Abstract
Background/Aim:
Plumbago indica (PI) L. and its active constituent, plumbagin, has been traditionally claimed for several pharmacological activities; however, there is little information regarding their toxicity. The present study aims to examine the effects of plumbagin and PI extract (PI) on hepatic histomorphology and antioxidative system in mice.
Materials and Methods:
Adult male intelligent character recognition mice were intragastrically administered plumbagin (1, 5, and 15 mg/kg/day) or PI (20, 200, and 1,000 mg/kg/day) consecutively for 14 days. Hepatic histomorphology was examined. Plasma alanine transaminase (ALT) and aspartate transaminase (AST) levels, hepatic lipid peroxidation, superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities, and the ratio of reduced to oxidized glutathione (GSH/GSSG) were determined.
Results:
Plumbagin and PI concentration-dependently induced hepatic injury based on histopathological changes via imbalance of antioxidative system. Plumbagin and PI significantly increased plasma ALT and AST levels, hepatic lipid peroxidation, and GPx activity but significantly decreased hepatic SOD and CAT activities. The GSH/GSSG ratio was significantly reduced by plumbagin.
Conclusion:
Plumbagin and PI caused hepatotoxic effects in the mice by unbalancing of the redox defense system. Therefore, plumbagin and PI-containing supplements should be used cautiously, especially when consumed in high quantities or for long periods.
Keywords: Antioxidative system, glutathione, hepatotoxicity, plumbagin, Plumbago indica, transaminase
INTRODUCTION
Plumbago indica (PI) L., a shrub belonging to the family Plumbaginaceae, is widely distributed in tropical and subtropical regions of Africa, Australia, and Asia including Thailand [1]. PI root has been used as an ingredient in ayurvedic remedies for diarrhea, indigestion, and several skin diseases and has also been applied as an anthelmintic, appetite stimulant, and rubefacient [2-4]. In addition, PI extract has been reported to possess antibacterial activity [5-7]. While all Plumbago species have been claimed to have antifertility properties [8], PI was exceptionally used in the ancient remedies for abortion [2].
Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone), a yellowish quinonoid compound, is the major constituent that contributes to the medicinal properties of PI [3,9]. The root contains the highest amount of plumbagin (0.17% w/w) among Plumbago species and is the part mostly contained in the medicinal remedies [10]. Plumbagin has been claimed for various biological activities, i.e., anthelmintic [4,9,11], antimalarial [12], antibacterial [6], antifungal [13], anti-inflammatory [14,15], immunosuppressive [16], abortifacient [8], anticancer [14,17-20], and antidiabetic activities [21].
Nowadays, traditional herbal remedies are often perceived as harmless due to the belief that they are natural [22]. Potential toxicities in these remedies are often overlooked due to a lack of scientific studies regarding safe-dosage range, safety windows of effective and toxic doses [23]. Recent evidence indicates that herbal supplements are regularly consumed as alternative medicines by approximately 20% of the adults in the United States [24]. Hence, any scientific information regarding undesirable effects and toxicity of these herbal supplements would be of great significance.
Plumbagin has been reported to exhibit several side effects including diarrhea, skin rashes, leukocytosis, and increased serum phosphatase levels [25]. In addition, there are reports of plumbagin-induced hepatotoxicity [3,25,26], cardiotoxicity [27], cytotoxicity toward human keratinocytes [26], and radiomimetic nucleotoxic and cytotoxic effects [3]. These effects are possibly due to the activity of plumbagin as a strong inducer of reactive oxygen species (ROS) and a depleting agent of cellular glutathione (GSH) [26,27].
Antioxidative enzymes, such as superoxide dismutase (SOD), catalase (CAT), and GSH peroxidase (GPx), are essential for regulating and balancing ROS [28-30]. Therefore, they can be employed as biomarkers to determine the levels of ROS production and oxidative stress [30]. In addition, drug-induced liver injury subsequent to long-term use of drugs or herbal supplements can be evaluated via histomorphology. Common histopathological patterns for drug-induced liver injury include acute hepatitis, with or without cholestasis [31].
In the current study, plumbagin and the methanolic crude extract of PI were examined for their influences on the antioxidative enzymes and GSH profile along with the plasma transaminase levels and hepatic histomorphology to assess hepatotoxic potential and their possible adverse effects in mice.
MATERIALS AND METHODS
Chemicals and Reagents
Plumbagin was obtained from the LKT Laboratories (St. Paul, Minnesota, USA). Bradford solution was a product of Bio-Rad (Hercules, CA, USA). Eosin Y 1% aqueous solution and Mayer’s hematoxylin were from Bio Optica (Milan, Italy). Xylene and Permount® were bought from Fisher Scientific (Loughborough, UK). Alanine transaminase (ALT) and aspartate transaminase (AST), bovine serum albumin, β-nicotinamide adenine dinucleotide phosphate reduced form (NADPH), xanthine oxidase, nitroblue tetrazolium (NBT), 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), SOD, CAT, GSH reductase, malondialdehyde (MDA), thiobarbituric acid (TBA), and 4-vinylpyridine (4-VP) were supplied by Sigma-Aldrich Chemical (St. Louis, Missouri, USA). Ammonium molybdate and hydrogen peroxide (H2O2) were purchased from Ajax Finechem (Melbourne, Australia). Trichloroacetic acid (TCA) was a product of Loba Chemie (Mumbai, India). All other laboratory chemicals were of the highest purity from commercial suppliers.
Preparation of the PI Methanolic Crude Extract
The root of PI was purchased from Mor Tong-In Thai Traditional Medicine (Mahasarakham, Thailand) in June 2014. Plant materials were identified by Dr. Waraporn Putalun, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand. Reference specimens (PANPB-PI 2014-002) were deposited at the Herbarium of the Faculty of Pharmaceutical Sciences, Khon Kaen University. It was dried at 50°C in an oven then shredded and extracted with methanol for 3 h using a Soxhlet apparatus. The extract was then evaporated and freeze-dried into powder. The percentage yield of the PI extract was 33.40%.
Determination of the Plumbagin Content in the PI Extract
The PI extract was analyzed for the plumbagin content using high-performance liquid chromatography (HPLC) which was carried out on a Hypersil ODS column (Agilent Technologies, CA, USA, particle size 5 μm, 250 × 4 mm) coupled with an Agilent 1260 Infinity system and a UV detector (Agilent Technologies), before being eluted by an isocratic linear solvent system of acetonitrile and water (50:50 by volume) with a flow rate of 1 mL/min. The chromatogram was monitored at a wavelength of 410 nm and analyzed by the ChemStation software (Agilent Technologies). Identification and quantification of plumbagin were performed on the basis of retention time and peak area of the authentic standard of plumbagin (LKT Laboratories).
Animal Treatments
7-week-old male intelligent character recognition (ICR) mice were obtained from the National Laboratory Animal Center (Mahidol University, Nakhon Pathom, Thailand) and housed in the Animal Unit of Faculty of Pharmaceutical Sciences, Khon Kaen University (Khon Kaen, Thailand) under a controlled temperature (23 ± 2°C) and humidity (45 ± 2%). The protocol for animal handling and treatment was approved by the Animal Ethics Committee for Use and Care of Khon Kaen University (Approval No. AEKKU04/2558). At all times, the mice were housed on wood shaved bedding in polysulfone cages with ad libitum access to water and commercial rodent diet (SmartHeart® from Perfect Companion Pet Care Company, Bangkok, Thailand). The mice were intragastrically administered plumbagin (1, 5, and 15 mg/kg/day) or the PI extract (20, 200, and 1,000 mg/kg/day) in a 0.5% carboxymethyl cellulose solution (CMC) consecutively for 14 days (n = 5 for each group). The control group was daily given the vehicle (0.5% CMC) for the same period. At 24 h after the last treatment, the mice were sacrificed. The mouse plasma and livers were collected and immediately stored at –20°C for further analysis.
Hepatic Histomorphological Examination
Liver tissues were collected and examined their histomorphology under a light microscope. The tissues were fixed by immersion in 10% neutral buffered formalin overnight before being dehydrated by ethanol then embedded in paraffin. The paraffin pieces were sectioned using a microtome before being placed on microscopic slides. The slides with embedded liver tissue were stained with hematoxylin and eosin (H&E) and further evaluated for their hepatic histomorphological patterns at ×20 magnification using Nikon Eclipse TS100 inverted microscope with ELWD condenser: N.A. 0.3 (W.D. 75 mm). The image was analyzed and displayed on the screen using NIS-Elements D software [32].
Assessment of Plasma ALT and AST
The blood samples were centrifuged at 5,000×g at 4°C for 10 min. The supernatant was collected and incubated with ALT or AST substrates at 37°C for 5 min before further incubation with a 2,4-dinitrophenylhydrazine for 20 min. The reaction was stopped by adding 4 N NaOH before measuring the absorbance at a wavelength of 505 nm. The ALT and AST levels were determined using pyruvate as a standard [32].
Determination of Protein Content
The liver homogenate was diluted 50 folds with distilled water before determination of protein content using the Bradford method [33]. The absorbance of the mixture of the liver homogenate and Bradford solution was measured at a wavelength of 595 nm. The protein content was calculated using bovine serum albumin as a standard.
Assessment of SOD Activity
SOD activity was assessed as previously described [34]. Briefly, a mixture containing liver homogenate, chloroform, and ethanol was centrifuged at 13,000×g, 4°C for 30 min. The supernatant was collected and incubated with a mixture of xanthine, xanthine oxidase, and NBT at 25°C for 20 min. The reaction was stopped by adding CuCl2, and the absorbance was measured at a wavelength of 550 nm. The SOD activity was calculated compared to the SOD standard.
Assessment of CAT Activity
CAT activity was assessed by the colorimetric method of Goth (1991) with some modifications [34]. The liver homogenate was incubated with H2O2 at 37°C for 1 min before adding ammonium molybdate to stop the reaction. The absorbance was measured at a wavelength of 405 nm. The CAT activity was calculated compared to the CAT standard.
Determination of the Lipid Peroxidation
The lipid peroxidation was determined by measuring the level of MDA formation [34]. In brief, TCA and TBA were added to the liver homogenate pre-incubated at 37°C for 1 h, followed by boiling at 100°C for 15 min. The reaction was immediately cooled down by immersion in an ice-bath before adding excess TCA to stop the reaction. The reaction mixture was then centrifuged at 1,123×g, 4°C for 5 min. The spectrofluorometric intensity of the supernatant was measured with excitation and emission wavelengths of 520 and 590 nm, respectively. The thiobarbituric acid-reactive substances (TBARS) content was calculated compared with the standard MDA.
Determination of Total GSH Content, the Ratio of Reduced to Oxidized GSH, and GSH Peroxidase Activity
The determination of total GSH content was performed as previously described [34]. Briefly, the liver homogenate was deproteinized by 5-sulfosalicylic acid (SSA) and kept at 2-8°C for 10 min before centrifugation at 10 000×g, 4°C for 10 min. The supernatant was then mixed with GSH reductase and DTNB before adding NADPH to start the reaction. The absorbance was measured at a wavelength of 405 nm every 60 s for 5 min. The slope of absorbance per min was plotted and compared to the standard GSH to calculate the total GSH content.
The oxidized GSH (GSSG) content was determined by the same procedure used for the determination of total GSH content after treatment of the sample with 4-vinylpyridine [34]. The reduced GSH content was calculated by subtracting the GSSG content from the total GSH content.
The GPx activity was assessed as described previously [34]. The liver homogenate was incubated with sodium azide at 30°C for 10 min before starting the reaction by adding the GSH substrate and H2O2. The reaction was stopped with SSA before centrifugation at 330 × g for 15 min. The GPx activity was measured as μmol of GSSG produced per min at 30°C, pH 7.4.
Statistical Analysis
All data were expressed as means ± SD and analyzed by one-way ANOVA followed by least significant difference (LSD) post hoc test using the Statistical Package for Social Sciences statistical program. P < 0.05 was considered to be significant.
RESULTS
The Content of Plumbagin in the PI Extract
The PI extract was quantitatively determined for the content of plumbagin using the HPLC method, in which the validation of the method was achieved within a linearity range of 1-100 mg/mL (R2 = 0.9990). The precision of the method expressed as the coefficient of variation within the linearity range were 0.69 ± 0.29% (for within-day) and 0.87 ± 0.42% (for between-day). The accuracy was 102.16 ± 1.40% with the limit of determination and the limit of quantification of 10 and 34 ng/mL, respectively. The chromatograms of the plumbagin standard (retention time of 5.538 min, Figure 1a) and the PI extract (retention time of 5.494 min, Figure 1b) revealed that the major constituent in the PI extract was plumbagin. The content of plumbagin in the PI extract was 0.18 ± 0.01% dry weight extract.
Figure 1.

High-performance liquid chromatography chromatogram of the plumbagin standard and the Plumbago indica extract. (a) Chromatogram of the plumbagin standard at a concentration of 25 mg/mL, (b) Chromatogram of the P. indica extract at a concentration of 24.25 mg/mL
Effect of Plumbagin and the PI extract on Hepatic Histomorphology
The histomorphological findings of the H&E-stained livers were shown in Figure 2. The control group demonstrated normal hepatic histomorphology [Figure 2a]. Mice fed with the lowest dose of plumbagin (1 mg/kg/day) [Figure 2b] and the PI extract (20 mg/kg/day) [Figure 2e] were not found any change in hepatic histomorphological anatomy compared to the control group. However, at the higher doses of both plumbagin and the PI extract, the evidence of liver injury was observed. The livers of mice received plumbagin (5 and 15 mg/kg/day) [Figure 2c and d] and the PI extract (200 and 1,000 mg/kg/day) [Figure 2f and g] were presented the nuclear shrinkage, where the nuclear chromatin materials were condensed and clumped together. In addition, at the highest doses of plumbagin (15 mg/kg/day) [Figure 2d] and the PI extract (1,000 mg/kg/day) [Figure 2g], the hepatic hydropic cell swelling, where the cytoplasm of hepatocyte was filled with vacuoles, and nuclear fading along with some hepatocyte nuclei disappearance were extensively observed. These hepatic histopathological findings revealed that plumbagin and the PI extract induced hepatotoxicity in the dose-dependent pattern.
Figure 2.
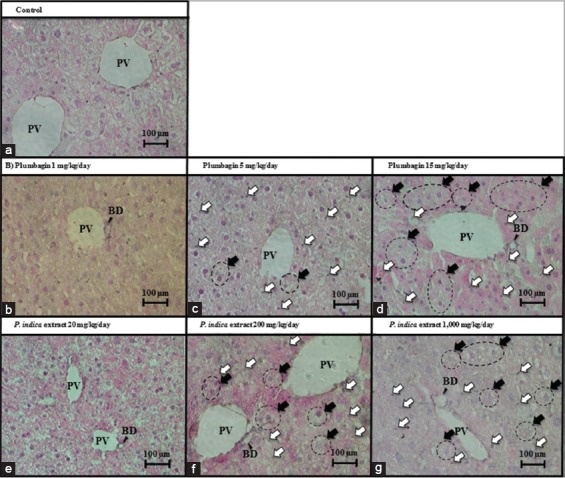
Liver histomorphology. Hepatic histomorphology of the mice treated with (a) control, 0.5% carboxymethyl cellulose (CMC), i.g., for 14 days, (b-d) Plumbagin in 0.5% CMC at the doses of 1, 5, and 15 mg/kg/day, i.g., for 14 days, (e-g) P. indica extract in 0.5% CMC at the doses of 20, 200, and 1,000 mg/kg/day, i.g., for 14 days. BD: Bile duct; PV: Portal vein. Open arrows indicate nuclear shrinkage. Closed arrows and circles indicate hydropic cell swelling
Effect of Plumbagin and the PI Extract on ALT and AST Levels
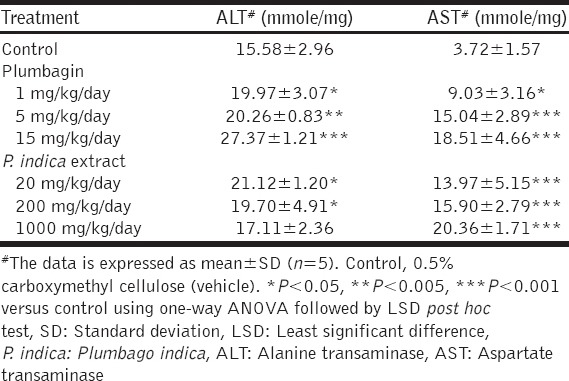
Plumbagin and the PI extract had no significant influence on the profile of mouse body weight [Figure 3]. However, the treatments with plumbagin and the PI extract significantly elevated the levels of ALT and AST, compared with the control mice [Table 1]. While plumbagin significantly increased both plasma ALT and AST levels in a dose-dependent manner, the PI extract only increased the AST levels dose-dependently; the ALT levels were increased in a dose-independent manner.
Figure 3.
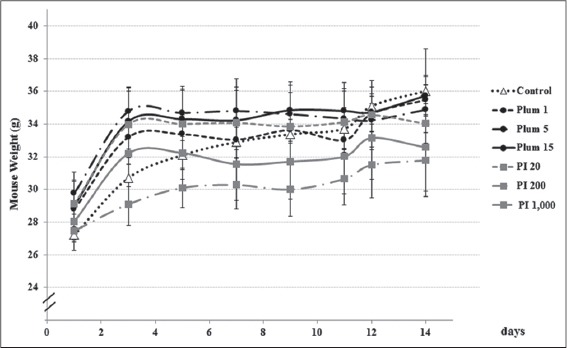
The weight profiles of mice during the treatments with plumbagin and the Plumbago indica extract. Mice were treated as described in the method (n = 5). Plum 1, 5, and 15, plumbagin in 0.5% carboxymethyl cellulose (CMC) at the doses of 1, 5, and 15 mg/kg/day, i.g., for 14 days; P. indica 20, 200, and 1,000, P. indica extract in 0.5% CMC at the doses of 20, 200, and 1,000 mg/kg/day, i.g., for 14 days
Table 1.
The levels of ALT and AST in the mice after the treatments of plumbagin or the P. indica extract for 14 days
Effect of Plumbagin and the PI Extract on Lipid Peroxidation in the Mouse Livers
The levels of MDA formation were significantly increased in a dose-dependent pattern by both plumbagin and the PI extract [Figure 4], corresponding with the increases of ALT and AST levels.
Figure 4.
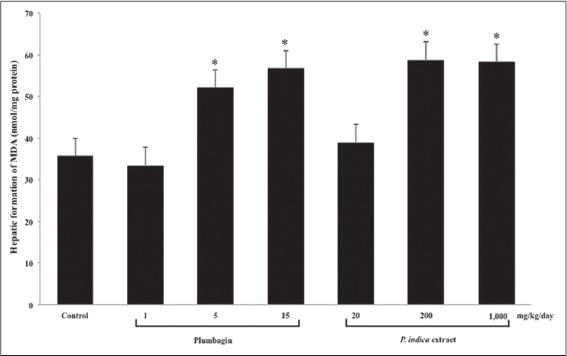
The hepatic lipid peroxidation in the mouse livers after the treatments with plumbagin and the Plumbago indica extract. Mice were treated, and the levels of malondialdehyde (MDA) formation were measured as described in the method (n = 5). *P < 0.001 versus Control using one-way ANOVA with least significant different post hoc test
Effect of Plumbagin and the PI Extract on SOD and CAT Activities in the Mouse Livers
Both of plumbagin and the PI extract significantly lowered SOD activity in the mouse livers to at least 2 times lesser than the control group [Figure 5]. The CAT activity was significantly decreased by both plumbagin and the PI extract in a dose-dependent pattern [Figure 6].
Figure 5.
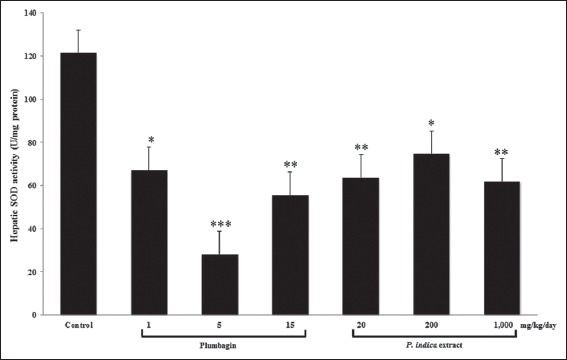
The superoxide dismutase (SOD) activity in the mouse livers after the treatments with plumbagin and the Plumbago indica extract. Mice were treated, and the SOD activity was measured as described in the method (n = 5). *P<0.05, **P<0.01, ***P<0.001 versus control using one-way ANOVA with least significant different post hoc test
Figure 6.
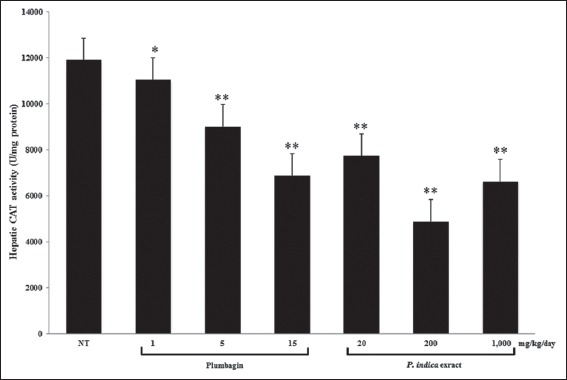
The catalase (CAT) activity in the mouse livers after the treatments with plumbagin and the Plumbago indica extract. Mice were treated, and the CAT activity was measured as described in the method (n = 5). *P<0.05, **P<0.001 versus control using one-way ANOVA with least significant different post hoc test
Effect of Plumbagin and the PI Extract on the GSH Profile and GPx Activity in the Mouse Livers
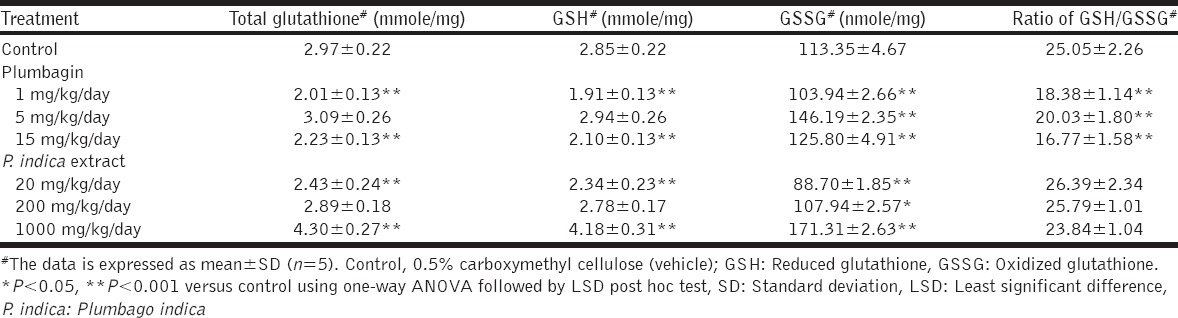
Either an increase or decrease in the total GSH, GSH, and GSSG contents was noted [Table 2] in accord with the reduction of SOD and CAT activities [Figures 5 and 6]. Total GSH and GSH contents were significantly reduced or even unchanged by plumbagin, whereas those levels were modified, increased, decreased, or unchanged by the PI extract. Regarding the GSSG content, increase or a decrease was observed by either plumbagin or the PI extract. The ratio of GSH to GSSG was significantly reduced by plumbagin but unchanged by the PI extract.
Table 2.
The glutathione profiles in the mouse livers after the treatments of plumbagin or the P. indica extract for 14 days
The GPx activity was significantly induced by plumbagin at the dose of 5 mg/kg/day and the PI extract at the dose of 1,000 mg/kg/day [Figure 7]. These findings corresponded with the increases in the GSSG contents [Table 2].
Figure 7.
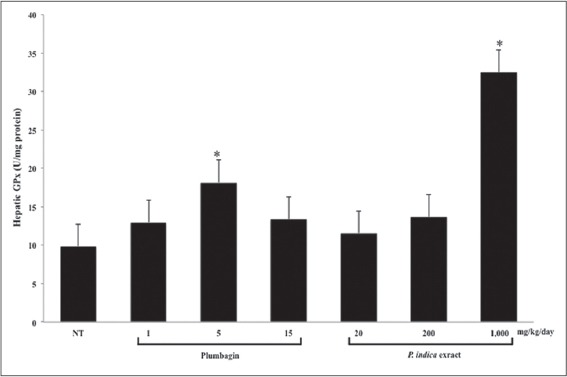
The glutathione peroxidase (GPx) activity in the mouse livers after the treatments with plumbagin and the Plumbago indica extract. Mice were treated, and the GPx activity was measured as described in the method (n = 5). *P < 0.001 versus control using one-way ANOVA with least significant difference post hoc test
DISCUSSION
In our study, the yield of plumbagin in the PI extract was 0.18 ± 0.01% dry weight which well correlated with the previous studies of the chloroform extract of PI root of 0.17% [10] and the ethanolic extract of PI root of 0.20% [35].
PI is extensively used in Ayurvedic and Thai traditional remedies, especially as a hematinic agent and for post-partum women [2]. Besides, the numerous pharmacological properties, the active constituent of PI, plumbagin, have been reported to possess many undesirable effects [25] principally due to its capability to generate ROS, leading to an imbalance of the oxidant-antioxidant system [26,27]. The accumulation of ROS induces excessive lipid oxidation, further along with oxidative attack to lipids, proteins, and DNA materials. This results in damage of cellular components including cellular membranes, functional and structural proteins, and DNA components [29]. In addition to non-specific tissue damage, ROS, particularly H2O2, was able to activate transcription factors of the nuclear factor kappa B family, which activates the inflammatory process [36,37]. Further study reported that H2O2 acted as a second messenger which activated protein kinase cascades coupled to inflammatory gene expression [36]. This information indicates that ROS not only produces non-specific damage to cellular components but also activates inflammation of the tissue. However, there is currently very little toxicological information regarding either plumbagin or the PI extract. In one study, Sumsakul et al. (2014) performed acute and subacute toxicity screening of plumbagin in ICR mice [12]. In the acute toxicity screening, a single dose of 100 mg/kg plumbagin showed no significant behavioral change, but a single dose of plumbagin at either 200 or 500 mg/kg led to anxiety and agitation and resulted in death within 24 h [12]. The subacute toxicity was investigated by oral administration of plumbagin (25, 50, and 100 mg/kg/day) for 14 days. The mice given with the two higher doses died within 8-11 and 4-8 days, respectively [12]. In another subacute toxicity study in rats, increases in serum alkaline phosphatase and ALT levels were noted following daily intraperitoneal administration of the PI root extract (50 mg/kg/day) for 30 days [38]. Our study is the first time to reveal the hepatotoxic impacts of the oral administration of plumbagin and the PI extract through the increased levels of transaminases in plasma and modification of antioxidative system in the mouse livers, including the increase in lipid peroxidation, the modulatory activities of antioxidative enzymes (SOD, CAT, and GPx), and related GSH profiles with lowering level of the GSH/GSSG ratio.
The LD50 of plumbagin in mice was reported at 16 mg/kg [25] with the oral bioavailability of 38.7% [39], and the LD50 of the ethanolic extract of PI root in mice was noted at 1,250 mg/kg [38,40]. Therefore, the doses of plumbagin of 1, 5, and 15 mg/kg/day and the PI extract of 20, 200, and 1,000 mg/kg/day were presently employed to examine their hepatotoxicity.
The mouse livers were collected and examined under a light microscope to evaluate hepatic histomorphological changes after the administration of plumbagin and the PI extract. Liver injury induced by drugs or herbs can be presented with all kinds of patterns representing primary liver diseases [31]. The most common histopathological sign of drug-induced liver injury is acute hepatitis, with or without cholestasis. The hallmarks of acute hepatitis include portal and parenchymal inflammation and hepatocellular injury with or without necrosis [31]. In the present study, the lowest doses of either plumbagin (1 mg/kg/day) or the PI extract (20 mg/kg/day) did not change the histomorphological anatomy of the mouse liver while the higher doses of plumbagin and the PI extract underwent serious hepatic histomorphological changes. At the low concentrations of plumbagin (1 mg/kg/day) and PI extract (20 mg/kg/day), ROS production had only risen slightly which likely did not disturb the balance in the antioxidative system, a known cause of liver tissue damage. Nuclear shrinkage is a phenomenon where the nuclei are shrunken and dark, but still intact [Figure 2c and d, f and g]. This happens as an early stage of cellular degeneration as a sequence of chromatin clumping before the nuclear envelope is ruptured and the nucleus is disappeared, as known as nuclear fragmentation and nuclear fading, respectively [41]. The proposed mechanism behind these phenomena is the activation of caspase enzymes due to alterations of mitochondrial status, which, in turn, activates the intrinsic apoptotic pathway, resulting in DNA fragmentation and proteolysis of nuclear polypeptides [42,43]. In this study, the liver tissues of the mice receiving plumbagin of 5 mg/kg/day and the PI extract of 200 mg/kg/day were presented with hepatic nuclear shrinkage [Figure 2c and f] while those receiving the highest doses of both plumbagin and the PI extract demonstrated hepatic nuclear fading [Figure 2d and g], indicating an increase in cellular injury severity with the dose. Hepatic hydropic cell swelling was additionally observed [Figure 2d and g]. This was due to the accumulation of water in the cytoplasm, indicating a severe but reversible hepatic injury, which is not yet lethal but can result in liver lytic necrosis if the causative is continued [44]. To date, there is no study of the PI extract regarding its toxicological effects inducing hepatic histopathology. There is only one study claimed for the hepatoprotective effect of PI extract in Wistar albino rats induced hepatotoxicity using paracetamol. The PI extract at 200 and 400 mg/kg/day for 14 days were reported to prevent hepatic histopathological injury caused by paracetamol [45]. However, in the present study, the hepatic histopathology caused by the PI extract was indicated from the dose of 200 up to 1,000 mg/kg/day. The differences in experimental species and strains possibly affect the bioavailability of the PI extract including their susceptibility to toxicity. In addition, the solvent and extraction condition which were different between the studies of Rajasekaran et al. [45] (Soxhlet extraction using ethanol at 68°C for 72 h) and our study (soxhlet extraction using methanol at its boiling temperature for 3 h) might influence on the amounts of constituents in the extracts. Hence, the outcomes are possibly not the same.
Hepatocytes contain intracellular ALT and AST enzymes [46]. AST is additionally found in skeletal muscles and erythrocytes. Therefore, AST is a biomarker indicating the damage of liver and other organs [47], whereas ALT is rather quite specific to liver injury [48]. Elevations of these two intracellular transaminase enzymes in the serum indicate leakage of hepatocytes, therefore suggesting liver injury [49]. Plumbagin and the PI extract significantly induced both ALT and AST in the mouse plasma suggesting the occurrence of liver injury. In addition, elevation of the plasma AST level was more prominent than that of the ALT, indicating a possibility of injury in extrahepatic organs.
TBARS assay measures formation of MDA, a product resulting from oxidative damage of lipid [50]. The dose-dependent increase in the MDA levels observed in the mouse livers after the treatment with either plumbagin or the PI extract suggested that these treatments lead to lipid peroxidation in the hepatic tissues. By contrast, a single intraperitoneal dose of plumbagin at <4 mg/kg was reported to decrease hepatic MDA levels, protecting against oxidative damage in adult male Wistar rats [51]. In addition, Plumbago zeylanica (white leadwort) extract provided a hepatoprotective effect in alcohol intoxicated Wistar albino rats [52]. It is likely that these different findings result from differences in species and strains of experimental animals, cultivation and harvesting of the herb, the method of extraction, and the dosage regimens employed in the different studies. Our investigation observed the effects of plumbagin and the PI extract in the ICR mice at a stronger dosage regimen with a longer period of administration, representing the Thai traditional regimens of PI.
The antioxidative enzymes, SOD, CAT, and GPx, provide the first line of defense against oxidative damage [53]. SOD is the first enzyme in the antioxidant armory, catalyzing superoxide into H2O2, which is then converted into oxygen and water by GPx and CAT [53]. GPx is responsible for the detoxification process when low amounts of H2O2 are present, followed by CAT after saturation of GPx [54]. We observed an inhibitory effect of plumbagin and the PI extract on the hepatic SOD and CAT activities, whereas the GPx activity was provoked. Correspondingly, plumbagin was reported to modify the expression of genes involved in ROS metabolism; down-regulation of CAT and mitochondrial Mn-SOD expression while up-regulating the expression of GPx in LNCaP cells [55].
GSH is a non-enzymatic intracellular antioxidant present in all mammalian tissues; however, it is most abundant in the liver which is the main site of GSH synthesis [56]. Total GSH content consists of the reduced form (GSH) plus the oxidized form (GSSG). During oxidative stress, GSH is oxidized to GSSG, which is then converted back to its reduced form by GSH reductase (GR). Over-capacity of GR leads to the export of excess GSSG out of the cell [56]. In our hands, both plumbagin and the PI extract depleted the hepatic GSH content while increased the hepatic GSSG content, resulting in a decrease in the GSH/GSSG ratio and imbalance of the oxidant-antioxidant system.
This imbalance in the cellular redox status ultimately results in hepatic oxidative stress as demonstrated by the elevation of hepatic MDA levels. Prolong oxidative stress then results in chronic inflammation and cellular injury [29]. Hence, we propose a model for plumbagin- and the PI-induced hepatotoxicity; plumbagin and the PI extract initiated oxidative stress in the hepatic tissue through an imbalance of the hepatic antioxidative system: A decrease in SOD and CAT activity along with an increase in GPx activity and deterioration of the GSH antioxidant system. These findings resulted in increases in the hepatic lipid peroxidation and hepatocyte injury as demonstrated by the increases in plasma ALT and AST levels, including hepatic histopathological changes.
CONCLUSION
Herewith the specific hepatotoxic properties of plumbagin and the PI extract were underlined. The findings suggest that practitioners should be cautious for the use of plumbagin or PI-containing supplements, especially if they are consumed at high quantity or for a long period. In addition, it is worth for further study on the pharmacology and toxicology of plumbagin and the PI extract in depth at the molecular mechanism.
ACKNOWLEDGMENTS
Nadta Sukkasem sincerely thanks the Research Group for Pharmaceutical Activities of Natural Products using Pharmaceutical Biotechnology (PANPB), Khon Kaen University, Thailand, for scholarship and research grant, Faculty of Pharmaceutical Science, Khon Kaen University, for providing research facilities. Dr. Glenn Borlace, Khon Kaen University, is deeply acknowledged for the critical review and editorial evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Saha D, Paul S. Antibacterial activity of Plumbago indica. Turk J Pharm Sci. 2014;11:217–22. [Google Scholar]
- 2.Dutt UC, King G. The Materia Medica of the Hundus: Compiled from Sanskrit Medical Works. Calcutta: Thacker Spink and Co; 1877. [Google Scholar]
- 3.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012;32:1131–58. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]
- 4.Lorsuwannarat N, Piedrafita D, Chantree P, Sansri V, Songkoomkrong S, Bantuchai S, et al. The in vitro anthelmintic effects of plumbagin on newly excysted and 4-weeks-old juvenile parasites of Fasciola gigantica. Exp Parasitol. 2014;136:5–13. doi: 10.1016/j.exppara.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Paul AS, Islam A, Yuvaraj P. Anti - Helicobacter pylori and cytotoxic activity of detoxified root of Plumbago auriculata, Plumbago indica and Plumbago zeylanica. J PhytoPharm. 2013;2:4–8. [Google Scholar]
- 6.Kaewbumrung S, Panichayupakaranant P. Antibacterial activity of plumbagin derivative-rich Plumbago indica root extracts and chemical stability. Nat Prod Res. 2014;28:835–7. doi: 10.1080/14786419.2013.879585. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim M, Baura J, Ahasan Q, Islam T, Homa Z, Chowdhury MU, et al. Preliminary phytochemical and pharmacological investigations of Alpinia conchigera Griff and Plumbago indica L. Bangladesh Pharm J. 2012;15:153–7. [Google Scholar]
- 8.Sheeja E, Joshi SB, Jain DC. Antiovulatory and estrogenic activity of Plumbago rosea leaves in female albino rats. Indian J Pharmacol. 2009;41:273–7. doi: 10.4103/0253-7613.59927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorsuwannarat N, Saowakon N, Ramasoota P, Wanichanon C, Sobhon P. The anthelmintic effect of plumbagin on Schistosoma mansoni. Exp Parasitol. 2013;133:18–27. doi: 10.1016/j.exppara.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Ariyanathan S, Saraswathy A, Rajamanickam GV. Quality control standards for the roots of three Plumbago species. Indian J Pharm Sci. 2010;72:86–91. doi: 10.4103/0250-474X.62254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SM, Coultas KA. Identification of plumbagin and sanguinarine as effective chemotherapeutic agents for treatment of schistosomiasis. Int J Parasitol Drugs Drug Resist. 2013;3:28–34. doi: 10.1016/j.ijpddr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumsakul W, Plengsuriyakarn T, Chaijaroenkul W, Viyanant V, Karbwang J, Na-Bangchang K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Complement Altern Med. 2014;14:15. doi: 10.1186/1472-6882-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuete V, Tangmouo JG, Meyer JJ, Lall N. Diospyrone, crassiflorone and plumbagin: Three antimycobacterial and antigonorrhoeal naphthoquinones from two Diospyros spp. Int J Antimicrob Agents. 2009;34:322–5. doi: 10.1016/j.ijantimicag.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B, et al. Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem Toxicol. 2014;64:177–83. doi: 10.1016/j.fct.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Checker R, Sharma D, Sandur SK, Khanam S, Poduval TB. Anti-inflammatory effects of plumbagin are mediated by inhibition of NF-kappaB activation in lymphocytes. Int Immunopharmacol. 2009;9:949–58. doi: 10.1016/j.intimp.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 16.McKallip RJ, Lombard C, Sun J, Ramakrishnan R. Plumbagin-induced apoptosis in lymphocytes is mediated through increased reactive oxygen species production, upregulation of Fas, and activation of the caspase cascade. Toxicol Appl Pharmacol. 2010;247:41–52. doi: 10.1016/j.taap.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Wang CC, Chiang YM, Sung SC, Hsu YL, Chang JK, Kuo PL. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008;259:82–98. doi: 10.1016/j.canlet.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Xu KH, Lu DP. Plumbagin induces ROS-mediated apoptosis in human promyelocytic leukemia cells in vivo. Leuk Res. 2010;34:658–65. doi: 10.1016/j.leukres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Lai L, Liu J, Zhai D, Lin Q, He L, Dong Y, et al. Plumbagin inhibits tumour angiogenesis and tumour growth through the Ras signalling pathway following activation of the VEGF receptor-2. Br J Pharmacol. 2012;165:1084–96. doi: 10.1111/j.1476-5381.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafeez BB, Zhong W, Fischer JW, Mustafa A, Shi X, Meske L, et al. Plumbagin, a medicinal plant (Plumbago zeylanica)-derived 1,4-naphthoquinone, inhibits growth and metastasis of human prostate cancer PC-3M-luciferase cells in an orthotopic xenograft mouse model. Mol Oncol. 2013;7:428–39. doi: 10.1016/j.molonc.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunil C, Duraipandiyan V, Agastian P, Ignacimuthu S. Antidiabetic effect of plumbagin isolated from Plumbago zeylanica L. root and its effect on GLUT4 translocation in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2012;50:4356–63. doi: 10.1016/j.fct.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 22.Ernst E. Harmless herbs? A review of the recent literature. Am J Med. 1998;104:170–8. doi: 10.1016/s0002-9343(97)00397-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Onakpoya IJ, Posadzki P, Eddouks M. The safety of herbal medicine: From prejudice to evidence. Evid Based Complement Alternat Med. 2015;2015:316706. doi: 10.1155/2015/316706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu CH, Wang CC, Tsai MT, Huang WT, Kennedy J. Trend and pattern of herb and supplement use in the United States: Results from the 2002 2007, and 2012 national health interview surveys. Evid Based Complement Alternat Med. 2014;2014:872320. doi: 10.1155/2014/872320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SivaKumar V, Prakash R, Murali MR, Devaraj H, Niranjali Devaraj S. In vivo micronucleus assay and GST activity in assessing genotoxicity of plumbagin in Swiss albino mice. Drug Chem Toxicol. 2005;28:499–507. doi: 10.1080/01480540500263019. [DOI] [PubMed] [Google Scholar]
- 26.Jeong SH, Choi JS, Ko YK, Kang NS. The discovery of bioisoster compound for plumbagin using the knowledge-based rational method. J Mol Struct. 2015;1085:84–9. [Google Scholar]
- 27.Shimada H, Yamaoka Y, Morita R, Mizuno T, Gotoh K, Higuchi T, et al. Possible mechanism of superoxide formation through redox cycling of plumbagin in pig heart. Toxicol In Vitro. 2012;26:252–7. doi: 10.1016/j.tiv.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Zangar RC, Davydov DR, Verma S. Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol. 2004;199:316–31. doi: 10.1016/j.taap.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Zhang H, Zhang T, Zhang R, Liu R, Chen Y. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int J Biol Macromol. 2015;77:59–67. doi: 10.1016/j.ijbiomac.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62:481–92. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- 32.Sinthorn W, Chatuphonprasert W, Chulasiri M, Jarukamjorn K. Thai red rice extract provides liver protection in paracetamol-treated mice by restoring the glutathione system. Pharm Biol. 2015:1–10. doi: 10.3109/13880209.2015.1079725. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 34.Jearapong N, Chatuphonprasert W, Jarukamjorn K. Miroestrol, a phytoestrogen from Pueraria mirifica, improves the antioxidation state in the livers and uteri of ß-naphthoflavone-treated mice. J Nat Med. 2014;68:173–80. doi: 10.1007/s11418-013-0788-6. [DOI] [PubMed] [Google Scholar]
- 35.Unnikrishnan KP, Raja SS, Balachandran I. A Reverse Phase HPLC-UV and HPTLC Methods for Determination of Plumbagin in Plumbago indica and Plumbago zeylanica. Indian J Pharm Sci. 2008;70:844–7. doi: 10.4103/0250-474X.49142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–62. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 37.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–58. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon FE, Sharada AC, Devi PU. Toxic effects of crude root extract of Plumbago rosea (Rakta chitraka) on mice and rats. J Ethnopharmacol. 1993;38:79–84. doi: 10.1016/0378-8741(93)90081-f. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh YJ, Lin LC, Tsai TH. Measurement and pharmacokinetic study of plumbagin in a conscious freely moving rat using liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844:1–5. doi: 10.1016/j.jchromb.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Arambewela L, Silva R. Sri Lankan Medicinal Plant Monographs and Analysis: Plumbago Indica. Columbo: National Science Foundation; 1999. [Google Scholar]
- 41.Myers RK, McGavin MD, Zachary JF. Cellular adaptations, injury, and death: Morphologic, biochemical, and genetic bases. In: Zachary JF, McGavin MD, editors. Pathologic Basis of Veterinary Disease. 2nd ed. Missouri, United States: Mosby; 2012. pp. 1–59. [Google Scholar]
- 42.Martelli AM, Zweyer M, Ochs RL, Tazzari PL, Tabellini G, Narducci P, et al. Nuclear apoptotic changes: An overview. J Cell Biochem. 2001;82:634–46. doi: 10.1002/jcb.1186. [DOI] [PubMed] [Google Scholar]
- 43.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dancygier H, editor. Microscopic anatomy. Clinical Hepatology, Principles and Practice of Hepatobiliary Diseases. Vol. 1. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 2010. pp. 51–5. [Google Scholar]
- 45.Rajasekaran A, Periasamy M. Protective effect of ethanolic root extract of Plumbago indica L. on paracetamol induced hepatotoxicity in rats. Afr J Pharm Pharmacol. 2011;5:2330–4. [Google Scholar]
- 46.Kang KS. Abnormality on liver function test. Pediatr Gastroenterol Hepatol Nutr. 2013;16:225–32. doi: 10.5223/pghn.2013.16.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vroon DH, Israil Z. Aminotransferases. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworth Publishers; 1990. pp. 492–3. [PubMed] [Google Scholar]
- 48.Oh RC, Hustead TR. Causes and evaluation of mildly elevated liver transaminase levels. Am Fam Physician. 2011;84:1003–8. [PubMed] [Google Scholar]
- 49.Benedetti AL, Vituri Cde L, Trentin AG, Domingues MA, Alvarez-Silva M. The effects of sub-chronic exposure of Wistar rats to the herbicide Glyphosate-Biocarb® . Toxicol Lett. 2004;153:227–32. doi: 10.1016/j.toxlet.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Dawn-Linsley M, Ekinci FJ, Ortiz D, Rogers E, Shea TB. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J Neurosci Methods. 2005;141:219–22. doi: 10.1016/j.jneumeth.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Sankar R, Devamanoharan PS, Raghupathi G, Krishnasamy M, Devi CS. Lipid peroxidation in plumbagin administered rats. J Biosci. 1987;12:267–71. [Google Scholar]
- 52.Goyal R, Sharma PL. Possible mechanism of Plumbago zeylanica in prevention of hepatic damage in Wistar rat. Am J Pharmacol Toxicol. 2012;7:101–8. [Google Scholar]
- 53.Araújo MB, Moura LP, Junior RC, Junior MC, Dalia RA, Sponton AC, et al. Creatine supplementation and oxidative stress in rat liver. J Int Soc Sports Nutr. 2013;10:54. doi: 10.1186/1550-2783-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussain AL, Jakoniuk JM. Liver catalase, glutathione peroxidase and reductase activity, reduced glutathione and hydrogen peroxide levels in acute intoxication with chlorfenvinphos, an organophosphate insecticide. Pol J Environ Stud. 2004;13:303–9. [Google Scholar]
- 55.Powolny AA, Singh SV. Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm Res. 2008;25:2171–80. doi: 10.1007/s11095-008-9533-3. [DOI] [PubMed] [Google Scholar]
- 56.Nobili V, Pastore A, Gaeta LM, Tozzi G, Comparcola D, Sartorelli MR, et al. Glutathione metabolism and antioxidant enzymes in patients affected by nonalcoholic steatohepatitis. Clin Chim Acta. 2005;355:105–11. doi: 10.1016/j.cccn.2004.12.022. [DOI] [PubMed] [Google Scholar]


