid leucine is a primary regulator of muscle protein synthesis (12, 75), as it acts not only as a substrate for protein synthesis but also as a signaling molecule to enhance protein synthesis in muscle (1, 15) and other tissues (57). This effect appears to be unique to leucine, as other branched-chain amino acids do not have this effect (35). Our laboratory has demonstrated that short-term administration of leucine provided either parenterally or enterally increases skeletal muscle protein synthesis in neonatal pigs, and this effect is associated with an increase in the activation of mTORC1 and its downstream targets (34, 59). Recently, we showed that parenteral administration of leucine pulses every 4 h for 24 h during continuous orogastric feeding enhances protein synthesis by triggering the activation of mTORC1-regulated translation initiation in a pulsatile manner, similar to that which occurs with intermittent bolus feeding (8). The long-term effects of leucine have been studied largely for the maintenance of lean body mass in adults and for the treatment of muscle loss in the elderly (3), but its efficacy is still controversial because of contradictory results. Recently, we showed that enteral leucine supplementation of a milk-based formula with restricted protein content had modest anabolic effects on muscle mass in neonatal pigs (13), but the effectiveness of parenteral leucine pulses in piglets fed a complete protein formula has not been studied.
Despite numerous short-term studies demonstrating the positive effects of leucine on muscle anabolism (1, 15, 33–36, 45, 59, 77, 79, 85, 86), it is not clear whether this effect can be translated into long-term outcomes, i.e., gain in body weight and especially lean mass. Our objective was to determine whether the increase in protein synthesis we observed previously after 24 h of leucine pulses during continuous feeding (8) can be sustained in the long term to promote lean growth in neonates. We hypothesized that administration of leucine pulses during 21 days of continuous orogastric feeding would enhance lean mass in neonatal pigs by stimulating the amino acid signaling pathway that regulates protein synthesis in skeletal muscle.
MATERIALS AND METHODS
Animals and surgeries.
This study was approved by the Animal Care and Use Committee at the Baylor College of Medicine and was conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. Twenty-three 2-day-old female piglets (2.0 ± 0.2 kg; Agricultural Headquarters of the Texas Department of Criminal Justice) were weaned from sows and housed in individual stainless-steel cages in an environmentally controlled room. Piglets were fed a milk replacement formula ad libitum (Soweena Litter Life; Merrik's, Middleton, WI) for 6 days and weighed every 3 days during the experiment. Three days before the beginning of the study, piglets underwent surgery to have indwelling catheters placed in their jugular veins and carotid arteries for infusion and sampling and a gastrostomy tube for feeding. Surgical procedures were performed using sterile techniques under general anesthesia, as described previously (16).
Diet and infusion.
Piglets (8 days old, 2.3 ± 0.2 kg) were continuously fed by gastrostomy tube the same amount of a sow milk replacer formulated to meet the National Research Council's (60) requirements for neonatal pigs (240 ml·kg body wt−1·day−1 with a rate of 10 ml·kg body wt−1·h−1, providing 13.5 g crude protein·kg body wt−1·day−1) for 21 days (Table 1). Piglets were randomly assigned to one of two groups: 1) continuous feeding with leucine pulses (CON + LEU; n = 11) or 2) continuous feeding with alanine pulses (CON + ALA; n = 12) using a syringe pump. The pulses of leucine and alanine provided equimolar amounts of nitrogen and were infused into the jugular vein (800 μmol·kg body wt−1·h−1) for 1 h every 4 h. Alanine was used as the isonitrogenous control because it does not affect protein metabolism (46, 89).
Table 1.
Composition of the experimental diet
| CON + ALA | CON + LEU | |
|---|---|---|
| Whey protein concentrate | 16.8 | 16.8 |
| Lactose | 2.16 | 2.16 |
| Fat pak 80† | 1.44 | 1.44 |
| Corn oil† | 7.49 | 7.49 |
| Minerals§ | 2.16 | 2.16 |
| Vitamins§ | 0.48 | 0.48 |
| Xanthan gum | 0.24 | 0.24 |
| Water | 209 | 209 |
Values are expressed in g·kg body wt−1·day−1. CON + ALA, alanine; CON + LEU, leucine. *Hilmar Ingredients, Hilmar, CA.
Milk Specialties Global Animal Nutrition, Carpentersville, IL.
Dyets, Bethlehem, PA.
Experimental protocol.
Body composition was determined before the beginning of the experiment and after 18 days of feeding on anesthetized pigs by dual-energy X-ray absorptiometry using a fan-beam densitometer (Hologic QDR4500A, 414 Hologic) in the infant whole body scan mode. After 21 days of enteral continuous feeding and infusion of either leucine or alanine, blood samples were collected from the carotid artery into heparinized tubes over the course of a 4-h infusion period (every 15 min for 1.5 h of infusion, every 30 min until 2 h, and every hour until 4 h of infusion) to document hormone and substrate profiles. Plasma was frozen at −20°C for subsequent analyses. Tissue protein synthesis rates were measured (38) from 45 to 75 min after the beginning of the infusion of LEU or ALA by injecting piglets with a flooding dose of l-[4-3H]phenylalanine (1.5 mmol/kg body wt, 0.5 mCi/kg; American Radiolabeled Chemicals, St. Louis, MO) 30 min before tissue collection (16). Plasma samples were collected 5, 15, and 30 min after [3H]phenylalanine infusion and frozen for analysis. Piglets were euthanized 30 min after [3H]phenylalanine infusion and tissues samples obtained from the longissimus dorsi (LD), gastrocnemius, and soleus muscles; heart, jejunum, liver, and kidney were removed promptly, rinsed, weighed, frozen in liquid nitrogen, and stored at −70°C until analysis.
Plasma insulin, glucose, IGF-I, and amino acid concentrations.
Plasma radioimmunoreactive insulin concentrations were analyzed using a porcine insulin radioimmunoassay kit (Linco, St. Louis, MO), and plasma concentrations of insulin-like growth factor I were determined using a human IGF-I ELISA kit (R & D Systems, Minneapolis, MN). Plasma glucose concentrations were determined with the glucose oxidase method (model 2300; Yellow Springs Instruments, Yellow Springs, OH). Amino acid concentrations were determined in plasma with a high-performance liquid chromatography method (PICO-TAG reverse-phase column; Waters, Milford, MA) after deproteinization and derivatization with phenylisothiocyanate (10).
Tissue fractional protein synthesis rates.
The specific radioactivity of free and protein-bound phenylalanine in tissues was measured by high-performance liquid chromatography using an anion exchange column (PA1 column; Dionex, Sunnyvale, CA) after sample preparation, as described previously (19). Fractions were collected, and the radioactivity associated with the phenylalanine peak was measured using Ultima gold (Perkin-Elmer, Waltham, MA) in a liquid scintillation counter (Tri-Carb 2500TR; Packard Instrument, Meriden, CT). Fractional rates of protein synthesis (KS; %protein mass synthesized in 1 day) in tissues were calculated as KS = [(SAbound phe/SAfree phe) × 1,440/t] × 100, where SAbound phe and SAfree phe (in disintegrations·min−1·nmol−1) are the specific radioactivity of the protein-bound and the tissue-free phenylalanine, respectively, t is the time of labeling in minutes, and 1,440 is the minutes-to-days conversion. Protein content was determined by Pierce bicinchoninic acid assay (55) and total RNA by the method of Munro and Fleck (58) for the determination of both protein synthetic capacity (CS) and efficiency (KRNA). CS (in μg RNA/mg protein) was estimated as RNA/protein ratio because the majority of RNA in tissues is ribosomal. KRNA (in g protein·day−1·g RNA−1) is the total protein synthesized in 1 day per total RNA: KRNA = CS/KS.
Reverse transcriptase and real-time quantitative PCR.
Analysis of ribosome distribution between polysomal and nonpolysomal fractions was determined by sucrose density gradient centrifugation, as described previously (51). Isolated RNA was reverse transcribed to cDNA and quantitated by qRT-PCR. The resulting cDNAs were normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The proportion of ribosomal protein S4 (rpS4), rpS8, and ornithine decarboxylase (ODC) mRNAs in the polysomal fraction was determined in LD muscle and liver. The primers used for rpS4, rpS8, ODC, and GAPDH were described previously (39).
Protein immunoblot analysis.
Proteins from LD muscle homogenates were subjected to Western blot analysis, as described previously (76). Immunoblotting was performed using the following primary antibodies: PKB [total (Santa Cruz Biotechnology, Dallas, TX) and phosphorylated Thr308 (Cell Signaling Technology, Danvers, MA)], S6K1 [total (Santa Cruz Biotechnology) and phosphorylated Thr389 (Millipore, Bedford, MA)], 4E-BP1 [total (Bethyl, Montgomery, TX) and phosphorylated Thr46 (Invitrogen, Carlsbad, CA)], eIF2α (total and phosphorylated Ser51; Cell Signaling Technology), eEF2 (total and phosphorylated Thr56; Cell Signaling Technology), LAT1 (MBL International, Woburn, MA), SNAT2 (Santa Cruz Biotechnology), PRAS40 (total and phosphorylated Thr246; Cell Signaling Technology), total Deptor, mTOR, Raptor, Rictor, RagA, RagC, LAMTOR1, and LAMTOR2 (Cell Signaling Technology), v-ATPase (V0d1 subunit; GeneTex, Irvine, CA), Rheb (R & D Systems, Minneapolis, MN), SLC38A9 (Antibody Verify, Las Vegas, NV), and GAPDH (Abgent, San Diego, CA). Blots were developed as described previously (31). Values for the protein phosphorylation were normalized by their corresponding protein abundance. Values for total protein abundance were corrected and normalized by GAPDH as an internal control.
Quantification of eIF4E·eIF4G complex.
The complex was immunoprecipitated as described previously (31). Amounts of eIF4G were corrected for the amount of eIF4E (Cell Signaling Technology and EMD Millipore) recovered in the immunoprecipitate.
Phosphorylation of mTORC1 and mTORC2 and RagC-mTOR complex abundance.
mTORC1- and mTORC2-associated autophosphorylation on Ser2481 and RagC-mTOR association were determined by immunoprecipitating the homogenates with Raptor, Rictor, and mTOR (Cell Signaling Technology), respectively (22). The immunoprecipitated samples were run on SDS-PAGE and probed with phospho-mTOR Ser2481 (Bio legend, San Diego, CA) or RagC (Cell Signaling Technology). The phosphorylation of mTORC1, mTORC2, and the RagC-mTOR complex abundance was normalized with Raptor, Rictor, and mTOR abundance, respectively, in the precipitate.
Statistics.
Data are expressed as means ± SE. The effects of the treatments (Tt) were analyzed using one-way ANOVA (SAS 9.1; SAS Institute, Cary, NC) for body composition, tissue weight, fractional protein synthesis rates, and Western blots. Differences between groups for all other results were analyzed using mixed models for repeated-measures analysis, with the treatment and the time as independent fixed factors and piglets as a random factor (version 9.1; SAS Institute). For each variable, the most appropriate matrix of covariance structures for random statements was selected based on Sawa's Bayesian Information Criteria (BIC). The model with the smallest BIC among all competing models was deemed the best model. Post hoc tests were performed by using contrast analysis. A probability value of 0.05 was considered statistically significant.
RESULTS
Body weight and composition and tissue weight.
Body weight increased over the 21-day treatment to a greater value in CON + LEU than in CON + ALA pigs (P < 0.01; Fig. 1A). The lean gain was higher (Fig. 1B) and the fat gain lower (Fig. 1C) in the CON + LEU than in the CON + ALA group (P < 0.04).
Fig. 1.
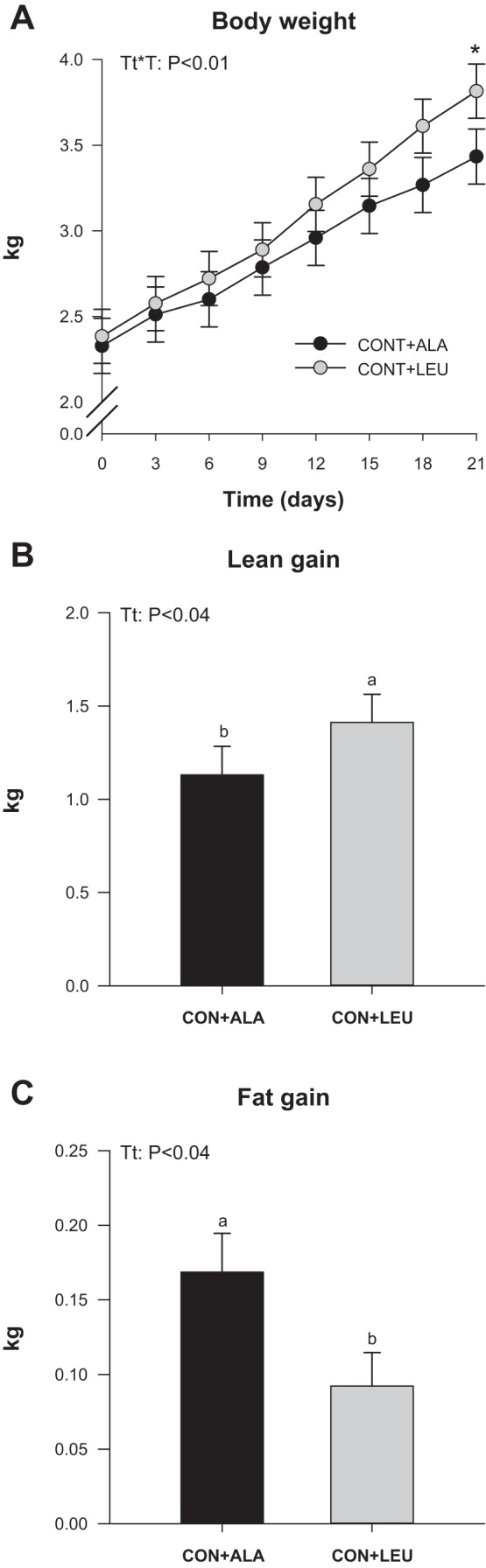
Body weight (A), lean gain (B), and fat gain (C) of neonatal pigs continuously fed and pulsed with either alanine (CON + ALA) or leucine (CON + LEU) for 21 days. Values are means ± SE (n = 11–12). Time is given as days from initiation of treatment. The statistical effects [interaction of treatment (Tt) by time, (Tt*T)] from a mixed model for repeated measurements over time and treatment effects are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
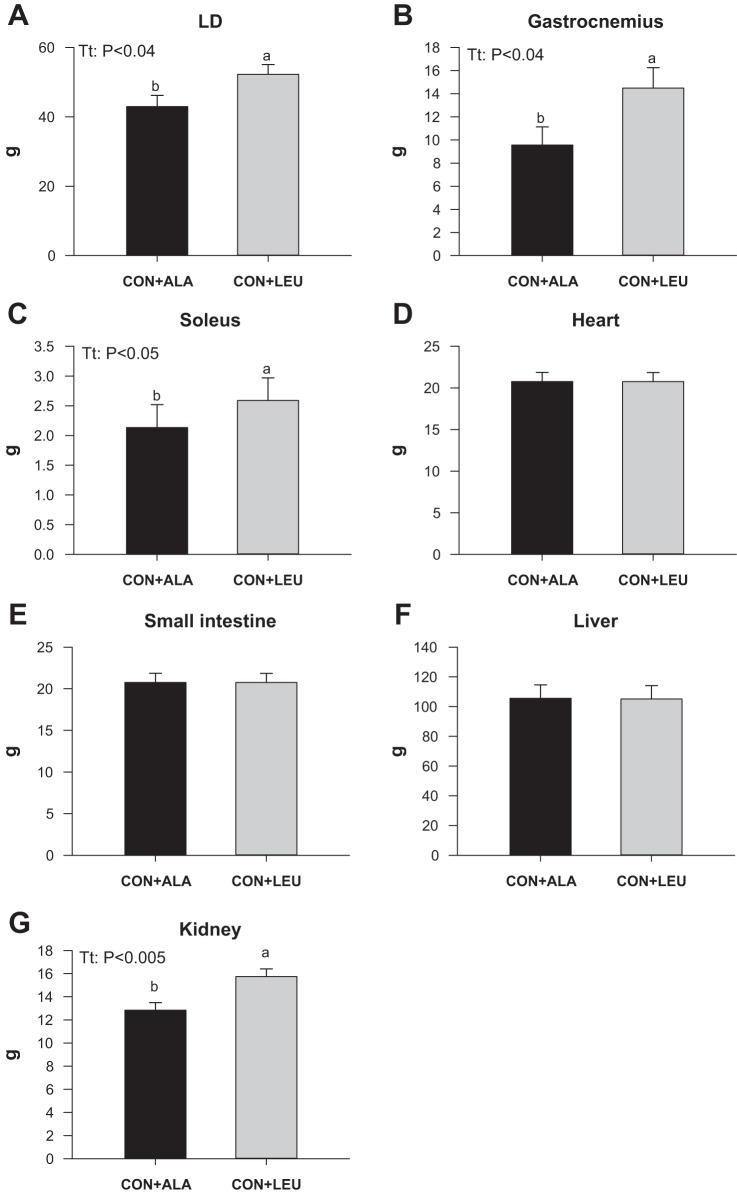
The weights of the LD (Fig. 2A), gastrocnemius (Fig. 2B), and soleus (Fig. 2C) muscles and kidney (Fig. 2G) were heavier in the CON + LEU than in the CON + ALA group (P < 0.05). There was no effect of treatment on heart (Fig. 2D), liver (Fig. 2E), or small intestine (Fig. 2F) weights.
Fig. 2.
Weights of longissimus dorsi (LD; A), gastrocnemius (B), and soleus (C) muscles, heart (D), small intestine (E), liver (F), and kidney (G) in neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
Plasma glucose, insulin, IGF-I, and amino acid concentrations.
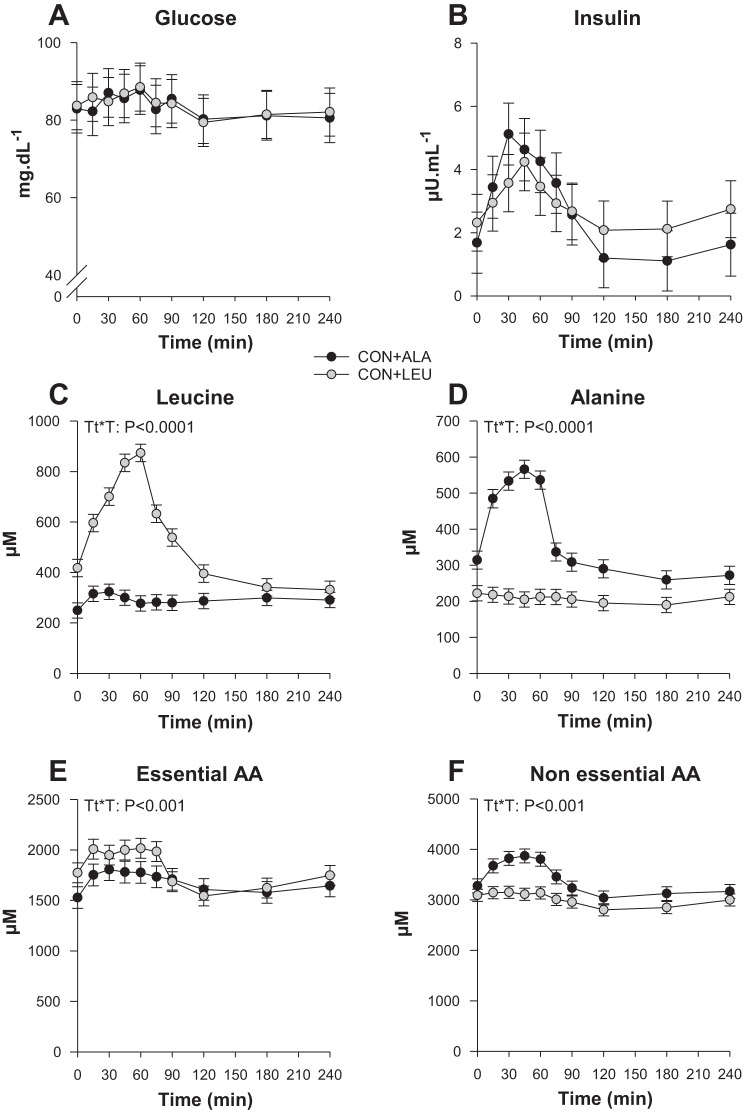
Circulating hormone and substrate concentrations were determined over the last 4 h of the infusion. There were no significant differences between the CON + ALA and CON + LEU groups for plasma glucose (Fig. 3A) or insulin concentrations (Fig. 3B). IGF-I concentrations were 50.1 ± 19.8 and 51.7 ± 15.4 ng/ml after 1-h LEU infusion for the CON + ALA and CON + LEU groups, respectively, and 49.2 ± 16.6 and 50.0 ± 18.8 ng/mL after 4-h LEU infusion for the CON + ALA and CON + LEU groups, respectively. There was no effect of the treatment. Plasma leucine (Fig. 3C) and alanine (Fig. 3D) concentrations were increased in the CON + LEU and CON + ALA groups with infusion of leucine and alanine, respectively (P < 0.05). The responses were rapid and apparent by 15 min after the initiation of the infusion, with a maximum peak at 75 min. Plasma essential (Fig. 3E) and nonessential (Fig. 3F) amino acid concentrations were higher from 45 to 75 min of infusion in the CON + LEU and CON + ALA groups, respectively (P < 0.05), reflecting the presence of the leucine and alanine infusion. There were no significant differences between groups for the other amino acids (data not shown).
Fig. 3.
Plasma glucose (A), insulin (B), leucine (C), alanine (D), essential amino acid (E), and nonessential amino acid (F) concentrations in neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). Time is given as minutes from initiation of infusion. The statistical effects (Tt*T) from a mixed model for repeated measurements over time are reported for each variable when P < 0.05.
Protein synthesis rates in tissues.
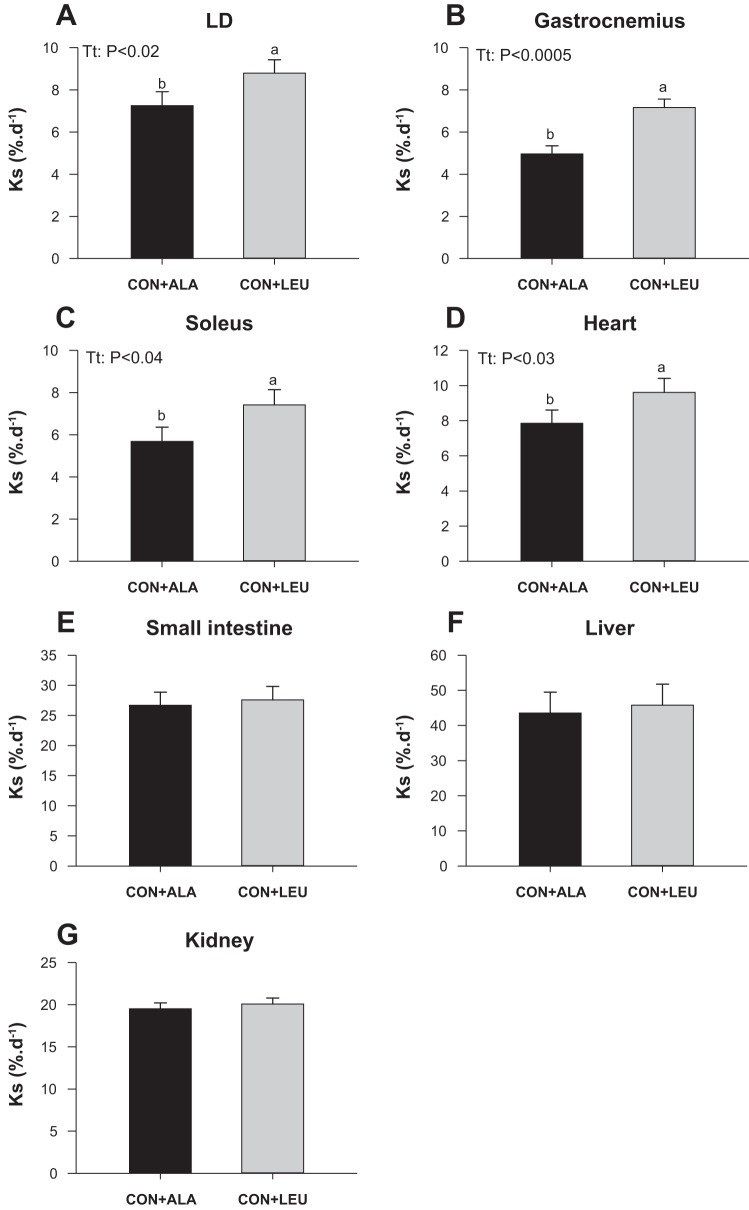
Fractional protein synthesis rates in the LD (Fig. 4A), gastrocnemius (Fig. 4B), and soleus (Fig. 4C) muscles and the heart (Fig. 4D) were greater in the CON + LEU compared with the CON + ALA group (P < 0.05). The fractional protein synthesis rates in the small intestine (Fig. 4E), liver (Fig. 4F), and kidney (Fig. 4G) were not significantly different between groups.
Fig. 4.
Protein synthesis (KS) in LD (A), gastrocnemius (B), and soleus (C) muscles, heart (D), small intestine (E), liver (F), and kidney (G) in neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
The efficiency with which the ribosomes translated mRNA into protein (KRNA) in the gastrocnemius muscle and heart was greater (P < 0.05) and in the LD tended to be greater (P < 0.08) in the CON + LEU compared with CON + ALA group (Table 2). In the soleus, small intestine, liver, and kidney, KRNA did not differ significantly between groups. CS was not affected by treatment (Table 2).
Table 2.
KRNA and CS in neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days
| CON + ALA | CON + LEU | P Value* | |
|---|---|---|---|
| LD | |||
| KRNA | 2.77 ± 0.19 | 3.32 ± 0.21 | 0.08 |
| CS | 25.4 ± 0.66 | 25.6 ± 0.73 | NS |
| Gastrocnemius | |||
| KRNA | 1.60 ± 0.15b | 2.55 ± 0.15a | <0.0002 |
| CS | 30.8 ± 1.86 | 28.1 ± 0.84 | NS |
| Soleus | |||
| KRNA | 2.26 ± 0.25 | 2.50 ± 0.28 | NS |
| CS | 27.6 ± 0.82 | 28.4 ± 1.57 | NS |
| Heart | |||
| KRNA | 3.29 ± 0.20b | 4.74 ± 0.33a | <0.002 |
| CS | 20.2 ± 0.72 | 20.4 ± 0.57 | NS |
| Small intestine | |||
| KRNA | 5.69 ± 0.35 | 6.00 ± 0.57 | NS |
| CS | 49.4 ± 0.67 | 49.9 ± 0.84 | NS |
| Liver | |||
| KRNA | 5.84 ± 0.50 | 6.05 ± 0.55 | NS |
| CS | 71.2 ± 2.01 | 73.9 ± 2.83 | NS |
| Kidney | |||
| KRNA | 2.38 ± 0.26 | 2.27 ± 0.14 | NS |
| CS | 84.6 ± 3.00 | 89.2 ± 2.52 | NS |
Results are expressed as means ± SE (n = 11–12). LD, longissimus dorsi; KRNA, protein synthetic efficiency; CS, protein synthetic capacity; NS, not significant. Values are expressed in μg RNA/mg protein for CS and in g protein·day−1·g RNA−1 for KRNA.
One-way ANOVA. Means without a common superscripted letter differ.
Ribosomal proteins.
The expression of ODC was used as a control. There were no differences in the proportion of muscle and liver rpS4 or rpS8 mRNA in the polysomal fraction in the CON + LEU or CON + ALA group (Table 3). The polysomal/nonpolysomal fraction was not affected by treatment in muscle and liver.
Table 3.
Proportion of rpS4, rpS8, and ODC mRNAs in the polysomal fraction and P/NP fractions in LD muscle and liver of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days
| CON + ALA | CON + LEU | P Value* | |
|---|---|---|---|
| LD | |||
| rpS4 | 40.9 ± 6.24 | 40.0 ± 4.36 | NS |
| rpS8 | 28.5 ± 5.20 | 28.2 ± 5.21 | NS |
| ODC | 80.4 ± 3.19 | 80.6 ± 2.57 | NS |
| P/NP | 0.67 ± 0.05 | 0.64 ± 0.05 | NS |
| Liver | |||
| rpS4 | 16.3 ± 4.22 | 17.9 ± 2.70 | NS |
| rpS8 | 9.8 ± 2.71 | 16.3 ± 3.22 | NS |
| ODC | 56.0 ± 7.98 | 59.0 ± 6.45 | NS |
| P/NP | 1.16 ± 0.09 | 1.38 ± 0.08 | NS |
Results are expressed as means ± SE (n = 11–12). rpS4, ribosomal protein S4; rpS8, ribosomal protein S8; ODC, ornithine decarboxylase; P/NP, polysomal/nonpolysomal. Values are expressed as %mRNA in polysome fraction for rpS4, rpS8, and ODC and in arbitrary units for P/NP.
One-way ANOVA.
Protein synthetic machinery and amino acid sensing components.
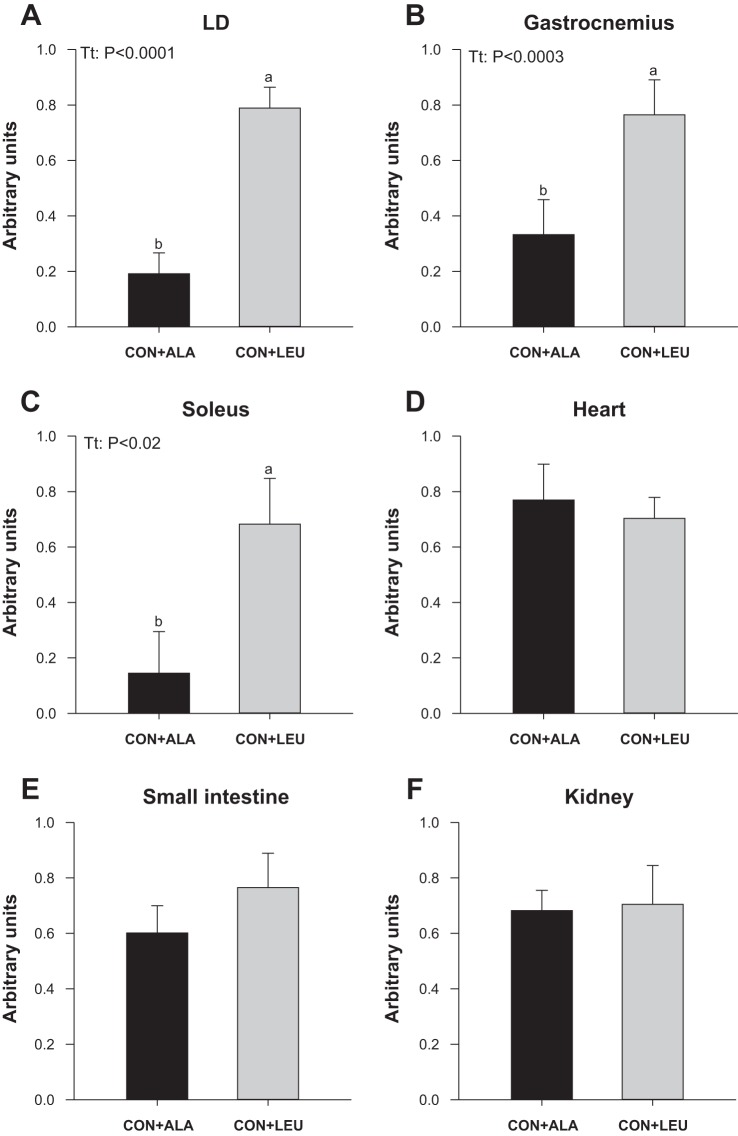
The active eIF4E·eIF4G complex formation was higher in LD (Fig. 5A), gastrocnemius (Fig. 5B), and soleus (Fig. 5C) muscles in the CON + LEU compared with the CON + ALA group (P < 0.05). There were no significant differences between groups in eIF4E·eIF4G complex formation in the heart (Fig. 5D), small intestine (Fig. 5E), or kidney (Fig. 5F).
Fig. 5.
Eukaryotic initiation factor (eIF)4E·eIF4G complex abundance in LD (A), gastrocnemius (B), and soleus (C) muscles, heart (D), small intestine (E), and kidney (F) of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
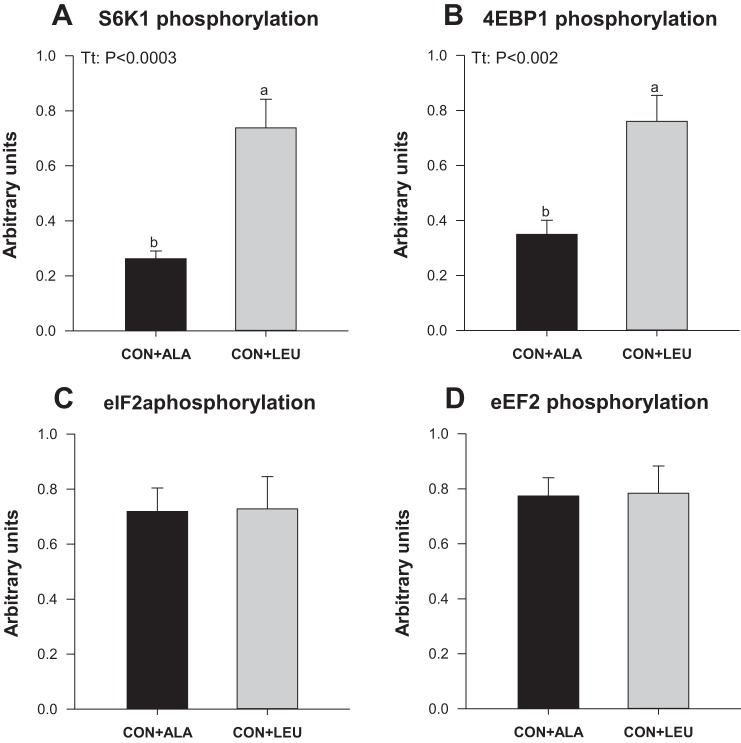
To examine the mechanism by which leucine promotes lean accretion, the abundance and the activation of signaling components of the amino acid-sensing and insulin pathways leading to protein synthesis were examined in LD muscle as a representative of tissues that were affected by leucine treatment. PKB Thr308 phosphorylation was 0.72 ± 0.10 and 0.59 ± 0.11 (arbitrary units) for the CON + ALA and CON + LEU groups, respectively. There was no effect of treatment. The phosphorylation of S6K1 (Fig. 6A) and 4E-BP1 (Fig. 6B) was higher in the CON + LEU compared with the CON + ALA group (P < 0.05). There were no significant differences between groups in the phosphorylation of eIF2α (Fig. 6C) or eEF2 (Fig. 6D).
Fig. 6.
Phosphorylation of S6K1 (A), eIF4E-binding protein 1 (4E-BP1; B), eIF2α (C), and eukaryotic elongation factor 2 (eEF2; D) in LD muscle of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
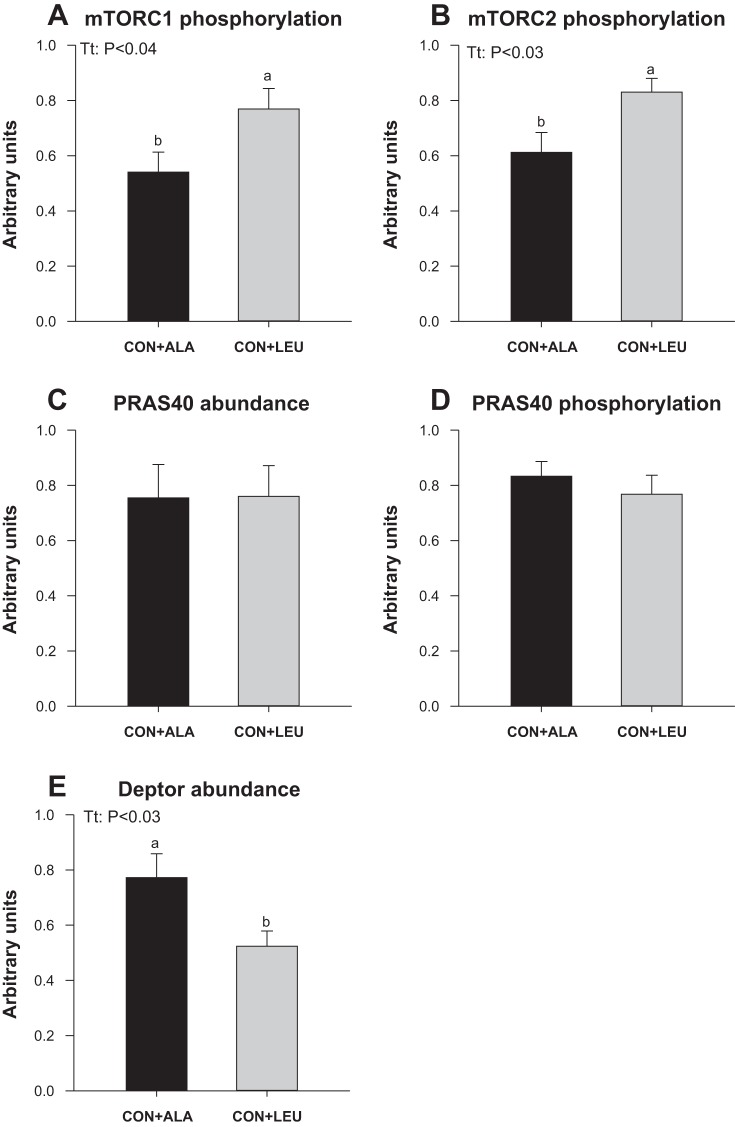
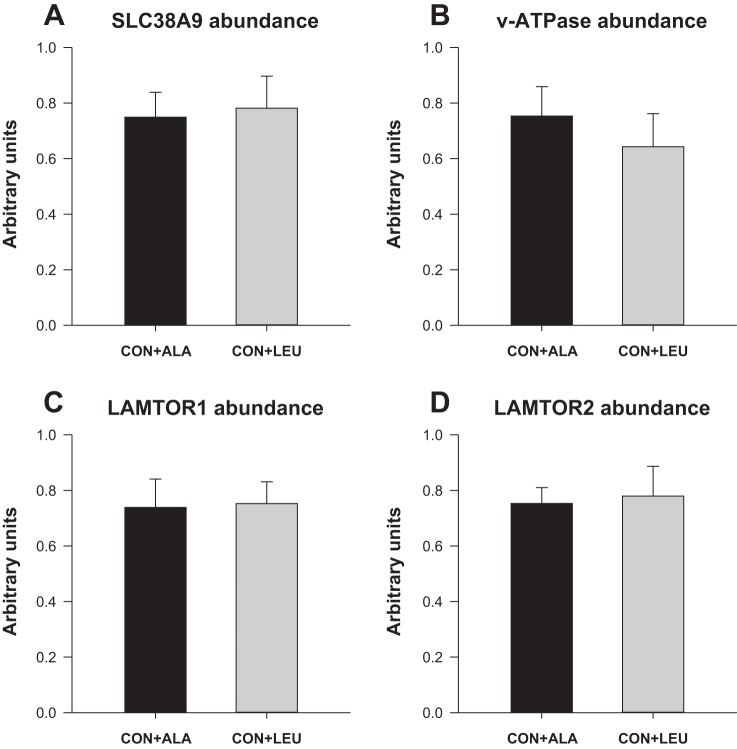
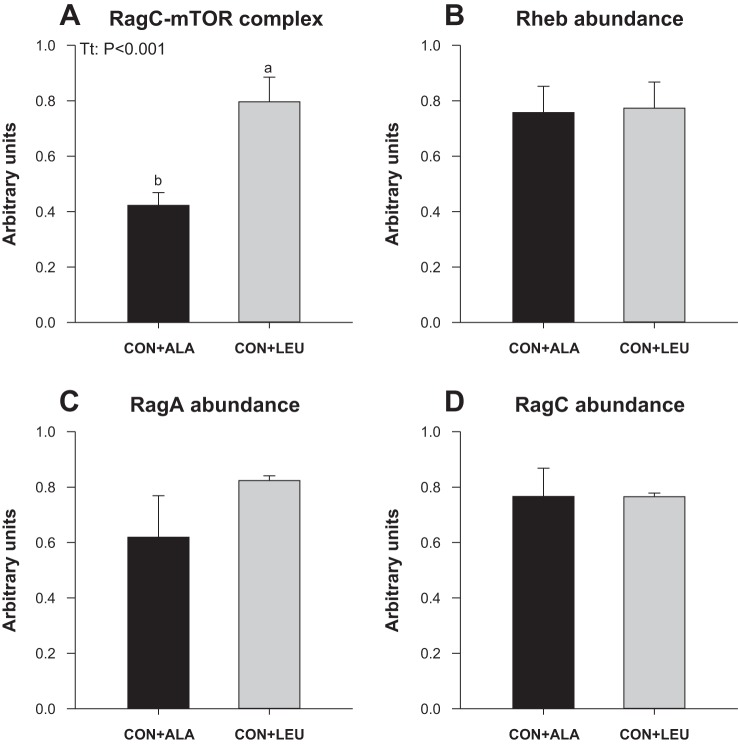
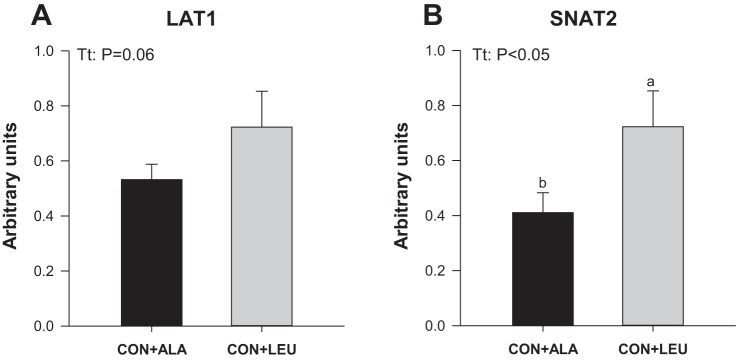
To measure the activation of amino acid-sensing pathways, we analyzed the abundance and the activation of signaling components upstream of mTOR. The phosphorylation of mTOR in both complex 1 (Fig. 7A) and complex 2 (Fig. 7B) was higher in the CON + LEU compared with the CON + ALA group (P < 0.05). The abundance (Fig. 7C) and the phosphorylation of PRAS40 (Fig. 7D) were unaffected by treatment. The abundance of Deptor (Fig. 7E) was lower in the CON + LEU than in the CON + ALA group (P < 0.05). The abundance of SL38A9, v-ATPase, LAMTOR1, and LAMTOR2 was not affected by treatment (Fig. 8). The abundance of the RagC-mTOR complex (Fig. 9A), but not Rheb (Fig. 9B), RagA (Fig. 9C), or RagC (Fig. 9D), was greater in the CON + LEU than in the CON + ALA group (P < 0.05). Expression of LAT1 (Fig. 10A) tended to be higher (P = 0.06), and SNAT2 (Fig. 10B) was significantly higher (P < 0.05) in the CON + LEU compared with the CON + ALA group.
Fig. 7.
Phosphorylation of mechanistic (a.k.a. mammalian) target of rapamycin (mTORC1; A), phosphorylation of mTORC2 (B), PRAS40 abundance (C), proline-rich Akt substrate of 40 kDa (PRAS40) phosphorylation (D), and Deptor abundance (E) in LD muscle of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
Fig. 8.
Abundance of SL38A9 (A), v-ATPase (B), lysosome and activate v-ATPase and Ragulator (LAMTOR)1 (C), and LAMTOR2 (D) in LD muscle of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12).
Fig. 9.
Abundance of RagC·mTOR complex (A), Rheb (B), RagA (C), and RagC (D) in LD muscle of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
Fig. 10.
Abundance of amino acid transporters L-type amino acid transporter 1 (LAT1; A) and small neutral amino acid transporter 2 (SNAT2; B) in LD muscle of neonatal pigs continuously fed and pulsed with either CON + ALA or CON + LEU for 21 days. Values are means ± SE (n = 11–12). The statistical effects (Tt) are reported for each variable when P < 0.05. Means without a common letter differ (P < 0.05).
DISCUSSION
Clinical studies have not been able to discern whether intermittent bolus or continuous feeding is more beneficial for promoting growth in the neonate due to the frequent presence of confounding factors and methodological limitations that are present in human studies. Recent findings in our neonatal piglet model have demonstrated that continuous feeding by orogastric tube reduces protein synthesis and blunts protein deposition compared with intermittent bolus feeding for 24 h (31, 32, 40). This reduced response to the same nutrient load is due to the relatively suppressed and constant hormone and substrate patterns incurred with continuous feeding that attenuate muscle protein synthesis. On the other hand, the pulsatile pattern of circulating insulin and amino acids that occurs with intermittent bolus feeding stimulates protein synthesis. Because continuous feeding is still indicated in some infants when there is meal feeding intolerance, strategies to enhance the efficient use of dietary nutrients administered this way would be beneficial. Previous studies have demonstrated that the stimulatory effect of amino acids can be reproduced largely by leucine (12, 75) and that supplementation with leucine, either parenterally or enterally, stimulates protein synthesis, provided that an adequate supply of amino acids is available (33–36, 59, 74, 75, 77). Thus, a solution that could be used to promote protein deposition for infants fed continuously is to provide pulses of leucine that mimic the pulsatile pattern of amino acids that occurs with intermittent bolus feeding. Indeed, we recently found that leucine pulses delivered parenterally every 4 h for 24 h in neonatal pigs fed continuously enhanced muscle protein synthesis more than with continuous feeding alone (8). Whether this strategy and the resulting response can be sustained long term to affect positive changes in growth and/or body composition has not been determined. The findings from our current study revealed that pulsatile parenteral delivery of a leucine supplement during 21 days of continuous enteral feeding in neonatal pigs improved body weight, lean gain, and skeletal muscle mass compared with pulses of the isonitrogenous control alanine. These effects were associated with stimulation of protein synthesis rates and activation of the mTORC1 signaling pathway.
In the present study, the delivery of intermittent parenteral leucine pulses for 21 days with continuous feeding of a diet that met the piglets' dietary requirements increased weight gain by 28% and lean gain by 25% and reduced fat gain by 48% compared with alanine pulses. This is the first study showing a long-term beneficial effect of parenteral supplementation with leucine on growth and suggests a repartitioning of nutrients toward lean growth and away from fat deposition. A 6% increase in body weight in suckling pigs administered leucine orally has been reported (72), although our recent study of oral leucine supplementation of a protein-restricted diet found only a trend for an increase in body weight and lean mass (13). The long-term effect of leucine supplementation on body composition has been assessed largely in selected physiological conditions such as exercise, malnutrition, or aging, all situations where protein metabolism is challenged, and both positive and no effects have been reported (5, 14, 27, 37, 71, 80). A recent meta-analysis concluded that leucine supplementation exerts beneficial effects on body weight and lean body mass in older persons already prone to sarcopenia (49). Beneficial effects on lean body mass and/or muscle mass in rats (11) and humans (7, 24) have also been reported with supplementation of leucine-enriched amino acid mixtures. A reduction in body fat mass has been reported with long-term leucine supplementation in animals fed a high-fat diet (6, 91), during food restriction (27), and in aging rats (81), but these findings have not been confirmed in humans (7, 24, 80). Whether the reduction in fat gain in the current study involves differences in energy expenditure or fatty acid metabolism, as has been reported in leucine supplementation studies in rodents (6, 91), is not known but is at least in part attributable to the increased utilization of amino acids for protein synthesis in muscle. Moreover, these results do not support the purported causative link between increased circulating branched-chain amino acids and obesity or insulin resistance (61) but instead suggest a positive impact of leucine supplementation on body composition in growing animals.
In the current study, the greater lean gain resulting from the pulsed leucine protocol is consistent with the 20% greater gain in the weight of the muscles regardless of fiber type, i.e., the LD (composed of primarily fast-twitch glycolytic fibers), the gastrocnemius (composed of mixed glycolytic and oxidative fibers), and the soleus (composed mainly of slow-twitch oxidative fibers). However, there was no effect on the mass of other tissues measured. The lack of effect on visceral tissue mass is consistent with recent studies in suckling (72) and restricted-protein formula-fed neonatal pigs provided enteral leucine supplementation (13). The leucine-induced increase in muscle mass can be explained by the increased rates of protein synthesis in the three muscles measured. The 30% increase in skeletal muscle protein synthesis is consistent with our previously demonstrated stimulatory effect of leucine pulses during 24 h of continuous feeding (8) and with acute effect of parenteral and enteral leucine administration (34, 59). Less is known about the effect in visceral tissues. In mature rats, leucine supplementation increases protein synthesis in liver (57) and in cardiac muscle (79). Studies in neonatal (59, 77) and weanling pigs (90) suggest that enteral leucine supplementation of a low-protein diet may increase protein synthesis in vital organs. However, 24-h continuous parenteral infusion of leucine (86) and leucine pulses during 24-h continuous feeding (8) had no effect in visceral tissue, except if the leucine was infused with other essential amino acids (86). In the current study, leucine pulses during 21 days of continuous feeding increased protein synthesis in the heart but had no effect in visceral tissues. These differences in protein synthesis were driven by an increase in KRNA with no change in CS, an estimate of ribosome number (42). This is consistent with the lack of effect of leucine pulses on the abundance of ribosomal protein S4 and S8 mRNA in polysomes, suggesting that leucine pulses may not stimulate ribosomal biogenesis. Although intermittent bolus feeding increases the aggregation of ribosomes on mRNA in skeletal muscle more than continuous feeding (40), in this study we could not detect an effect of leucine pulses on the proportion of ribosomes in polysomes. Since protein synthesis rates were increased in muscle, this could indicate proportional increases in translation initiation and elongation rates, although other factors may be involved.
In the current study, the leucine-induced stimulation of skeletal muscle protein synthesis in the CON + LEU group compared with the CON + ALA group can be explained by the pulse in the circulating levels of leucine. Previously, we have shown that an increase in the circulating level of LEU can be accompanied by a reduction in the other branched-chain amino acids isoleucine and valine (8, 33, 77) due to increased branched-chain amino acid oxidation (70). These amino acids may become rate limiting for protein synthesis, as demonstrated by our previous observation that the leucine-induced stimulation of protein synthesis can be sustained if the resulting reduction in circulating amino acids is prevented (85). In the current study, there was no effect of leucine pulses on the circulating levels of isoleucine and valine, which may be explained by the provision of these amino acids by the formula. Indeed, in a recent study in which a low-protein formula was supplemented with leucine (13), the anabolic effects of leucine were more muted than in the current study.
To understand the molecular mechanism by which leucine pulses during 21 days of continuous feeding regulates protein synthesis, we determined the protein abundance and the activation of signaling components involved in translation initiation, focusing largely on the LD muscle as a representative of leucine-sensitive tissues. Our results suggest that administration of leucine pulses increased translation initiation by inducing the phosphorylation of 4E-BP1 and S6K1 as well as the association of the eIF4E·eIF4G active complex. These results are consistent with previous findings in piglets administered leucine parenterally and enterally (8, 34, 36, 59, 74, 77, 85, 86). For comparison we also measured eIF4E·eIF4G complex abundance in other tissues and found that leucine administration increased eIF4E·eIF4G complex formation in gastrocnemius and soleus muscles but not in heart, liver, or kidney. In all tissues except heart, there were parallel changes in the abundance of the eIF4E·eIF4G complex and protein synthesis. In the heart, leucine had no effect on tissue weight and eIF4E·eIF4G complex abundance but enhanced protein synthesis; the reason for the discrepancy is currently unknown.
In agreement with other studies in rats (1) and piglets (8, 74, 77), our results showed that leucine pulses had no effect in muscle on the phosphorylation of eIF2α, which is involved mainly in the critical step of protein synthesis that responds to stress (50) or leucine deprivation (2). It has been reported that leucine deprivation in myoblast and myotubes increased (78) and leucine administration in cell culture (44, 66), rats (87, 88), and humans (29, 41) decreased the phosphorylation of eEF2, a key component of the elongation process. In the current study, phosphorylation of eEF2 did not differ between the CON + LEU and the CON + ALA groups. Indeed, we have previously shown no effect of LEU supplementation on eEF2 phosphorylation in neonatal pigs (8, 74, 77, 85), suggesting that the elongation process is not a rate-limiting step in the leucine-induced stimulation of protein synthesis in neonatal muscle.
Recently, there have been breakthroughs in our understanding of how intracellular amino acids promote the activation of mTORC1 (23). In this study, we determined the effects of long-term administration of leucine pulses on the protein abundance and protein-protein interaction of purported members of the amino acid-sensing pathway. We found no effect of leucine administration on the abundance of the lysosomal amino acid transporter SLC38A9 or on proteins associated with activation of mTORC1 on the lysosome, i.e., v-ATPase, LAMTOR1 and -2, RagA, RagC, and Rheb. Some of our results are in agreement with Laufenberg et al. (54), who reported that leucine administration did not affect the protein abundance of RagA–D, LAMTOR1 and -2, or Rheb in mature rat muscle. In agreement with a recent study in the rat (44), we found that leucine increased the association between RagC and mTOR. In addition, leucine pulses enhanced the abundance of the glutamine transporter SNAT2, which is critical for leucine transport and tended to increase the abundance of the leucine transporter LAT1, as observed previously in human skeletal muscle after essential amino acid ingestion (30).
In this study, we found that both mTORC1 and mTORC2 phosphorylation were increased in the CON + LEU group compared with the CON + ALA group. To the best of our knowledge, this is the first study showing a positive effect of leucine on the activation of both mTORC1 and mTORC2 in vivo. The results suggest that the leucine-induced stimulation of muscle growth is in part due to activation of mTORC1 and mTORC2. Although mTORC2 is considered a regulator of PKB (48), we found no effect of leucine on the phosphorylation of PKB, which is consistent with the lack of effect of leucine administration on insulin, a modulator of PKB activity (47). Furthermore, we found that the abundance of Deptor, an inhibitor of both mTORC1 and mTORC2 (65), was suppressed by leucine administration. However, leucine pulses had no effect on the abundance or phosphorylation of PRAS40, a specific inhibitor of mTORC1 (81), although others have reported that leucine administration in rodents enhances PRAS40 phosphorylation in skeletal (45) and cardiac muscles (68).
In conclusion, the results of the current study suggest that pulsatile parenteral delivery of a leucine supplement, compared with an alanine supplement, during 21 days of continuous orogastric feeding had beneficial effects on growth by increasing body weight and promoting lean growth while reducing fat gain. This anabolic effect can be explained by the leucine-induced increase in protein synthesis in skeletal muscle, which lead to an increase in muscle mass. Our detailed study of the mechanisms involved in this leucine-induced enhancement of lean growth has implicated upregulation of amino acid transporter expression, increased association of mTOR with the Rag proteins, and stimulation of the activation of signaling proteins associated with mTORC1-dependent translation initiation. No evidence of involvement of proteins in the lysosomal-Ragulator-mTORC1 complex was found. These results are of direct relevance for the feeding of infants and children that need continuous tube feeding, as they suggest that pulsatile delivery of a leucine supplement can enhance the efficiency with which nutrients are metabolized to improve lean growth. Future studies are needed to determine the effectiveness of leucine supplementation in preterm neonates and especially in those born small for gestational age.
GRANTS
This work is a publication of the US Department of Agriculture/Agricultural Research Service (USDA/ARS) Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. Financial support for this project was provided by National Institute of Child Health and Human Development Grant HD-072891, USDA National Institute of Food and Agriculture Grant 2013-67015-20438, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-044474 and AR-046308, and USDA Current Research Information System Grant 6250-51000-055. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US Government.
DISCLOSURES
None of the authors has a conflict of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
C.B., M.L.F., and T.A.D. conception and design of research; C.B., S.W.E.-K., A.S., J.S.-W., B.S., R.A.O., H.V.N., S.R.K., and M.L.F. performed experiments; C.B., A.S., H.V.N., S.R.K., M.L.F., and T.A.D. analyzed data; C.B., A.S., M.L.F., and T.A.D. interpreted results of experiments; C.B. prepared figures; C.B. drafted manuscript; C.B., S.W.E.-K., A.S., J.S.-W., S.R.K., M.L.F., and T.A.D. edited and revised manuscript; C.B., S.W.E.-K., A.S., J.S.-W., B.S., R.A.O., H.V.N., S.R.K., M.L.F., and T.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully thank D. G. Burrin, W. C. Heird, and M. W. Haymond for helpful discussions and R. D. Almonaci and S. J. Koo for expert technical assistance.
REFERENCES
- 1.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130: 139–145, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Anthony TG, McDaniel BJ, Byerley RL, McGrath BC, Cavener DR, McNurlan MA, Wek RC. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 279: 36553–36561, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Balage M, Dardevet D. Long-term effects of leucine supplementation on body composition. Curr Opin Clin Nutr Metab Care 13: 265–270, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr 23: 588S–595S, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, McMurdo ME, Mets T, Seal C, Wijers SL, Ceda GP, De Vito G, Donders G, Drey M, Greig C, Holmbäck U, Narici M, McPhee J, Poggiogalle E, Power D, Scafoglieri A, Schultz R, Sieber CC, Cederholm T. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 16: 740–747, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Binder E, Bermudez-Silva FJ, Andre C, Elie M, Romero-Zerbo SY, Leste-Lasserre T, Belluomo I, Duchampt A, Clark S, Aubert A, Mezzullo M, Fanelli F, Pagotto U, Laye S, Mithieux G, Cota D. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS One 8: e74705, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr 27: 189–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab 305: E620–E631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269: 5360–5368, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37: 593–599, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Chen Scarabelli C, McCauley RB, Yuan Z, Di Rezze J, Patel D, Putt J, Raddino R, Allebban Z, Abboud J, Scarabelli GM, Chilukuri K, Gardin J, Saravolatz L, Faggian G, Mazzucco A, Scarabelli TM. Oral administration of amino acidic supplements improves protein and energy profiles in skeletal muscle of aged rats: elongation of functional performance and acceleration of mitochondrial recovery in adenosine triphosphate after exhaustive exertion. Am J Cardiol 101: 42E–48E, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 47: 259–270, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Columbus DA, Steinhoff-Wagner J, Suryawan A, Nguyen HV, Hernandez-Garcia A, Fiorotto ML, Davis TA. Impact of prolonged leucine supplementation on protein synthesis and lean growth in neonatal pigs. Am J Physiol Endocrinol Metab 309: E601–E610, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowe MJ, Weatherson JN, Bowden BF. Effects of dietary leucine supplementation on exercise performance. Eur J Appl Physiol 97: 664–672, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135: 376–382, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol 257: R1141–R1146, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Davis TA, Fiorotto ML, Suryawan A. Bolus vs. continuous feeding to optimize anabolism in neonates. Curr Opin Clin Nutr Metab Care 18: 102–108, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279: E1226–E1234, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 6: 8287–8296, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol 15: 555–564, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon EL, Sheffield-Moore M, Paddon-Jones D, Gilkison C, Sanford AP, Casperson SL, Jiang J, Chinkes DL, Urban RJ. Amino acid supplementation increases lean body mass, basal muscle protein synthesis, and insulin-like growth factor-I expression in older women. J Clin Endocrinol Metab 94: 1630–1637, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 302: E1329–E1342, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Dollberg S, Kuint J, Mazkereth R, Mimouni FB. Feeding tolerance in preterm infants: randomized trial of bolus and continuous feeding. J Am Coll Nutr 19: 797–800, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Donato J Jr, Pedrosa RG, Cruzat VF, Pires IS, Tirapegui J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition 22: 520–527, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2α kinases: their structures and functions. Cell Mol Life Sci 70: 3493–3511, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294: E392–E400, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298: E1011–E1018, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 302: E674–E686, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Kadi SW, Gazzaneo MC, Suryawan A, Orellana RA, Torrazza RM, Srivastava N, Kimball SR, Nguyen HV, Fiorotto ML, Davis TA. Visceral and muscle protein synthesis in neonatal pigs is increased more by intermittent bolus than by continuous feeding. Pediatr Res 74: 154–162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293: E1615–E1621, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288: E914–E921, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140: 1418–1424, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao X, Tian F, Wang X, Zhao J, Wan X, Zhang L, Wu C, Li N, Li J. Leucine supplementation improves acquired growth hormone resistance in rats with protein-energy malnutrition. PLoS One 10: e0125023, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazzaneo MC, Orellana RA, Suryawan A, Tuckow AP, Kimball SR, Wilson FA, Nguyen HV, Torrazza RM, Fiorotto ML, Davis TA. Differential regulation of protein synthesis and mTOR signaling in skeletal muscle and visceral tissues of neonatal pigs after a meal. Pediatr Res 70: 253–260, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gazzaneo MC, Suryawan A, Orellana RA, Torrazza RM, El-Kadi SW, Wilson FA, Kimball SR, Srivastava N, Nguyen HV, Fiorotto ML, Davis TA. Intermittent bolus feeding has a greater stimulatory effect on protein synthesis in skeletal muscle than continuous feeding in neonatal pigs. J Nutr 141: 2152–2158, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 140: 1970–1976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henshaw EC, Hirsch CA, Morton BE, Hiatt HH. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem 6: 436–446, 1971. [PubMed] [Google Scholar]
- 43.Hizli AA, Chi Y, Swanger J, Carter JH, Liao Y, Welcker M, Ryazanov AG, Clurman BE. Phosphorylation of eukaryotic elongation factor 2 (eEF2) by cyclin A-cyclin-dependent kinase 2 regulates its inhibition by eEF2 kinase. Mol Cell Biol 33: 596–604, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol 302: C1557–C1565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kazi AA, Pruznak AM, Frost RA, Lang CH. Sepsis-induced alterations in protein-protein interactions within mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. Shock 35: 117–125, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SW, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134: 625–630, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Kimball SR, Jefferson LS. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J Biol Chem 285: 29027–29032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinert M, Sylow L, Fazakerley DJ, Krycer JR, Thomas KC, Oxboll AJ, Jordy AB, Jensen TE, Yang G, Schjerling P, Kiens B, James DE, Ruegg MA, Richter EA. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Mol Metab 3: 630–641, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komar B, Schwingshackl L, Hoffmann G. Effects of leucine-rich protein supplements on anthropometric parameter and muscle strength in the elderly: a systematic review and meta-analysis. J Nutr Health Aging 19: 437–446, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22: 7405–7416, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem 280: 7570–7580, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Kuh D, Bassey J, Hardy R, Aihie Sayer A, Wadsworth M, Cooper C. Birth weight, childhood size, and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol 156: 627–633, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126: 1713–1719, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laufenberg LJ, Pruznak AM, Navaratnarajah M, Lang CH. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 46: 2787–2798, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 56.Lucas A, Morley R, Lister G, Leeson-Payne C. Effect of very low birth weight on cognitive abilities at school age. N Engl J Med 326: 202–203, 1992. [DOI] [PubMed] [Google Scholar]
- 57.Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab 283: E824–E835, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Munro HN, Fleck A. Analysis of tissues and body fluids for nitrogenous constituents. In: Mammalian Protein Metabolism, edited by Munro HN. New York: Academic, 1969, p. 465–483. [Google Scholar]
- 59.Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140: 2145–2152, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Research Council. Nutrient Requirements of Swine (10th Revised Edition). Washington, DC: National Academies, 1998. [Google Scholar]
- 61.Newgard CB, An J, Bain JR, Muehbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wennr BR, Yancy WS, Elsenson H, Musant G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 285: E40–E53, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr 95: 904–908, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Parker P, Stroop S, Greene H. A controlled comparison of continuous versus intermittent feeding in the treatment of infants with intestinal disease. J Pediatr 99: 360–364, 1981. [DOI] [PubMed] [Google Scholar]
- 65.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun 313: 429–436, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Rebsamen M, Pochini L, Stasyk T, de Araújo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519: 477–481, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez Canedo C, Demeulder B, Ginion A, Bayascas JR, Balligand JL, Alessi DR, Vanoverschelde JL, Beauloye C, Hue L, Bertrand L. Activation of the cardiac mTOR/p70(S6K) pathway by leucine requires PDK1 and correlates with PRAS40 phosphorylation. Am J Physiol Endocrinol Metab 298: E761–E769, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Anderson FH, Cooper C. Falls, sarcopenia, and growth in early life: findings from the Hertfordshire cohort study. Am J Epidemiol 164: 665–671, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimomura Y, Harris RA. Metabolism and physiological function of branched-chain amino acids: discussion of session 1. J Nutr 136: 232S–233S, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Sugawara T, Ito Y, Nishizawa N, Nagasawa T. Supplementation with dietary leucine to a protein-deficient diet suppresses myofibrillar protein degradation in rats. J Nutr Sci Vitam 53: 552–555, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Sun Y, Wu Z, Li W, Zhang C, Sun K, Ji Y, Wang B, Jiao N, He B, Wang W, Dai Z, Wu G. Dietary l-leucine supplementation enhances intestinal development in suckling piglets. Amino Acids 47: 1517–1525, 2015. [DOI] [PubMed] [Google Scholar]
- 73.Suryawan A, Nguyen HV, Almonaci RD, Davis TA. Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids 45: 523–530, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suryawan A, Nguyen HV, Almonaci RD, Davis TA. Differential regulation of protein synthesis in skeletal muscle and liver of neonatal pigs by leucine through an mTORC1-dependent pathway. J Anim Sci Biotechnol 3: pii: 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suryawan A, Orellana RA, Fiorotto ML, Davis TA. Triennial Growth Symposium: leucine acts as a nutrient signal to stimulate protein synthesis in neonatal pigs. J Anim Sci 89: 2004–2016, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71: 324–331, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talvas J, Obled A, Fafournoux P, Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr 136: 1466–1471, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Vary T. Oral leucine enhances myocardial protein synthesis in rats acutely administered ethanol. J Nutr 139: 1439–1444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verhoeven S, Vanschoonbeek K, Verdijk LB, Koopman R, Wodzig WK, Dendale P, van Loon LJ. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am J Clin Nutr 89: 1468–1475, 2009. [DOI] [PubMed] [Google Scholar]
- 81.Vianna D, Resende GF, Torres-Leal FL, Pantaleao LC, Donato J Jr, Tirapegui J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition 28: 182–189, 2012. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Harris TE, Roth RA, Lawrence JC Jr. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J Biol Chem 282: 20036–20044, 2007. [DOI] [PubMed] [Google Scholar]
- 83.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL, Sabatini DM. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347: 188–194, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson FA, Suryawan A, Orellana RA, Kimball SR, Gazzaneo MC, Nguyen HV, Fiorotto ML, Davis TA. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J Nutr 139: 1873–1880, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140: 264–270, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson FA, Suryawan A, Orellana RA, Gazzaneo MC, Nguyen HV, Davis TA. Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids 40: 157–165, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab 301: E1236–E1242, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson GJ, Moulton CJ, Garlick PJ, Anthony TG, Layman DK. Post-meal responses of elongation factor 2 (eEF2) and adenosine monophosphate-activated protein kinase (AMPK) to leucine and carbohydrate supplements for regulating protein synthesis duration and energy homeostasis in rat skeletal muscle. Nutrients 4: 1723–1739, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao K, Yin YL, Chu W, Liu Z, Deng D, Li T, Huang R, Zhang J, Tan B, Wang W, Wu G. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr 138: 867–872, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Yin Y, Yao K, Liu Z, Gong M, Ruan Z, Deng D, Tan B, Liu Z, Wu G. Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39: 1477–1486, 2010. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 56: 1647–1654, 2007. [DOI] [PubMed] [Google Scholar]
- 92.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]