Abstract
Background:
Hibiscus sabdariffa L. is a plant native to tropical Africa and intensively cultivated in Sudan. Its calyces are widely consumed with many uses in Sudanese folk medicine.
Materials and Methods:
The dried calyces of H. sabdariffa were subjected to soak in 80% v/v methanol to get the methanolic extract, which was tested against five Gram-negative and three Gram-positive referenced bacterial strains using disc diffusion method. Selected bioactive phytochemical compounds were also investigated using qualitative methods.
Results:
The results of the antibacterial test indicate that the methanol extract of H. sabdariffa calyces contained effective antibacterial agent(s), revealed a considerable zone of inhibition against all tested Gram-negative and Gram-positive bacteria, and it was a competitor to gentamicin and greatly higher than penicillin which showed weak or no effect.
Conclusion:
The results of current investigation support the folk medicine application of this plant against different microbial ailments and suggest it as a promising source for new antibacterial agents.
Keywords: Antibacterial, disk diffusion, Hibiscus sabdariffa, methanol extract, phytochemical
INTRODUCTION
The battle with diseases started since the advent of man on earth and it will continue in an endless warfare. The discovery of antibiotics in the 1950s has turned the result of this war in favor of man, but few years later microbes returned with mutant strains, resistant to almost all inventive antibiotics. This forced scientists searching for new alternatives to be used against these adaptable microorganisms. Stupendous plants were the main renewable source for medications since times immemorial. Yet, many drugs from the modern medicine are originally derived from ancient herbal medicine. Currently, the dramatic increase in resistance of pathogens to current antibiotics leads to the requisite need for new antimicrobial agents [1,2].
Plants, particularly those prescribed against microbial infections since a long time in traditional and folk medicine from different societies could be promising sources for new antimicrobials [3]. In Sudan, the majority of Sudanese people, like many African countries, are still relying on traditional or folk medicine in treatment of diseases which are an integral part of an informal healthcare system, although this popular folk medicine has roots from Islamic and West African medicine [4]. Roselle (Hibiscus sabdariffa L) belongs to Malvaceae family, it is an annual tropical small shrub native to Africa and also distributed in Southeast Asia and Central America [5,6], it is Known locally as Karkadeh. The macerate of the red calyces of this plant [Figure 1] is one of the most famous public Sudanese beverages all over this country and all Sudanese people know and use, which are consumed as hot or cold drinks for the treatment of respiratory tract infections, hypertension, colds, and fever. It is also mixed with other plants to treat malaria [7,8].
Figure 1.

The dried calyces of Hibiscus sabdariffa
Internationally, H. sabdariffa is well known, many parts of Hibiscus sabdariffa is employed and prescribed in traditional medicine in many countries such as African countries, India, Mexico, Brazil, China, and Iran [9]; leaves which eaten as vegetables are diuretic, antiseptic, digestive, purgative, sedative, demulcent, and astringent [10]; calyces are used for treating of hypertension, liver disorders, diuretic, digestive, and sedative [5,11,12]; seeds are rarely mentioned in traditional medicine compared to the other parts of Hibiscus, but seeds are roasted and consumed as food, also used traditionally as debility, diuretic, laxative, and tonic [13]. Although Sudan is the biggest African countries cultivated H. sabdariffa but scientific studies on this product is not adequate. The red Hibiscus calyces were found to have a pigment called anthocyanins; it is also rich in polyphenolic compounds such as flavonoids and phenolic acids such as gallic and protocatechuic acid [14]. The objective of this study was to evaluate the antibacterial potential of the calyces of the Sudanese Roselle (H. sabdariffa) which intensively used in Sudanese folk medicine.
MATERIALS AND METHODS
Plant Material
Calyces of H. sabdariffa were purchased from local herbal markets in Khartoum, Sudan [Figure 1]; Dried calyces were crushed into fine powders, kept dry in a clean, well-tightened glass container until used.
Preparation of Methanol Extract
A total of 100 g of the dried powder of H. sabdariffa calyces was weighted and put in a sterile glass container, 500 ml of 80% methanol (to serve as hydroalcoholic solvent) was added gradually and soaked, and then the container was well tightened and kept in the refrigerator to avoid any microbial contamination or fungal growth. The closed container was subjected to frequent shaking (2-3 times a day) and macerated for up to 3 days. Then, the macerate was filtered using Whatman filter papers No.1; the filtrate was put in the incubator and allowed to evaporate at 45°C for up to 10 days to get a semi-solid extract. For antibacterial testing, the extract was reconstituted in absolute methanol to get a working concentration (500 mg/ml) and kept in a closed dark container until used.
Microorganisms
Eight referenced bacterial strains were used in testing the antibacterial activity of the methanol extract of H. sabdariffa calyces. These bacteria are representing five Gram-negatives (Escherichia coli ATCC 25922, Salmonella enteric ATCC 5174, Klebsiella pneumonia ATCC 27736, Proteus vulgaris ATCC 49132, and Pseudomonas aeruginosa ATCC 27853) and three Gram-positives (Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 49461, and Bacillus cereus ATCC 10876). Bacterial strains were purchased from Watin-Biolife, KSA.
Inoculum Preparation
Bacterial strains were cultured following the manufacturer’s instructions. Bacterial cultures were identified microscopically and biochemically, then sub-cultured in sterile bottles containing Nutrient Broth (Scharlab, S.L., and Spain) and incubated overnight at 37°C. Prior of the antibacterial experiment, the working bacterial samples were adjusted to 0.5 McFarland to be equivalent to about 1-2 × 108 CFU/ml.
Antibacterial Assay
The antibacterial activity of methanol extract of H. sabdariffa calyces was evaluated by disc diffusion method as mentioned in Abdallah and Al-Harbi [15]; 100 μl from each working bacterial cultures were mixed with 20 ml warm autoclaved Mueller-Hinton agar (Watin-Biolife, KSA) in glass bottles size 50 ml, tighten and mixed well and then poured directly into 90 mm sterile plastic disposable plates (Jalil Medicals) and left to solidify at room temperature. 6 mm discs were previously prepared using Whatman No.1 filter paper, sterilized and saturated with 300 mg/ml of the methanol extract of H. sabdariffa calyces, the pre-experimental test showed that the blank disk (6 mm) could carry 20 μl of the extract at a concentration of 500 mg/ml, which equivalent to 10 mg/disc. Then, the wet, saturated disks were directly loaded on the cultured Mueller-Hinton Agar plate in aseptic conditions. On the same plate, 6 mm antibiotic discs; penicillin G 10 units and gentamicin 10 μg (Oxoid) were also loaded, which served as positive controls. Cultured plates with extract and antibiotic discs were incubated for about 24 h at 37°C. The mean zone of inhibition of two replicated disks on the same plate was taken in millimeter (mm) using a ruler, 6 mm zone diameter considered as no inhibition.
Preliminary Phytochemical Analysis
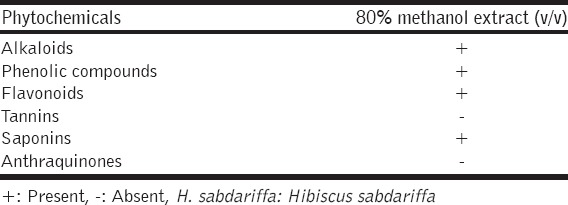
The phytochemical constituents of the same methanol extract which used in antibacterial testing were evaluated qualitatively as described elsewhere, for alkaloids (Mayer’s test) [16], saponins (Foam test), tannins (Ferric chloride test), anthraquinones (Bornträger test) [17], phenolic compounds, and flavonoids were also investigated [18].
Statistical Analysis
All the statistical analyzes were performed with the SPSS 17.0 (SPSS Inc., Chicago, USA) statistical package, variables considered significant at P < 0.05.
RESULTS AND DISCUSSION
The current study revealed the considerable antibacterial activity of methanol extract of H. sabdariffa calyces against all tested bacteria, particularly the Gram-positives, as shown in Tables 1 and 2, respectively. Disc inhibition zones above 10 mm for the tested crude extract considered a good antibacterial activity (Blank disc diameter 6 mm). Interestingly, the investigated extract was found to inhibit the growth of all tested Gram-positives and Gram-negatives, giving clear, obvious zones by disc diffusion method compared with the antibiotics [Figure 2], being statistically significant (P < 0.05). The highest antibacterial activity of H. sabdariffa calyces was recorded by S. aureus (18.5 ± 0.5 mm), followed by S. epidermidis (17.5 ± 1.5 mm), S. enteric (17.5 ± 1.5 mm), K. pneumonia (17.5 ± 0.5 mm), P. aeruginosa (15.5 ± 0.5 mm), E. coli (14.5±0.5 mm), P. vulgaris (14.5±0.5 mm), and B. cereus (13.5 ± 1.5 mm), respectively. Similar several previous studies were reported on the calyces of H. sabdariffa using different solvents, concentrations, and bacterial strains (clinical and referenced strains); Borrás-Linares et al. [19] published that the ethanol extract from 25 varieties of mexican H. sabdariffa calyces was effective against all Gram-negatives (E. coli and S. enteritidis) and Gram-positives (S. aureus and Micrococcus luteus), the greater effect was against the Gram-positive bacteria. Its ethanol extract was potent against bacteria isolated from wastewater, particularly P. aeruginosa [20]. Aqueous, ethanol, and methanol extracts H. sabdariffa calyxes’ revealed good antibacterial activity against Salmonella cultures [6]. Its 80% methanol extract was also found active against Escherichia coli O157:H7, a major foodborne pathogen [21] and suggested as potential antibacterial in foods [22]. The current investigation and the majority of previous published studies unanimously agreed that H. sabdariffa calyces which collected from different localities around the world have effective antibacterial properties; this feature makes it a unique and promising antibacterial plant. It is known that the bioactivity of the extracts from the same plant species may vary according to the plant extraction process which affect greatly by many factors such as type of solvents, method of extraction, temperature (hot or cold liquids), age of the plant and season of harvesting [23], this stability of H. sabdariffa calyces as distinguished antibacterial agent is very interesting. Moreover, as represented in Figure 3, the antibacterial activity of the crude methanol extract of H. sabdariffa calyces (10 mg/disc) was significantly higher than penicillin G (10 units), and non-significant statistically when compared with the gentamicin (10 μg/disc), meaning that this plant extract is competitor to gentamicin. This is interesting since antibiotics are suffering from deterioration in its effectiveness around the globe [24], S. aureus is one of the most prevalent antibiotics resistant pathogens worldwide, particularly the methicillin-resistant S. aureus [25]. More antibacterial studies on the effects of the calyces of H. sabdariffa on this bacterium in particular are recommended. These considerable antibacterial properties of the methanol extract of H. sabdariffa calyces (80% v/v) are attributed to some bioactive phytochemical constituents present in this extract. Table 3 shows that the methanolic extract is rich in phytochemicals such as alkaloids, phenolic compounds, flavonoids, and saponins while tannins and anthraquinones did not detect. This is in agreement with Djeussi et al. [26], who found many phytochemicals in the methanol extract of H. sabdariffa calyces such as alkaloids, flavonoids, phenols, polyphenols, saponins, triterpenes, and sterols but no anthraquinones, tannins, or anthocyanins. On the other side, Suliman et al. [27] figure out many bioactive phytochemicals in the aqueous extract of H. sabdariffa calyces such as saponins, phlobatannins, terpenoids, anthraquinones, tannins, steroids, and phenolic compounds. However, the antibacterial agent(s) in the calyces are not determined yet, it was believed that its antibacterial activity could be attributed to anthocyanins [28], but it was found that this antibacterial activity was high with H. sabdariffa calyces lacking totally these anthocyanins [6]. Accordingly, more future studies should be conducted to find out and isolate a single compound or group of compounds which could serve as an antibacterial drug. Certainly, additional pharmacological, microbiological, and toxicological studies are recommended.
Table 1.
The antibacterial activity of methanol extract of H. sabdariffa calyces by disc diffusion method (10 mg/disc) against Gram-positive bacteria
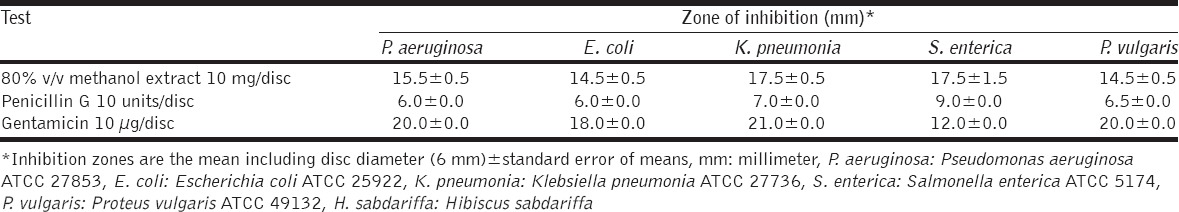
Table 2.
The antibacterial activity of methanol extract of H. sabdariffa calyces by disc diffusion method (10 mg/disc) against Gram-negative bacteria
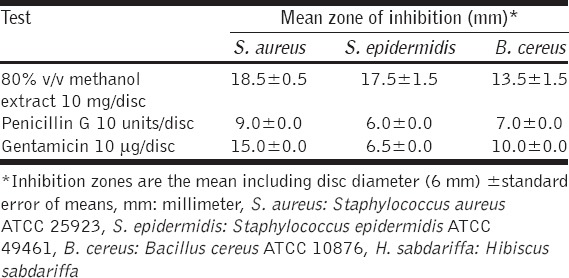
Figure 2.
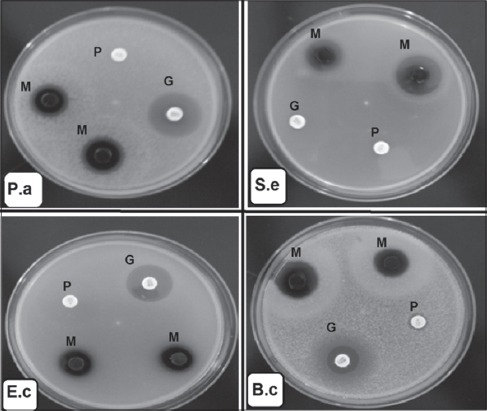
Representative photos showing the antibacterial activity of the methanol extract of Hibiscus sabdariffa calyces at concentration 500 mg/ml (10 mg/disc). P: Penicillin G 10 units/disc, G: Gentamicin 10 μg/disc, M: 80% Methanol extract of Hibiscus sabdariffa calyces 10 μg/disc, P.a: Pseudomonas aeruginosa ATCC 27853, S.e: Staphylococcus epidermidis ATCC 49461, E.c: Escherichia coli ATCC 25922, B.c: Bacillus cereus ATCC 10876
Figure 3.
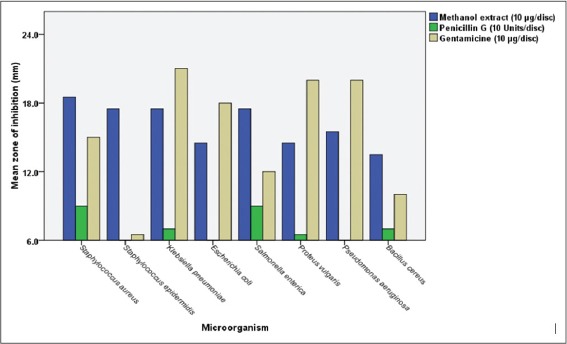
Comparison between the antibacterial effects of methanol extract Hibiscus sabdariffa and antibiotics
Table 3.
The phytochemical analysis of 80% methanol extract (v/v) of H. sabdariffa calyces
CONCLUSION
The results of the current study support the widespread use of this popular plant in Sudanese folk medicine, particularly against some illnesses related to microbial infections. The output of this investigation also introduces the calyces of H. sabdariffa as one of the most promising sources for new natural and effective antibacterial drugs competitor to antibiotics.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Falagas ME, Bliziotis IA. Pandrug -resistant gram-negative bacteria: The dawn of the post-antibiotic era? Int J Antimicrob Agents. 2007;29:630–6. doi: 10.1016/j.ijantimicag.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Viens AM, Littmann J. Is Antimicrobial resistance a slowly emerging disaster? Public Health Ethics. 2015;8:255–265. doi: 10.1093/phe/phv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdallah EM. Plants: An alternative source for antimicrobials. J Appl Pharm Sci. 2011;1:16–20. [Google Scholar]
- 4.WHO. Legal Status of Traditional Medicine and Complementary Alternative Medicine: A Worldwide Review. Geneva: World Health Organization; 2001. [Google Scholar]
- 5.Voon HC, Bhat R, Rusul C. Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Compr Rev Food Sci Food Safe. 2012;11:34–55. [Google Scholar]
- 6.Cabrera MM, Morales JH, Rúelas GL, Moreno YS, Rojas LS, Rosas JC. Influence of variety and extraction solvent on antibacterial activity of Roselle (Hibiscus sabdariffa L.) calyxes. J Med Plants Res. 2013;7:2319–22. [Google Scholar]
- 7.Khalid H, Abdalla WE, Abdelgadir H, Opatz T, Efferth T. Gems from traditional North-African medicine: Medicinal and aromatic plants from Sudan. Nat Prod Bioprospect. 2012;2:92–103. [Google Scholar]
- 8.El-Kamali HH, Mohammed MF. Antibacterial activity of Hibiscus sabdariffa Acacia seyal var. seyal and Sphaeranthus suaveolens var suaveolens against upper respiratory tract pathogens. Sudan J Med Sci. 2006;1:121–7. [Google Scholar]
- 9.Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M. Hibiscus sabdariffa L. A phytochemical and pharmacological review. Food Chem. 2014;165:424–43. doi: 10.1016/j.foodchem.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Obouayeba AP, Djyh NB, Diabate S, Djaman AJ, N'Guessan JD, Kone M, et al. Phytochemical and antioxidant activity of Roselle (Hibiscus Sabdariffa L.) petal extracts. Res J Pharm Bio Chem Sci. 2014;5:1453–65. [Google Scholar]
- 11.Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP, Tseng TH. Protective effect of Hibiscus anthocyanins against tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food Chem Toxicol. 2000;38:411–6. doi: 10.1016/s0278-6915(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 12.Ewansiha JU. Evaluation of the antimicrobial activity of Roselle (Hibiscus sabdariffa L.) leaf extracts and its phytochemical properties. Peak J Med Plant Res. 2014;2:1–5. [Google Scholar]
- 13.Ismail A, Ikram EH, Nazri HS. Roselle (Hibiscus sabdariffa L.) seeds-nutritional composition, protein quality and health benefits. Food. 2008;2:1–16. [Google Scholar]
- 14.Higginbotham KL, Burris KP, Zivanovic S, Davidson PM, Stewart CN. Antimicrobial activity of Hibiscus sabdariffa aqueous extracts against Escherichia coli O157: H7 and Staphylococcus aureus in a microbiological medium and milk of various fat concentrations. J Food Prot. 2014;77:262–8. doi: 10.4315/0362-028X.JFP-13-313. [DOI] [PubMed] [Google Scholar]
- 15.Abdallah EM, Al-Harbi KA. Phytochemical screening and antibacterial activity of crude aqueous and ethanol extracts of Salvadora persica L. stem (Miswak) from Saudi Arabia. J Phytopharm. 2015;4:243–7. [Google Scholar]
- 16.Deshpande SN. Preliminary phytochemical analysis and in vitro investigation of antibacterial activity of Acacia nilotica against clinical isolates. J Pharmacogphytochem. 2013;1:23–7. [Google Scholar]
- 17.Stahl E. Drug analysis by Chromatography and Microscopy. Michigan, USA: Ann Arbor Scientific Publishers, Inc; 1973. [Google Scholar]
- 18.Solihah MA, Rosli WI, Nurhanan AR. Phytochemical screening and total phenolic content of Malaysian Zea mays hair extracts. Int Food Res J. 2012;19:1533–8. [Google Scholar]
- 19.Linares IB, Arroyo SF, Roman DA, Suárez PA, Díaz RD, Gonzáles IA, et al. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa) Ind Crops Prod. 2015;69:385–94. [Google Scholar]
- 20.Khalaphallah R, Soliman WS. Effect of henna and Roselle extracts on pathogenic bacteria. Asian Pac J Trop Dis. 2014;4:292–6. [Google Scholar]
- 21.Fullerton M, Khatiwada J, Johnson JU, Davis S, Williams LL. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Escherichia coli O157: H7 isolated from food, veterinary, and clinical samples. J Med Food. 2011;14:950–6. doi: 10.1089/jmf.2010.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaroni D, Ravishankar S. Bactericidal effects of Roselle (Hibiscus sabdariffa) against foodborne pathogens in vitro and on romaine lettuce and alfalfa sprouts. Qual Assur Safe Crops Foods. 2012;4:33–40. [Google Scholar]
- 23.Ogu GI, Tanimowo WO, Nwachukwu PU, Igere BE. Antimicrobial and phytochemical evaluation of the leaf, stem bark and root extracts of Cyathulaprostrata (L) Blume against some human pathogens. J Intercult Ethnopharmacol. 2012;1:35–43. [Google Scholar]
- 24.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013;13:1057–98. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 25.Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, et al. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents. 2012;39:273–82. doi: 10.1016/j.ijantimicag.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 26.Djeussi DE, Noumedem JA, Seukep JA, Fankam AG, Voukeng IK, Tankeo SB, et al. Antibacterial activities of selected edible plants extracts against multidrug-resistant gram-negative bacteria. BMC Compl Altern Med. 2013;13:164. doi: 10.1186/1472-6882-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulaiman FA, Kazeem Mo, Waheed AM, Temowo SO, Azeez IO, Zubair FI, et al. Antimicrobial and toxic potential of aqueous extracts of Allium sativum Hibiscus sabdariffa and Zingiberofficinale in Wistar rats. J Taibah Univ Sci. 2014;8:315–22. [Google Scholar]
- 28.Cahliková L, Ali BH, Havliková L, Locárek M, Siatka T, Opletal L, et al. Anthocyanins of Hibiscus sabdiffera calyces from Sudan. Nat Prod Commun. 2015;10:77–9. [PubMed] [Google Scholar]


