Abstract
Background:
Makaradhwaja a gold containing mercurial preparation used for diabetes mellitus in indigenous system of medicine. It is a popular aphrodisiac and rejuvenator traditional medicine. It is prepared by using processed gold, mercury and sulfur in different ratios by applying intermittent heating pattern in Valuka Yantra.
Objectives:
The aim of the study was to evaluate anti-diabetic effect of Shadguna Balijarita Makaradhwaja (SBM) on streptozotocin (STZ) induced diabetic rats.
Materials and Methods:
Diabetes was induced to normal rats by injecting STZ in dose 40 mg/kg. Powdered SBM and dried extract of Tinospora cordifolia were mixed with honey and administered orally for 20 days at dose 2.63 mg/kg and 42.34 mg/kg body weight, respectively. The effects of treatment on body weight changes and blood glucose levels were quantified on day 1, 5, 10, 15 and 21 of the experiments. On the 21st day, animals were sacrificed and gross histopathological changes in liver, kidney and pancreas were illustrated. Blood sugar level, glyacated hemoglobin, blood urea, serum cholesterol, serum creatinine, serum triglyceride and serum protein were estimated with standard methods. The study was conducted in the year 2011.
Results:
Test drug observed significant decrease (P < 0.001) in glyacated hemoglobin level compared to diabetic control rats. Blood sugar level of test drug group shown a significant decrease (279.11 ± 57.95) compared with diabetic rats.
Conclusion:
The present study demonstrates that SBM and dried extract of T. cordifolia with honey significantly reduces the blood glucose level and shows anti-diabetic effect.
Keywords: Anti-diabetic activity, cinnabar gold, Kupipakwa Rasayana, mercury sulfide Shadguna Balijarita Makaradhwaja
INTRODUCTION
Diabetes mellitus is threatening for the 21st century. It will be leading cause of morbidity and mortality in near future due to increasing incidence worldwide. Herbo-mineral-metallic drugs of Ayurveda having potential of decreasing blood sugar levels and found efficient on experimental animal models. Makaradhwaja [1] is such herbo-mineral-metallic composition.
Medieval classical texts of Ayurveda quoted that Makaradhwaja is one of the best rejuvenator and aphrodisiac [2] agent. It is prepared by using processed gold, mercury and sulfur in the ratio of 1:8:24 or 1:8:48 by sublimation in the traditional system of heating device Valuka Yantra [1,2]. Nowadays, it is prepared in modified vertical electrical muffle furnace (modified Valuka Yantra) [3]. It gives synergistic action with different herbs in the various disorders. It is therapeutically efficient in disorders such as Madhumeha (diabetes mellitus), Jwara (remittent fever), Kushta (skin disorders), and Raktaja Vikara (blood disorders) [2]. Previous studies claimed its safe use without any untoward effect and toxicity [4]. Specific action on cell-mediated immunity is proved by its immune-modulator action [5]. In experimental and clinical studies, it is found effective in diabetes [6].
Guduchi (Tinospora cordifolia Linn.) is well-known Madhumehahar (anti - hyperglycemic) herb described throughout ayurvedic classics [7]. Most commonly, its stem is used for medicinal purposes. It is well-known anti-oxidant, anti-hyperglycemic, immune-modulatar, and rejuvenator [8]. Its different formulations are quoted in the ayurvedic classics like juice, decoction, powder and Ghana (dried extract) as per diseases.
Madhu (honey) is mentioned as Madhumehahar (~anti-diabetic) agent within classical texts of Ayurveda [9]. Its anti-diabetic activity is reported by Erejuwa et al. [10]. It is used as a vehicle drug (Yogavahi) in the numerous herbo mineral formulations. It is mentioned in the reference to Makaradhwaja as a vehicle drug [11]. In the present study, it was used as a vehicle drug as prescribed in texts of Rasashastra.
The present study was planned to evaluate anti-diabetic activity of Shadguna Balijarita Makaradhwaja (SBM) and T. cordifolia Linn with honey in streptozotocin (STZ) induced diabetic rats.
MATERIALS AND METHODS
Preparation of SBM and Guduchi Ghana
Test drug SBM was prepared as per the classical text reference in the Department of Rasashastra and Bhaishajya Kalpana, Institute of Post Graduate Teaching and Research in Ayurveda (IPGT and RA), Gujarat Ayurved University, Jamnagar in 2011 [1]. Raw Material Swarna (gold) was purchased from the local market, and Hingula (cinnabar) and Gandhaka (sulfur) were collected from Pharmacy of Gujarat Ayurved University, Jamnagar. Gold was subjected to Shodhana and after Shodhana, its foils were prepared. Cinnabar was processed to Shodhana (purification) and Parada (mercury) was procured from its sublimation by adopting Nada Yantra method [12]. Gandhaka (sulfur) was subjected to Shodhana by melting it and pouring in cow milk and continuously heated in same milk for 3 h [13]. Processed gold foils, mercury and sulfur were brought in ratio of 1:8:48 in weight. Amalgamation was done by adding gold foils to mercury. Fine lusterless powder Kajjali was prepared by triturating sulfur with above prepared amalgam. Kajjali was levigated with juice of Kumari (Aloe barbadensis Mill.) and Japa (Hibiscus rosa-sinensis) for 3 h consecutively. Levigated Kajjali was dried and powdered. The fine powder was filled in seven-layer mud smeared cotton cloth wrapped glass bottle (Kacha Kupi) and heated for 12 h in specially designed electrical muffle furnace. The heat was provided in controlled and gradually increasing temperature in modified electrical muffle furnace (modified Valuka Yantra). After the desired characteristic features of product preparation, the mouth of glass bottle was sealed; furnace was switched off and subjected for self-cooling. The highest recorded temperature during procedure was 600°C. Sublimed product was procured from neck of glass bottle, it was powdered and used for further analysis and study [14]. In analytical studies, it was observed that Makaradhwaja is consisted of red sulfide of mercury and having an empirical formula of HgS (mercury sulfide). Inductively coupled plasma optical emission spectrometry was observed that it contains 1.2% of gold with mercury and sulfur in finished product as a major element.
T. cordifolia’s stems were collected from the herbal garden of Gujarat Ayurved University, Jamnagar. Stems of T. cordifolia were cut into small pieces and crushed. These crushed pieces were cooked with 4 times of potable water ant reduced at 1/4th of the same to prepare decoction. The decoction was sieved, cooked to semisolid consistency. The semisolid mass was dried in hot air oven at 45°C to prepare dry extract [15]. Average 5.21% yield was obtained from the stem extract. The Guduchi Ghana (dried extract) was powdered and stored [16].
Honey was collected from the local forest of Jamnagar.
Experimental Animals
Albino rats (160-220 g) of either sex were selected for this study. Animals were procured from the Animal house of Pharmacology laboratory of IPGT and RA, Gujarat Ayurved University, Jamnagar. Permission for the experiment was granted by Animal Ethical Committee of the Same Institute (IAEC-06/09-11/02). The animals were fed pellet diet and water. As per the guidelines of National Institute of Health’s guide for the Care and Use of Laboratory Animals, the study was conducted [17].
Collection of Blood Samples
Tail veni-puncture method was applied for the collection of blood sample in the rats. For investigation Glucometer strip was used, and reading was noted down. By adopting this procedure blood glucose level of animals was estimated, prior injection of STZ, 5th, 10th, 15th, and 20th day during trials.
The sacrifice was carried out on the 21st day after completion of the study. Animals were anesthetized and stroked over tiles for sacrifice. The blood sample was collected by the dissection of jugular veins of animals. Biochemical parameters were investigated at pathology laboratory, IPGT, and RA, Jamnagar.
Experimental Induction of Diabetes
Diabetes was induced to rats by single intra peritoneal injection of STZ (40 mg/kg). STZ was weighed individually for each animal, according to its weight it was solubilized with 0.2 mL saline (154 mM NaCl) just prior to injection. After 72 h of STZ injection, rats with diabetic hyperglycemia (blood glucose more or equal to 250 mg/dL) were selected for experiment. Suspension of Makaradhwaja and T. cordifolia with honey was started fed to selected diabetic rats.
Anti-diabetic Activity
Anti-diabetic activity was evaluated by the effect of test drugs on the ponderal and biochemical parameters. Ponderal parameters like gross body and different body organs weight were evaluated. Biochemical variables such as blood sugar, glycated hemoglobin (HbA1C), serum total cholesterol, serum high-density lipoprotein (HDL), serum triglyceride, serum creatinine, blood urea, serum glutamic-pyruvic transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), total protein, albumin, and globulin were estimated. Sacrificed animals gross and histological appearance of vital organs (liver, pancreas and kidney) were examined at the end of the study.
Experimental Design
Diabetic animals were divided into four groups. Food and water were provided to the animals.
Group 1: Normal control (NC)
Group 2: Diabetic control (DC)
Group 3: DC + SBM and Guduchi Ghana with honey suspension (SBM)
Group 4: DC + glibenclamide (reference standard [RS])
Dose Fixation
The dose of the drug was calculated by extrapolating the therapeutic dose to rat on the basis of body surface area ratio by referring to the table of Paget and Barnes (1964) [18].
Dose
Powdered SBM 2.63 mg/kg rat and dried extract of T. cordifolia 42.34 mg/kg rat were grinded with honey to prepare suspension. This suspension was administered orally to test drug group SBM diabetic animals. Glibenclamide, in the dose 0.45 mg/kg was administered to RS drug control group.
Statistical Analysis
All the values of were expressed as mean ± standard error mean. A statistical analysis was performed by using Student’s t-test. It was calculated by using Microsoft excel programmer.
RESULTS
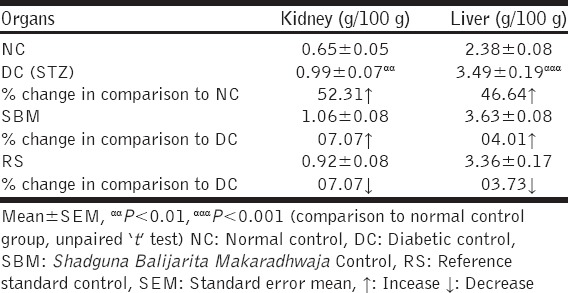
In NC rats, during the course of 21 days, 13.90% weight was increased. Insignificant decrease in body weight was observed in SBM and RS group in comparison to DC rats [Table 1]. In DC rats, weight of kidney and liver was increased up to significant extent. Treatment with test drug did not affect the weight of these organs to significant extent in comparison to the DC group [Table 2].
Table 1.
Effect of test drug on body weight in STZ diabetic Wister strain albino rats
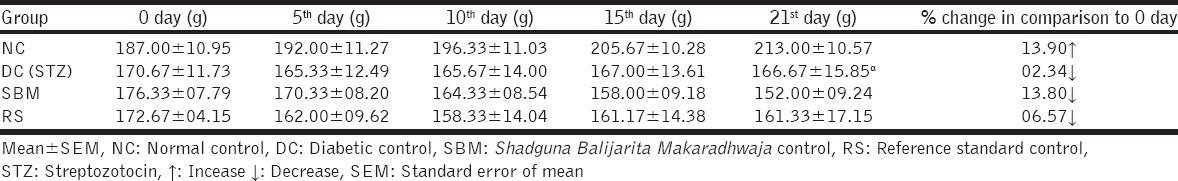
Table 2.
The effect of test drugs on weight of liver and kidney in streptozotocin induced diabetic Wistar strain albino rats
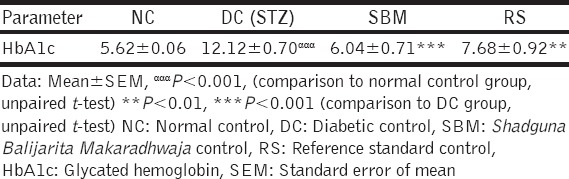
Initial blood sugar level was decreased by 44.27% and 44.04% in SBM and RS treated groups respectively [Table 3]. Raised levels of HbA1C were significantly attenuated by test drug SBM and RS [Table 4]. Increased blood sugar levels were insignificantly decreased by SBM and RS test drugs.
Table 3.
The effect of test drugs on blood sugar level in STZ induced diabetic rats at various intervals
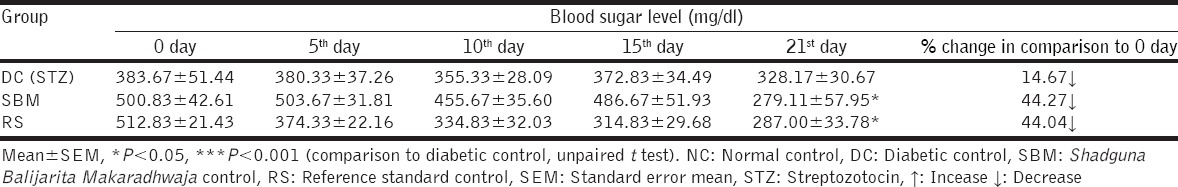
Table 4.
The effect of test drugs on HbA1c in STZ induced diabetic Wistar strain albino rats
Non-significant rise in serum cholesterol, triglyceride and HDL were moderately decreased by administration of SBM. Blood urea levels decreased significantly in group SBM and moderately decreased in RS group. Elevated serum creatinine levels non-significantly decreased by administration of SBM which was non-significantly increased in RS group in comparison to DC group. Decreased SGOT parameter in DC group was significantly attenuated by SBM and RS drugs. Increased levels of SGPT were decreased up to significant extent by SBM and RS drugs [Table 5].
Table 5.
The effect of test drugs on various serum biochemical parameters
The accumulation of fat in liver was observed in histopathological study. SBM and RS treated animals restored the histological changes [Figure 1a-d]. Inflammation in blood vessels, increase in the thickness of bowman capsules, fat deposition, and change in size of the glomerulus were found in the kidney of diabetic rats. The treatment with SBM showed the normal histopathology of the kidney without any inflammatory vessels and fat deposition [Figure 2a-d]. Decreased Islets of Langerhans, smashed size of β cells and extensive necrosis, fibrosis and atrophy were observed in the pancreas of diabetic rats. STZ induced diabetic rat treated with SBM and glibenclamide restored the necrotic and fibrotic changes and up to moderate levels, raised the number of β cells. [Figure 3a-d].
Figure 1.
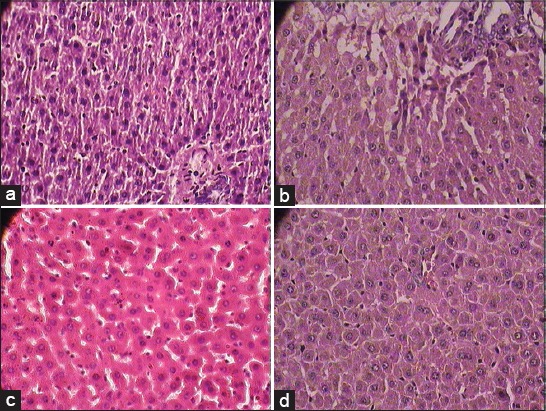
(a) Normal cytoarchitecture of liver in normal control group (1 × 400 magnification), (b) Photomicrographs of representative section of Liver. Macro and micro fatty changes, cell infiltration in almost all the sections streptozotocin control group (1 × 400 magnification) (c) Shadguna Balijarita Makaradhwaja treated rats shows almost normal cytoarchitecture of liver in comparison with streptozotocin control group diabetic rats (1 × 400 magnification), (d) Glibenclamide treated rat showed almost normal cytoarchitecture of liver sections in comparison with streptozotocin control group diabetic rats (1 × 400 magnification)
Figure 2.
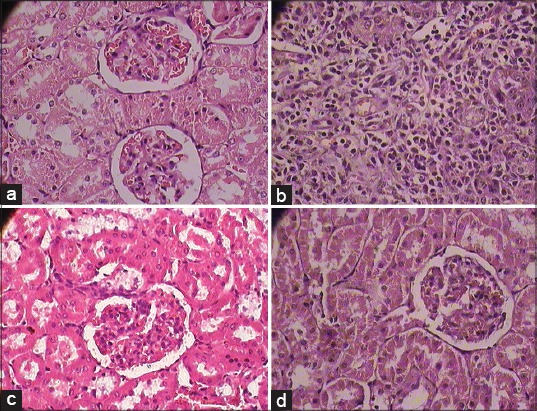
(a) Normal cytoarchitecture of sections of kidney in normal control group (b). (1 × 400 magnification), (b) Photomicrographs of representative section of kidney. Cell infiltration and micro-fatty changes in all the sections of streptozotocin control treated diabetic rats (1 × 400 magnification), (c) Shadguna Balijarita Makaradhwaja treated rat showed almost normal cytoarchitecture in comparison with streptozotocin control group diabetic rats (1 × 400 magnification), (d): Glibenclamide treated rat showed almost normal cytoarchitecture in comparison with streptozotocin control group diabetic rats (1 × 400 magnification)
Figure 3.
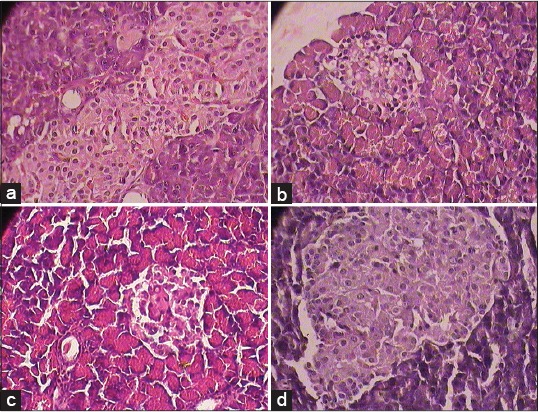
(a) Normal cytoarchitecture in normal control group, (b) Photomicrographs of representative section of Pancreas. Marked degeneration of Islets of Langerhans and degranulation of streptozotocin control treated diabetic rats (1 × 400 magnification), (c) SBM treated rats shows comparatively less degeneration of Islets of Langerhans with intact granules in comparison with streptozotocin control group diabetic rats (1 × 400 magnification), and (d) Glibenclamide treated rat showed normal cyto-architecture with intact granules in comparison with streptozotocin control group diabetic rats (1 × 400 magnification)
DISCUSSION
Excessive breakdown of tissue proteins decreases body weight in diabetes. It was observed in DC rats in comparison to normal rats [19-21]. Treatment with SBM improved body weight to a certain extent, indicating that control over muscle wasting resulted from glycemic control.
Previous studies claimed that STZ destructs beta cells, which leads to cells less active that makes poor utilization by tissues [22,23]. This suggests that test drug may possess as insulin-like effect on peripheral tissues by inhibiting hepatic gluconeogenesis by promoting glucose uptake or metabolism [24]. Or it might be possible that test drug increases absorption of glucose into the muscles and adipose tissues [25] by stimulation of regeneration process and revitalization of remaining beta cells [26]. Hence, the hypoglycemic activity of SBM may be due to its protective action against damaged pancreatic beta cells and possibly because of increased insulin release or secretion or regeneration of damaged beta cell. Makaradhwaja is a well-known therapeutic medicine of rejuvenation in Indian system of medicine. Its immune-modulatory action was also established [5]. The observed effect may be attributed to the rejuvenation property of Makaradhwaja. The previous study supports its action too [27]. Moderate but insignificant decrease in blood sugar levels were also observed in test drug-treated animals.
Higher levels of HbA1C were observed in the diabetic rats compared with those in normal rats, it might be due to poor glycemic control. SBM treated diabetic rats significantly decreased the level of HbA1C, may be glucose metabolism was improved. This action represents that SBM has an ability to prevent the development of diabetes associated complications.
Elevated levels of SGPT indicating impaired liver function due to hepato-cellular necrosis. Due to elevated transaminase activities leads to diabetic complications like increased ketogenesis and glucogenesis [28], test drug significantly restored this parameter toward normal levels.
Elevation of urea and creatinine levels results due to renal dysfunction caused by free radical generation mediated stress in diabetes, persistent hyperglycemia, and hemo-dynamic changes within the kidney tissue [29-31]. Administration of SBM and glibenclamide to the diabetic rats significantly reduced the creatinine and urea levels, which represent the preventive action of SBM on kidney damages in diabetic condition perhaps due to the anti-oxidant properties. Blood urea level was significantly increase in diabetic rats compared to normal rats due to excessive breakdown of protein. This elevation was significantly decreased by test drug.
STZ induced diabetic rat’s increases the level of lipid peroxidation, as an indirect evidence of production of free radical [32]. Hyperlipidemia commonly associated with diabetes [33]. Test drug significantly attenuated serum lipid profiles in diabetic rats. STZ induced diabetic groups treated with glibenclamide and SBM brought back the increased level of total cholesterol and triglyceride near to the normal levels.
Histopathological changes in liver, kidney and pancreas were well restored by the test drug in comparison with DC group. The observed results show anti-diabetic potential of SBM. Observed results may be due to synergistic action of Makaradhwaja with adjuvant T. cordifolia and honey.
CONCLUSION
The present study demonstrates that SBM and dried extract of T. cordifolia with honey significantly reduces the blood glucose level and shows anti-diabetic effect. Restoration histopathological changes in different organs support safe and effective anti-diabetic action of test drug. A significant decrease in glycated hemoglobin shows effect of Makaradhwaja on diabetes-related complications.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sen G. In: Bhaishajya Ratnavali. Mishra S, editor. Varanasi: Chaukhamba Surabharti Prakashan; 2007. p. 194. [Google Scholar]
- 2.Sen G. In: Bhaishjyaratnavali. Mishra sS., editor. Varanasi: Chaukhamba Surabharti Prakashan; 2007. p. 1135. [Google Scholar]
- 3.Khedekar S, Prajapati PK. Standard manufacturing process of Shadguna Balijarita Makaradhwaja. Ayu. 2014;35:428–32. doi: 10.4103/0974-8520.159011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prajapati PK, Joshi D, Dube GP, Kumar M, Prakash B. Pharmaceutical and Experimental Study on Makaradhwaja. MD Dissertation. Varanasi: BHU; 1994. [Google Scholar]
- 5.Patgiri BJ, Prajapati PK. A Pharmaceutical and Toxicity Study of Makaradhwaja Prepared by Astasmsakarita Parada. PhD Thesis. IPGT & RA. Jamnagar: Gujarat Ayurved University; 2005. [Google Scholar]
- 6.Khedekar S, Patgiri BJ, Ravishankar B, Prajapati PK. A pharmaceutico-pharmacoclinical study of Makaradhwaja Prepared by Swarna Patra-Varkha and Bhasma wsr to Madhumeha (Diabetes Mellitus). MD Dissertation. IPGT & RA. Jamnagar: Gujarat Ayurved University; 2009. [Google Scholar]
- 7.Bhavaprakasha . In: Bhavprakash Samhita. Part I. Vaidya L, editor. New Delhi: Motilal Banarasidas; 1986. p. 156. [Google Scholar]
- 8.Saha S, Ghosh S. Tinospora cordifolia: One plant, many roles. Anc Sci Life. 2012;31:151–9. doi: 10.4103/0257-7941.107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhavaprakasha . In: Bhavprakash Samhita. Part I. Vaidya L, editor. New Delhi: Motilal Banarasidas; 1986. p. 329. [Google Scholar]
- 10.Erejuwa OO, Sulaiman SA, Ab Wahab MS. Honey-A novel anti-diabetic agent. Int J Biol Sci. 2012;8:913–34. doi: 10.7150/ijbs.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakar C. Rasachikitsa. Varanasi: Chowkhamba Vidya Bhawan; 1956. p. 356. [Google Scholar]
- 12.Bhatta K. In: Siddhabheshajamanimala. Bhatta RK, editor. Varanasi: Chaukhambha Krishnadas Academy; 2008. p. 355. [Google Scholar]
- 13.Vagbhata . In: Vidhnyanbodhini Hindi Commentary. Rasaratnasamucchaya. Kulkarni DA Prof, editor. New Delhi: Meherchanda Lachamandasa Publications; 2006. p. 45. [Google Scholar]
- 14.Khedekar SB, Prajapati PK. Standard manufacturing procedure of Shadguna Balijarita Makaradhwaja. Ayu. 2014;35:428–32. doi: 10.4103/0974-8520.159011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharangdhara . In: Sharangadhara Samhita. Parashar R, editor. Nagpur: Baidyanath Ayurveda Bhavan Ltd; 1994. p. 189. [Google Scholar]
- 16.Khedekar S, Ravishankar B, Prajapati PK. Role of Gandhaka Jarana in the Preparation of Makaradhwaja. PhD Thesis. IPGT & RA. Jamnagar: Gujarat Ayurved University; 2012. [Google Scholar]
- 17.Guide for the Care and Use of Laboratory Animals. National Institute of Health, Offices of Science and Health Reports. DRR/NIH. Bethesda, MD, USA: DHEW Publication No (NIH) 86-23; 1985. [Google Scholar]
- 18.Paget GE, Barnes JM. Evaluation of drug activities. In: Lawrence DR, Bacharach AL, editors. Pharmacometrics. Vol. 1. New York: Academic Press New York; 1964. p. 161. [Google Scholar]
- 19.Mishra SB, Verma A, Mukerjee A, Vijayakumar M. Anti-hyperglycemic activity of leaves extract of Hyptis suaveolens L. Poit in streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2011;4:689–93. doi: 10.1016/S1995-7645(11)60175-2. [DOI] [PubMed] [Google Scholar]
- 20.Sajeesh T, Arunachalam K, Parimelazhagan T. Antioxidant and antipyretic studies on Pothos scandens L. Asian Pac J Trop Med. 2011;4:889–99. doi: 10.1016/S1995-7645(11)60214-9. [DOI] [PubMed] [Google Scholar]
- 21.Salahuddin M, Jalalpure SS. Antidiabetic activity of aqueous fruit extract of Cucumis trigonus Roxb. in streptozotocin-induced-diabetic rats. J Ethnopharmacol. 2010;127:565–7. doi: 10.1016/j.jep.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Junod A, Lambert AE, Stauffacher W, Renold AE. Diabetogenic action of streptozotocin: Relationship of dose to metabolic response. J Clin Invest. 1969;48:2129–39. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netchiporouk LI, Shram NF, Jaffrezic-Renault N, Martelet C, Cespuglio R. In vivo brain glucose measurements: Differential normal pulse voltammetry with enzyme-Modified carbon fiber microelectrodes. Anal Chem. 1996;68:4358–64. doi: 10.1021/ac960190p. [DOI] [PubMed] [Google Scholar]
- 24.Gray AM, Wahab A, Flatt PR. The traditional plant treatment Sambuscus nigra (Elder), exhibits insulin-like and insulin releasing actions in vitro. J Nutr. 2000;130:15–20. doi: 10.1093/jn/130.1.15. [DOI] [PubMed] [Google Scholar]
- 25.Kamanyi A, Diamen D, Nkeh B. Hypoglycemic properties of the aqueous roots extract of Morinda lucida (Rubiaceae) study in mouse. Phytother Res. 1994;8:369–71. [Google Scholar]
- 26.Shanmugasundaram ER, Gopinath KL, Shanmugasundaram KR, Rahjendran VM. Possible regeneration of the islets of Langerhans in Streptozotocin diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–79. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]
- 27.Khedekar S, Patgiri BJ, Ravishankar B, Prajapati PK. Antihyperglycemic effect of Makaradhwaja on Streptozotocin induced diabetes in rats. J Glob Pharm Tech. 2012;4:16–24. [Google Scholar]
- 28.Ghosh S, Suryawanshi SA. Effect of Vinca rosea extracts in treatment of alloxan diabetes in male albino rats. Indian J Exp Biol. 2001;39:748–59. [PubMed] [Google Scholar]
- 29.Aurell M, Björck S. Determinants of progressive renal disease in diabetes mellitus. Kidney Int Suppl. 1992;36:S38–42. [PubMed] [Google Scholar]
- 30.Shokeen P, Anand P, Murali YK, Tandon V. Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol. 2008;46:3458–66. doi: 10.1016/j.fct.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Prabhu KS, Lobo R, Shirwaikar A. Antidiabetic properties of the alcoholic extract of Sphaeranthus indicus in streptozotocin-nicotinamide diabetic rats. J Pharm Pharmacol. 2008;60:909–16. doi: 10.1211/jpp.60.7.0013. [DOI] [PubMed] [Google Scholar]
- 32.Maritim AC, Sanders RA, Watkins JB., rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 33.Chase HP, Glasgow AM. Juvenile diabetes mellitus and serum lipids and lipoprotein levels. Am J Dis Child. 1976;130:1113–7. doi: 10.1001/archpedi.1976.02120110075010. [DOI] [PubMed] [Google Scholar]


