Abstract
Aims:
The present study was undertaken to evaluate the effect of methanolic leaf extract of Gymnema sylvestre (MLGS) on glucose transport (GLUT) and insulin resistance in vitro.
Materials and Methods:
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) and GLUT-4 expression were assessed in L6 myotubes for concluding the GLUT activity, and adiponectin and leptin expression was studied in 3T3 L1 murine adipocyte cell line to determine the effect of MLGS (250-750 μg/ml) on insulin resistance.
Results:
The findings of the experiments have demonstrated a significant and dose-dependent increase in glucose uptake in all the tested concentrations of MLGS, further the glucose uptake activity of MLGS (750 μg/ml) was at par with rosiglitazone (50 μg/ml). Concomitantly, MLGS has shown enhanced GLUT-4 and PPAR-γ gene expressions in L6 myotubes. Furthermore, cycloheximide (CHX) had completely abolished the glucose uptake activity of MLGS when co-incubated, which further confirmed that glucose uptake activity of MLGS was linked to enhanced expression of GLUT-4 and PPAR-γ. In addition, in another experimental set, MLGS showed enhanced expression of adiponectin and leptin, thus confirms the ameliorative effect of MLGS on insulin resistance.
Conclusion:
These findings suggest that MLGS has an enhanced glucose uptake activity in L6 myotubes, and ameliorate the insulin resistance in 3T3 L1 murine adipocyte cell line in vitro.
Keywords: Adiponectin, antidiabetic activity, diabetes, glucose transport, Gymnema sylvestre, leptin, L6 myotubes, peroxisome proliferator-activated receptor-gamma, 3T3 L1 murine adipocyte cell line
INTRODUCTION
Diabetes is a metabolic disorder of diverse etiology, manifested as hyperglycemia, polyuria, polydipsia, and polyphagia, characterized by disturbed carbohydrate, fat, and protein metabolism, due to defects in insulin secretion, and/or insulin action, or both [1]. Various classes of synthetic drugs are available for the treatment of diabetes; however, the inherent deleterious effects associated with these drugs have made a massive scope for discovering better and safe drug for the treatment of diabetes. In this perspective, plant-based medicines are believed to have great avenue over synthetic classes of drugs, and in recent times, many researchers have found numerous herbal medicines as highly promising in the treatment of diabetes [2]. In this context, Gymnema sylvestre is a very popular plant well known for its antidiabetic property, and it was scientifically validated for antidiabetic and hypoglycemic activity [3-6]. However, the molecular mechanism behind the hypoglycemic and antidiabetic activity of G. sylvestre remain uncertain, in this context we thought to evaluate the effect of G. sylvestre on glucose transporter-4 (GLUT-4), peroxisome proliferator-activated receptor-gamma (PPAR-γ), adiponectin, and leptin gene expression levels in vitro. GLUT-4 is one of the major GLUTs known to facilitate the glucose uptake in major tissues such as skeletal muscle, thus abnormal and/or decreased expression of GLUT-4 leads to diminished glucose uptake into the target tissues [7,8]. Further, PPAR-γ is a transcription factor belonging to the nuclear receptor superfamily, which is known to play a vital role in insulin signaling induced glucose uptake, and the role PPAR-γ in the development of insulin resistance has been scientifically well demonstrated, and therefore, the drugs that enhance PPAR-γ expression and/or acts as PPAR-γ agonists play a key role in reversing the insulin resistance [9-13].
In addition, adiponectin and leptin are the fundamental insulin-sensitizing adipokines secreted by adipocytes [14]. Adiponectin enhances insulin sensitivity and helps in controlling dyslipidemia through AMPK activation and increased fatty acid (FA) oxidation [15,16]. Scientifically, it is ascertained that abnormal and/or diminished expression of adiponectin leads to insulin resistance, dyslipidemia, and atherosclerosis in rodents and humans [17]. Similarly, leptin is an another adipocytokine produced by adipocytes, and it is known to play a remarkable role in controlling body weight, metabolism, and reproductive functions [18], Leptin presents in central nervous system acts as a checkpoint to control the food consumption, in short low levels of leptin leads to increased food consumption, and it is very clearly demonstrated in leptin knockout animals, and further leptin levels are observed to be significantly low in obese individuals compared to healthy volunteers [19,20]. Concisely, the available scientific literature suggests that GLUT-4, PPAR-γ, adiponectin, and leptin levels have a positive correlation in controlling the blood glucose levels and insulin resistance in patients suffering from diabetes and its complications.
Considering the potential anti-diabetic property of G. sylvestre, we thought to explore the possible correlation between the anti-diabetic activity of G. sylvestre and GLUT-4, PPAR-γ, adiponectin, and leptin gene expressions in vitro.
With the above hypothesis, the present study was designed to explore the possible mechanism behind the hypoglycemic and antidiabetic activity of G. sylvestre, through evaluating its effect on GLUT-4, PPAR-γ, adiponectin, and leptin gene expression in vitro.
MATERIALS AND METHODS
Reagents, Chemicals, and Cell Lines
Avian moloney leukemic virus (AMLV) reverse transcriptase (RT) and taq polymerase (dNTP) were purchased from Thermo Scientific, USA. Insulin, rosiglitazone, TRI reagent, cell culture supplements, and reagents used were of molecular biology grade and purchased from Sigma-Aldrich, St. Louis, USA.
Collection of Plant Material
The leaves of G. sylvestre were collected from Hospete (Bellary District, Karnataka, India) in the month of May-June 2013, considering the seasonal conditions for obtaining maximum phytoconstituents, and were authentified by Dr. K. P. Sreenath, Professor, Department of Botany, Bangalore University, Bengaluru, Karnataka, India. A voucher specimen of the plant material is preserved in the department with specimen no. 2013-14/GS/BT-01.
Plant Material Preparation
The shade dried leaf powder of G. sylvestre was subjected to extraction process as follows. Exactly 50 g of the plant material was extracted with various organic solvents successively in the ascending order of polarity (hexane, dichloromethane, ethylacetate, and methanol) in Soxhlet apparatus. In brief, 50 g of the plant material was initially extracted with 1000 ml hexane at 60°C for 24 h, the marc obtained was completely dried and extracted with 1 L of dichloromethane for 24 h, and subsequently, the marc obtained was extracted with 1 L of ethyl acetate and followed by 1 L of methanol.
Extracts were concentrated by Rotary evaporator (Rotavap-Remi Instrument) under reduced pressure at room temperature, and 4 mg of dried extract was reconstituted to 4 ml with respective solvents and diluted to attain the final concentration 750 μg/ml, 500 μg/ml, and 250 μg/ml for glucose uptake studies.
Propagation and Maintenance of L6 Cells and 3T3 L1
L6 cells (Rat skeletal muscle), cell culture was procured from National Centre for Cell Sciences (NCCS), Pune, Maharashtra, India. L6 cells were cultured and maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% inactivated fetal bovine serum (FBS) along with penicillin (100 μg/ml), streptomycin (100 μg/ml), and amphotericin B (5 μg/ml) in an humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with trypsin phosphate versene glucose solution (0.2% trypsin, 0.02% EDTA, and 0.05% glucose in phosphate buffered saline). The stock cultures were grown in 25 cm2 culture flasks, and the experiments were carried out in 60 mm petri dishes and 96 well microtiter plates (Tarsons India Pvt., Ltd., Kolkata, West Bengal, India) [21].
3T3 L1 adipocyte cell culture was procured from NCCS, Pune, Maharashtra, India. The cells were grown to confluence in DMEM containing glucose. The monolayer of the 3T3 L1 cells was trypsinized and resuspended at 1 × 104 cells/ml in DMEM with 10% FBS. 0.5 ml of cell suspension was seeded per well in 24 well plates (Tarsons India Pvt. Ltd., Kolkata, India); the plates were incubated and allowed to attain the confluent monolayer. Growth medium was removed and followed by the addition of adipogenesis initiation media containing 0.5 mm 3-iso butyl-1-methyl xanthine, 10% FBS and 1 μm dexamethasone in DMEM. Initially, the cell lines were incubated for 48 h at 5% CO2 at 37°C, after incubation adipogenesis initiation media was replaced by adipogenesis progression media (DMEM with 10% FBS and 10 μm/ml insulin). Subsequently, the plate was incubated for 48 h at 5% CO2 at 37°C, and then adipogenesis progression media was removed, and the cells were treated with various concentrations of test extracts along with controls in adipogenesis maintenance media (DMEM with 10% FBS). The test drugs samples were dissolved in DMEM media and incubated with adipogenesis maintenance media [22].
Glucose Uptake Assay
The fully differentiated L6 myotubes were serum starved overnight and washed with HEPES in KREB’s Ringer phosphate solution (KRP buffer) and incubated with KRP buffer with 0.1% bovine serum albumin for 30 min at 37°C. Myotubes were treated with various concentrations of test drug, and standard along with vehicle controls in 60 mm Petri plates, D-glucose solution was added to all the plates and incubated for 30 min at 37°C. Subsequently, the liquid medium was aspirated from all the plates to terminate the glucose uptake, and the cells were washed thrice with ice-cold KRP buffer solution. Further, the cells were lysed with 0.1 M NaOH solution and an aliquot of the cell lysates were used to measure the cell-associated glucose. Glucose uptake was estimated by Biovision Kit Inc., USA. Three independent experimental values in duplicates were taken to determine the percentage enhancement of glucose uptake over controls [23,24].
RT-Polymerase Chain Reaction (RT-PCR)
RT-PCR was carried out as per Kumar et al., 2015 [21,23]. In short, after the completion of the incubation period, the cells were lysed in TRI reagent, proteins were extracted with chloroform, and the total RNA was precipitated with isopropanol. The RNA precipitate was washed with 70% ethanol and resuspended in 50 μl of DEPC-treated water. Reverse transcription was carried out using 200 units of avian RT and 200 ng/μl oligo d(T)18. The primers used were as follows, GLUT-4: Sense, 5’-CGG GAC GTG GAG CTG GCC GAG GAG-3’; anti-sense, 5’CCC CCT CAG CGA GTG A-3’ (318-bp), [12]; PPAR-γ; sense, 5’-GGA TTC ATG ACC AGG GAG TTC CTC-3’; anti-sense, 5’-GCG GTC TCC ACT GAG AAT AAT GAC-3’ (155-bp), [12]; glyceraldehydes-3-phosphate dehydrogenase (GAPDH); sense, 5’CCA CCC ATG GCA AAT TCC ATG GCA-3’; anti-sense, 5’-TCT AGA CGG CAG GTC AGG TCC ACC-3’ (588-bp) [12]. For PCR reaction, 1 μl of cDNA mixture was added to a PCR reaction mix containing ×10 PCR buffer, 2 mM dNTP, 10 pM of paired primers, 2 units of dNTP. PCR products were run on 1.5% agarose gels, stained with ethidium bromide and photographed.
Total RNA was obtained from cultured 3T3-L1 adipocytes (Day 8) as per previously mentioned procedures. cDNA was generated from total RNA and synthesized using a high capacity cDNA reverse transcription kit. The PCR conditions were as follows: For GAPDH, 25 cycles of 95°C for 30 s, 55° C for 30 s.
The primers used are adiponectin: Sense, 5’-GTTCTACTGCAACATTCCGG-3’; antisense, 5’-TACACCTGGAGCCAGACTTG-3’; leptin: Sense, 5’-TGTGCTGCAGATAGCCAATG-3’; Antisense, 5’-AGGGAGCAGCTCTTGGAGAAG-3’. To facilitate comparisons between sample groups, quantities of all targets in test samples were normalized to GAPDH. PCR products were run on 1.5% agarose gels, stained with ethidium bromide and photographed [12].
Statistical Analysis
All the values were expressed as mean ± standard error of mean. The results were analyzed statistically using one-way ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism version 4.0 for Windows, GraphPad Software, San Diego California USA. The minimum level of significance was fixed at P < 0.05.
RESULTS
Extraction of Plant Material
The leaf powder of G. sylvestre was successively extracted with dichloromethane, hexane, ethyl acetate, and methanol. The extractive values of dichloromethane, hexane, ethylacetate, and methanol were found to be 0.52, 0.7, 2.5, and 3.3% w/w (gram per gram), respectively. In a pilot study, all the extracts were subjected for glucose uptake assay using L6 myotube and based on the findings methanolic extract was found to be promising; hence, only methanolic extract was subjected for further studies.
Evaluation of Glucose Uptake
Glucose uptake activity of MLGS was assessed using rosiglitazone (50 μg/ml) as the reference standard, and the dose of rosiglitazone was selected based on the pilot plant study performed in house (unpublished data).
The MLGS at concentrations ranging from 250 to 750 μg/ml showed dose-dependent stimulation of glucose uptake. Noteworthy, MLGS at 750 μg/ml concentration has offered approximately 2-fold increase in glucose uptake from basal glucose concentration, similarly, rosiglitazone also showed a substantial increase in glucose uptake. The results are given in Figure 1.
Figure 1.
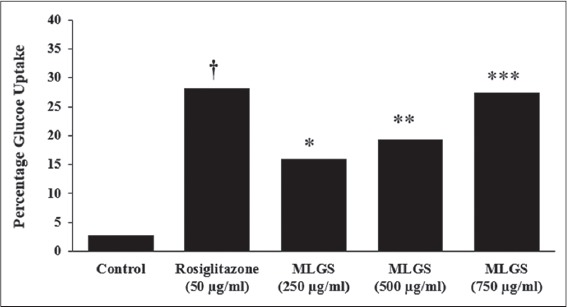
Glucose uptake activity of methanolic leaf extract of Gymnema sylvestre (MLGS) in L6 myotubes. All of the values are expressed as x ± standard error of mean (n = 3); means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph Pad version 4.0. †P < 0.001 corresponds to rosiglitazone versus control; *P < 0.05, **P < 0.01, ***P < 0.01 corresponds to MLGS versus control
Further, the outcome of MLGS on glucose uptake was thought to be intervened through increased expression of GLUT-4 and PPAR-γ synthesis and thereby enhanced protein synthesis. To authenticate this hypothesis, another experiment was carried out connected to the glucose uptake activity of MLGS, in presence and absence of a protein synthesis inhibitor cycloheximide (CHX) in L6 myotubes. The findings have revealed complete blockade of glucose uptake activity of MLGS in the presence of CHX; these findings propose that the elevated glucose uptake activity of MLGS is due to enhanced protein synthesis [Figure 2].
Figure 2.
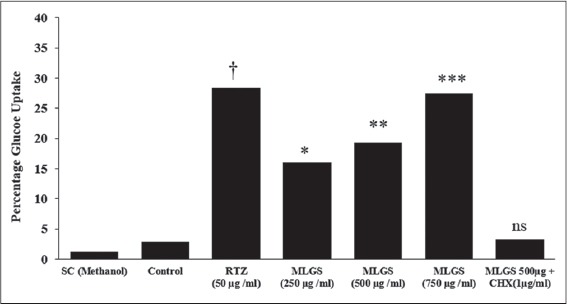
Effect of cycloheximide on methanolic leaf extract of Gymnema sylvestre (MLGS) intervening glucose uptake in L6 myotubes. ns: Not significant. The values are x̄ ± Standard error of mean (n = 3) of independent experiments, means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph Pad version 4.0. †P < 0.001 corresponds to rosiglitazone versus control; *P < 0.05, **P < 0.01, ***P < 0.01 corresponds to MLGS versus control. nsP > 0.05 corresponds to MLGS 500 μg/ml + cycloheximide versus MFMC 500 μg/ml
Effect of MLGS on GLUT-4, PPAR-γ, Adiponectin, and Leptin Transcription Level
We determined the outcome of MLGS on GLUT-4, PPAR-γ, adiponectin, and leptin mRNA expression by semi-quantitative RT-PCR. MLGS has significantly elevated the GLUT-4 transcript level compared to control. A depictive image of agarose gel [Figure 3a and b] and The corresponding densitometry scanning [Figure 3c] confirms approximately 7.1-fold increase in GLUT-4 expression in MLGS (750 μg/ml) group and 4.6-fold increase in rosiglitazone group compared to control [Figures 3a-c].
Figure 3.
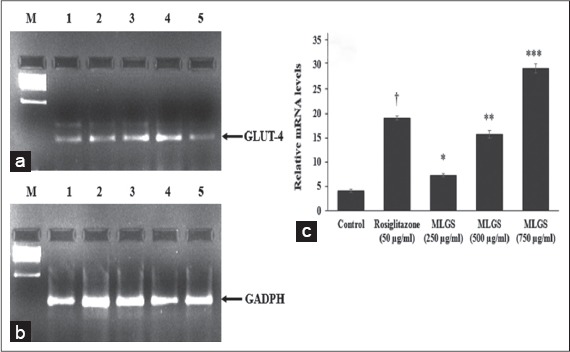
Effect of the methanolic leaf extract of Gymnema sylvestre (MLGS) on glucose transport-4 transcripts in L6 myotubes. (a and b) - M: Ikbp marker, Lane 1: 250 μg/ml MLGS, Lane 2: 500 μg/ml MLGS, Lane 3: 750 μg/ml MLGS, Lane 4: 50 μg/ml rosiglitazone, Lane 5: Control. (c) The values are x ± standard error of mean (n = 3) of independent experiments, means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph Pad version 4.0. †P < 0.001 corresponds to rosiglitazone versus control; *P < 0.05, **P < 0.01, ***P < 0.01 corresponds to MLGS versus control
As a second step, we studied the role of PPAR-γ in GLUT activity exhibited by MLGS. In findings, the MLGS has shown significant and dose-dependent upregulation of PPAR-γ compared to control, the higher dose of MLGS (750 μg/ml) showed approximately ~ 2.5-fold increase in PPAR-γ expression compared to control, similarly, the reference standard rosiglitazone has also shown a significant increase in PPAR-γ compared to control. The results are shown in Figure 4a-c.
Figure 4.
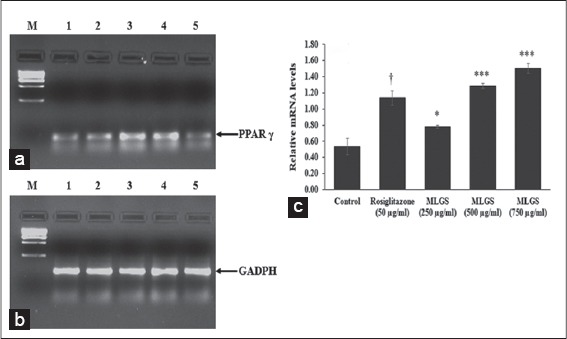
Effect of the methanolic leaf extract of Gymnema sylvestre (MLGS) on peroxisome proliferator-activated receptor-gamma transcripts in L6 myotubes. (a and b) - M: Ikbp marker, Lane 1: 250 μg/ml MLGS, Lane 2: 500 μg/ml MLGS, Lane 3: 750 μg/ml MLGS, Lane 4: 50 μg/ml rosiglitazone, Lane 5: Control. (c) The values are x ± standard error of mean (n = 3) of independent experiments, means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph Pad version 4.0. †P < 0.001 corresponds to rosiglitazone versus control; *P < 0.05, ***P < 0.01 corresponds to MLGS versus control
Based on the above findings, it was concluded that MLGS could upregulate the expression of GLUT-4 and PPAR-γ, and thereby increased the glucose uptake in vitro. Further, the glucose uptake activity of MLGS was completely abolished in the presence of CHX (1 μg/ml), which is a protein synthesis inhibitor, thus it was confirmed that glucose uptake activity of MLGS is mediating through enhanced expression of GLUT-4 and PPAR-γ. Furthermore, in another experimental set, MLGS has shown enhanced expression of adiponectin, leptin by 3.9-fold and 7.1-fold, respectively, compared to control [Figures 5a-c and 6a-c] Collectively, it can be concluded that MLGS shows hypoglycemic activity through enhanced expression of GLUT-4, and reverses insulin resistance by enhancing the expression of PPAR-γ, adiponectin, and leptin, respectively.
Figure 5.
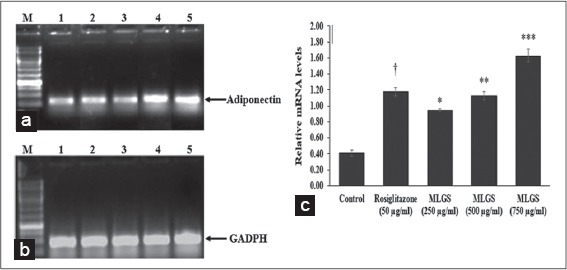
Effect of the methanolic leaf extract of Gymnema sylvestre (MLGS) on Adiponectin transcripts in 3T3 L1 cell lines. (a and b) - M: Ikbp marker, Lane 1: Control, Lane 2: 50 μg/ml rosiglitazone, Lane 3: 250 μg/ml MLGS, Lane 4: 500 μg/ml MLGS, Lane 5: 750 μg/ml MLGS, (c) The values are x ± standard error of mean (n = 3) of independent experiments, means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph Pad version 4.0. †P < 0.001 corresponds to rosiglitazone versus control; *P < 0.05, **P < 0.01, ***< 0.01 corresponds to MLGS versus control
Figure 6.
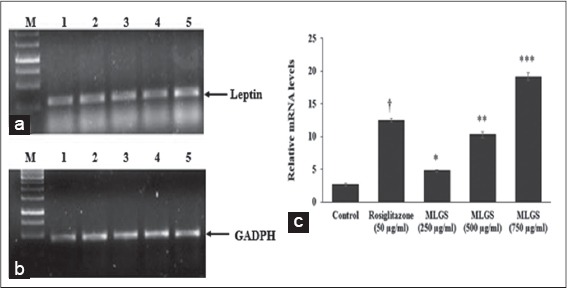
Effect of the methanolic leaf extract of Gymnema sylvestre (MLGS) on leptin transcripts in 3T3 L1 cell lines. (a and b) M: Ikbp marker, Lane 1: Control, Lane 2: 50 μg/ml rosiglitazone, Lane 3: 250 μg/ml MLGS, Lane 4: 500 μg/ml MLGS, Lane 5: 750 μg/ml MLGS, (c) The values are x ± standard error of mean (n = 3) of independent experiments, means of various groups were statistically compared by ANOVA followed by Tukey’s multiple comparison test using Graph Pad version 4.0. †P < 0.001 corresponds to rosiglitazone versus control; *P < 0.05, **P < 0.01, ***P < 0.01 corresponds to MLGS versus control
DISCUSSION
Diabetes is a metabolic disorder with varied etiology, many important biochemical entities involved in the development of abnormal GLUT. In this context, the markers such as GLUT-4, PPAR-γ, adiponectin, and leptin are well known to play a crucial role in insulin-dependent glucose transport [25]. The declined GLUT-4 translocation and defective insulin signaling cascade were demonstrated as one among the prevalent factors in type 2 diabetes and insulin resistance [25]. Thus, the agents that enhance the GLUT-4 expression could decrease the circulating blood glucose levels by enhancing cellular glucose uptake. In this context, L6 muscle cell line is a relevant in vitro model to study the GLUT activity; moreover, skeletal muscle is the main site for primary glucose clearance and glucose utilization [26]. Earlier reports of L6 myotubes manifested the maximum glucose uptake activity by troglitazone and rosiglitazone at 10 and 100 μM concentrations, respectively [10]. In addition, the enhanced glucose uptake in L6 cells is majorly intervened through increased GLUT-4 level [26,10]. In these lines, the findings in the present study are in compliance with the literature reports where there is a coincidental increase in glucose uptake along with enhanced GLUT-4 levels in L6 myotubes, the MLGS has shown increased glucose uptake, and facilitated the enhanced GLUT-4 expression. In literature, CHX was used as a protein synthesis inhibitor to completely abolish the troglitazone-mediated glucose uptake [26]. Similarly, in the present study, the glucose uptake activity of MLGS was completely blocked in the presence of CHX, which clearly confirmed the role of new protein synthesis compatible to GLUT.
Altogether, the above findings proved that glucose uptake is dependent on increased or decreased expression of GLUT-4, further enhanced glucose uptake in the present study was concomitant with the increased expression of GLUT-4 encoding mRNA, in L6 myotubes on incubation with MLGS [27].
Furthermore, PPAR-γ is a nuclear transcriptional factor known for its pivotal role in the insulin receptor signaling cascade in glucose uptake, also the levels of PPAR-γ is noted to be less in insulin resistance. In this context, the PPAR-γ agonists belong to the chemical class of thiazolidinedione’s (TDZs) (such as rosiglitazone and pioglitazone) play a major role in reversing the insulin resistance [28]. Further, PPAR-γ activation leads to enhanced glucose-stimulated insulin secretion and increased insulin sensitivity [29].
In continuation, adiponectin is an abundantly expressed adipokine that exerts a potent insulin-sensitizing effect through binding to its receptors AdipoR1 and AdipoR2, leading to activation of AMPK, PPAR-a, and presumably other yet-unknown signaling pathways. Thus, the serum levels of adiponectin have a positive correlation with insulin sensitivity [30]. Adiponectin is known to increase insulin sensitivity by three major mechanisms, First, in skeletal muscle adiponectin increases the expression of CD36 and acyl-coenzyme-A oxidase molecules involved in transport and combustion of FA, and also increases uncoupling protein 2 required during energy dissipation. These changes led to decreased triglyceride content in skeletal muscle, which contributes to improved insulin signaling transduction [17]. Furthermore, adiponectin stimulates phosphorylation of acetyl coenzyme-A carboxylase, increases fatty-acid combustion, glucose uptake, and lactate production in myocytes, through activation of AMPK. These changes reduce gluconeogenesis in the liver; thus, adiponectin shows acute glucose lowering effect [17]. Remarkably, the PPAR-γ agonist belongs to TZDs class are well known to raise the circulating adiponectin levels, this is well demonstrated by exposing 3T3-L1 cells to TZDs [31].
In line with the above reports, in the present study, the effect of MLGS on adiponectin levels was evaluated using 3T3-L1 cell lines, and the outcomes of the study showed a significant increase in expression of adiponectin in MLGS and rosiglitazone-treated groups.
In obesity-linked insulin resistance, both adiponectin and adiponectin receptors are downregulated. Apart from adiponectin, Leptin is another very important adipocytokine majorly secreted by adipocytes, the net action of leptin is to inhibit appetite, stimulate thermogenesis, decrease blood glucose, and reduce body weight and fat. Leptin also directly affects glucose metabolism in the liver, by inhibiting gluconeogenesis mediated through phosphatidylinositol 3-kinase (PI3K)-dependent activation of phosphodiesterase 3B [32], interferes with lipid metabolism mediated through activation of PI3K [33]. Further, many studies have confirmed a complex interaction between leptin and insulin-signaling pathways on glucose metabolism. The observations from in vitro and in vivo studies suggest that leptin promotes energy dissipation and decreases lipid deposition in adipose tissues [33].
Leptin receptor-mediated JAK–STAT signaling is essential for regulation of food intake and body weight. It also limits the accumulation of triglycerides in the liver and skeletal muscle through a combination of direct activation of AMPK and indirect actions mediated through central neural pathways, thereby improving insulin sensitivity [34]. In addition, leptin regulates pancreatic β-cells function through direct actions [35] and indirectly through central neural pathways [36].
On the whole, leptin enhances peripheral (hepatic and skeletal muscle) insulin sensitivity and modulates pancreatic β cell function. Leptin resistance/deficiency leads to reduced leptin-mediated JAK–STAT signaling and upregulation of suppressor of cytokine signaling-3 (SOC-3). In contrary, expression of leptin is directly related to insulin sensitization and activation of JAK-STAT signaling thereby reducing SOC-3. Altogether, expression of leptin has profound positive relation with the glucose metabolism and insulin signaling.
In this regard, in the present study, the effect of MLGS was examined on the expression of leptin in 3T3-L1 cells in vitro using rosiglitazone as the reference standard. A significant increase in leptin levels was observed in MLGS and rosiglitazone-treated groups. These findings observed are in constituent with the GLUT-4, PPAR-γ, adiponectin and leptin gene expressions to link with the hypoglycemic and antidiabetic activity of MLGS.
CONCLUSION
The role of GLUT-4, PPAR-γ, adiponectin, and leptin in insulin dependent glucose transport and insulin resistance is well understood with available scientific literature, and thus, these factors have a key role in etiology of diabetes and related complications.
In the present study, an attempt was made to investigate the mechanism involved in hypoglycemic and antidiabetic activity of methanolic leaf extract of G. sylvestre.
The findings of the study showed that MLGS could dose-dependently enhance the expression of GLUT-4, PPAR-γ, adiponectin, and leptin, and thereby shows potential antidiabetic activity. Interestingly, the actives responsible for hypoglycemic and antidiabetic activity of MLGS needs to be isolated, in this regard, further studies are in the pipeline to identify and isolate the phytoconstituent responsible for antidiabetic activity of MLGS.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mohan CG, Viswanatha GL, Savinay G, Rajendra CE, Halemani PD. 1,2,3,4,6 Penta-O-galloyl-ß-d-glucose, a bioactivity guided isolated compound from Mangifera indica inhibits 11ß-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine. 2013;20:417–26. doi: 10.1016/j.phymed.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 3.Baskaran K, Kizar Ahamath B, Radha Shanmugasundaram K, Shanmugasundaram ER. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J Ethnopharmacol. 1990;30:295–300. doi: 10.1016/0378-8741(90)90108-6. [DOI] [PubMed] [Google Scholar]
- 4.Prakash AO, Mathur S, Mathur R. Effect of feeding Gymnema sylvestre leaves on blood glucose in beryllium nitrate treated rats. J Ethnopharmacol. 1986;18:143–6. doi: 10.1016/0378-8741(86)90026-7. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugasundaram ER, Gopinath KL, Radha Shanmugasundaram K, Rajendran VM. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–79. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugasundaram KR, Panneerselvam C, Samudram P, Shanmugasundaram ER. Enzyme changes and glucose utilisation in diabetic rabbits: The effect of Gymnema sylvestre R.Br. J Ethnopharmacol. 1983;7:205–34. doi: 10.1016/0378-8741(83)90021-1. [DOI] [PubMed] [Google Scholar]
- 7.Ziel FH, Venkatesan N, Davidson MB. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988;37:885–90. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]
- 8.Fukumoto H, Kayano T, Buse JB, Edwards Y, Pilch PF, Bell GI, et al. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989;264:7776–9. [PubMed] [Google Scholar]
- 9.Kellerer M, Lammers R, Häring HU. Insulin signal transduction: Possible mechanisms for insulin resistance. Exp Clin Endocrinol Diabetes. 1999;107:97–106. doi: 10.1055/s-0029-1212082. [DOI] [PubMed] [Google Scholar]
- 10.Ciaraldi TP, Huber-Knudsen K, Hickman M, Olefsky JM. Regulation of glucose transport in cultured muscle cells by novel hypoglycemic agents. Metabolism. 1995;44:976–81. doi: 10.1016/0026-0495(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 11.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 13.Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–6. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- 14.Sethi JK, Vidal-Puig AJ. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 18.Flier JS. Obesity wars: Molecular progress confronts an expanding epidemic. Cell. 2004;116:337–50. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi IS, Matarese G, Lord GM. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar PM, Venkataranganna MV, Manjunath K, Viswanatha GL, Ashok G. Methanolic extract of Momordica cymbalaria enhances glucose uptake in L6 myotubes in vitro by up-regulating PPAR-γ and GLUT-4. Chin J Nat Med. 2014;12:895–900. doi: 10.1016/S1875-5364(14)60132-1. [DOI] [PubMed] [Google Scholar]
- 22.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Isoproterenol inhibits resistin gene expression through a G(S)-protein-coupled pathway in 3T3-L1 adipocytes. FEBS Lett. 2001;500:60–3. doi: 10.1016/s0014-5793(01)02588-1. [DOI] [PubMed] [Google Scholar]
- 23.Hall LR, Mehlotra RK, Higgins AW, Haxhiu MA, Pearlman E. An essential role for interleukin-5 and eosinophils in helminth-induced airway hyperresponsiveness. Infect Immun. 1998;66:4425–30. doi: 10.1128/iai.66.9.4425-4430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yonemitsu S, Nishimura H, Shintani M. Troglitazone induces GLUT-4 translocation in L6 myotubes. Diabetes. 2001;50:1093–101. doi: 10.2337/diabetes.50.5.1093. [DOI] [PubMed] [Google Scholar]
- 25.Laville M, Auboeuf D, Khalfallah Y, Vega N, Riou JP, Vidal H. Acute regulation by insulin of phosphatidylinositol-3-kinase, Rad, Glut 4, and lipoprotein lipase mRNA levels in human muscle. J Clin Invest. 1996;98:43–9. doi: 10.1172/JCI118775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koivisto UM, Martinez-Valdez H, Bilan PJ, Burdett E, Ramlal T, Klip A. Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem. 1991;266:2615–21. [PubMed] [Google Scholar]
- 27.Garvey WT, Maianu L, Zhu JH, Hancock JA, Golichowski AM. Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes Heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes. 1993;42:1773–85. doi: 10.2337/diab.42.12.1773. [DOI] [PubMed] [Google Scholar]
- 28.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: A potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 29.Maryam A, Jae MS, Nasun H, Christopher L, Annette RA, Michael D, et al. PPARg signaling and metabolism: The good, the bad and the future. Nat Med. 2013;99:557–66. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotta K, Funahashi T, Arita Y. Plasma concentrations of a novel, adipo sespecific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 31.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–9. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 32.Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, et al. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–54. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]
- 33.Huang W, Dedousis N, Bhatt BA, O'Doherty RM. Impaired activation of phosphatidylinositol 3-kinase by leptin is a novel mechanism of hepatic leptin resistance in diet-induced obesity. J Biol Chem. 2004;279:21695–700. doi: 10.1074/jbc.M401546200. [DOI] [PubMed] [Google Scholar]
- 34.Kondo H, Shimomura I, Matsukawa Y, Kumada M, Takahashi M, Matsuda M, et al. Association of adiponectin mutation with type 2 diabetes: A candidate gene for the insulin resistance syndrome. Diabetes. 2002;51:2325–8. doi: 10.2337/diabetes.51.7.2325. [DOI] [PubMed] [Google Scholar]
- 35.Karpichev IV, Cornivelli L, Small GM. Multiple regulatory roles of a novel Saccharomyces cerevisiae protein, encoded by YOL002c, in lipid and phosphate metabolism. J Biol Chem. 2002;277:12152–62. doi: 10.1074/jbc.M202045200. [DOI] [PubMed] [Google Scholar]
- 36.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic ßcells. Biochem Biophys Res Commun. 2003;312:1118–22. doi: 10.1016/j.bbrc.2003.11.042. [DOI] [PubMed] [Google Scholar]


