Abstract
Background/Aim:
Elevated uric acid level, an index of gout resulting from the over-activity of xanthine oxidase (XO), increases the risk of developing hypertension. However, research has shown that plant-derived inhibitors of XO and angiotensin 1-converting enzyme (ACE), two enzymes implicated in gout and hypertension, respectively, can prevent or ameliorate both diseases, without noticeable side effects. Hence, this study characterized the polyphenolics composition of guava leaves extract and evaluated its inhibitory effect on XO and ACE in vitro.
Materials and Methods:
The polyphenolics (flavonoids and phenolic acids) were characterized using high-performance liquid chromatography (HPLC) coupled with diode array detection (DAD). The XO, ACE, and Fe2+-induced lipid peroxidation inhibitory activities, and free radicals (2,2-diphenylpicrylhydrazyl [DPPH]* and 2,2´-azino-bis-3-ethylbenzthiazoline-6-sulphonic [ABTS]*+) scavenging activities of the extract were determined using spectrophotometric methods.
Results:
Flavonoids were present in the extract in the order of quercetin > kaempferol > catechin > quercitrin > rutin > luteolin > epicatechin; while phenolic acids were in the order of caffeic acid > chlorogenic acid > gallic acids. The extract effectively inhibited XO, ACE and Fe2+-induced lipid peroxidation in a dose-dependent manner; having half-maximal inhibitory concentrations (IC50) of 38.24 ± 2.32 μg/mL, 21.06 ± 2.04 μg/mL and 27.52 ± 1.72 μg/mL against XO, ACE and Fe2+-induced lipid peroxidation, respectively. The extract also strongly scavenged DPPH* and ABTS*+.
Conclusion:
Guava leaves extract could serve as functional food for managing gout and hypertension and attenuating the oxidative stress associated with both diseases.
Keywords: Angiotensin I-converting enzyme, Fe2+-induced lipid peroxidation, oxidative stress, xanthine oxidase
INTRODUCTION
Gout and hypertension are two degenerative diseases that are capable of reducing the quality of life of individuals suffering from them. Research has shown that elevated uric acid level, an index of gout resulting from the over-activity of xanthine oxidase (XO), increases the risk of developing hypertension [1], indicating a link between gout and hypertension. In fact, hypertension is an established predictor of gout, in addition to other risk factors such as serum uric acid level and obesity [2]. The incidences of both diseases are increasing globally and both increases with advancing age. Whereas gout is a more prevalent in men over 30 years of age and in women above 50 years [3], hypertension is estimated to affect about 65% of the population in developed countries within the age group of 65-74 years [4]. It is projected that the number of adults with hypertension will increase to a total of 1.56 billion by the year 2025, which translates to about 60% increase [5]. The two diseases are degenerative in nature, being capable of reducing the quality of life of individuals suffering from them [5,6]. Hypertension is also known risk factor for other diseases, including arteriosclerosis, myocardial infarction, stroke, and end-stage renal disease, that also contribute to reduce the quality of life [5].
The level of uric acid in the cell depends on the activity of XO. XO catalyzes the terminal steps in the catabolism of purines in which hypoxanthine is converted to xanthine, and eventually to uric acid, during which molecular oxygen is reduced to superoxide, a reactive oxygen species (ROS) [1]. The over-activity of XO leads to hyperuricemia (increased concentration of uric acid), and the subsequent deposition of monosodium urate monohydrate crystals in tissue, especially joints, thereby resulting in gouty arthritis, or uric acid nephrolithiasis [3,7]. Hence, gout is a chronic inflammatory arthritis in which there is a high concentration of uric acid in body fluids, due to the over-activity of XO [8].
The activity of XO is linked with elevated arteriolar tone and consequently, hypertension [9]. Indeed, the activity of endothelial-bound XO rises by over 200% in patients with chronic heart failure [10]. The ROS produced by XO-catalyzed reactions are known to promote cardiovascular pathologies such as endothelial dysfunction, atherosclerosis, and hypertension [11]. The oxidative stress on endothelial cells resulting from the intracellular production of uric acid-derived radicals has the potential to activate the renin/angiotensin pathway, and consequently, the development of renal arteriolar disease [12]. In the rennin/angiotensin pathway, angiotensin 1-converting enzyme (ACE) (EC: 3.4.15.1) catalyzes the conversion of angiotensin I to angiotensin II, a known vasoconstrictor that increases blood pressure, by activating aldosterone secretion, and inactivating bradykinin, a vasodilator and hypotensive peptide [13]. ACE therefore plays a vital role in the regulation of blood pressure and normal cardiovascular function.
Thus, the inhibition of XO and ACE is an important strategy for the treatment and management of gout and hypertension, and this informs why chemically synthesized inhibitors of XO (including allopurinol) and ACE (including captopril, enalapril, and ramipril), are used clinically for the treatment of gout and hypertension, respectively [7,14]. Incidentally, synthetic inhibitors of XO and ACE both present with some adverse effects. The XO inhibitors are associated with the risk of developing hypersensitivity syndrome, characterized by side effects such as hepatic dysfunction, renal impairment, fever, rashes, and leukocytosis [15], whereas the ACE inhibitors have such adverse effects as skin rashes, cough, proteinuria, and hypotension [16]. However, research has shown that plant-derived inhibitors of both enzymes, including the polyphenolics, could be relatively safe and effective. Previous studies have also shown that several food and medicinal plants with a high level of flavonoids and other phenolic compounds are able to inhibit XO [17,18]; and ACE [19].
Guava (Psidium guajava Linn.), belonging to the family Myrtaceae, is found in the tropical and subtropical regions of the world including Nigeria. The plant has versatile applications ranging from food to folk medicine. In fact, it has been regarded as “a plant of multipurpose medicinal applications” [20], as different parts of it, including the roots, leaf and stem bark, have plenty of medicinal values. In particular, the leaf aqueous extracts were reported to possess anti-inflammatory and analgesic effects [21]; as well as anti-diabetic and anti-hypertensive activities [22] in rats. Other biological activities of the leaf extract include anti-microbial [23]; antioxidant, antibacterial and anti-tumor [24]; and several others as reviewed by Barbalho et al. [20]. In this study, we characterized the polyphenolics (flavonoids and phenolic acids) of guava leaves, and evaluated its inhibitory effect on XO and ACE; and it antioxidant effects in vitro, with a view to elucidating the possible mechanism of its anti-gout and antihypertensive activities.
MATERIALS AND METHODS
Samples Collection and Preparation
About 800 g of fresh leaves sample was collected from a guava plant in Akingbile, Moniya, Ibadan, Nigeria. The sample was botanically identified and authenticated at the herbarium of the Department of Botany, University of Ibadan, Nigeria. Thereafter, the sample was air-dried for 7 days and later ground finely to a particle size of 0.5 mm. The powdery sample was stored in air-tight plastic vials at –4°C until analysis.
Chemicals and Reagents
Methanol, formic acid, gallic acid, chlorogenic acid, caffeic acid, and ellagic acid purchased from Merck (Darmstadt, Germany). Catechin, epicatechin, quercetin, rutin, apigenin, and luteolin; porcine pancreatic lipase, Hippuryl-histidyl- leucine (Bz-Gly-His-Leu), Rabbit lung ACE, Xanthine, Allopurinol, 2,2-diphenylpicrylhydrazyl (DPPH), 2,2´-azino-bis-3-ethylbenzthiazoline-6-sulphoni (ABTS), and Trolox, L-ascorbic acid (Vitamin C) were acquired from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals used for analysis were of analytical grade.
Preparation of Polyphenolics-rich Extract
Polyphenolics-rich extract of guava leaves was prepared as described by Kuo et al. [25]. A portion of the leaves powder (100 g) was extracted three successive times with 300 mL of methanol at 50°C for 3 h, and the sample was filtered after each extraction with Whatman (No. 2) filter paper. The combined extract was partitioned with 200 mL hexane in a separatory funnel to get rid of the lipids and some pigments. The aqueous phase was extracted 3 times with 180 mL ethyl acetate and evaporated to dryness at 45°C under reduced pressure in a rotary evaporator. The residue obtained was used for the assays.
Quantification of Flavonoids and Phenolic Acids by High Performance Liquid Chromatography Diode Array Detection (HPLC-DAD)
The guava leaves extract was injected by means of Auto-sampler (Shimadzu, model SIL-20A) at a concentration of 15 mg/mL. Separations of phenolics were carried out using Phenomenex C18 column (4.6 mm × 250 mm × 5 μm particle size). The mobile phase comprised solvent A (water: formic acid [98:2, v/v]) and solvent B (acetonitrile), at a flow rate of 0.6 mL/min and injection volume of 40 μL. Gradient program was started with 95% of A and 5% of B until 2 min and changed to obtain 25%, 40%, 50%, 70% and 80% B at 10, 20, 30, 50 and 70 min, respectively, following the method described by Boligon et al. [26], with slight modifications. The extract and mobile phase were filtered through a 0.45 μm membrane filter (Millipore) and then degassed in an ultrasonic bath before use. Standards references stock solutions were prepared in the HPLC mobile phase at a concentration range of 0.025-0.300 mg/mL. Quantifications of the flavonoids and phenolic acids in the extract were carried out by integration of the peaks using the external standard method at the following wavelengths: 254 nm for gallic acid and ellagic acid; 280 nm for catechin and epicatechin; 325 nm for caffeic acid and chlorogenic acid; and 366 nm for quercetin, quercitrin, kaempferol, luteolin and rutin. The identification of the individual chromatography peaks and quantification of the corresponding phenolic compounds were based on a combination of retention time and spectral matching with those of reference standards. The chromatography analysis was carried out at ambient temperature and in triplicate.
Limit of Detection (LOD) and Limit of Quantification (LOQ) of Flavonoids and Phenolic Acids
LOD and LOQ were calculated based on the standard deviation (SD) of the responses and the slope using three independent analytical curves, as previously defined by Khaliq et al. [27]. LOD and LOQ were calculated as 3.3 and 10 s/S, respectively, where s is the SD of the response and S is the slope of the calibration curve.
Handling of Experimental Animal
Adult male Wister strain albino rats weighing 200-250 g were procured from the experimental animal breeding unit of Department of Veterinary Medicine, University of Ibadan, Nigeria. To ensure the protection of animals’ welfare during experiments, the guidelines outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institute of Health (USA) [28], were followed. The rats were acclimatized in cages under ambient laboratory conditions for 7 days, during which they had free access to food and water.
Preparation of Liver Homogenates for XO and Lipid Peroxidation Inhibition Assays
The method described by Nakamura et al. [29] was followed to prepare the liver tissue homogenates used for XO and lipid peroxidation inhibition assays. The liver tissue was rapidly excised after decapitation of the rats under mild ether anesthesia. The tissue was washed in cold 0.15 M KCl, and blotted dry. Then, 1 g of it was homogenized in 9 volumes of ice-cold 50 mM Tris-HCl buffer (pH 7.4) containing 1 mM ethylenediaminetetraacetic acid. Thereafter, a portion of the homogenate was centrifuged for 10 min at 1400 × g to yield a low-speed supernatant that was used for the lipid peroxide assay. For the XO assay, another portion of the homogenate was sonicated twice on ice for 30 s and then centrifuged at 10,000 × g for 20 min at 4°C to obtain the supernatant fraction used.
XO Inhibition Assay
The ability of the extract to inhibit XO was tested according to the method reported by Umamaheswari et al. [18] with slight modification. The reaction mixture contained 300 μL of 50 mM sodium phosphate buffer (pH 7.5), 100 μL of the extract at different concentrations (15, 30, 45 and 60 μg/mL) in dimethyl sulfoxide (DMSO), 100 μL of freshly prepared tissue enzyme preparation (liver homogenate) and 100 μL of distilled water. The test mixture was pre-incubated at 37°C for 15 min. Thereafter, 200 μL of 0.15 mM of xanthine solution (substrate) was added to the mixture and it was further incubated at 37°C for 30 min. Next, 200 μL of 0.5 M HCl was added to terminate the reaction. Allopurinol was used as a positive control for the assay; a reference test containing 100 μL of DMSO instead of the extract was also carried out so as to obtain the maximum uric acid formed. The absorbance was measured at 295 nm on a UV/VIS spectrophotometer against a blank prepared in the same way except that the liver homogenate was replaced with the phosphate buffer. One unit (U) of this enzyme is defined as the amount of enzyme required to form 1 mmol of uric acid per min at the reaction conditions. The XO inhibitory ability of the extract was calculated as percentage inhibition as follows:
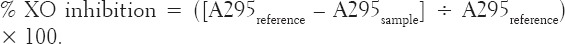
Where A295reference is the reference without the extract, and A295sample is the absorbance of test containing the extract.
Lipid Peroxidation Inhibition Assay
The ability of the extract to inhibit Fe2+-induced lipid peroxidation was tested according to the modified method of Ohkawa et al. [30]. Briefly, to a reaction mixture containing 100 μL of the homogenate supernatant, 30 μL of 0.1 M Tris-HCl buffer (pH 7.4) and different concentrations (15, 30, 45 and 60 μg/mL) of the extract, 30 μL of freshly prepared 25 μM solution of ferric sulfate was added to initiate lipid peroxidation. The volume was made up to 300 μL with deionized water before incubation at 37°C for 1 h. The color reaction was initiated by adding 300 μL of 81 g/L sodium duodecyl sulfate to the reaction mixture, followed by the addition of 600 μL of acetic acid/HCl (pH 3.4) and 600 μL of 0.8% (v/v) TBA (thiobarbituric acid). This mixture was incubated at 100°C for 1 h. The absorbance of thiobarbituric acid reactive species (TBARS) produced were measured at 532 nm in a UV-visible spectrophotometer. A reference test without the plant extract was carried out to obtain the maximum TBARS formation. The ability of the extract to inhibit Fe2+-induced lipid peroxidation was expressed as percentage inhibition thus:
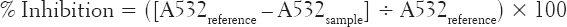
Where A532reference is the absorbance of the reference without the extract, and A532sample is the absorbance of test containing the extract.
ACE Inhibition Assay
ACE inhibition was assayed using a spectrophotometric method described by Cushman and Cheung [31]. ACE from rabbit lung (EC 3.4.15.1) and the substrate (Hippuryl-histidyl-leucine [Bz-Gly-His-Leu]) were used. In this assay, the Bz-Gly-His-Leu is cleaved by ACE to form hippuric acid, which is measured spectrophotometrically. Different dilutions (15, 30, 45 and 60 μg/mL) of the extract amounting to 50 μL and 50 μL ACE solutions (4 mU/mL) were pre-incubated at 37°C for 15 min. After pre-incubation, 150 μL of 8.33 mM of the Bz-Gly-His-Leu in 125 mM Tris-HCl buffer (pH 8.3) was added to the mixture to initiate the enzymatic reaction, and this was incubated at 37°C for 30 min. Next, the reaction was terminated by adding 250 μL of 1 M HCl. The hippuric acid produced by the reaction was extracted with 1.5 mL ethyl acetate. Subsequently, the ethyl acetate layer was separated from the mixture by centrifugation, and 1 mL of it was transferred to a clean test tube and evaporated to dryness in a hot-air oven. The resulting residue was redissolved in distilled water and its absorbance was measured at 228 nm. A reference test (without the extract), and a positive control test (containing 64 nmol/L of captopril) were carried out simultaneously with the test extract. 1 unit (U) of ACE activity is defined as the amount of enzyme required to catalyze the formation of 1 μmol of hippuric acid from hippuryl-histidyl-leucine per minute at 37°C. The ability of the extract to inhibit ACE activity was expressed as percentage inhibition thus:

Where A228reference is the absorbance of the reference without the extract, and A228sample is the absorbance of test containing the extract.
Estimation of ABTS*+ Scavenging Ability
The ABTS*+ scavenging ability of the extract was determined according to the method described by Re et al. [32]. To generate the ABTS*+, an equal volume of 7 mM ABTS*+ aqueous solution was incubated with 2.45 mM K2S2O8 for 16 h at room temperature in the dark; then its absorbance (at 734 nm) was adjusted to 0.7 ± 0.02 with 95% ethanol. Thereafter, appropriate dilution of the extract amounting to 0.2 mL was mixed with 2.0 mL ABTS*+ solution. The test mixture was kept in the dark for 15 min, after which its absorbance was measured at 734 nm. The ABTS*+ scavenging ability of the extract was subsequently calculated from a standard curve prepared using Trolox and expressed in Trolox equivalent (TE).
Determination of DPPH free Radical Scavenging Ability
The DPPH* scavenging ability of the extract was determined as described by Cervato et al. [33]. Appropriate dilutions (10, 20, 30 and 40 μg/mL) of the extract, amounting to 1.0 mL was mixed with 3.0 mL of DPPH* (60 μM). The test mixture was kept in the dark for 30 min, after which the absorbance was measured at 517 nm. A reference test (containing the DPPH* solution without the extract), and a reference standard (containing the DPPH* solution and ascorbic acid) were included in the assay. The DPPH* percentage scavenging ability of the extract was calculated as follows:
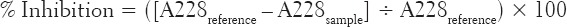
Where A517reference is the absorbance of the reference test; and A517sample is the absorbance of the test containing the extract.
Statistical Analysis
Results of replicate experiments were expressed as mean ± SD. Independent samples t-test was performed on the result data at 95% confidence level using SPSS statistical software package, version 17. IC50 was calculated from the % inhibition versus extract concentration non-linear regression curve of the extract.
RESULTS
Characterization of the phenolics composition of the guava leaf extract using HPLC-DAD showed that the extract was rich in flavonoids and phenolic acid, and the result is presented in Table 1. Flavonoids were present in the extract in the order of quercetin > kaempferol > catechin > quercitrin > rutin > luteolin > epicatechin; while phenolic acids were in the order of caffeic acid > chlorogenic acid > gallic acids. A representative HPLC chromatogram of the extract is shown in Figure 1.
Table 1.
Flavonoids and phenolics acids composition of guava leaves extract
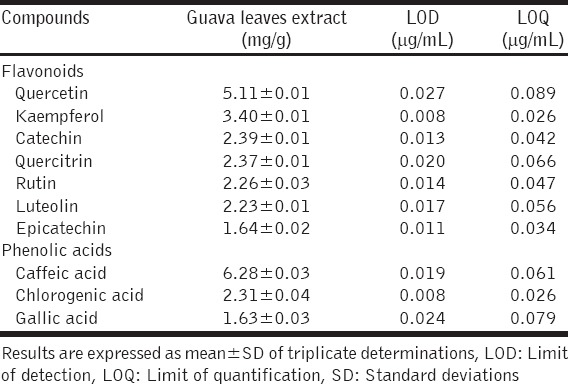
Figure 1.
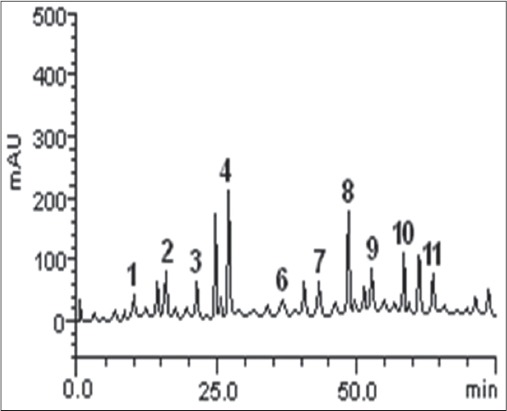
Representative high performance liquid chromatography profile of guava leaves extract. Detection UV was at 325 nm. Gallic acid (peak 1), catechin (peak 2), chlorogenic acid (peak 3), caffeic acid (peak 4), ellagic acid (peak 5), epicatechin (peak 6), rutin (peak 7), quercitrin (peak 8), quercetin (peak 9), kaempferol (peak 10) and luteolin (peak 11)
The inhibitory effects of the extract on the activities of XO and ACE, and Fe2+-induced lipid peroxidation, expressed as IC50, are presented in Table 2. The extract inhibited XO, having IC50 of 38.24 ± 2.32 μg/mL, in comparison with the IC50 of 5.78 ± 0.25 μg/mL, observed for the standard reference XO inhibitor (allopurinol). The extract also inhibited ACE, with IC50 of 21.06 ± 2.04 μg/mL in relation to the IC50 of 4.96 ± 0.18 μg/mL, recorded for the captopril that was used as the standard reference ACE inhibitor. The result showed that the extract had an IC50 of 27.52 ± 1.72 μg/mL against Fe2+-induced lipid peroxidation. The patterns of the inhibitory effects of the extract on XO, ACE and Fe2+-induced lipid peroxidation were dose-dependent as depicted in Figure 2.
Table 2.
IC50 of guava leaves extract against XO, ACE activities, and Fe2+-induced lipid peroxidation
Figure 2.
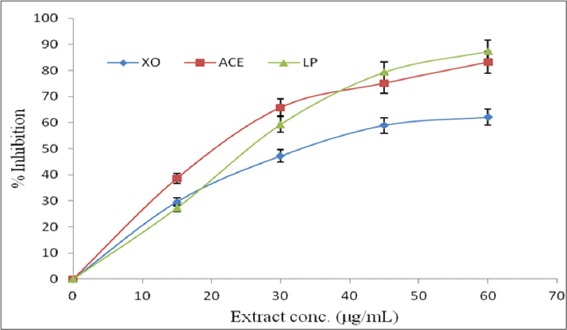
% inhibition-extract concentration curves showing the dose-dependent inhibition of xanthine oxidase (XO), angiotensin 1-converting enzyme (ACE) and Fe2+-induced lipid peroxidation (LP) by guava leaves extract
The ability of the extract to scavenge free radicals was tested using DPPH* and ABTS*+ assays, and the results are presented in Table 3. The extract strongly scavenged DPPH*, having half-maximal scavenging concentration (SC50) of 13.38 ± 0.86 μg/mL, relative to that of ascorbic acid (7.38 ± 0.27 μg/mL), the standard reference antioxidant. In addition, the extract scavenged DPPH* in a dose-dependent manner [Figure 3]. Similarly, the extract strongly scavenged ABTS*+ as shown by its TE antioxidant capacity (TEAC) value of 3.20 ± 0.14 mmol TEAC/g.
Table 3.
DPPH* IC50 and ABTS*+ scavenging ability of guava leaves extract
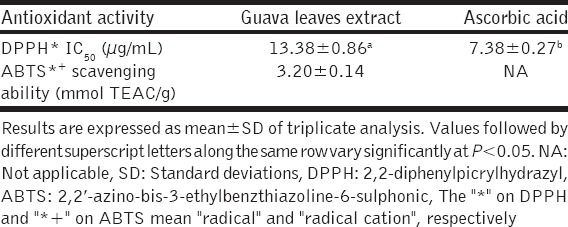
Figure 3.
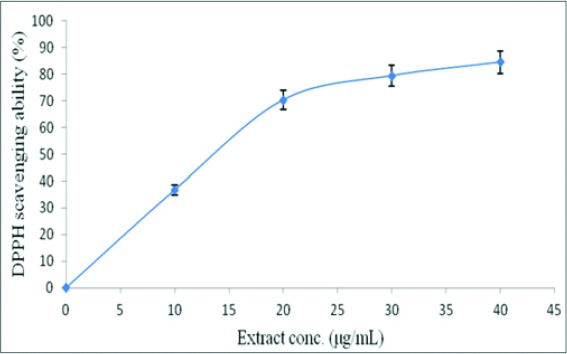
2,2-diphenylpicrylhydrazyl % scavenging ability-extract concentration curve of guava leaves extract
DISCUSSION
Despite advances in medicine, the incidences of gout and hypertension continue to increase, partly due to the high cost of the available synthetic inhibitors of the enzymes (including XO and ACE) associated with both diseases, and their attendant side effects. Unfortunately, both diseases are degenerative, and are so, capable of reducing the quality of life of those suffering from them. This development has aroused research for natural inhibitors of these enzymes that could be effective, safe and more affordable. In this regard plant phenolics, particularly flavonoids and phenolic acids, have emerged to be promising candidates. Recent studies have revealed that various plants extracts rich in flavonoids and phenolic acids can inhibit one of these two enzymes [17,19]. Existing literature also indicated that guava leaves extracts possessed anti-inflammatory [21], and anti-hypertensive [22] activities in rats. Hence, we characterized the flavonoids and phenolics acids of guava leaves extract, and evaluated its XO and ACE inhibitory effects, and antioxidant activity; with a view to elucidating the possible mechanism(s) of its anti-gout and anti-hypertensive effects.
The phenolics composition of the guava leaves extract revealed that it contained the flavonoids: Quercetin, kaempferol, catechin, quercitrin, rutin, luteolin, and epicatechin; with quercetin being the most abundant flavonoid, followed by kaempferol. The result further revealed that the extract contained the phenolic acids: Caffeic acid, chlorogenic acid, and gallic acids; with caffeic acid, followed by chlorogenic acid as the most abundant. This result is in conformity with the report of Jang et al. [34], who identified the presence of gallic acid, catechin, chlorogenic acid, catechin, caffeic acid, rutin, and other phenolic compounds in guava leaf extract using HPLC. However, there were differences in the quantities of the phenolic compounds we detected and those they earlier reported. For instance, the quantities we observed for gallic acid, catechin, chlorogenic acid, caffeic acid and rutin (1.63 ± 0.03, 2.39 ± 0.01, 2.31 ± 0.04, 6.28 ± 0.03 and 2.26 ± 0.03 mg/g, respectively), were generally higher than the values (gallic acid: 0.09 ± 0.00 mg/g; catechin: 0.72 ± 0.04 mg/g; chlorogenic acid: 0.19 ± 0.01 mg/g; caffeic acid: 0.14 ± 0.00 mg/g; rutin: 0.34 mg/g) they reported [34]. These differences may partly be due to the variation in the detection system of the HPLC used. Whereas we used HPLC coupled with diode array detector (HPLC-DAD), Jang et al. [34] used HPLC coupled with a UV-vis multi-wavelength detector (HPLC-UV-vis). In addition, variations in biotic and abiotic factors relative to the regions where plants were grown; as well as variations in the period of sample collection, are also known to contribute to differences in the levels of phytochemicals in plants [24].
Previous reports have shown that various plant extracts rich in flavonoids and phenolic acids were potent in inhibiting some enzymes implicated in certain pathological conditions, including XO for gout [17], ACE for hypertension [19], α-amylase and α-glucosidase for Type 2 diabetics [35], in addition to possessing antioxidant activity, and other health benefits. The inhibition of XO is an important clinical strategy for treating hyperuricemia and gouty arthritis; as it helps in decreasing the amount of uric acid in circulation, and vascular oxidative stress [36]. In this respect, the plant-derived polyphenolics have been shown to be effective in inhibiting XO and alleviating the resultant hyperuricemia. As natural components of plant foods, they are considered to be safer than synthetic XO inhibitors including allopurinol [37]. The structure of the flavonoids promotes their ability to inhibit XO. In particular, the C-5 and C-7 hydroxyl groups of flavones and flavonols can replace the C-2 and C-6 ones of xanthine in the XO active site [38,39]. This effect is made possible by the mutual inter-convertibility of the carboxyl structures of xanthine to hydroxyl groups [40]. The IC50 of the guava leaves extract against XO in this study (38.24 ± 2.32 μg/mL) is lower than the 45.71 ± 1.44 μg/mL we recently reported for phenolics extract of Tetrapleura tetraptera fruit [17], and the 42 μg/mL reported for Olea europaea leaf extract [41]. The lower IC50 of the guava leaves extract indicates a stronger XO inhibitory activity than T. tetraptera fruit and O. europaea leaf extracts. By inhibiting XO, the guava leaves extract could be useful in managing hyperuricemia, the index of gout, and preventing the downstream events, including increased production of ROS, activation of the renin/angiotensin pathway and inactivation of bradykinin, that link hyperuricemia to hypertension.
The inhibition of ACE, the enzyme that catalyzes the conversion of angiotensin I to angiotensin II in the renin–angiotensin–aldosterone system, has become a strategic target for the treatment of hypertension and other cardiovascular diseases [42]. Angiotensin II is a known vasoconstrictor that activates the aldosterone secretion, and inactivates bradykinin, a vasodilator and hypotensive peptide [13]; thereby increasing the blood pressure. The angiotensin II is also capable of increasing the superoxide production activity of the endothelial cells. However, research has shown that plant-derived polyphenolics can inhibit ACE. Among the polyphenolic compounds, flavonoids and phenolic acids are prominent for their potent anti-hypertensive activity, and are therefore promising active principles for non-pharmacological nutraceutical intervention in hypertension [43]. The inhibition of ACE is a striking anti-hypertensive mechanism of flavonoids. The flavonoids have a combination of sub-structures on their skeleton that favors their ACE inhibitory effect. These sub-structures include the catechol group in the B-ring, the double bond between C2 and C3 at the C-ring, and the cetone group in C4 at the C-ring of the flavonoids [44]. Other mechanisms of their anti-hypertensive activity include improvement of endothelial function, modulation of vascular smooth muscle, cell signaling, and gene expression; and their antioxidant effect [45]. Interestingly, quercetin, the most abundant flavonoid in the guava leaves extract, has been reported to have anti-hypertensive activity which it mediates via several mechanisms including ACE inhibition, which is considered as the most important mechanism [46]. The salient inhibitory effect of quercetin on ACE activity could be attributed to its optimum binding affinity with the ACE, as it has a binding energy of –8.5 kcal/mol relative to the standard value of –7.0 kcal/mol, as revealed by its in silico analysis [45].
Phenolic acids also mediate their anti-hypertensive effect by inhibiting ACE and maintaining vascular endothelial function [47]. The ACE inhibitory effect of phenolic acids is due to the net contribution of their functional groups (COO– and OH–); the ability of the oxygen atom of their carboxylate moiety to form charge-charge interactions with the Zn2+ present in the ACE active site; and their ability to form a stable complex with ACE, through their interaction with the amino acids residues at the active site of ACE [46]. It is also noteworthy that caffeic acid, the most abundant phenolic acid in the guava leaves extract in this study, was reported to exhibit strong anti-hypertensive effect in both in vitro [48] and in vivo [49] studies. Bhullar et al. [50] further demonstrated that caffeic acid and its derivatives exhibit a strong anti-hypertensive effect through the inhibition of ACE.
Vascular oxidative stress is a common feature of both gout and hypertension. Oxidative stress sets in when the oxidant burden (free radicals, ROS and reactive nitrogen species) of the body cells outweighs the available antioxidant defense system, both enzymic and non-enzymic. Hence, improving the antioxidant status of the body is important for the management of gout and hypertension. The guava leaves extract exhibited antioxidant activity by inhibiting Fe2+-induced lipid peroxidation and scavenging free radicals (DPPH* and ABTS*+). Previous reports have indicated that the activities of XO [51] and angiotensin II [52] enhance the peroxidation of lipids. Oxidative damage to the membrane lipids produces an array of cytotoxic products, especially aldehydes such as malondialdehyde (MDA) [53]. These cytotoxic aldehydes play a role in a number of oxidative stress-induced inflammatory diseases [54], including gouty arthritis. Oxidative damage to the cell membrane caused by the peroxidation of membrane lipids has the tendency to disrupt the functions of membrane transport proteins and ionic channels; deactivate membrane-bound enzymes; and increase the permeability of the membrane lipid bilayer [37], to ions and other molecules that may be toxic to the cell. The ability of the guava leaves extract to inhibit Fe2+-induced lipid peroxidation indicates that it could mitigate the oxidative damage to cell membrane lipids caused by ROS produced by the over-activity of XO in gout patience, and by angiotensin II in hypertensive patients. Interestingly, the extract had a lower IC50 (27.52 ± 1.72 μg/mL) against Fe2+-induced lipid peroxidation, in comparison with the 36.97 ± 2.06 μg/mL (in rat liver homogenate) reported for T. tetraptera fruit extract [17].
The DPPH* SC50 (13.38 ± 0.86 μg/mL) observed in this study for the guava leaves extract is about twice higher than the IC50 of 6.25 μg/ml of its ethanolic extract earlier reported by Thephinlap et al. [55]. On the other hand, the ABTS*+ scavenging ability (3.20 ± 0.14 mmol TEAC/g) of the extract in this study is lower than the TEAC value (4.908 ± 0.050 mM/mg) of its ethanol extract reported by Tachakittirungrod et al. [56]. These variations could be attributed to differences in biotic and abiotic factors affecting the phytochemical level and bioactivities of the plant in the different regions where the leaves sample was collected, and variations in the period of sample collection [24] as earlier stated. The antioxidant activities of the guava leave extract could be attributed to the flavonoids and phenolic acids. These polyphenols have redox properties, which enable them to function as reducing agents, hydrogen donors, and singlet oxygen quenchers [57]; hence, their antioxidant activity. Thus, the guava leaves extract could help in scavenging the free radicals and ROS generated by the activities of XO and angiotensin II. Interestingly, using XO inhibitors is suggested to be a viable antioxidant approach in pathophysiologic conditions such as hypertension in which ROS production is exacerbated [11]. Our results confirm the report of Braga et al. [24], who observed high levels of antioxidant properties in guava leaves ethanol extract, and inferred that it could be used as functional food. It has earlier been stated that the activity of XO is linked with elevated arteriolar tone and consequently, hypertension [9]. One of the ways by which this occurs is through the formation of peroxynitrite (ONOO−) by the reaction of the O2 •− generated by the activity of XO in the endothelial cells, with endothelial nitric oxide (NO); thereby lowering the vasorelaxant effect of NO [11]. It is important to recall that the NO is produced from L-arginine by the catalytic function of endothelial nitric oxide synthase (eNOS) [58]. The ONOO− so-formed is a much more potent oxidant and cytotoxic agent, as experimental evidence suggests that its formation and the concomitant activation of downstream signaling pathways eventually result in injury of the endothelial cell [59,60]. The oxidative stress on endothelial cells resulting from the intracellular production of uric acid-derived radicals has the potential to activate the renin/angiotensin pathway [12], and consequently, elevate the blood pressure.
Thus, the mechanism of the anti-hypertensive activity of guava leaves extract could be viewed to be tripartite; the first is the inhibition of XO with a concomitant decrease in ROS generation; the second is the inhibition of ACE resulting in the decreased production of angiotensin II (a vasoconstrictor), with a concomitant activation of bradykinin (a vasodilator); the third is the scavenging of the ROS generated by the two pathways (XO- and ACE-catalyzed pathways), thereby attenuating the formation of the cytotoxic ONOO− from the reaction of the O2 •− with NO, and maintaining the vasorelaxant effect of the NO. A proposed scheme of this tripartite mechanism of action is shown in Figure 4.
Figure 4.
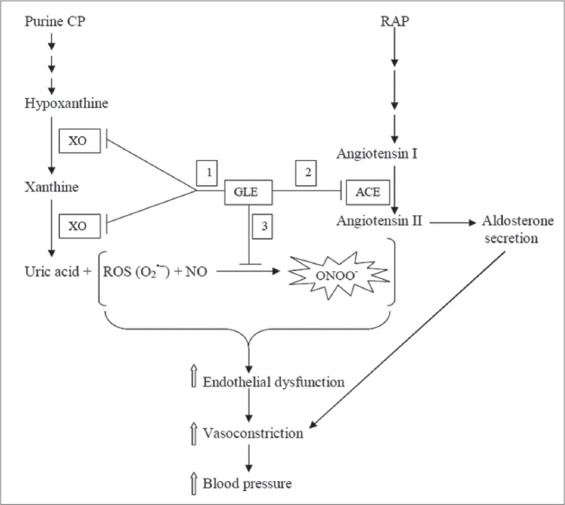
Schematic diagram of proposed tripartite mechanism of anti-hypertensive effect of guava leaves extract. Purine CP: Purine catabolic pathway, XO: Xanthine oxidase, RAP: Rennin-angiotensin pathway, ACE: Angiotensin I-converting enzyme, GLE: Guava leaves extract, O2 •−: superoxide anion radical, ONOO−: Peroxynitrite, ↑: Increase. The numbers: 1, 2 and 3 in rectangular boxes indicate the XO inhibition, ACE inhibition; and scavenging of reactive oxygen specie (respectively) by guava leaves extract
CONCLUSION
The inhibition of XO and angiotensin 1-converting; and scavenging of free radicals might be the possible mechanisms of the anti-gout and anti-hypertensive effects of guava leave extract. These effects could be attributed to the combined effect of the flavonoids and phenolic acids present in the extract. Guava leaves extract may, therefore, serve as a functional food for managing gout and hypertension and attenuating the oxidative stress associated with both diseases.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: Molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terkeltaub RA. Clinical practice Gout. N Engl J Med. 2003;349:1647–55. doi: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- 3.Kramer HM, Curhan G. The association between gout and nephrolithiasis: The National Health and Nutrition Examination Survey III 1988-1994. Am J Kidney Dis. 2002;40:37–42. doi: 10.1053/ajkd.2002.33911. [DOI] [PubMed] [Google Scholar]
- 4.Duprez D, Van Helshoecht P, Van den Eynd W, Leeman M. Prevalence of hypertension in the adult population of Belgium: Report of a worksite study: Attention hypertension. J Hum Hyper. 2002;16:47–52. doi: 10.1038/sj.jhh.1001293. [DOI] [PubMed] [Google Scholar]
- 5.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 6.Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R. Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol. 2004;31:1582–7. [PubMed] [Google Scholar]
- 7.Emmerson BT. The management of gout. N Engl J Med. 1996;334:445–51. doi: 10.1056/NEJM199602153340707. [DOI] [PubMed] [Google Scholar]
- 8.Burke A, Smyth E, FitzGerald GA. Analgesic-antipyretic agents;pharmacotherapy of gout. In: Brunton LL, Lazo JS, Parker KL, editors. The Pharmacological Basis of Therapeutics. 11th ed. New York: McGraw-Hill Medical Publishing Division; 2006. pp. 706–10. [Google Scholar]
- 9.DeLano FA, Parks DA, Ruedi JM, Babior BM, Schmid-Schönbein GW. Microvascular display of xanthine oxidase and NADPH oxidase in the spontaneously hypertensive rat. Microcirculation. 2006;13:551–66. doi: 10.1080/10739680600885152. [DOI] [PubMed] [Google Scholar]
- 10.Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: Role of xanthine-oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073–8. doi: 10.1161/01.cir.0000041431.57222.af. [DOI] [PubMed] [Google Scholar]
- 11.Weseler AR, Bast A. Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr Hypertens Rep. 2010;12:154–61. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–6. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson U, Danilczyk U, Penninger JM. Just the beginning: Novel functions for angiotensin-converting enzymes. Curr Biol. 2002;12:R745–52. doi: 10.1016/s0960-9822(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 14.Thurman JM, Schrier RW. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on blood pressure and the kidney. Am J Med. 2003;114:588–98. doi: 10.1016/s0002-9343(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 15.Khoo BP, Leow YH. A review of inpatients with adverse drug reactions to allopurinol. Singapore Med J. 2000;41:156–60. [PubMed] [Google Scholar]
- 16.Vyssoulis GP, Karpanou EA, Papavassiliou MV. Side effects of antihypertensive treatment with ACE inhibitors. Am J Hypertens. 2001;14:114–5. [Google Scholar]
- 17.Irondi AE, Oboh G, Agboola SO, Boligon AA, Athayde ML. Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney, liver, and lungs tissues of rats in vitro. Food Sci Hum Wellness. 2015 http://dx.doi.org/10.1016/j.fshw.2015.11.001. [Google Scholar]
- 18.Umamaheswari M, AsokKumar K, Somasundaram A, Sivashanmugam T, Subhadradevi V, Ravi TK. Xanthine oxidase inhibitory activity of some Indian medical plants. J Ethnopharmacol. 2007;109:547–51. doi: 10.1016/j.jep.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Ademosun AO, Oboh G, Passamonti S, Tramer F, Ziberna L, Boligon AA, et al. Phenolics from grapefruit peels inhibit HMG-CoA reductase and angiotensin-I converting enzyme and show antioxidative properties in endothelial EA. Hy 926 cells. Food Sci Hum Wellness. 2015;4:80–5. [Google Scholar]
- 20.Barbalho SM, Farinazzi-Machado FM, de Alvares Goulart R, Brunnati AC, Otoboni AM, Ottoboni B, et al. Psidium guajava (Guava): A plant of multipurpose medicinal applications. Med Aromat Plants. 2012;1:104. [Google Scholar]
- 21.Ojewole JA. Antiinflammatory and analgesic effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rats and mice. Methods Find Exp Clin Pharmacol. 2006;28:441–6. doi: 10.1358/mf.2006.28.7.1003578. [DOI] [PubMed] [Google Scholar]
- 22.Ojewole JA. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find Exp Clin Pharmacol. 2005;27:689–95. doi: 10.1358/mf.2005.27.10.948917. [DOI] [PubMed] [Google Scholar]
- 23.Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int J Microbiol. 2013;2013:746165. doi: 10.1155/2013/746165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braga TV, Dores RG, Ramos CS, Evangelista FC, Tinoco LM, Varotti F, et al. Antioxidant, antibacterial and antitumor activity of ethanolic extract of the Psidium guajava leaves. Am J Plant Sci. 2014;5:3492–500. [Google Scholar]
- 25.Kuo C, Kao E, Chan K, Lee H, Huang T, Wang C. Hibiscus sabdariffa L. extracts reduce serum uric acid levels in oxonate-induced rats. J Funct Foods. 2012;4:375–81. [Google Scholar]
- 26.Boligon AA, Piana M, Kubiça TF, Mario DN, Dalmolin TV, Bonez PC, et al. HPLC analysis and antimicrobial, antimycobacterial and antiviral activities of Tabernaemontana catharinensis A. DC. J Appl Biomed. 2015;13:7–18. [Google Scholar]
- 27.Khaliq A, Sabir SM, Ahmad SD, Boligon AA, Athayde ML, Jabbar A, et al. Antioxidant activities and phenolic composition of Olive (Olea europaea) leaves. J Appl Bot Food Qual. 2015;88:16–21. [Google Scholar]
- 28.Public Health Service (PHS) Public Health Service Policy on Humane Care and Use of Laboratory Animals, (PL 99-158 Health Research Extension Act 1985) Washington, DC: US Department of Health and Human Services; 1996. [Google Scholar]
- 29.Nakamura T, Ohta Y, Ikeno K, Ohashi K, Ikeno T. Protective effect of repeatedly pre-administered Brazilian Propolis ethanol extract against stress-induced gastric mucosal lesions in rats. Evid Based Complement Alternat Med. 2014;2014:383482. doi: 10.1155/2014/383482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Cushman DW, Cheung HS. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem Pharmacol. 1971;20:1637–48. doi: 10.1016/0006-2952(71)90292-9. [DOI] [PubMed] [Google Scholar]
- 32.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 33.Cervato G, Carabelli M, Gervasio S, Cittera A, Cazzola R, Cestaro B. Antioxidant properties of oregano (Origanum vulgare) leaf extracts. J Food Biochem. 2000;24:453–65. [Google Scholar]
- 34.Jang M, Jeong SW, Cho SK, Yang HJ, Yoon DS, Kim JC, et al. Improvement in the anti-inflammatory activity of guava (Psidium guajava L.) leaf extracts through optimization of extraction conditions. J Funct Foods. 2014;10:161–8. [Google Scholar]
- 35.Irondi EA, Oboh G, Akindahunsi AA, Boligon AA, Athayde ML. Phenolic composition and inhibitory activity of Mangifera indica and Mucuna urens seeds extracts against key enzymes linked to the pathology and complications of Type 2 diabetes. Asian Pac J Trop Biomed. 2014;4:903–10. [Google Scholar]
- 36.Ngoc TM, Khoi NM, Ha do T, Nhiem NX, Tai BH, Don DV, et al. Xanthine oxidase inhibitory activity of constituents of Cinnamomum cassia twigs. Bioorg Med Chem Lett. 2012;22:4625–8. doi: 10.1016/j.bmcl.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 37.Gawlik-Dziki U. Dietary spices as a natural effectors of lipoxygenase, xanthine oxidase, peroxidase and antioxidant agents. LWT-Food Sci Technol. 2012;47:138–46. [Google Scholar]
- 38.Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–6. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- 39.Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem. 1999;63:1787–90. doi: 10.1271/bbb.63.1787. [DOI] [PubMed] [Google Scholar]
- 40.Takahama U, Koga Y, Hirota S, Yamauchi R. Inhibition of xanthine oxidase activity by an oxathiolanone derivative of quercetin. Food Chem. 2011;126:1808–11. doi: 10.1016/j.foodchem.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Flemmig J, Kuchta K, Arnhold J, Rauwald HW. Olea europaea leaf (Ph.Eur.) extract as well as several of its isolated phenolics inhibit the gout-related enzyme xanthine oxidase. Phytomedicine. 2011;18:561–6. doi: 10.1016/j.phymed.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Ma TK, Kam KK, Yan BP, Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: Current status. Br J Pharmacol. 2010;160:1273–92. doi: 10.1111/j.1476-5381.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y, Wang J, Ballevre O, Luo H, Zhang W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens Res. 2012;35:370–4. doi: 10.1038/hr.2011.195. [DOI] [PubMed] [Google Scholar]
- 44.Guerrero L, Castillo J, Quiñones M, Garcia-Vallvé S, Arola L, Pujadas G, et al. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS One. 2012;7:e49493. doi: 10.1371/journal.pone.0049493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hügel HM, Jackson N, May B, Zhang AL, Xue CC. Polyphenol protection and treatment of hypertension. Phytomed. 2016 doi: 10.1016/j.phymed.2015.12.012. http://dx.doi.org/10.1016/j.phymed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 46.Shukor NA, Camp JV, Gonzales GB, Staljanssens D, Struijs K, Zotti MJ, et al. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J Agric Food Chem. 2013;61:11832–9. doi: 10.1021/jf404641v. [DOI] [PubMed] [Google Scholar]
- 47.Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L. A phytochemical and pharmacological review. Food Chem. 2014;165:424–43. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Actis-Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem. 2006;54:229–34. doi: 10.1021/jf052263o. [DOI] [PubMed] [Google Scholar]
- 49.Li PG, Xu JW, Ikeda K, Kobayakawa A, Kayano Y, Mitani T, et al. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2005;28:369–77. doi: 10.1291/hypres.28.369. [DOI] [PubMed] [Google Scholar]
- 50.Bhullar KS, Lassalle-Claux G, Touaibia M, Rupasinghe HP. Antihypertensive effect of caffeic acid and its analogs through dual renin-angiotensin-aldosterone system inhibition. Eur J Pharmacol. 2014;730:125–32. doi: 10.1016/j.ejphar.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 51.Arhan E, Serdaroglu A, Ozturk B, Ozturk HS, Ozcelik A, Kurt N, et al. Effects of epilepsy and antiepileptic drugs on nitric oxide, lipid peroxidation and xanthine oxidase system in children with idiopathic epilepsy. Seizure. 2011;20:138–42. doi: 10.1016/j.seizure.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest. 2003;112:1383–94. doi: 10.1172/JCI18212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shalaby EA, Shanab SM. Antioxidant compounds, assays of determination and mode of action. Afr J Pharm Pharmacol. 2013;7:528–39. [Google Scholar]
- 54.Yadav UC, Ramana KV. Regulation of NF-μ-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid Med Cell Longev. 2013;2013:690545. doi: 10.1155/2013/690545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thephinlap C, Pangjit K, Suttajit M, Srichairatanakool S. Anti-oxidant properties and anti-hemolytic activity of Psidium guajava, Pandanous odorus and Rhinacanthus nasutus. J Med Plants Res. 2013;7:2001–9. [Google Scholar]
- 56.Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007;103:381–8. [Google Scholar]
- 57.Chang ST, Wu JH, Wang SY, Kang PL, Yang NS, Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001;49:3420–4. doi: 10.1021/jf0100907. [DOI] [PubMed] [Google Scholar]
- 58.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–60. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 59.Radi R, Rubbo H, Bush K, Freeman BA. Xanthine oxidase binding to glucosaminoglycans: Kinetics and superoxide dismutase interactions of immobilized xanthine oxidase-heparin complexes. Arch Biochem Biophys. 1997;339:125–35. doi: 10.1006/abbi.1996.9844. [DOI] [PubMed] [Google Scholar]
- 60.Ungvári Z, Gupte SA, Recchia FA, Bátkai S, Pacher P. Role of oxidative-nitrosative stress and downstream pathways in various forms of cardiomyopathy and heart failure. Curr Vasc Pharmacol. 2005;3:221–9. doi: 10.2174/1570161054368607. [DOI] [PMC free article] [PubMed] [Google Scholar]


