Abstract
Aim/Background:
The development of resistance to synthetic drugs by target organisms is a major challenge facing medicine, yet locked within plants are phytochemicals used in herbal medicine (especially in the Arabian Peninsula) that may find application in this regard. In pursuit of unlocking these “hidden treasures,” the methanol extracts of leaves, aerial parts, fruits, and resins of 17 plants used in the Arabian Peninsula were screened for antimicrobial activities.
Materials and Methods:
The nematicidal, antibacterial, and antifungal activities were determined using appropriate assays. Steinernema feltiae, Staphylococcus carnosus, Escherichia coli, and Saccharomyces cerevisiae were used as test organisms. Concentrations of the extracts ranging from 0.5 to 20 mg/ml were tested and appropriate statistical tests performed on the data generated.
Results:
The results show that extracts from Solanum incanum, Chenopodium murale, Commiphora myrrha, Anthemis nobilis, and Achillea biebersteinii were the most active and had very high activities against two or more of the test organisms at low concentrations. Extracts of the leaves of S. incanum and resins of Ferula asafoetida were the most active nematicides, with significant activity at 0.5 mg/ml. Extracts of C. myrrha and C. murale had the most active antibacterial activity with inhibition zones of 12-15 mm and minimum inhibitory concentrations (MICs) of 2.5 mg/ml for both bacteria. Extracts of the leaves of A. biebersteinii were the most active fungicide, giving an MIC of 1.5 mg/ml.
Conclusion:
The results validate the use of these plants in ethnopharmacology, and open new vistas of opportunities for the development of cheap but effective agents that may be useful against infectious diseases.
Keywords: Antimicrobials, Commiphora myrrha, medicinal plants, nematicides, phytochemicals, Solanum incanum
INTRODUCTION
Although significant progress has been achieved during the last 50 years in fighting infectious diseases, they still remain an important cause of morbidity and mortality globally [1]. Infections cause an estimated 50% of all deaths in tropical countries, where as much as three million preschool children die each year solely due to infections of the gastrointestinal tract [2]. Besides bacteria and fungi, nematodes transmitted from the soil cause diseases which affect 25% of the world’s population, again mostly in the tropics. They are known to lead to anemia and to cause retarded physical and mental growth [3,4]. The negative effects of nematodes on agricultural livestock are also well-documented [5,6]. As for bacteria enteropathogenic strains of Gram-negative Escherichia coli are known to cause acute and chronic diarrhea, vomiting, and fever in infants [7]. The Gram-positive bacterium Staphylococcus aureus can multiply and spread widely in tissues resulting in enteric infections, boils, skin sepsis, endocarditis, and pneumonia. Their heat-stable endotoxins cause diarrhea, fever, abdominal cramps, and vomiting with an attendant electrolyte imbalance [8]. Owing to their ability to thrive better in warm, humid environments, fungal infections are equally problematic and more rampant in the tropics and sub-tropics than any other place in the world. They cause diseases ranging from superficial mycoses, cutaneous mycoses, sub-cutaneous mycoses, and systemic mycoses; and are usually very difficult to treat [9]. These organisms also cause diseases in domestic and farm animals resulting in massive economic losses.
Unfortunately, many drugs currently available for the treatment of infections are expensive and often not readily available or are easily counterfeited. Furthermore, the development of resistances to these drugs is a major setback to their continued use in humans and livestock [10-12].
Interestingly, the tropics where most of these infections are rampant are also amazingly rich in a diversity of plants and fungi. Given the WHO report that medicines derived from plants serve the health needs of approximately 80% of people globally [13], it is important to screen plants that are used in ethnopharmacology and ethnomedicine for activities against nematodes, bacteria, and fungi. Such plants may provide new and, above all, inexpensive and locally available drugs and improve the health of people in economically under-developed or developing countries.
In the light of the above, the nematicidal and antimicrobial properties of methanol extracts of 17 plants used in ethnopharmacology and ethnomedicine around the tropics and sub-tropics, and particularly in Saudi Arabia and Yemen were investigated [Figure 1]. The primary aim of this investigation has been to uncover phytochemical products that can be produced locally and in sufficient commercial quantities to be used in improving Medicine and Agriculture, especially in some of the developing economies of the world. Details of the plants, the parts harvested and their uses in folk medicine have been obtained from published literature, and traditional users of the plants [14-16] are summarized in Table 1.
Figure 1.

Map of Arabian Peninsula indicating the regions of plant collection
Table 1.
Medicinal plants selected as part of this study, ethnobotanical information and relevant characteristics

MATERIALS AND METHODS
Plant Materials
The plant materials were collected between the months of March and April 2014 at different locations in Al Baha town, and its outskirts, Saudi Arabia. Dendrosicyos socotrana and Dracaeana cinnabari were collected from the island of Socotra between November and December 2014. Those plants were identified taxonomically at the Department of Botany, Faculty of Science, Aden University, Republic of Yemen. Voucher specimens of the plant materials were deposited at the Pharmacognosy Department, Faculty of Clinical Pharmacy, Al Baha University, Saudi Arabia for the Saudi plants and at the Department of Botany, Faculty of Science, Aden University, Yemen for D. socotrana and D. cinnabari.
Preparation of Plant Extracts
The plant parts harvested were air-dried under the shade at ambient temperature and powdered with a blender. The powdered plant material (10 g) was extracted with absolute methanol (4 × 100 ml). The extractions were carried out at room temperature with the constant shaking of the extraction set-up. Thereafter, the mixtures were filtered, and the filtrate evaporated to dryness in vacuo at 40°C to yield the methanol extracts subsequently used as a part of our studies. The yields of each dried extract were calculated in %. The resulting dried crude extracts were stored at 4°C until they were analyzed for nematicidal and antimicrobial properties.
Nematicidal Activity
Steinernema feltiae was purchased from Sautter and Stepper GmbH (Ammerbuch, Germany), as a powder cake product and stored in the dark at 4°C. Fresh samples were ordered before each experiment, and each opened batch was discarded after 6 days. Prior to each experiment, a homogeneous mixture of nematodes was prepared by suspending 200 mg of powder cake in 50 ml of distilled water at 27°C to revive the nematodes. Contained therein, the suspension was allowed to stand at room temperature though with occasional rocking and in moderate light for 30 min. Thereafter, the viability of the nematodes in suspension was determined with a microscope at four-fold magnification (TR 200, VWR International, Belgium). A viability of more than 80% was considered optimal and seen as a prerequisite for each experiment.
Each plant extract (100 mg) was dissolved in 5 ml of 2% DMSO to yield a 20 mg/ml stock solution. From this stock solution, a series of dilutions in water was prepared with 0.5, 1, 3, 5, 10, and 15 mg/ml solutions which were then used for the experiments. To each well in the 96-well plate, 10 ml of the nematode suspension was added (which usually contains 30-40 nematodes per well). Thereafter, 100 ml of each concentration of the plant extracts was added to each well. The control experiment was performed with the DMSO/water vehicle in place of the extracts. The well plates were then assessed immediately for viability under the microscope before incubation in the dark at room temperature for 24 h. After 24 h, 50 ml of distilled water at 50°C was added to each well to stimulate the movement of the nematodes. Thereafter, live and dead nematodes were counted under the microscope (magnification × 4). Each concentration was tested in three different wells per experiment, and each experiment was repeated three times to yield a total of nine repeats per individual experiment.
The viability of the nematodes was expressed as percentages. The viability values were calculated using the equation:
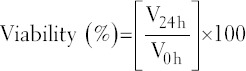
Where, V24 h is number of live nematodes after 24 h and V0 h is the number of live nematodes at 0 h.
Antimicrobial Activity
Two bacterial strains, Staphylococcus carnosus TM 300 and E. coli K2 (representing Gram-positive and Gram-negative bacteria species, respectively) as well as the fungus Saccharomyces cerevisiae were used as representative model organisms for the antimicrobial investigations.
The disc diffusion assay [17] was used to determine antimicrobial activities of the extracts investigated. Nutrient Luria-Bertani and Yeast Extract-Peptone-Dextrose (YPD)(Sigma-Aldrich, Steinheim, Germany) were used as media. Sterile plates were inoculated evenly using sterile swab sticks. Sterile qualitative filter paper discs of 6 mm diameter (VWR International GmbH-Darmstadt, Deutschland, ref. No. 601110, lot. 06513) were impregnated with 20 ml of each extract solution (equivalent to 4 mg/disc). The paper discs were allowed to dry before being gently placed on the surface of the inoculated agar plates, at positions that were equidistant from each disc. The plates were kept for 3 h in a refrigerator to enable pre-diffusion of the substances into the media. A mixture of Penicillin-Streptomycin-Amphotericin-B was used as positive control while the solvent (methanol) was used as negative control. Plates inoculated with bacteria and yeasts were incubated for 18-24 h at 37°C. Inhibition zone diameters around each disc (diameter of inhibition zone plus diameter of the disc) were measured and recorded at the end of the incubation time [18]. An average zone of inhibition was calculated for the three replicates.
The MIC was determined using the broth microdilution method of Mann and Markham [19], with slight modifications. Fresh cultures of bacteria on LB agar and yeast on YPD agar were prepared and incubated for 18-24 h. From these cultures, inocula were prepared by suspending colonies of the respective organisms in sterile 0.85% NaCl solution and then adjusted to 0.5 of the McFarland standard (1.5 × 108 CFU/ml for bacteria and 1.5 × 106 CFU/ml for yeast). Different concentrations (0.5, 1.0, 2.5, 5.0, and 10 mg/ml) of the plant extracts were added to the LB or YPD broth in 96-well plates, and the inocula were subsequently added to each well. Thereafter, the plates were incubated at 37°C for 18-24 h. Antibacterial activity was detected by adding 20 mL of 0.01% sodium resazurin (Sigma) and incubating the plates for 1 h. A change from blue to pink indicates a reduction of resazurin and, therefore, bacterial growth. The assay was conducted in triplicate, and three independent experiments were performed on different occasions. The MIC value was defined as the minimum concentration of test sample that inhibited the selected organism’s growth, i.e., at which no growth was observed. The reference antibiotics used were penicillin-streptomycin-amphotericin B (Sigma-Aldrich, Steinheim, Germany).
Statistics
The data generated was subjected to descriptive statistical analysis and the results are presented as mean ± standard error of mean. Differences between the means (test versus control) were assessed for statistical significance using the one-way ANOVA test with the significance threshold fixed at P < 0.05. The GraphPad Prism software (GraphPad Inc., USA) was used for all statistical analyzes. The results are presented in Tables 1-3 and Figures 1-3 and statistical significances in the Figures 1-3 are marked as *, **, or *** when the P < 0.05, < 0.01 or < 0.001, respectively.
Table 2.
Nematicidal activity of plant extracts against S. feltiae

Table 3.
Antimicrobial activity of plant extracts against S. carnosus and E. coli
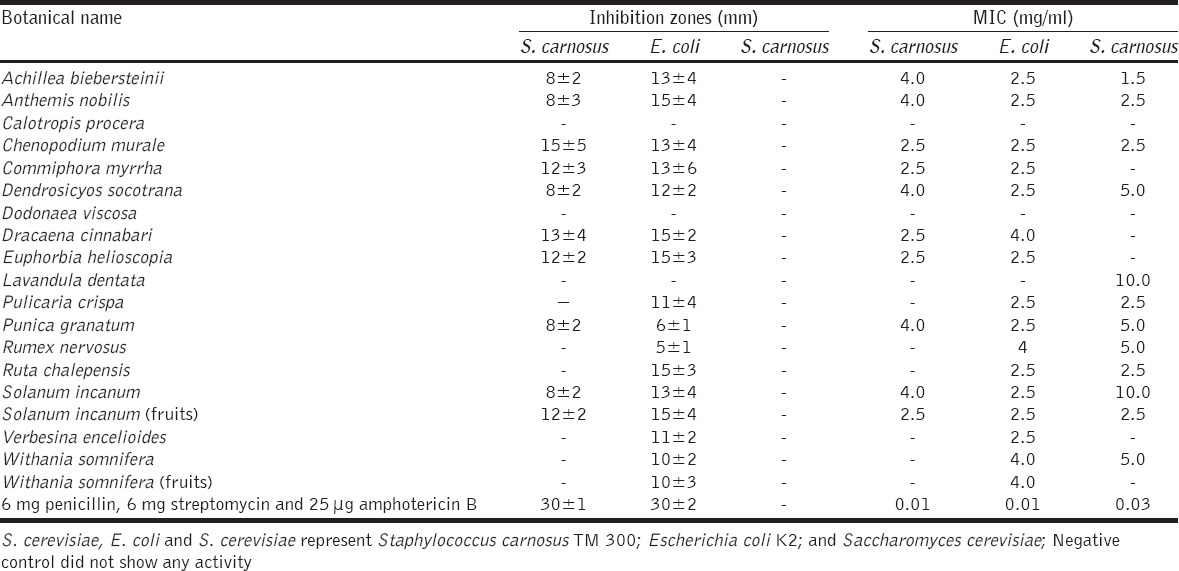
Figure 2.

Nematicidal activity of the most active plant extracts against the model nematode S. feltiae
Figure 3.

The plants which exhibited the highest toxicity against the selected microorganisms
RESULTS
For all plant materials under investigation, suitable extracts could be obtained in good quality and yield. Table 1 briefly summarizes the individual yields of extraction for the different extracts. It should be emphasized that the fruits of Withania somnifera yielded the most extract (8.5%) while the leaves of Achillea biebersteinii yielded the least (2.6%)[Table 1]. The average yield was approximately 5%. The extracts obtained were subsequently investigated for biological activity, first against the nematode S. feltiae, and subsequently against different bacteria and the fungus S. cerevisiae.
Nematicidal Activity
In the case of nematodes, a distinctively different activity could be observed for the methanol extracts of aerial parts of Chenopodium murale, Pulicaria crispa, Euphorbia helioscopia, and Lavandula dentata, leaves of Dodonaea viscosa, Verbesina encelioides, and A. biebersteinii; leaves of W. somnifera, Calotropis procera, D. socotrana, Rumex nervosus, Ruta chalepensis, and Solanum incanum; resins of C. myrrha and D. cinnabari; fruits of S. incanum and W. somnifera; flowers of Punica granatum, A. biebersteinii, and Anthemis nobilis. These activities are summarized in Table 2.
While some extracts showed considerable activity against S. feltiae, others were hardly active. The extract from the leaves of S. incanum was the most active as it resulted in statistically significant mortality of the nematodes at the lowest concentration tested (0.5 mg/ml) [Figure 2]. Purely for comparison: this concentration corresponds to 2 mM of a chemically pure compound with a molecular weight of 250 g/mol. The next most active extracts in order of activity were those from S. incanum and W. somnifera fruits, R. nervosus leaves, P. crispa aerial parts, and resins of C. myrrha, each showing statistically significant nematicidal activity at a concentration of 1 mg/ml. This was followed by extracts from E. helioscopia, D. viscosa, A. biebersteinii, P. granatum, D. socotrana, and D. cinnabari, each with statistically significant nematicidal activity at a concentration of 2.5 mg/ml. In contrast, extracts from C. murale (10 mg/ml), L. dentata (10 mg/ml), and C. procera (20 mg/ml) were hardly effective as nematicides.
Antimicrobial Activity
The extracts were then tested for their activity against two bacteria, S. carnosus and E. coli, which are representative of Gram-positive and Gram-negative bacteria, respectively. An activity profile similar to one in nematodes could be observed. In the case of S. carnosus, the extract from the fruits of S. incanum, but also extracts of the resins of D. cinnabari and C. myrrha and the aerial parts of E. helioscopia resulted in high zones of inhibition and low MIC values against this strain of Staphylococcus. E. coli appeared to be even more sensitive against a wider range of extracts, such as extracts obtained from the leaves of C. murale, S. incanum, D. socotrana, A. biebersteinii, and V. encelioides, from the flowers of P. granatum and A. nobilis, the resins of C. myrrha, and the aerial parts of P. crispa. All of these extracts resulted in high zones of inhibition and low MIC values against E. coli. Ultimately, extracts from the resins C. myrrha, the leaves of D. socotrana and S. incanum, and from the flowers of A. nobilis, provided the best antibacterial activity for both Gram-positive and negative bacteria [Table 3].
A very similar picture also emerged with the single-cell fungus S. cerevisiae [Table 3]. Since the broth microdilution method is known to be more reliable and useful in testing plant extracts for activity [19], we have collected data via both, the disc diffusion and the microdilution method. Indeed, in our hands, the latter was also more reliable with regard to toxicity against S. cerevisiae. Extracts of the leaves of A. biebersteinii, C. murale, and R. chalepensis, of the aerial parts of P. crispa, of the flowers of A. nobilis and of the fruits of S. incanum inhibited the growth of S. cerevisiae at low concentrations. Other extracts such as the ones of the leaves of R. nervosus and W. somnifera and the flowers of P. granatum also inhibited the growth of S. cerevisiae, yet to a lesser extent. The extracts from the other plants showed little or no effect with respect to the growth of yeast.
Overall, extracts from 14 of the 17 plants under investigation exhibited high activity against at least one organism tested while the other three plants, D. viscosa, C. procera, and L. dentata showed only limited activities against all of the organisms tested. Among the active plants, S. incanum clearly attracted the most attention. Intriguingly, extracts of the fruits of S. incanum appeared to possess high activity against all four organisms tested, while the leaves of this plant had limited activity against S. cerevisiae and S. carnosus. Preparations from other plants were also rather active such as extracts of the aerial parts of P. crispa and extracts of the resins of C. myrrha, which yielded high activities against three of the four organisms tested. Less active but still of interest due to possible harvesting and processing where the flowers of A. nobilis and leaves of A. biebersteinii, which showed high activities against two of the four organisms tested.
DISCUSSION
Although synthetic nematicides and antimicrobials used in Medicine and Agriculture are effective and rapid-acting, the challenges of resistance to these agents by microorganisms, and the concerns to human health and the environment raised by their use in Agriculture, have spurred research efforts at developing “green” plant-based or plant derived alternatives. In fact, various phytochemicals are known to be safe to both humans and the environment. When used in Agriculture, as in the case of nematicides, they are “biodegradable” and usually do not persist in the fields for longer periods of time than is really necessary [20]. Naturally, therefore, research efforts have been geared toward plants that are used in Folk Medicine and Agriculture from different cultures, with a view to identifying those that can be used to develop “green” phyto-protectants, antimicrobials, and pesticides. Many of these plants contain a cocktail of phytochemicals, true treasure chests for bio-activity against the myriad of microorganisms that pose challenges to Medicine and Agriculture, especially in developing countries of the tropics.
Our study has, therefore, investigated the nematicidal and antimicrobial properties of methanol extracts of leaves, aerial parts, and resins from 17 plants used in traditional ethnopharmacology and ethnomedicine in Saudi Arabia, Yemen and neighboring countries of the Arabian Peninsula. Deliberately, methanol was used as the solvent of extraction based on our experience over the years in working with plant products and reports from other researchers working on similar subjects [21]. Indeed, several studies have shown that methanol is the solvent of choice for the extraction of antimicrobial constituents of plants [22-25]. Compared to ethanol, methanol is also less controversial culturally.
Interestingly, extracts from five of the plants studied [Figure 3] - namely S. incaum, P. crispa, C. myrrha, A. biestersteinii, and A. nobilis - exhibited high activities at low concentrations against two or more of the organisms tested. S. incanum, in particular, which is also known as Jericho tomato, attracted our particular interest, as several parts of this plant seem to be extraordinarily toxic. Though there are reports of toxicity of the plant, such reports should spur on research especially as the plant is particularly promising for several reasons. First, it grows readily, widely and requires little care. Second, it can be harvested and processed easily. Third, it is not used for any other purposes, hence has little “value.” Finally, its extract is amenable to further purification and/or modification, which may improve activity significantly. Indeed, the literature available to date on the most active plants show that S. incanum is rich in phytochemicals such as incanumine, solasodine, carpesterol, β-sitosterol, stigmasterol, and khasianine [22]. Lin et al. [26] also reported the presence of quercetin, kaempferol, and astragalin in parts of the plant. Besides, members of the Solanaceae family have been known for a long time to possess antibiotic activity, which is likely due to the presence of glycosides and alkaloids [27].
Ultimately, a partially purified, stabilized, and appropriately conserved extract of the fruits and/or leaves of the Jericho tomato may well be suitable for applications in the field of Agriculture and possibly also Medicine. Within this context, possible toxic effects on humans and higher animals need to be considered and addressed in earnest, and the possibility of synergism in the action of the individual chemical components contained within the different extracts may have to be accounted for.
Indeed, the activity observed for extracts of S. incanum, and the various other extracts may arise from a variety of chemical components and biochemical mechanisms. Though we do not currently possess data on the mechanisms of action, it is known, for instance, that plant extracts often exert their lethal effects through the disruption of cell membrane permeability in organisms that come in contact with them [28]. Within this context, variations observed in the effectiveness of the extracts in killing the nematodes, bacteria, or fungi may indeed be explained by the biological differences which exist between the organisms, for instance, differences in cell wall structure and composition. In fact, it has been reported in other studies, and corroborated by this study, that plant extracts often show a higher activity against bacteria compared to fungi, and this may, in part, be due to differences in the cell wall synthesis and structure [25,29,30]. Specific phytochemicals, such as tannins, furthermore have the capacity to bind to and subsequently denature or disrupt proteins, and if such proteins are vital structural or catabolic proteins, would result in the death of the organism [31-33]. There are obviously many other possible mechanisms and mode(s) of action associated with the plethora of phytochemicals found in those plants.
While we cannot list all of the ingredients contained within our most active extracts and their suspected mode(s) of action, it is worth mentioning a few. Many potent phytochemicals have been found in P. crispa, especially sesquiterpene lactones and guaianolide sesquiterpenes [32,33]. Possibly, the in vitro antimicrobial activity and known anti-leishmanicidal activities of the methanol extract of this particular plant are due to the presence of these phytochemicals [34,35]. Similarly, the genus Commiphora in general and C. myrrha, in particular, is a true hot(s) pot of biologically active secondary metabolites, with more than 300 of them identified and many of them associated with a pronounced activity against a variety of different microorganisms [36,37]. Those include flavonoids, alkaloids, tannins, glycosides, steroids, saponins, and terpenoids, and among them biologically highly active molecules such as myrracadinol A, B, and C, and myrracalamene A, B, and C, and triacont-1-ene [37]. Similarly, flowers of A. nobilis, a plant referred to in German language as “Alles zutraut,” meaning “capable of anything” [38], have been used for a long time and are documented in more than 27 national pharmacopoeias. Indeed, this Chuck Norris of medicinal plants has been studied for centuries, and over a century ago, in 1914, Power and Jun [39] reported that the flowers contain essential oils, anthemene, anthemol, and anthesterol. More recently, the terpenoids: bisabolol, chamazulene, and sesquiterpenes; the flavonoids: apigenin, luteolin, and quercetin; and the coumarins: umbelliferone and scopoletin-7-glucoside, have been described as biologically active constituents of A. nobilis. Other active substances contained within that particular plant include angelic and tiglic acid esters, anthemic acid, choline, tannin, polysaccharides, phenolic, and fatty acids [40,41]. Finally, the extracts of A. biebersteinii contain large quantities of β-sitosterol, stigmasterol, sesquiterpene lactones, guaianolide, germacranolide, and flavonoids [42]. This list of phytochemicals for the five most active plants is clearly not exhaustive and is without prejudice to the apparent rich phytochemical constitution of the other ten plants that did show at least some activity against at least one of the organisms studied. Ultimately, it is, therefore, permissible to speculate that the nematicidal and antimicrobial activities observed in this study derive from the rich milieu of phytochemicals found in these very active plant extracts. These aspects now require further attention.
This study is limited by our inability to access a wider spectrum of organisms and test the activities of these extracts on them. It is hoped that these results will spur interest in these plants and provide the impetus to widen the screening and identification of the active constituents. Work on the latter is currently ongoing in our laboratory. Again, the nematicidal assays would have benefitted from a positive control. This would have afforded us a window to compare the data appropriately. We, however, feel that given the activity observed at concentrations as low as 1 mg/mL in these crude extracts, the purified extracts/fractions may have profound activity and testing them will necessarily require a positive control. In making the above assertion, we are mindful of interactions between individual constituents of plant extracts which may attenuate or accentuate the activity of their parent crude extract.
CONCLUSIONS
A future search for potent phytochemicals as an integral part of future “green” Medicine and Agriculture should consider the five plants identified by us as most active in more detail, with a particular focus on S. incanum. Isolating and characterizing the active compounds contained therein and elucidating the mode(s) of action will indeed be very important yet also challenging. It may also be useful to develop agents based on combinations of different extracts such as combined extracts of the fruits and leaves of S. incanum. Eventually, it may also be feasible to blend active phytochemicals from different plants such as S. incanum with A. biebersteinii and R. nervosus with C. myrrha. Moving on from natural products to synthetic chemistry, one may also envisage the design and development of synthetic analogues of the natural compounds, yet this task will be more challenging scientifically and also does not address the matter of local availability at a low cost. After all, the plants selected by us are readily available, easy to cultivate and harvest which renders their utilization cost effective in the Arabian Peninsula and parts of Africa.
ACKNOWLEDGMENTS/DECLARATIONS
The authors would like to thank the following institutions for providing financial support, without which this project would not have been possible: The University of Saarland in Germany; Thamar University in Yemen for financing the scholarship; the Dean of High Studies and Scientific Research in Al Baha University for funding the plant collection and extraction through funding the project number (56/1434); and The World Academy of Sciences (TWAS)- Deutsche Forschungsgemeinschaft (DFG) for the Cooperation Visit Fellowship awarded to CECCE. A special thank you also goes to the Academics International Network for numerous stimulating discussions. The authors have no real or potential conflicts of interest to declare
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moellering RC, Jr, Graybill JR, McGowan JE, Jr, Corey L. Antimicrobial resistance prevention initiative –An update: Proceedings of an expert panel on resistance. Am J Med. 2007;120:S4–25. doi: 10.1016/j.amjmed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Farthing MJ, Kelly P. Infectious diarrhoea. Medicine. 2007;35:251–6. [Google Scholar]
- 3.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA. 2008;299:1937–48. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 4.Waterman C, Smith RA, Pontiggia L, DerMarderosian A. Anthelmintic screening of Sub-Saharan African plants used in traditional medicine. J Ethnopharmacol. 2010;127:755–9. doi: 10.1016/j.jep.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Wolstenholme AJ, Fairweather I, Prichard R, von Samson-Himmelstjerna G, Sangster NC. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–76. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Behnke JM, Buttle DJ, Stepek G, Lowe A, Duce IR. Developing novel anthelmintics from plant cysteine proteinases. Parasit Vectors. 2008;1:29. doi: 10.1186/1756-3305-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(Suppl 5):S350–9. doi: 10.1086/533591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu LY, Wijaya L, Shu-Ting Ng E, Gotuzzo E. Tropical fungal infections. Infect Dis Clin North Am. 2012;26:497–512. doi: 10.1016/j.idc.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Geerts S, Gryseels B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207–22. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGaw LJ, Jäger AK, van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol. 2000;72:247–63. doi: 10.1016/s0378-8741(00)00269-5. [DOI] [PubMed] [Google Scholar]
- 12.Moellering RC., Jr Discovering new antimicrobial agents. Int J Antimicrob Agents. 2011;37:2–9. doi: 10.1016/j.ijantimicag.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. Geneva, Switzerland: WHO; 2001. p. 1. [Google Scholar]
- 14.Ali AN, Attif OA, Mohammed MI. Herbal medicine in two Yemeni provinces-ethnobotanical study. Yem Med J. 1999;3:13–23. [Google Scholar]
- 15.Ali NA, Jülich WD, Kusnick C, Lindequist U. Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol. 2001;74:173–9. doi: 10.1016/s0378-8741(00)00364-0. [DOI] [PubMed] [Google Scholar]
- 16.Al-Fatimi M, Friedrich U, Jenett-Siems K. Cytotoxicity of plants used in traditional medicine in Yemen. Fitoterapia. 2005;76:355–8. doi: 10.1016/j.fitote.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 18.Tukue M, Kasimala MB. Phytochemical screening and antibacterial activity of two common terrestrial medicinal plants Ruta chalepensis &Rumex nervosus. Caribb J Sci Technol. 2014;2:634–41. [Google Scholar]
- 19.Mann CM, Markham JL. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol. 1998;84:538–44. doi: 10.1046/j.1365-2672.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 20.Chitwood DJ. Phytochemical based strategies for nematode control. Annu Rev Phytopathol. 2002;40:221–49. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- 21.de Boer HJ, Kool A, Broberg A, Mziray WR, Hedberg I, Levenfors JJ. Anti-fungal and anti-bacterial activity of some herbal remedies from Tanzania. J Ethnopharmacol. 2005;96:461–9. doi: 10.1016/j.jep.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 22.Lin CN, Lu CM, Cheng MK, Gan KH, Won SJ. The cytotoxic principles of Solanum incanum. J Nat Prod. 1990;53:513–6. doi: 10.1021/np50068a041. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad I, Mehmood Z, Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J Ethnopharmacol. 1998;62:183–93. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 24.Parekh J, Jadeja D, Chanda S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk J Biol. 2005;29:203–10. [Google Scholar]
- 25.Tekwu EM, Pieme AC, Beng VP. Investigations of antimicrobial activity of some Cameroonian medicinal plant extracts against bacteria and yeast with gastrointestinal relevance. J Ethnopharmacol. 2012;142:265–73. doi: 10.1016/j.jep.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Lin YL, Wang WY, Kuo YH, Chen CF. Nonsteroidal constituents from Solanum incanum L. J Chin Chem Soc. 2000;47:247–51. [Google Scholar]
- 27.Beaman-Mbaya V, Muhammed SI. Antibiotic action of Solanum incanum Linnaeus. Antimicrob Agents Chemother. 1976;9:920–4. doi: 10.1128/aac.9.6.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhtar T, Kayani MZ, Hussain MA. Nematicidal activities of Cannabis sativa L. and Zanthoxylum alatum Roxb. Against Meloidogyne incognita. Ind Crops Prod. 2013;42:447–53. [Google Scholar]
- 29.Avato P, Vitali C, Mongelli P, Tava A. Antimicrobial activity of polyacetylenes from Bellis perennis and their synthetic derivatives. Planta Med. 1997;63:503–7. doi: 10.1055/s-2006-957751. [DOI] [PubMed] [Google Scholar]
- 30.Zavala SM, Perez GM, Perez GR. Antimicrobial screening of some medicinal plants. Phytother Res. 1997;11:368–71. [Google Scholar]
- 31.Athanasiadou S, Kyriazakis I, Jackson F, Coop RL. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep In vitro and in vivo studies. Vet Parasitol. 2001;99:205–19. doi: 10.1016/s0304-4017(01)00467-8. [DOI] [PubMed] [Google Scholar]
- 32.Dendougui H, Benayache S, Benayache F, Connoly JD. Sesquiterpene lactones from Pulicaria crispa. Fitoterapia. 2000;71:373–8. doi: 10.1016/s0367-326x(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 33.Stavri M, Mathew KT, Gordon A, Shnyder SD, Falconer RA, Gibbons S. Guaianolide sesquiterpenes from Pulicaria crispa (Forssk.) Oliv. Phytochemistry. 2008;69:1915–8. doi: 10.1016/j.phytochem.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Abdelah-Bogdadi HA, Kokoska L, Havlik J, Kloucek P, Rada V, Vorisek K. In vitro antimicrobial activity of some Libyan medicinal plant extracts. Pharm Biol. 2007;45:386–91. [Google Scholar]
- 35.El-On J, Ozer L, Gopas J, Sneir R, Enav H, Luft N, et al. Antileishmanial activity in Israeli plants. Ann Trop Med Parasitol. 2009;103:297–306. doi: 10.1179/136485909X440827. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed F, Ali M, Singh O. New compounds from Commiphora myrrha (Nees) Engl. Pharmazie. 2006;61:728–31. doi: 10.1002/chin.200652186. [DOI] [PubMed] [Google Scholar]
- 37.Shen T, Li GH, Wang XN, Lou HX. The genus Commiphora: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2012;142:319–30. doi: 10.1016/j.jep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Berry M. Herbal products. Part 6. Chamomiles. Pharm J. 1995;254:191–3. [Google Scholar]
- 39.Power FB, Jun HB. The constituents of the flowers of Anthemis nobilis. J Chem Soc Trans. 1914;105:1829–45. [Google Scholar]
- 40.Newall CA, Anderson LA, Phillipson JD. Herbal Medicines: A Guide for Health-care Professionals. London: Pharmaceutical Press; 1996. p. 296. [Google Scholar]
- 41.Gardiner P. Chamomile. Longwood Herbal Task Force. 1999. [Last accessed on 2015 Aug 23]. Available from: http://www.mcp.edu/herbal/default.htm .
- 42.Badahdah KO, El-Orfy HS. Phytchemical constituents of Achillea biebersteinii. J Saudi Chem Soc. 2004;8:115–20. [Google Scholar]


