Abstract
Background:
The problem of resistance of malarial parasites to available antimalarial drugs makes the development of new drugs imperative, with natural plant products providing an alternative source for discovering new drugs.
Aim:
To evaluate the antimalarial activity of eluted fractions of Acacia nilotica root extract and determine the phytochemicals responsible for its antimalarial activity.
Materials and Methods:
The extract was eluted successively in gradients of solvent mixture (hexane, ethyl acetate, and methanol) in multiples of 100 ml, and each fraction was collected separately. Eluates that showed similar thin layer chromatographic profiles and Rf values were combined to produce 4 main fractions (F-1, F-2, F-3, and F-4), which were tested separately for antimalarial activity using the curative test. Changes in body weight, temperature, and packed cell volume (PCV) were also recorded.
Results:
Fraction F-1 of A. nilotica at 50 and 100 mg/kg b/w produced significant and dose-dependent reduction in parasite count in Plasmodium berghei infected mice compared to the control, and also significantly increased the survival time of the mice compared to the control group. This fraction also ameliorated the malaria-induced anemia by improving PCV in treated mice.
Conclusion:
Antimalarial activity of extract of A. nilotica root is probably localized in the F-1 fraction of the extract, which was found to be rich in alkaloids and phenolics. Further study will provide information on the chemical properties of the active metabolites in this fraction.
Keywords: Acacia nilotica, antimalarial, fractions, medicinal plant
INTRODUCTION
Malaria is a preventable and curable disease, yet it remains a devastating tropical disease, with a high infection and mortality statistics. It is the most prevalent parasitic disease and the most common cause of hospital visitation in Nigeria [1]. Globally, there are approximately 214 million malaria cases in 97 countries with ongoing malaria transmission and 80% of these cases were reported in the Sub-Saharan Africa. The disease caused approximately 438,000 deaths, 90% of which were in Sub-Saharan Africa, and 78% of these deaths occur in children under 5 years [2]. The main challenge to the effective management of diagnosed malaria cases is the resistance of the plasmodium parasite to commonly used antimalarial drugs, which results in the nonresolution of symptoms and in treatment failure [3,4]. Cases of resistance are currently extending to the artemisinin combination therapy with report of resistance to artemisinin drugs coming from Thailand, Vietnam, and Cambodia [2,5-7]. Furthermore, no new class of antimalarial drug has been introduced in clinical practice in the last 10 years [8,9]. This strongly supports the need for further research into new products that could complement the existing antimalarial, bearing in mind that it takes minimum of 10 years to develop a drug from discovery stage to completion of clinical trials [10]. The development of safe and effective anti-malarial preparations by simple procedures from locally grown medicinal plants, may offer new and complimentary drugs for malaria control, especially in remote geographical locations and in rural areas where modern antimalaria drugs are not readily available and malaria mortality is higher [11].
Acacia nilotica (Linn.) Willd. Ex Del. (also called Gum Arabic tree; Prickly tree, ‘Gabaruwa’ in Hausa and ‘Igi Kasia/Booni’ in Yoruba) is a scented thorny tree native to Africa (grows in Egypt, South Africa, Kenya, and Nigeria) and Indian subcontinents [12]. It is a nitrogen-fixing tree that grows to 14-17 m in height and 2-3 m in diameter.
African Zulu use the bark of A. nilotica to treat cough, diarrhea, dysentery, and leprosy [13]. The Massai (Kenya) use the bark and root decoction as aphrodisiac to cure impotence. The fruit is used to treat tuberculosis [14], whereas the powdered pods are consumed by Egyptians to treat diabetes mellitus [15]. In Northern Nigeria, the root is used for the treatment of malaria [16,17]. A. nilotica is rich in many secondary metabolites, such as tannins, terpenes, alkaloids, flavonoids, and phenolic [17], with known pharmacological properties, thus making it relevant in the treatment of various ailments.
This research aims at evaluating the antimalarial activity of fractions obtained from crude aqueous extract of A. nilotica root and investigates the active antimalarial phytochemical in the crude extract.
MATERIALS AND METHODS
Plant Collection, Identification, and Authentication
Fresh root samples of A. nilotica were collected around 8.45 am in Bamburu-Chaza, Suleja, North-central part of Nigeria. They were identified and authenticated as A. nilotica root at the herbarium of the National Institute for Pharmaceutical Research and Development (NIPRD), Abuja by a taxonomist, Mrs. Grace Ugbabe. Voucher specimen (NIPRD/H/6401) was prepared and deposited at the herbarium for referencing.
Experimental Animals
Swiss albino mice (Mus musculus, 25.00 ± 2 g) used for this study, were obtained from Animal Facility Center, NIPRD, Abuja. They were housed in well ventilated cages, fed with rat pellets and water ad libitum, and maintained under standard laboratory conditions (temperature of 25 ± 3°C and 12 h light/12 h dark cycle), in accordance with the guidelines for the care and use of laboratory animals by National Academy of Science (1996). Ethical approval for animal studies was obtained from the Animal Ethics Committee of NIPRD.
Malaria Parasites
Chloroquine-sensitive rodent Plasmodium; P. berghei NK 65 strain, was obtained from the National Institute for Medical Research, Lagos, Nigeria, and maintained alive by serial intraperitoneal passage in mice, every 5 days.
The reinfected mice were kept at the Animal Facility Center of NIPRD where this study was carried out.
Reagents and Chemicals
The chloroquine phosphate and silica gel (70-230 mesh) were obtained from Sigma-Aldrich Corporation, Missouri, 63103, USA. All other chemicals and reagents used were of analytical grade and prepared using distilled water.
Preparation of Aqueous Extract of A. nilotica root
The root sample of A. nilotica was air dried to constant weight and pulverized using grinding machine. The powder was stored in an airtight container and kept in a cool, dry place. Aqueous extraction was carried out following the cold maceration method. Four hundred grams of the powdered root of A. nilotica was soaked in 1 L of distilled water and kept for 24 h with intermittent shaking. The suspension was filtered after 24 h, with muslin cloth followed by filtration with Whatman filter paper (No.1). The filtrate was freeze-dried using AMSCO/FINN-AQUA GT2 Freeze dryer (Germany). This extraction procedure was carried out thrice to obtain sufficient quantity of extract for the entire study.
Column Fractionation of A. nilotica
Wet silica gel (70-230 mesh) was loaded into a column, and the crude extract was added on the upper layer [18]. The extract was eluted successively in gradients of solvent mixture (hexane, ethyl acetate, and methanol) in multiples of 100 ml and each fraction was collected separately. Eluates that showed similar thin layer chromatographic profiles and Rf values were combined to produce 4 main fractions, which were tested separately for antimalarial activity using the curative test.
Phytochemical Screening
The four fractions obtained were subjected to phytochemical screening following the procedures described by Sofowora [19], to determine the predominant secondary metabolite in each fraction.
Antimalarial Test
Parasite inoculation
Each mouse used in the experiment was inoculated intraperitoneally with 0.2 ml of infected blood (containing about 1 × 107 parasitized erythrocytes) obtained from a single donor mouse previously infected with chloroquine-sensitive P. berghei (containing 25.5% parasitemia). This was prepared by calculating the percentage parasitemia of donor mouse and diluting the blood with physiological saline so that 0.2 ml of diluted blood contained 1 × 107 infected erythrocytes [20].
Grouping and Drug Administration
P. berghei-infected mice were randomly divided into five groups of 6 mice per group. Thirty mice were used for the antimalarial test of each of the four fractions. Group 1 was administered 10 ml/kg body weight distilled water (negative control), Groups 2, 3, and 4 were administered 25, 50, and 100 mg/kg b.w of each fraction. Group 5 was administered 5 mg/kg b/w chloroquine (positive control). Fractions of A. nilotica and chloroquine drug used in this study were administered orally using stainless metal oral cannula. Doses administered were calculated from the value of median lethal dose. Same procedure as above was repeated for the antimalarial test for fractions F-2, F-3, and F-4.
Evaluation of Antimalarial Activity of Fractions of A. nilotica
The Rane test was used to evaluate the schizonticidal activity of the fractions of A. nilotica in established malaria infection.
The evaluation of the curative potential of fractions obtained from A. nilotica root against established infection was carried out as described by Ryley and Peters [21]. Thirty mice were inoculated as earlier described on day 0, and left untreated for 72 h. The mice were weighed and randomized into five groups of six mice each. Group 1 was administered 10 ml/kg body weight of distilled water (negative control); Groups 2, 3, and 4 received fraction of A. nilotica at doses of 25, 50, and 100 mg/kg body weight/day orally, respectively, whereas mice in Group 5 (positive control) received 5 mg/kg body weight/day of chloroquine orally for 4 days (D4-D7). On day-8, each mouse was tail-bled, and a thin blood film was made on a microscope slide.
The films were stained with Giemsa stain and examined microscopically to monitor the parasitemia level [22,23]. The mean survival time of the mice in each treatment group, monitored over a period of 30 days was calculated using the expression:
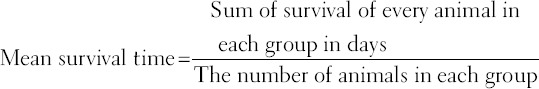
Determination of Percentage Parasitemia
Thin blood films were made from the tail blood of each infected mouse, stained with Giemsa and examined under the microscope at ×100 magnification (oil immersion), to determine the level of parasitemia. Percentage parasitemia was calculated using the formula below:
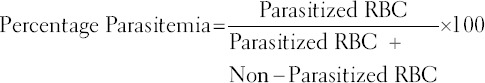
RBC = Red blood cells.
Determination of Packed Cell Volume (PCV)
PCV of the mice was measured before infecting them with parasite and after treatment. PCV was done to determine the effectiveness of the fractions in preventing hemolysis resulting from increasing parasitemia level associated with malaria. Heparinized capillary tubes were used for collection of blood from tail of each mouse. The capillary tubes were filled with blood up to 75% of their volume and sealed at the dry end with sealing clay. The tubes were placed in a microhematocrit centrifuge (Hawksley, England) with the sealed end outward and centrifuged for 5 min at 11,000 rpm [24]. The tubes were then taken out of the centrifuge and PCV was determined using a standard Micro-Hematocrit Reader (Hawksley, England).
Monitoring of Body Weight and Temperature Changes
Body weight of each mouse was measured before infection (day 0) and on day 8, using a sensitive digital weighing balance (OHAUS, USA). Rectal temperature was also measured with a digital thermometer before infection, and then daily. In order to evaluate the effect of the fractions on body weight and temperature, the fractions were administered to healthy (uninfected) mice at the doses of 25, 50, and 100 mg/kg b.w for 4 days.
Data Analysis
Graphpad prism Version 5.02 was used to analyze the data obtained and these were expressed as mean ± standard error of mean (SEM). The differences between means were compared using one-way Analysis of Variance, followed by Dunnet’s test. P < 0.05 was considered statistically significant.
RESULTS
Phytochemical Screening
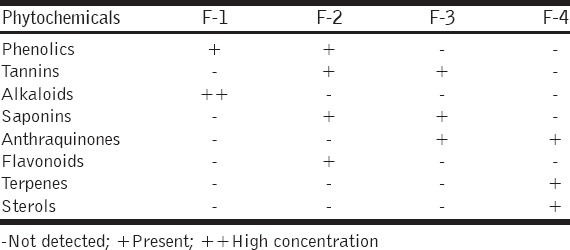
The summary of the phytochemical constituents of fractions of A. nilotica root extract, shown in Table 1, revealed that the fractions gave positive reactions to phenolic, tannins, alkaloids, anthraquinones, flavonoids, terpenes, and sterols. Fraction F-1 showed high amount of alkaloids compared with other three fractions [Table 1].
Table 1.
Phytochemical constituents of fractions (F-1, F-2, F-3, and F-4) of Acacia nilotica root
Antimalarial Activity of Fractions of A. nilotica; (F-1, F-2, F-3, and F-4)
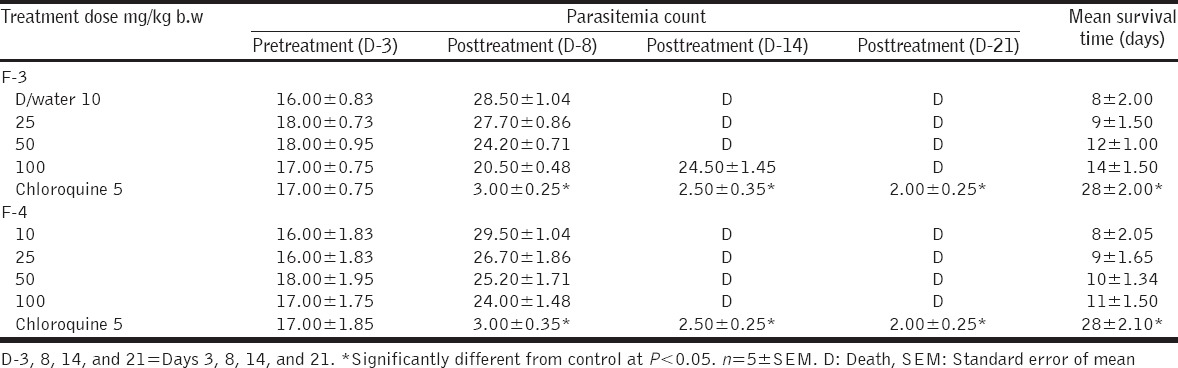
Fractions F-1 and F-2 caused significant and dose-dependent reduction in the mean parasite count at doses of 50 and 100 mg/kg b/w when compared with the control, although the reduction was less than chloroquine used as a standard drug [Table 2]. However, F-3 and F-4 did not produce significant reduction in parasite count at all the doses administered [Table 3]. Survival time was also prolonged by fraction 1 and 2, with fraction 1 producing a significantly prolonged survival time that was comparable to chloroquine [Table 2], unlike F-3 and F-4 that did not prolong the survival time.
Table 2.
Curative effect of fractions F-1 and F-2 of Acacia nilotica in Plasmodium berghei infected mice
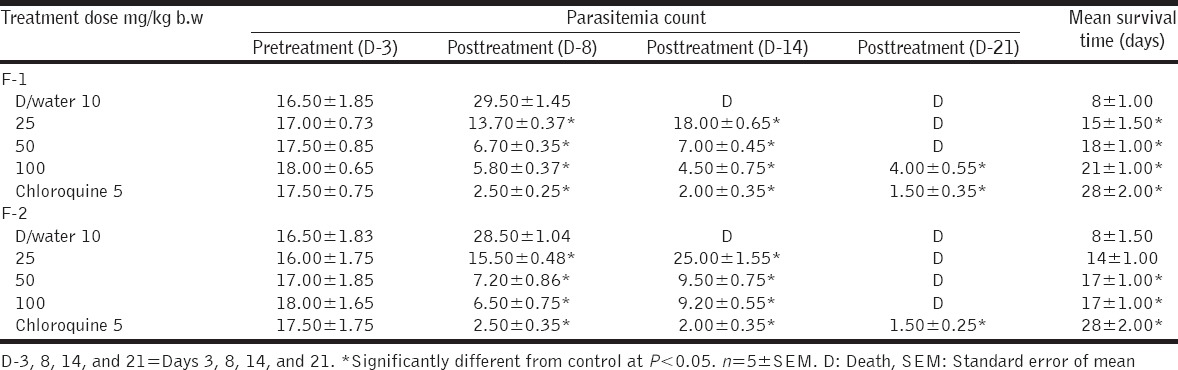
Table 3.
Curative effect of fractions F-3 and F-4 of Acacia nilotica in Plasmodium berghei infected mice
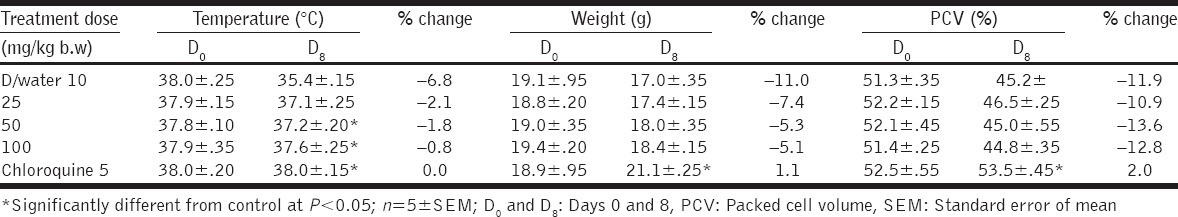
Fraction F-1 produced moderate weight gain and increased PCV at the dose of 100 mg/kg b/w; however, it did not prevent temperature lowering effects of malaria infection in mice [Table 4]. Fraction F-2 did not prevent weight loss, reduction in rectal temperature, and PCV in the treated mice [Table 5].
Table 4.
Temperature, weight, and PCV of Plasmodium berghei-infected mice treated with fraction F-1 of Acacia nilotica
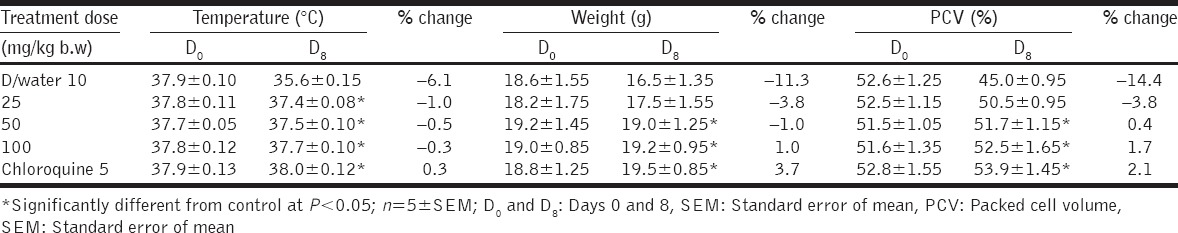
Table 5.
Temperature, weight, and PCV of Plasmodium berghei-infected mice treated with fraction F-2 of Acacia nilotica
DISCUSSION
P. berghei NK 65 was used in this study for inoculation and to predict antimalarial treatment outcome because of its ability to produce rodent model of malaria similar to human malaria infection [25,26]. Chloroquine, though no longer a first-line drug in the treatment of malaria, is used as control drug in this study because the Plasmodium parasite used for inoculation in this study is a chloroquine-sensitive strain. The Rane’s test is commonly used in antimalarial screening for evaluating the curative capability of extracts/drugs on established infections. This screening test was previously employed for the antiplasmodial screening of the crude extract of A. nilotica [17]. This study was carried out in-vivo, in order to factor in the possible effects of prodrug and involvement of immune system in the eradication of infection in a living host.
From the result of the antimalarial activity of the fractions in Table 2, fraction F-1 produced significant and dose-dependent antimalarial activity which was comparable to that of chloroquine, the control drug used. At the highest dose of 100 mg/kg b/w, F-1 was able to reduce parasite count from 18.0 to 4.0 (77.7% inhibition) compared to the standard drug which reduced the parasite count from 17.5 to 1.5 (91.4% inhibition). In the infected and untreated group, parasite count increased from 16.5 to 29.5. The percentage inhibition of the F-1, which is the closest (among the four fractions) to the standard drug, suggests that the active antimalarial compound in the crude extract of A. nilotica is localized in the F-1 fraction. Alkaloids and phenolic found in F-1 may possibly, individually or synergistically, account for its significant antimalarial activity, when compared with other three fractions. Many alkaloids derived from plants such as quinine and quinidine have been demonstrated to exhibit antimalarial activity [27,28]. Although fraction F-2 contain phenolic which may also account for some antimalarial activity observed (albeit lower than fraction F-1), the presence of saponin (phytodetergent) in this fraction, may account for the destruction of erythrocyte membrane manifesting as reduced PCV [29], which may also be responsible for the reduction in survival time of the F-2 infected mice [Table 2].
Anemia, change in body (rectal) temperature, and reduction in body weight are the general features of malarial infection in rodent (mice) and human malarial infection [30]. A potent antimalarial is expected to ameliorate anemia, prevent body weight loss, and stabilize temperature in infected mice with increasing parasitemia.
PCV was measured in this study to determine the effectiveness of fractions of A. nilotica in preventing malaria-induced hemolysis alongside its antimalarial activity. Malaria-induced hemolysis, either in human or rodents, may be due to rapid destruction/clearance of infected erythrocytes and/or sequestration of infected erythrocytes [30]. Fraction F-1 at doses of 50 and 100 mg/kg b/w produced significant increase in PCV [Table 4] when compared with the control, which suggests that this fraction of the extract could ameliorate anemia associated with malaria infection. The ability of this fraction to reverse reduction in PCV may be due to rapid clearance of parasite from infected erythrocytes before hemolysis occur or via enhanced erythropoiesis [29]. There was no reduction in PCV of mice at all the doses of fraction F-2 administered [Table 5]. The inability of fraction F-2 to improve the PCV of mice may be due to the presence of saponin in this fraction. Saponin is a phytodetergent known to destroy cell membrane by inducing cholesterol liberation from the cell membrane [31], resulting in erythrocyte hemolysis as manifested in the reduced PCV after administration of fraction F-2.
There was reduced rectal temperature with increasing parasitemia in the infected mice, in all the doses administered. The reduced rectal temperature could be attributed to reduced basal metabolic rate (observed during ongoing infection) or hypothermic effects of parasite multiplication. The highest dose of fraction F-1 at 100 mg/kg b.w, showed the best temperature stabilizing effect with the least reduction in rectal temperature in mice [Table 4]. Fraction F-2 could not stabilize the body temperature with increasing parasitemia as rectal temperature was significantly lower at all the doses of F-2 administered [Table 5].
Weight loss was recorded in all the doses of fractions F-1 and F-2 of A. nilotica administered to the mice except at 100 mg/kg b/w of F-2. This could either be due to reduced feed intake which may be attributed to appetite suppressing component of the extract such as saponin and tannins [32].
Our previous study showed that the crude aqueous extract of A. nilotica possess antimalarial activity [17]. Although the active antimalarial component of A. nilotica is not yet identified, the antimalarial activity of fractions F-1 of A. nilotica could be due to high proportion of alkaloid found in this fraction or synergistic effect of the alkaloids with the phenolic component. These phytochemicals have been documented to possess varying degree of antimalarial activity in plant extracts [10,16,32].
CONCLUSION
The results of this study showed that fraction F-1 of A. nilotica has significant antimalarial activity. This fraction was also able to improve the PCV of treated mice either by prevention of malaria-induce hemolysis or by enhancing erythropoiesis. However, F-1 could not reverse the reduced body temperature and weight loss associated with rodent malaria. The active antimalarial component of fraction F-1 of A. nilotica may be due to the alkaloid alone or combination of the alkaloid and the phenolic acting synergistically. This result also validate the antiplasmodial activity reported on the crude extract and the use of the A. nilotica root extract for treatment of malaria by local communities in Northern Nigeria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Federal Ministry of Health, (FMH) National Antimalarial Treatment Guidelines. Nigeria: Publication of Federal Ministry of Health Abuja; 2014. pp. 25–30. [Google Scholar]
- 2.World Health Organization (WHO) World Malaria Report. Geneva: World Health Organization; 2015. pp. 10–6. [Google Scholar]
- 3.Turschner S, Efferth T. Drug resistance in Plasmodium: Natural products in the fight against malaria. Mini Rev Med Chem. 2009;9:206–2124. doi: 10.2174/138955709787316074. [DOI] [PubMed] [Google Scholar]
- 4.Wells TN, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–91. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 5.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: A multisite prospective cohort study. Lancet Infect Dis. 2016;16:357–65. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 9.Kaur K, Jain M, Kaur T, Jain R. Antimalarials from nature. Bioorg Med Chem. 2009;17:3229–56. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira AB, Dolabela MF, Braga FC, Jácome RL, Varotti FP, Póvoa MM. Plant-derived antimalarial agents: New leads and efficient phythomedicines. Part I. Alkaloids. An Acad Bras Cienc. 2009;81:715–40. doi: 10.1590/s0001-37652009000400011. [DOI] [PubMed] [Google Scholar]
- 11.Bodeker G, Willcox M. New research initiative on plant-based antimalarials. Lancet. 2000;355:761. doi: 10.1016/s0140-6736(05)72181-4. [DOI] [PubMed] [Google Scholar]
- 12.Bargal K, Bargali SS. Acacia nilotica: A multipurpose leguminous plant. Nat Sci. 2009;7:11–9. [Google Scholar]
- 13.Van Wyk P. Field Guide to trees of Southern Africa. Vol. 2. Struik, Cape Town: Penguin Random House South Africa; 2000. pp. 25–7. [Google Scholar]
- 14.Oladosu P, Samuel BB, Okhale SE, Ibrahim K, Okogun JI. Anti-tubercular activity of the dried fruits of Acacia nilotica. J. Phytomed Ther. 2007;12:76–9. [Google Scholar]
- 15.Ali SI, Faruqi SA. Hybridization in Acacia nilotica complex. Pak J Bot. 1969;1:119–28. [Google Scholar]
- 16.Adebayo JO, Krettli AU. Potential antimalarials from Nigerian plants: A review. J Ethnopharmacol. 2011;133:289–302. doi: 10.1016/j.jep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Alli LA, Adesokan AA, Salawu OA, Akanji MA, Tijani AY. Anti-plasmodial activity of aqueous root extract of Acacia nilotica. Afr J Biochem Res. 2011;5:214–9. [Google Scholar]
- 18.Adzu B, Haruna AK, Salawu OA, Sule A. Bioassay-guided evaluation of the antidiarrhoeal potentials of Zizyphus spina-christi root bark in rats. Int J Biol Chem Sci. 2007;1:15–20. [Google Scholar]
- 19.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. Ibadan, Nigeria: Spectrum Books Limited; 1993. pp. 151–3. [Google Scholar]
- 20.Salawu OA, Chindo BA, Tijani AY, Adzu B. Analgesic, anti-inflammatory, antipyretic and antiplasmodial effects of the methanolic extract of Crossopteryx febrifuga. J Med Plant Res. 2008;2:213–8. [Google Scholar]
- 21.Ryley JF, Peters W. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 1970;64:209–22. doi: 10.1080/00034983.1970.11686683. [DOI] [PubMed] [Google Scholar]
- 22.Saidu K, Onah J, Orisadipe A, Olusola A, Wambebe C, Gamaniel K. Antiplasmodial, analgesic and anti-inflammatory activities of the aqueous stem bark of Erythrina senegalensis. J. Ethnopharmacol. 2000;71:275–80. doi: 10.1016/s0378-8741(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 23.Adzu B, Salawu OA. Screening Diospyros mespiliformis extract for antimalarial potency. Int J Biol Chem Sci. 2009;3:271–6. [Google Scholar]
- 24.Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hochst. (Euphorbiaceae) against plasmodium berghei in mice. BMC Complement Altern Med. 2014;14:79. doi: 10.1186/1472-6882-14-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomas AM, van der Wel AM, Thomas AW, Janse CJ, Waters AP. Transfection systems for animal models of malaria. Parasitol Today. 1998;14:245–9. doi: 10.1016/s0169-4758(98)01248-4. [DOI] [PubMed] [Google Scholar]
- 26.Willcox ML, Bodeker G. Traditional herbal medicines for malaria. BMJ. 2004;329:1156–9. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman DJ. Natural products as leads to potential drugs: An old process or the new hope for drug discovery? J Med Chem. 2008;51:2589–99. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]
- 28.Yang ZG, Sun HX, Fang WH. Hemolytic activities and adjuvant effect of Astragalus membranaceus saponins on the immune responses to ovalbumin in mice. Vaccine. 2005;23:5196–203. doi: 10.1016/j.vaccine.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Lamikanra AA, Brown D, Potocnik A, Casals-Pascual C, Langhorne J, Roberts DJ. Malarial anemia: Of mice and men. Blood. 2007;110:18–28. doi: 10.1182/blood-2006-09-018069. [DOI] [PubMed] [Google Scholar]
- 30.Böttger S, Melzig MF. The influence of saponins on cell membrane cholesterol. Bioorg Med Chem. 2013;21:7118–24. doi: 10.1016/j.bmc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Yun JW. Possible anti-obesity therapeutics from nature - A review. Phytochemistry. 2010;71:1625–41. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod. 2003;66:1022–37. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]


