Abstract
Background:
Currently available therapeutic options for thromboembolic disorders are often very expensive and are associated with unfavorable side effects.
Aim:
To establish the anticoagulant effect and safety profile of an extract made from of the root bark of Pseudocedrela kotschyi (Schweinf.) Harms and the aerial part of Adenia cissampeloides (Planch. ex Hook.) Harms (PAE).
Materials and Methods:
PAE (0.5-2.0 g/L) effect on prothrombin time (PT) and activated partial thromboplastin time (aPTT) were evaluated on whole blood drawn from the marginal ear vein of New Zealand White rabbits. Effect of PAE (250-2000 mg/kg) on bleeding time (BT) and clotting time (CT) in Sprague-Dawley rats were also assessed. Histopathological, hematological, and liver function studies were also carried out to assess the safety for use of PAE (250-2000 mg/kg).
Results:
PAE had no significant effect (P > 0.05) on PT, but resulted in a significant increase (P ≤ 0.05-0.0001) in aPTT. The PAE treatment resulted in a significant increase (P ≤ 0.05-0.0001) in BT and CT in vivo compared with control. Safety studies indicated no deaths with PAE treatment with hematological and liver function tests being normal. Histological studies revealed pathological changes in the liver at a PAE treatment dose of 2000 mg/kg but all doses had no detrimental effect on kidney and stomach tissue. The no-observed-adverse-effect-level was <2000 mg/kg when given orally.
Conclusion:
PAE has anticoagulant effect in vitro and is safe to use at oral doses <2000 mg/kg.
Keywords: Activated partial thromboplastin time, Adenia cissampeloides, bleeding time, clotting time, prothrombin time, Pseudocedrela kotschyi
INTRODUCTION
Thromboembolic disorders have become a leading cause of morbidity and mortality and an important challenge confronting physicians and surgeons, the world over [1]. The cause of thromboembolism is usually unknown but is by convention attributed to Virchow’s triad: Hemodynamic changes (stasis and turbulence), hypercoagulability, and endothelial injury [2]. Despite it being a preventable condition, thromboembolism remains a leading cause of morbidity and mortality globally, and Ghana is no exception. Deep vein thrombosis (DVT) and pulmonary embolism (PE) occur in 1 in 1000 patients annually in adult patients with the frequency of occurrence increasing with old age [3]. About 6% and 10% of patients die within a month following an episode of DVT and PE, respectively [4].
The impact of DVT and PE goes beyond the increased risk of mortality. For patients who survive these conditions, they sometimes will have to live with the dilapidating effect, e.g., loss or impairment of bodily function due to stroke, and post-thrombotic syndromes such as ulcers due to months of being bed ridden and impaired blood circulation resulting in gangrene [5], just to mention a few.
Anticoagulants remain the mainstay in the pharmacological management of thromboembolic disorder and have proven over the years to be effective and safe [6]. Despite the effectiveness of conventional pharmacological agents in the management of thromboembolic disorders, they are often associated with unfavorable side effects such as increased risk of bleeding and gastric ulceration and also prohibitive high treatment cost [7].
With the high cost and unfavorable side effects of orthodox medicines, there is the need to search for alternative therapeutic agents and the plant kingdom continues to show huge promise.
Pseudocedrela kotschyi (Schweinf.) Harms of the family Meliaceae, and Adenia cissampeloides (Planch. ex Hook.) Harms of the family Passifloraceae have found numerous uses in traditional medicine [8-11], e.g., P. kotschyi traditionally has been used in the management of several diseases and ailments. Decoctions and macerations of the stem and root bark have found use in the management of ulcers, sores, rheumatism, leprosy, syphilis and gingivitis [12]. Crushed leafy twigs and leaf decoctions have also been employed in the management of edema, rash and compound fractures with young stems and roots used as chewing stick to keep the teeth healthy. Research into the medicinal properties of P. kotschyi such as antimicrobial, antiprotozoal, and antidiabetic effect of P. kotschyi and its constituents have shed more light and sought to justify the folkloric medicinal use of the plant [13-16].
Traditionally, A. cissampeloides has found several uses in folkloric medicinal practice in infusions and decoctions of the stem, leaves or root have been employed in the management of gastrointestinal disorders such as, constipation, stomach ache, diarrhea, and dysentery. Such infusions have also been used in the management of rheumatic pain, numbness, malaria, wound dressing and worm infestations [17]. Studies that have been carried out on the medicinal properties of A. cissampeloides include: the antiplasmodial activity, antimicrobial properties and effect on blood pressure and serum analytes [18].
In traditional medicinal practice in Ghana, mixtures of extracts of the root bark of P. kotschyi and the aerial part of A. cissampeloides are currently employed in the management of circulatory disorders such as numbness.
This current study, therefore, sought to investigate the anticoagulant properties and safety profile of an extract made from the root bark of P. kotschyi and the aerial part of A. cissampeloides using in vitro and in vivo experimental protocols.
MATERIALS AND METHODS
Plant Collection and Authentication
A. cissampeloides and P. kotschyi [Figure 1a and b] were collected from Tetrem near Offinso and Ayigya near KNUST, respectively; in the Ashanti Region of Ghana in February 2015. Plant materials were verified at the Department of Herbal Medicine, KNUST, where voucher specimen (numbers: KNUST/HM1/2013/S043 and KNUST/HM1/2013/S048) have been kept.
Figure 1.

(a) The root bark of Pseudocedrela kotschyi and (b) the aerial part of Adenia cissampeloides
Preparation of P. kotschyi and A. cissampeloides extract (PAE)
PAE was prepared by boiling 1.5 kg of root bark of P. kotschyi and 1.0 kg of the aerial part of A. cissampeloides in 10 L of distilled water. The mixture was boiled for 45 min and cooled. The supernatant solution was decanted and dried, using a freeze drier (YK-118 Vacuum Freeze Drier, True Ten Industrial Company, Taiwan), to obtain solid pellets (percentage yield = 0.45%). The solid pellets were weighed and labeled PAE. Concentrations of PAE were prepared by weighing the required amount of pellets on an electronic balance (Sartorious AG, Germany) and dissolving the pellets in the required amount of distilled water.
Drugs and Chemicals
The drugs and chemicals used in this study included: Heparin sodium (Rotexmedica, Germany), soluble aspirin tablets 75 mg (Aspar Pharmaceuticals, London), rivaroxaban tablet 10 mg (Bayer, Germany), and dalteparin sodium (Pharmacia, USA).
Qualitative Phytochemical Analysis
The standard method for qualitative phytochemical screening described by Sofowora (1982) [19] was employed.
Animals
Adult New-Zealand White rabbits (weight: 1-2.6 kg), and Sprague-Dawley rats (weighing 225-300 g) were used in this study. Animals were housed in roomy cages with ambient day/night cycle, and room temperature (25±3°C). Animals were fed normal pellet chow and tap water for drinking water. They were allowed to acclimatize to the laboratory environment before use in experiments. All animals were kept according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH, Department of Health and Human Services publication no. 85-23, revised 1985) and was approved by the Departmental Ethics Committee (Committee on Animal Research, Publication and Ethics (CARPE); Ethics Reference No: FPPS/PCOL/0006/2013).
In vitro Clotting Profile
Briefly, 3.5 ml quantities of whole blood, drawn from the marginal ear vein of rabbits were added to 0.2 ml of 0.5, 1.0, 1.5 or 2.0 g/L PAE in sodium citrate tubes and analyzed by a semi-automated coagulation analyzer, to determine the effect of the extract on prothrombin time (PT) and activated partial thromboplastin time (aPTT). The tests were repeated using 0.2 ml of distilled water (negative control), reference standards rivaroxaban (0.1, and 0.01 mg/ml), aspirin (0.75, and 0.075 mg/ml), heparin (5, and 50 IU/L) and dalteparin (5, and 50 IU/L).
Effect of PAE on PT
Pre-warmed platelet poor plasma (prepared by centrifuging test samples at 1500 g for 15 minutes; 37°C), was added to thromboplastin (tissue factor)-calcium reagent which activates the coagulation cascade at Factor VII. The time taken for clot formation (PT) was determined using a semi-automated coagulation analyzer (CoaDATA 504, Labitec, Germany).
Effect of PAE on aPTT
Platelet-poor plasma (prepared by centrifuging test samples at 1500 g for 15 min) was added to an equal volume of partial thromboplastin reagent (phospholipid plus contact activator, e.g., Silica) and warmed to 37°C for an exact incubation time. Pre-warmed (37°C) calcium chloride reagent (0.025 M) was added to this mixture to activate the coagulation cascade. Time required for clot formation (aPTT) was determined using a semi-automated analyzer.
Bleeding Time (BT) Determination
The BT determination described by Gadi et al., (2009) [20] was employed with slight modifications. Briefly, Sprague-Dawley rats (225-300 g) were divided into four groups of 5 animals each and labeled groups A to F. Groups A, B, C, and D were treated orally with 250, 500, 1000 or 2000 mg/kg daily dose of PAE by gastric lavage, respectively. Groups E and F were given orally 75 mg/kg acetylsalicylic acid (ASA) and 2 ml/kg distilled water as positive and negative controls respectively. Rats were slightly anesthetized, kept in a restrainer and tails disinfected. Very small pieces of (1-2 mm) from the tip of the tail was SNIPPED off by a pair of sterilized scissors and blotted with filter paper (at 15 s intervals) until no blood flow was observed. The period between the tail snip and the cessation of bleeding was taken as the BT (in seconds).
Determination of Clotting Time (CT)
Sprague-Dawley rats (225-300 g) were divided into four groups of 5 animals each and labeled Groups A to F. Groups A, B, C, and D were treated orally with PAE dose of 250, 500, 1000 and 2000 mg/kg daily, respectively. Groups E and F were treated with acetylsalicylic acid 75 mg/kg and 2 ml/kg distilled water orally, serving as positive and negative controls, respectively. Tails of rats were disinfected and a snip taken off the tail at 1-2 mm from the tip. A drop of blood from the tail was placed in the middle of a clean clear glass slide (CAT.No.7102, Meno Int., Dunstable, England) and the timer started. A clean hypodermic needle was passed through the drop of blood carefully every 30 s. This was repeated carefully observing for the formation of the first fibrin strand. The time that elapsed for the formation of the first fibrin strand was taken as the CT.
Safety Studies
The rats were grouped into four (n = 6) and given PAE at doses of 250, 500, 1000 or 2000 mg/kg daily by gastric gavage, for a period of 2-weeks. The animals were observed continuously during the first hour, and then every hour for 6 h, then after 12 and 24 h, and finally after every 24 h, up to 2 weeks, for any physical signs of toxicity such as writhing, gasping, bleeding, palpitation and respiratory rate, or mortality. A sample of blood was obtained for liver function test and hematological assessment. Histopathological studies on the liver, kidney and stomach were carried out.
Hematological Profile
Rats were sacrificed by decapitation, blood samples collected into MediPlus vacutainer K3 EDTA tubes (Sunphoria Co. Ltd., Taiwan) and analyzed using a hemato-analyzer (Sysmex XP – 300TM Automated Hematology Analyzer, USA) for the hematological profile.
Liver Function Test
Rats from the various groups were sacrificed by decapitation at the end of the treatment period and blood samples collected into serum separating gel (BD Vacutainer® Blood Collection Tube Product, USA). This was allowed to stand in test tube rack for 30 min for the blood to clot and then centrifuged at 1800 rpm for 10 min to obtain the serum. Serum was then analyzed using a clinical chemistry analyzer (Vital Scientific N. V, Netherlands) to determine levels of liver enzymes, i.e., alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and total protein.
Histopathological Studies
At the end of the safety study, liver, kidney, and stomach from each sacrificed rat were dissected out and cleared of excess fat. Isolated organs were then fixed in 10% buffered formalin and processed for paraffin sectioning by dehydration in varying concentrations of alcohol, cleared with xylene and embedded in paraffin blocks. Sections of about 5 μm in thickness were stained with hematoxylin and eosin (H&E) for histological study.
Statistical Analysis
Data were expressed as a mean ± standard error of mean. Statistical analyses were performed using both one- and two-way Analysis of Variance (ANOVA) followed by Sidak post-hoc test with confidence interval of 95%.
RESULTS
Qualitative Phytochemical Analysis
The phytochemical analysis confirmed the presence of flavonoids, alkaloids, saponins, condensed tannins, phytosetrols, triterpenoids, and general glycosides.
In vitro clotting Profile
PT
Baseline PT was 10.51 ± 0.13 s. There was no significant difference in PT between treatment groups, i.e., aspirin, heparin, dalteparin and PAE-treated groups compared to control. However, rivaroxaban (0.1 mg/ml) showed a significant increase (P ≤ 0.0001) in PT as compared with control [Figure 2].
Figure 2.
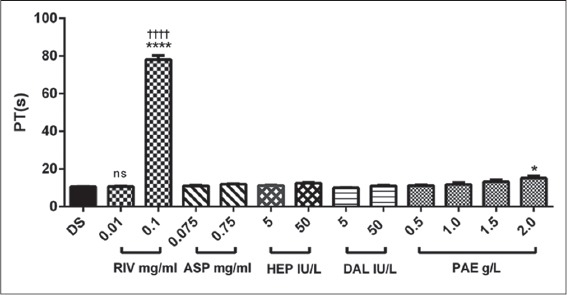
The effect of rivaroxaban, aspirin, heparin, dalteparin and PAE on prothrombin time. Values plotted are means ± standard error of the mean; (n = 5). ****P ≤ 0.0001; *P ≤ 0.05; ns P > 0.05 compared to control (One-way ANOVA followed by Sidak’s post hoc test). ††††P ≤ 0.0001 comparison between two doses of same treatment
aPTT
Positive controls (rivaroxaban 0.01 mg/ml, aspirin 0.075 mg/ml, heparin 5 IU/L and dalteparin 5 IU/L) and PAE (0.5 g/L) showed no significant difference in aPTT compared with control, baseline aPTT was 85.91 ± 1.25 s. There was, however, an increase in aPTT resulting in a significant difference compared with control at treatment doses of 0.1 mg/ml rivaroxaban, 0.75 mg/ml aspirin, 50 IU/l heparin and 50 IU/L dalteparin. PAE at (1.0, 1.5 and 2.0 g/L) also showed a significant increase (P ≤ 0.05 - 0.0001, respectively) compared with control [Figure 3].
Figure 3.
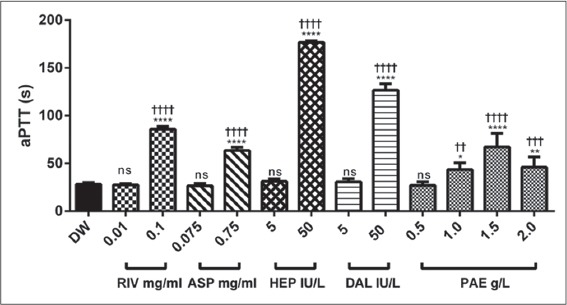
The effect of rivaroxaban, aspirin, heparin, dalteparin and PAE on activated partial thromboplastin time (aPTT). Values plotted are means ± standard error of the mean; (n = 5), ****P ≤ 0.0001; **P ≤ 0.01; *P ≤ 0.05; ns P > 0.05 compared to control (One-way ANOVA followed by Sidak’s post hoc test). ††††P ≤ 0.0001; †††P = 0.0001; ††P ≤ 0.01 comparison between the two doses of same treatment
Bleeding and CT
PAE treatment (250, 500, 1000 and 2000 mg/kg) showed a dose-dependent increase (P ≤ 0.0001) in bleeding and CT compared to the control. There were dose-dependent significant increases (P ≤ 0.0001) in bleeding and CT between PAE and aspirin treatments [Figures 4 and 5]. There was a significant increase (P ≤ 0.0001) in CT as compared with BT in both aspirin and PAE treated groups. This difference between bleeding and CT was significantly higher (P ≤ 0.0001) in the treatment groups compared with the untreated group (control) [Figure 6].
Figure 4.
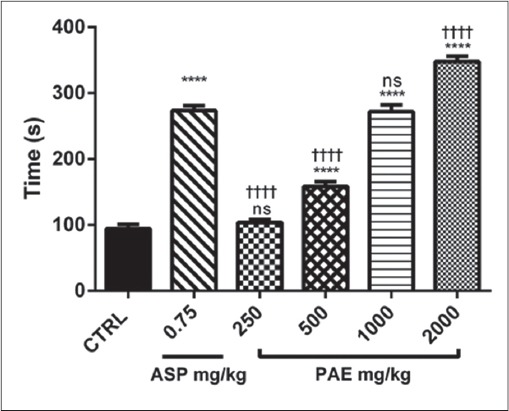
The effect of PAE and Aspirin on bleeding time in Sprague-Dawley rats. Values plotted are means ± standard error of the mean; (n = 5). ****P ≤ 0.0001; ns P > 0.05 compared to control (One-way ANOVA followed by Sidak’s post-hoc test). ††††P ≤ 0.0001; ns P > 0.05 comparison between aspirin and the various doses of Pseudocedrela kotschyi and Adenia cissampeloides extract
Figure 5.
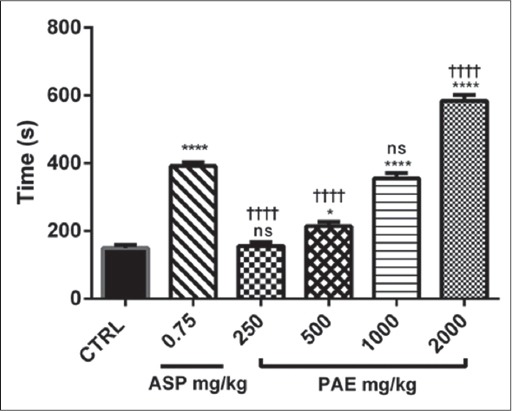
The effect of Pseudocedrela kotschyi and Adenia cissampeloides extract and aspirin on clotting time in Sprague-Dawley rats. Values plotted are means ± standard error of the mean; (n = 5). ****P ≤ 0.0001; *P ≤ 0.05; ns P > 0.05 compared to control. (One-way ANOVA followed by Sidak’s post hoc test). ††††P ≤ 0.0001; ns P > 0.05 comparison between aspirin and the various doses of Pseudocedrela kotschyi and Adenia cissampeloides extract
Figure 6.
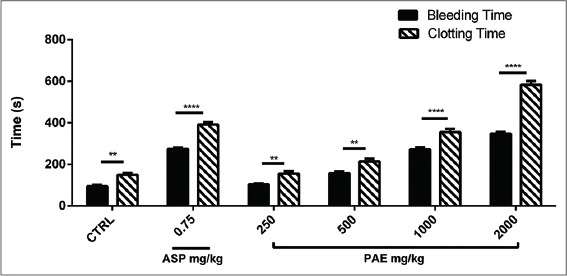
The effect of Pseudocedrela kotschyi and Adenia cissampeloides extract and Aspirin on Bleeding and clotting time in Sprague-Dawley rats. Values plotted are means ± standard error of the mean; (n = 5). ****P ≤ 0.0001; **P ≤ 0.01 compared to control (Two-way ANOVA followed by Sidak’s post hoc test)
Safety Studies
PAE was devoid of any toxic effects in rats in doses up to 2000 mg/kg when given orally; no deaths were also recorded in any of the treatment groups and control group. Cage-side observations also revealed no change in grooming habits, consistency of stool and respiratory rate in the treatment groups compared with the control. Locomotor and lachrymatory activities appeared normal with the absence of piloerection in both control and treatment groups.
Liver Function Test
Liver function tests appeared normal with ALT being slightly elevated in the PAE-treated groups compared to control. There was a dose-dependent increase in ALT from 250 mg/kg to 1000 mg/kg of PAE. However at 2000 mg/kg of PAE, ALT levels dropped slightly but was still higher compared to the control [Table 1].
Table 1.
Effect of a 14-day PAE treatment on serum liver enzymes in Sprague-Dawley rats
Hematological Profile
Hematological analysis of PAE-treated groups appeared normal compared with control except for platelet counts which were slightly higher in the PAE-treated groups (500, 1000, 2000 mg/kg) compared with the control [Table 2].
Table 2.
Effect of a 14-day PAE treatment on hematological profile on Sprague-Dawley rats
Histopathological Studies
The hepatic tissue of control group revealed normal architecture showing normal hepatocytes with nuclei, central and portal veins. Sinusoids appeared to radiate in a divergent fashion from a focal point towards the peripheries [Figure 7]. Treatment with 250 mg/kg PAE revealed no difference in the structural integrity of hepatic tissue compared with control [Figure 7]. PAE treatment at 500, 1000, and 2000 mg/kg also showed normal central and portal veins, hepatocytes, and nuclei; however, there was sinusoid dilatation, from minor to major, compared with the control. Portal veins also appeared dilated with 2000 mg/kg PAE treatment [Figure 7]. Renal tissue in control animals revealed normal tissue architecture, with normal sized glomerulus, urinary space, distal and proximal tubules with nuclei and Bowman’s capsule [Figure 8]. PAE treatments at 250, 500, 1000, and 2000 mg/kg showed no significant change in renal tissue architecture compared with the control [Figure 8]. The stomach also showed normal tissue architecture with normal gastric cells, mucosa and sub mucosa in all PAE treatments [Figure 9] compared with control [Figure 9]. Gastric epithelial cells appeared normal with no signs of erosion or tissue destruction and evenly spaced gastric pits.
Figure 7.
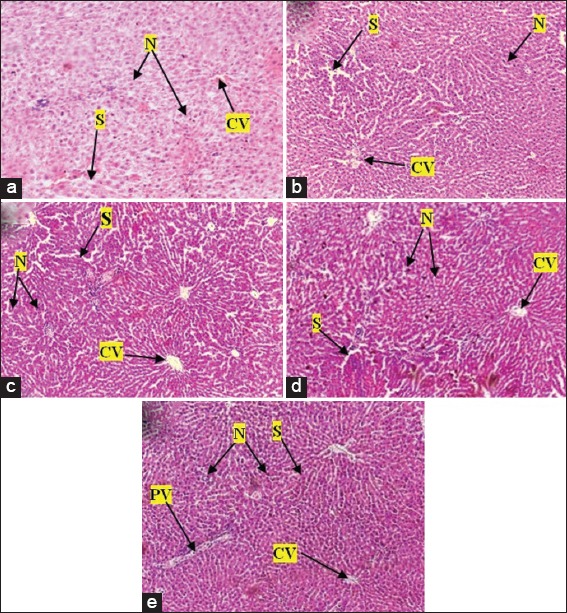
Photomicrographs of the liver of Sprague-Dawley rats after treatment with various doses of Pseudocedrela kotschyi and Adenia cissampeloides extract (a) control group showed normal hepatic architecture and hepatocytes, Nuclei (N), sinusoids (S) and central vein (CV), (b) 250 mg/kg PAE treatment showed hepatic tissue with normal hepatocytes, nuclei, sinusoids and central vein; (c) 500 mg/kg PAE treatment showed normal hepatocytes with nuclei, central vein and portal vein with slight dilatation of sinusoids (d) 1000 mg/kg PAE treatment showed normal hepatocytes with nuclei, central vein and portal vein. Sinusoids appear slightly dilated; (e) 2000 mg/kg PAE treatment showed normal hepatocytes with nuclei, central veins. Portal veins (PV) and sinusoids appear slightly dilated. H and E staining, Objective magnification: ×10
Figure 8.
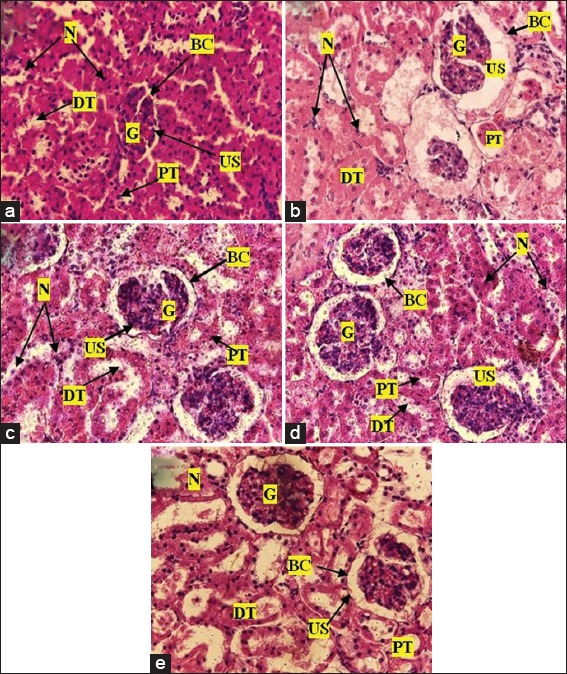
Photomicrographs of the kidney of Sprague-Dawley rats after treatment with various doses of Pseudocedrela kotschyi and Adenia cissampeloides extract: (a) the control showed normal appearance of glomerular (G), urinary space (US), Bowman’s capsule (BC), proximal tubule (PT), distal tubules (DT) with their nuclei (N); (b) 250 mg/kg PAE treatment showed normal sized glomerulus, nuclei, Bowman’s capsule, distal and proximal tubules. (c) 500 mg/kg PAE treatment showed normal renal architecture with normal cell distribution and cellular integrity; (d) 1000 mg/kg PAE treatment showed normal renal architecture i.e. renal corpuscles and renal tubules. Urinary space showing deposit of particles; (e) 2000 mg/kg PAE treatment, showed normal sized glomerulus, nuclei and renal tubules with little or no infiltration and deposits. H and E staining, Objective magnification: ×40
Figure 9.
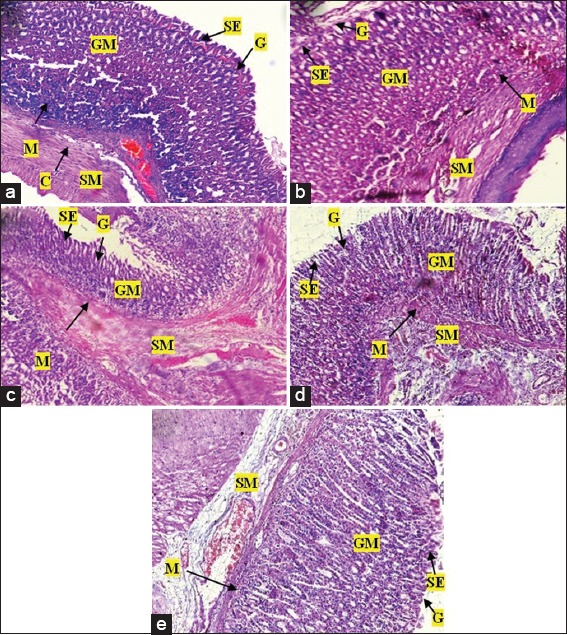
Photomicrographs of the stomach of Sprague-Dawley rats after treatment with various doses of Pseudocedrela kotschyi and Adenia cissampeloides extract. (a) Control showed normal architecture, intact gastric mucosa (GM), sub mucosa (SM), gastric pits (G) and surface epithelium (SE), and collagen fibres (C); (b) 250 mg/kg PAE treatment showed normal stomach architecture with no signs of erosion of epithelium or changes in gastric pits, intact mucosa and sub mucosa; (c) 500 mg/kg PAE treatment revealing normal stomach architecture with even distribution of cells and gastric pits. Intact epithelium, mucosa and sub mucosa; (d) 1000 mg/kg PAE treatment showed normal architecture with no superficial erosion. Gastric pits appeared normal with no unusual increase in depth or spacing, (e) 2000 mg/kg PAE treatment showed normal architecture with even distribution of cellular components and gastric pits, no visible signs of erosion of epithelium or changes in mucosa and sub mucosa. H and E staining, Objective magnification: ×10
DISCUSSION
Disturbances in the coagulation cascade often result in an increased rate of thrombus formation, i.e. venous or arterial thrombus and this has been associated with many cardiovascular diseases [21]. The anticoagulant effect of PAE was therefore evaluated by assessing its effect on PT, aPTT, BT, and CT. The safety profile of PAE was also assessed.
In the in vitro analysis PAE, heparin, aspirin, and dalteparin treatment did not have any significant effect on PT. However, rivaroxaban at 0.1 mg/ml and PAE treatment at 2.0 g/L resulted in an increase in the PT. PT, a measure of how long it takes the blood to clot, is an effective screening method for assessing the activity of the extrinsic coagulation pathway [22]. Rivaroxaban, a direct Factor Xa inhibitor [23], interferes with both the intrinsic and extrinsic pathway of the coagulation cascade (which could be as a result of direct inhibition or interference in synthesis of one or more clotting factors, i.e. factors I, II, V, VII, or X) thereby inhibiting the formation of thrombin and further development of thrombi [24].
Although lower concentrations of rivaroxaban, aspirin, heparin, dalteparin, and PAE did not show any significant effect on aPTT, higher concentrations increased aPTT significantly. aPTT is an important parameter in coagulation studies. Thromboplastin also known as thrombokinase is a plasma protein that affects blood coagulation by catalyzing the conversion of prothrombin to thrombin [25]. It is a combination of both phospholipids and tissue factor, both needed for the activation of the extrinsic pathway. The aPTT measures the activity of both the intrinsic pathway and the common pathways of the coagulation cascade by measuring the time taken for the formation of a fibrin clot [26]. It is often used to evaluate clotting abnormalities and as a monitoring parameter for the effect of anticoagulant treatments. The test is termed partial due to the absence of tissue factor in the reaction mixture.
The effects of PAE on PT and aPTT were similar to that observed for unfractionated heparin and the low-molecular-weight-heparin dalteparin. A prolonged aPTT with minimal effect on PT realized with PAE treatment, could be as result of PAE binding to the enzyme inhibitor antithrombin III (AT III) as seen with heparin. PAE binding to AT III could result in AT III activation thereby inactivating thrombin and Factor Xa; the end result being interference in the clotting ability of blood [27].
On safety, PAE was found not to be toxic in doses up to 2000 mg/kg when given orally with cage-side observations revealing no significant change in grooming habits and respiratory rate. No deaths were recorded in any of the groups. Acute toxicity is often depicted by reduced or increased activity, changes in grooming habits such as licking of fur or fur becoming unkempt and increased or slowed respiratory rate [28]. Such changes are often as a result toxic effects on normal physiological and biochemical processes, e.g., toxic effects on the parasympathetic system could be depicted by increased salivation and lacrimation symptoms [29]. The absence of these signs of toxicity in the safety model could mean the no-observed-adverse-effect-level of PAE is above 2000 mg/kg.
The liver function test results indicated a non-significant increase in ALT with 2000 mg/kg PAE treatment. Liver damage or injury is often characterized by an elevation of liver enzymes mainly ALT and AST [30]. ALT unlike AST, which is found in other organs like the heart and kidney, is mostly found in the liver and a good parameter for assessing liver injury [31]. PAE can, therefore, be said to be safe at lower doses of up to 2000 mg/kg. A. cissampeloides has been shown to contain substances that are hepatotoxic in vivo and excessive intake of it has been associated with liver complaints amongst the Zulu people in South Africa [32].
Hematological studies revealed a slight elevation of blood platelets albeit insignificant. An increase in platelet count after PAE treatment can be as a result of secondary thrombocytosis, which rarely results in an increased risk of thrombosis [33].
Histopathological studies of the liver, PAE treated groups at 2000 mg/kg showed slight dilatation of hepatic sinusoids and portal veins. Structural changes in the liver could be as a result of assault on the liver or inflammatory response. This could also account for the slight elevation in the ALT levels in PAE treated groups albeit insignificant. Histology of stomach tissue architecture also appeared normal with no visible signs of erosion of epithelium or disruption in the structural integrity of mucosa and submucosa. Use of conventional antithrombotic agents such as aspirin in thrombosis at risk populations has been associated with side effects such as gastric ulceration and bleeding. This has always proven a challenge of balancing benefit versus risk ratios in this category of patients. Gastric ulcerations are often depicted by erosion of the epithelium exposing the mucosa and sub-mucosa layers [34]. This was however notably absent after PAE treatment, and this could prove a significant advantage of PAE over conventional oral antithrombotic agents such as aspirin.
PAE treatment resulted in an increase in both bleeding and CTs compared with Aspirin. BT measures the time required for a clot to form and stop bleeding after a standardized skin incision, whilst CT, on the other hand, is the time required for a sample of blood to clot in vitro under standard conditions. BT albeit an old analytical parameter is one of the most important tests in coagulation studies and evaluates platelet function whilst CT gives an insight into the functioning or otherwise of the clotting cascade. Aspirin, an antiplatelet aggregating drug, irreversibly block the production of thromboxane A2 in platelets thereby exhibiting an inhibitory effect to platelet aggregation [35]. Aspirin’s antithrombotic action through its inhibitory effect on platelet aggregation is thought to be due to acetylation of platelet cyclooxygenase at the active site of the amino acid serine 529, thereby irreversibly inhibiting thromboxane A2 formation [36]. The significant increase in clotting and BT s caused by PAE treatment could, therefore, be as a result of PAE interfering with the clotting cascade by inhibiting the synthesis or function of clotting factors and also by inhibiting platelet aggregation thereby interfering with platelet plug formation (primary hemostasis), respectively. This effect could prove much beneficial in the management of thromboembolic disorders with reduced risk of bleeding.
Phytochemical screening of the aerial part of A. cissampeloides has confirmed the presence of tannins, saponins, flavonoids, phlobatannins, terpenoids, steroids, alkaloids, carbohydrates and glycosides. P. kotschyi root bark, on the other hand, has been found to contain tannins, coumarins, saponins and alkaloids. Several research has also focused on the possible effect of flavonoids on the circulatory system with a focus on the coagulation cascade and other factors affecting the process of hemostasis such as endothelial function and platelet aggregation [37]. The antiplatelet action of flavonoids has been thought to be receptor-mediated [38,39]. These receptor-mediated actions include inhibitory actions at adenosine receptors and also inhibition of von Willebrand binding to platelet glycoprotein Iba. Some flavonoids have also been shown to inhibit thromboxane A2 (TXA2) mediated response [40,41]. The anticoagulant effect of PAE could, therefore, be as a result of an antiplatelet effect due to the flavonoid constituent. Compounds known to suppress the extent of the coagulation cascade are the coumarins. Coumarins have been found to interfere with coagulation cascade by inhibiting the vitamin K epoxide reductase multiprotein complex [42]. Naturally occurring coumarins have been found not to have anticoagulant properties, however, enzymatic metabolism and fermentation processes could result in the activation to metabolites that have anticoagulant properties [43]. P. kotschyi root bark is known to contain coumarins [44].
In comparing the effect of PAE on PT and aPTT, it could be realized that PAE has a significant effect on aPTT than on PT. This effect is very similar to that demonstrated by heparin. This suggests that the anticoagulant effect of PAE has a more significant effect on the intrinsic pathway of coagulation, relative to the extrinsic pathway.
CONCLUSION
The extract made from the root bark of P. kotschyi (Schweinf.) Harms and the aerial part of A. cissampeloides (Planch. ex Hook.) Harms has anticoagulant effect in vitro. It thus justifies the folkloric use of PAE in the management of circulatory disorders such as numbness and also recommended in the management of thromboembolic disorders such as DVT and varicose veins. The extract is safe at doses lower than 2000 mg/kg.
ACKNOWLEDGMENTS
Authors wish to express our sincere gratitude to the laboratory technicians of the Department of Pharmacology especially Mr. Thomas Ansah, Mr. Fulgencious Somkang, and the entire staff of the department. We sincerely appreciate your commitment and dedication to duty.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64. doi: 10.1160/TH07-03-0212. [DOI] [PubMed] [Google Scholar]
- 2.Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. p. 396. [Google Scholar]
- 3.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 4.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: The longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 5.van Korlaar IM, Vossen CY, Rosendaal FR, Bovill EG, Cushman M, Naud S, et al. The impact of venous thrombosis on quality of life. Thromb Res. 2004;114:11–8. doi: 10.1016/j.thromres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet. 1960;1:1309–12. doi: 10.1016/s0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- 7.Werdan K, Braun-Dullaeus R, Presek P. Anticoagulation in atrial fibrillation: NOAC's the word. Dtsch Arztebl Int. 2013;110:523–4. doi: 10.3238/arztebl.2013.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchinson J, Dalziel JM. Flora of West Tropical Africa. 2nd ed. Part. 2. Millbank, London: Crown Agents for Overseas Governments and Administrations; 1958. p. 702. [Google Scholar]
- 9.Shahina G. Savanna Plants. An Illustrated Guide. London: MacMillan Publishers Ltd; 1989. p. 105. [Google Scholar]
- 10.Njoku OV, Obi C. Phytochemical constituents of some selected medicinal plants. Afr J Pure Appl Chem. 2009;3:228–33. [Google Scholar]
- 11.Ngarivhume T, Van't Klooster CI, de Jong JT, Van der Westhuizen JH. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J Ethnopharmacol. 2015;159:224–37. doi: 10.1016/j.jep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds MS. Ethnobotanical study of some Ghanaian anti-malarial plants. J Ethnopharmacol. 2005;99:273–9. doi: 10.1016/j.jep.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Georgewill UO, Georgewill OA. Effect of extract of Pseudocedrela kotschyi on blood glucose concentration of alloxan induced diabetic albino rats. East J Med. 2009;14:17–9. [Google Scholar]
- 14.Christian AG, Ahunna AG, Nwakaego EM, Chimsorom CK, Chile AE. Antimalarial potential of the ethanolic leaf extract of Pseudocedrala kotschyi. J Acute Dis. 2015;4:23–7. [Google Scholar]
- 15.Kassim OO, Loyevsky M, Amonoo H, Lashley L, Ako-Nai KA, Gordeuk VR. Inhibition of in-vitro growth of Plasmodium falciparum by Pseudocedrela kotschyi extract alone and in combination with Fagara zanthoxyloides extract. Trans R Soc Trop Med Hyg. 2009;103:698–702. doi: 10.1016/j.trstmh.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Adeniyi C, Odumosu BT, Ayelaagbe OO, Kolude B. In-vitro antimicrobial activities of methanol extracts of Zanthoxylum xanthoxyloides and Pseudocedrela kotschyi. Afr J Biomed Res. 2013;13:61–8. [Google Scholar]
- 17.Annan K, Sarpong K, Asare C, Dickson R, Amponsah K, Gyan B, et al. In vitro anti-plasmodial activity of three herbal remedies for malaria in Ghana: Adenia cissampeloides (Planch.). Harms. Terminalia ivorensis A. Chev, and Elaeis guineensis Jacq. Pharmacognosy Res. 2012;4:225–9. doi: 10.4103/0974-8490.102270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyarko AA, Addy ME. Effect of aqueous extract of Adenia cissampeloides on blood pressure and serum analytes of hypertensive patients. Phytother Res. 1990;4:25–8. [Google Scholar]
- 19.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. Chichester: John Willey and Sons; 1982. pp. 221–3. [Google Scholar]
- 20.Gadi D, Bnouham M, Aziz M, Ziyyat A, Legssyer A, Legrand C, et al. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J Ethnopharmacol. 2009;125:170–4. doi: 10.1016/j.jep.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Pifarré R. Thrombosis and cardiovascular disease. Med Clin North Am. 1998;82:511–22. doi: 10.1016/s0025-7125(05)70008-0. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E. Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with non-valvular atrial fibrillation: A systematic review and meta-analysis of subgroups. Thrombosis. 2013;2013:640723. doi: 10.1155/2013/640723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roehrig S, Straub A, Pohlmann J, Lampe T, Pernerstorfer J, Schlemmer KH, et al. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): An oral, direct factor Xa inhibitor. J Med Chem. 2005;48:5900–8. doi: 10.1021/jm050101d. [DOI] [PubMed] [Google Scholar]
- 24.Abdulsattar Y, Bhambri R, Nogid A. Rivaroxaban (xarelto) for the prevention of thromboembolic disease: An inside look at the oral direct factor Xa inhibitor. P T. 2009;34:238–44. [PMC free article] [PubMed] [Google Scholar]
- 25.Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112:e53–60. doi: 10.1161/CIRCULATIONAHA.104.478222. [DOI] [PubMed] [Google Scholar]
- 26.Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: From vascular endothelium to circulatory assist device surface. Circulation. 2010;122:2068–77. doi: 10.1161/CIRCULATIONAHA.110.936773. [DOI] [PubMed] [Google Scholar]
- 27.Korte W, Clarke S, Lefkowitz JB. Short activated partial thromboplastin times are related to increased thrombin generation and an increased risk for thromboembolism. Am J Clin Pathol. 2000;113:123–7. doi: 10.1309/g98j-ana9-rmnc-xlyu. [DOI] [PubMed] [Google Scholar]
- 28.Pramyothin P, Samosorn P, Poungshompoo S, Chaichantipyuth C. The protective effects of Phyllanthus emblica Linn. extract on ethanol induced rat hepatic injury. J Ethnopharmacol. 2006;107:361–4. doi: 10.1016/j.jep.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Organization for Economic Co-operation and Development (OECD) Guidance Notes for Analysis and Evaluation of Chronic Toxicity and Carcinogenicity Studies, Series on Testing and Assessment No. 35 and Series on Pesticides No. 14. OECD Environment, Health and Safety Publications, ENV/JM/MONO. 2002 [Google Scholar]
- 30.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74:663–71. doi: 10.1007/s12098-007-0118-7. [DOI] [PubMed] [Google Scholar]
- 32.Grace OM, Fowler D. Adenia cissampeloides (Planch ex Hook.) harms. In: Schmelzer GH, Gurib-Fakim A, editors. Prota 11(1): Medicinal Plants/Plantes médicinales 1. Wageningen, Netherlands: PROTA; 2007. [Google Scholar]
- 33.Gregg D, Goldschmidt-Clermont PJ. Cardiology patient page. Platelets and cardiovascular disease. Circulation. 2003;108:e88–90. doi: 10.1161/01.CIR.0000086897.15588.4B. [DOI] [PubMed] [Google Scholar]
- 34.Almadi MA, Barkun A, Brophy J. Antiplatelet and anticoagulant therapy in patients with gastrointestinal bleeding: An 86-year-old woman with peptic ulcer disease. JAMA. 2011;306:2367–74. doi: 10.1001/jama.2011.1653. [DOI] [PubMed] [Google Scholar]
- 35.Warner TD, Nylander S, Whatling C. Anti-platelet therapy: Cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72:619–33. doi: 10.1111/j.1365-2125.2011.03943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrör K. Aspirin and platelets: The antiplatelet action of aspirin and its role in thrombosis treatment and prophylaxis. Semin Thromb Hemost. 1997;23:349–56. doi: 10.1055/s-2007-996108. [DOI] [PubMed] [Google Scholar]
- 37.Tzeng SH, Ko WC, Ko FN, Teng CM. Inhibition of platelet aggregation by some flavonoids. Thromb Res. 1991;64:91–100. doi: 10.1016/0049-3848(91)90208-e. [DOI] [PubMed] [Google Scholar]
- 38.Polette A, Lemaitre D, Lagarde M, Véricel E. N-3 fatty acid-induced lipid peroxidation in human platelets is prevented by catechins. Thromb Haemost. 1996;75:945–9. [PubMed] [Google Scholar]
- 39.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 40.Jacobson KA, Moro S, Manthey JA, West PL, Ji XD. Interactions of flavones and other phytochemicals with adenosine receptors. Adv Exp Med Biol. 2002;505:163–71. doi: 10.1007/978-1-4757-5235-9_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mruk JS, Webster MW, Heras M, Reid JM, Grill DE, Chesebro JH. Flavone-8-acetic acid (Flavonoid) profoundly reduces platelet-dependent thrombosis and vasoconstriction after deep arterial injury in vivo. Circulation. 2000;101:324–8. doi: 10.1161/01.cir.101.3.324. [DOI] [PubMed] [Google Scholar]
- 42.Whitlon DS, Sadowski JA, Suttie JW. Mechanism of coumarin action: Significance of Vitamin K epoxide reductase inhibition. Biochemistry. 1978;17:1371–7. doi: 10.1021/bi00601a003. [DOI] [PubMed] [Google Scholar]
- 43.Bye A, King HK. The biosynthesis of 4-hydroxycoumarin and dicoumarol by Aspergillus fumigatus Fresenius. Biochem J. 1970;117:237–45. doi: 10.1042/bj1170237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adebayo OL, Osman K. A comparative evaluation of in vitro growth inhibitory activities of different solvent extracts of some medicinal plants in Northern Ghana against selected human pathogens. IOSR J Pharm. 2012;2:199–206. [Google Scholar]


