ABSTRACT
Autophagy is a process of bulk protein degradation and organelle turnover, and is a current therapeutic target in several diseases. The present study aimed to clarify the significance of myocardial autophagy of patients with dilated cardiomyopathy (DCM). Left ventricular endomyocardial biopsy was performed in 250 consecutive patients with DCM (54.9±13.9 years; male, 79%), initially presenting with decompensated heart failure (HF). The association of these findings with HF mortality or recurrence was examined. Myofilament changes, which are apparent in the degenerated cardiomyocytes of DCM, were recognized in 164 patients (66%), and autophagic vacuoles in cardiomyocytes were identified in or near the area of myofilament changes in 86 patients (34%). Morphometrically, fibrosis (odds ratio [OR], 0.96; 95% confidence interval [CI], 0.93 to 0.99) and mitochondrial abnormality (OR, 2.24; 95% CI, 1.23 to 4.08) were independently related with autophagic vacuoles. During the follow-up period of 4.9±3.9 y, 24 patients (10%) died, including 10 (4%) who died of HF, and 67 (27%) were readmitted for HF recurrence. Multivariate analysis identified a family history of DCM (hazard ratio [HR], 2.117; 95% CI, 1.199 to 3.738), hemoglobin level (HR, 0.845; 95% CI, 0.749 to 0.953), myofilament changes (HR, 13.525; 95% CI, 5.340 to 34.255), and autophagic vacuoles (HR, 0.214; 95% CI, 0.114 to 0.400) as independent predictors of death or readmission due to HF recurrence. In conclusion, autophagic vacuoles in cardiomyocytes are associated with a better HF prognosis in patients with DCM, suggesting autophagy may play a role in the prevention of myocardial degeneration.
KEYWORDS: Autophagy, dilated cardiomyopathy, electron microscopy, endomyocardial biopsy, heart failure, prognosis
Introduction
Macroautophagy (hereafter referred to as autophagy), a lysosomal degradation pathway, is a process of bulk protein decomposition and organelle turnover. It is a housekeeping process to degrade long-lived proteins and remove damaged organelles. Autophagy plays an important role in the developmental process and the physiological responses to aging, as well as human diseases such as cancer, neurodegeneration, and many other illnesses.1 Recently, an increasing number of studies have investigated the mediation and control of autophagy as a therapeutic target in a number of organs. However the pathologic basis of the role of autophagy remains controversial and it is unclear whether it works adaptively or maladaptively for human health.2
Heart failure (HF) is the most significant cardiovascular disorder encountered in clinical practice. It impairs cardiac function and carries increased risks of hospital admission and mortality.3 Dilated cardiomyopathy (DCM), which is a major cause of HF, is a severe disease characterized by enlarged ventricles with systolic dysfunction.4 Autophagic vacuoles, a morphological term describing autophagosomes or autolysosomes because it is not always possible using only morphological methods to confirm whether or not an autophagic structure has fused with a lysosome,5 have been easily recognized in the cardiomyocytes of patients with DCM by electron microscopy.6,7 However, it has not been fully investigated whether autophagy plays a role in cardioprotection or is just a sign indicating damage to cardiomyocytes in a failing human heart, as it has in other organs. The aim of this study was to clarify the significance of myocardial autophagy in patients with DCM presenting initially with decompensated HF.
Results
Autophagic vacuoles and ultrastructural findings in the cardiomyocytes of patients with DCM
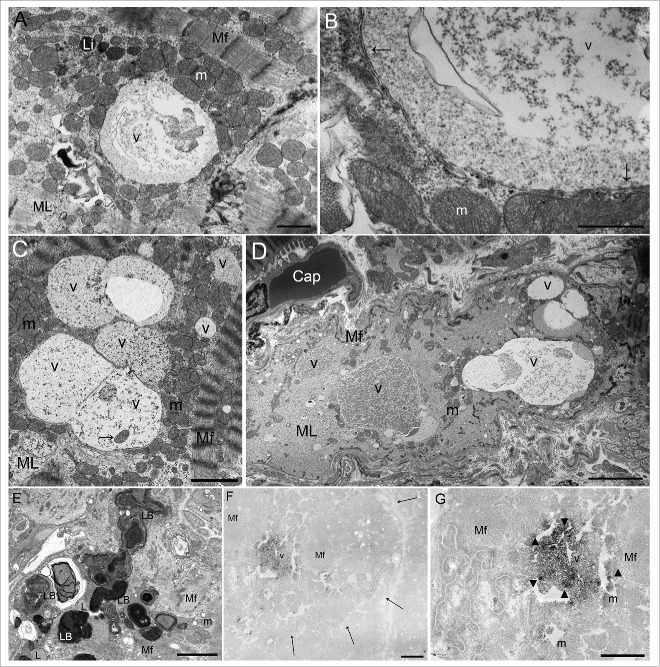
Baseline clinical characteristics of the patients, classified into 3 groups by the presence or absence of myofilament change and autophagic vacuoles, are summarized in Table 1. Autophagic vacuoles were defined as double-membrane-bound bodies containing glycogen granules, ribosomes, damaged mitochondria, and other degenerated organelles (Fig. 1A to D). The autophagic vacuoles were of various sizes (1 to 10 μm), and were identified only in the cardiomyocytes that were positive for myofilament change. Moreover, they were seen in or near the areas of myofilament changes, which were apparent in degenerated cardiomyocytes. Myofilament change was characterized by focal derangement of myofilaments, or by the disappearance of many myofilaments in cardiomyocytes with some poorly surviving myofilaments. Myofilament changes were recognized in 164 patients (66%), and autophagic vacuoles were present in 86 patients (34%). There was no patient positive for autophagy and negative for myofilament change.
Table 1.
Patient characteristics.
| Myofilament change (−)Autophagic vacuole (−) | Myofilament change (+)Autophagic vacuole (−) | Myofilament change (+)Autophagic vacuole (+) | ||
|---|---|---|---|---|
| (n = 86) | (n = 78) | (n = 86) | P Value | |
| Clinical characteristics | ||||
| Age (years) | 51.5 ± 14.3 | 55.7 ± 14.6 | 57.7 ± 12.3 | 0.011 |
| No. men | 62 (72%) | 66 (85%) | 69 (80%) | 0.136 |
| Body mass index, mean (kg/m2) | 26.2 ± 6.5 | 25.9 ± 7.3 | 24.0 ± 3.8 | 0.193 |
| Systolic blood pressure (mmHg) | 140.0 ± 33.2 | 130.5 ± 25.5 | 132.9 ± 27.7 | 0.237 |
| Diastolic blood pressure (mmHg) | 82.9 ± 22.5 | 81.8 ± 18.3 | 78.5 ± 19.0 | 0.377 |
| Heart rate (b.p.m.) | 89.4 ± 25.8 | 89.7 ± 28.2 | 96.4 ± 29.9 | 0.163 |
| NYHA scale III or IV | 36 (42%) | 37 (47%) | 41 (48%) | 0.692 |
| Family history of DCM | 9 (10%) | 19 (24%) | 11 (13%) | 0.034 |
| Comorbidities | ||||
| Atrial fibrillation | 24 (28%) | 37 (47%) | 32 (37%) | 0.036 |
| Hypertension | 39 (45%) | 42 (54%) | 47 (55%) | 0.407 |
| Diabetes | 30 (35%) | 26 (33%) | 34 (44%) | 0.687 |
| Renal dysfunctiona | 18 (21%) | 20 (26%) | 25 (29%) | 0.468 |
| Clinical chemistry | ||||
| B-type natriuretic peptide (pg/mL) | 573.3 ± 634.2 | 744.0 ± 660.4 | 873.3 ± 1036.4 | 0.025 |
| C-reactive protein (mg/dL) | 1.9 ± 4.1 | 1.6 ± 5.0 | 1.4 ± 2.5 | 0.937 |
| Fasting blood-sugar (mg/dL) | 108.6 ± 31.2 | 103.5 ± 28.6 | 114.6 ± 45.4 | 0.199 |
| Hemoglobin A1c (%) | 5.7 ± 0.9 | 5.8 ± 1.0 | 6.1 ± 1.4 | 0.074 |
| Creatinine (mg/dL) | 1.1 ± 1.2 | 1.2 ± 1.4 | 1.2 ± 1.6 | 0.307 |
| Hemoglobin (g/dL) | 14.2 ± 2.1 | 13.9 ± 2.1 | 13.6 ± 2.1 | 0.170 |
| Total bilirubin (mg/dL) | 1.0 ± 0.6 | 0.9 ± 0.5 | 0.9 ± 0.6 | 0.960 |
| Echocardiographic data | ||||
| Left atrial dimension (mm) | 44.1 ± 7.4 | 45.8 ± 7.3 | 43.5 ± 7.4 | 0.067 |
| Left ventricular ejection fraction (%) | 33.3 ± 14.1 | 29.6 ± 11.4 | 31.8 ± 13.2 | 0.245 |
| Left ventricular diastolic dimension (mm) | 60.8 ± 8.2 | 63.0 ± 11.4 | 60.7 ± 9.2 | 0.173 |
| Left ventricular systolic dimension (mm) | 50.9 ± 9.9 | 54.2 ± 9.0 | 51.5 ± 10.7 | 0.085 |
| Interventricular septum thickness (mm) | 10.2 ± 2.5 | 9.7 ± 2.1 | 9.7 ± 1.8 | 0.506 |
| Posterior wall thickness (mm) | 10.0 ± 2.1 | 9.6 ± 2.1 | 9.6 ± 1.6 | 0.313 |
| Catheterization data | ||||
| Left ventricular end diastolic pressure (mmHg) | 13.4 ± 8.3 | 14.6 ± 8.7 | 13.1 ± 7.2 | 0.517 |
| Mean pulmonary arterial pressure (mmHg) | 20.5 ± 8.1 | 21.7 ± 9.2 | 20.4 ± 9.1 | 0.513 |
| Right atrial pressure (mmHg) | 7.4 ± 3.8 | 8.4 ± 5.0 | 7.8 ± 5.9 | 0.445 |
| Pulmonary capillary wedge pressure (mmHg) | 14.0 ± 8.0 | 14.8 ± 8.8 | 13.1 ± 8.4 | 0.495 |
| Cardiac index (L/min/m2) | 2.9 ± 0.9 | 2.7 ± 0.7 | 2.7 ± 0.8 | 0.361 |
| Medication at admission | ||||
| ACE-Is or ARBs | 14 (16%) | 7 (9%) | 6 (7%) | 0.120 |
| Treatment in acute phase of heart failure | ||||
| Intra-aortic balloon pumping | 1 (1%) | 3 (4%) | 2 (2%) | 0.534 |
| Respirator support | 10 (12%) | 9 (12%) | 13 (15%) | 0.731 |
| Catecholamine infusion | 9 (10%) | 9 (15%) | 12 (14%) | 0.772 |
| Medication in follow up period | ||||
| ACE-Is or ARBs | 73 (85%) | 68 (87%) | 76 (88%) | 0.791 |
| Beta-blockers | 60 (70%) | 57 (73%) | 66 (77%) | 0.588 |
| Diuretics | 61 (71%) | 58 (74%) | 68 (79%) | 0.467 |
| Aldosterone receptor antagonists | 42 (49%) | 46 (58%) | 52 (60%) | 0.251 |
| Outcome of histopathological morphometry | ||||
| Cellular diameter (μm) | 16.9 ± 1.7 | 17.7 ± 1.9 | 17.6 ± 1.9 | 0.008 |
| Perinuclear halo | 33 (38%) | 43 (55%) | 43 (50%) | 0.087 |
| Nuclear diameter (μm) | 7.9 ± 0.8 | 8.0 ± 0.8 | 7.9 ± 0.8 | 0.483 |
| Proportion of fibrosis (%) | 11.2 ± 10.6 | 11.7 ± 10.3 | 8.4 ± 6.9 | 0.174 |
| Ultrastructural variables | ||||
| Mitochondrial abnormality | 21 (24%) | 14 (18%) | 32 (37%) | 0.017 |
| Aggregation of smooth endoplasmic reticulum | 22 (26%) | 15 (19%) | 29 (34%) | 0.107 |
| Lamellar body | 9 (10%) | 14 (18%) | 21 (24%) | 0.056 |
Data presented as mean ± SD or as the number of patients, with percentages in parentheses, as appropriate.
Continuous variables were evaluated by two-way repeated-measures analysis of variance in the case of normally distributed data and by the Kruskal-Wallis test if data were not normally distributed. Discontinuous variables were compared using the Chi-squared test.
Renal dysfunction was classified as glomerular filtration rate <60 mL/min/1.73 m2.
NYHA, New York Heart Association; DCM, dilated cardiomyopathy; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Figure 1.
Ultrastructural findings of autophagic vacuoles in cardiomyocytes. (A) An autophagic vacuole (v) in the myofilament (Mf) lysis area (ML); this vacuole contains abundant glycogen granules and ribosomes. Li, lipofuscin; m, mitochondria. Scale bar: 1 μm. (B) An enlarged photomicrograph of panel (A) showing an autophagic vacuole (v) surrounded by a double membrane (arrows). m, mitochondria. Scale bar: 0.5 μm. (C) Fusing autophagic vacuoles (v) contain a mitochondrion (arrow). m, mitochondrial area; Mf, myofilament; ML, myofilament lysis area. Scale bar: 2 μm. (D) There are several different sized autophagic vacuoles (v) apparent in a degenerated cardiomyocyte. Only a few myofilaments (Mf) are seen in the periphery. Cap, capillary; m, mitochondria; ML, myofilament lysis area. Scale bar: 5 μm. (E) Lamellar bodies (LB), irregular electron-dense concentric-layered structures, were found in degenerated cardiomyocytes. L, lipid droplet; m, mitochondria; Mf, myofilament. Scale bar: 2 μm. (F) To assess whether the membrane-bound vacuoles in dilated cardiomyopathy cardiomyocytes have autophagic function, immunohistochemical staining was performed using an antigen retrieval method and the avidin-biotin peroxidase complex technique using a labeled anti-human LC3 antibody.7 Peroxidase depositions in the vacuole (v) indicate immunohistochemical detection of LC3 expression, so these vacuoles were identified as autophagic vacuoles. Since formalin fixation is too weak to maintain the ultrastructure of tissue, and paraffin embedding often destroys most or all of the native LC3 immunopositivity, this combined method is useful. The limited membrane structure was unclear in this photomicrograph because this structure consists of lipoprotein, which may be destroyed at the ultrastructural level by inadequate formalin fixation, or melt during deparaffinization. However, peroxidase deposits can be recognized in the area of the vacuoles owing to the antigen retrieval method. Mf, myofilament. Scale bar: 1 μm. Arrows show the boundary of a cardiomyocyte. (G) An enlarged photomicrograph of panel (F). m, mitochondria; Mf, myofilament; v, vacuole. Scale bar: 1 μm.
In cardiomyocytes with myofilament changes, sarcomere-depleted areas were replaced by glycogen, ribosomes, increased mitochondria, and smooth endoplasmic reticulum.8 Mitochondria showed abnormalities of shape, rarefaction, and size, especially in the appearance of giant mitochondria. Smooth endoplasmic reticulum formed a branching and anastomosing network.8 Lamellar bodies, which are irregular electron-dense concentric-layered structures, were found in degenerated cardiomyocytes (Fig. 1E).
To confirm whether the vacuoles enclosed by double membranes that were easily observed using electron microscopy were autophagic vacuoles, combination method, based on antigen retrieval and avidin-biotin peroxidase complex (ABC) techniques was performed. Peroxidase depositions (Fig. 1F and G) in the vacuoles indicate microtubule-associated protein light chain 3 (MAP1LC3/LC3) expression, detected by immunohistochemistry,7 and therefore identify those vacuoles as autophagic vacuoles. Lamellar bodies were negative for LC3-labeled peroxidase.
Myocardial intercellular fibrosis (odds ratio [OR], 0.96; 95% confidence interval [CI], 0.93 to 0.99) and mitochondrial abnormality (OR, 2.24; 95% CI, 1.23 to 4.08) were independent predictors of autophagic vacuoles (Table 2). The average density of autophagic vacuoles in all patients was 0.23±0.48 (range, 0 to 3.08/cell).
Table 2.
Univariate and multivariate analyses for the relationship between autophagic vacuoles and histopathologic/ultrastructural variables.
| Univariate model | Multivariate model | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P value | OR | 95% CI | P value |
| Cellular diameter | 1.101 | 0.953–1.272 | 0.193 | |||
| Perinuclear halo | 1.158 | 0.687–1.952 | 0.582 | |||
| Nuclear diameter | 0.896 | 0.638–1.258 | 0.524 | |||
| Proportion of fibrosis | 0.962 | 0.932–0.994 | 0.020 | 0.961 | 0.930–0.993 | 0.018 |
| Mitochondrial abnormality | 2.184 | 1.229–3.882 | 0.008 | 2.235 | 1.225–4.079 | 0.009 |
| Aggregation of smooth endoplasmic reticulum | 1.768 | 0.980–3.112 | 0.059 | |||
| Lamellar body | 1.981 | 1.023–3.834 | 0.043 | 1.964 | 0.976–3.952 | 0.058 |
OR, odds ratio; CI, confidence interval.
Clinical outcome
During the mean follow-up time of 4.9 ± 3.9 y, 67 patients (27%) died due to HF or were readmitted due to HF, and 24 patients (10%) died (10 deaths were due to HF, 9 deaths due to malignancy, and 5 deaths due to infection). The 5-year event-free survival for the primary endpoint was 68%, and for the secondary endpoints (all-cause death and HF-related death) was 92% and 96%, respectively.
Predictors of events
Kaplan-Meier survival curves for death or readmission due to HF, all-cause death, and death due to HF in patients with and without myofilament changes and with/without autophagic vacuoles are shown in Fig. 2. DCM patients with myofilament changes had a lower event-free survival rate than those without myofilament changes (P < 0.001); however, in patients with myofilament changes, the existence of autophagic vacuoles produced a significantly higher event-free survival rate (P < 0.001), and this event-free survival rate was close to that of patients without myofilament changes. The results of univariate and multivariate analyses for candidate predictors of prognosis are given in Table 3. Positive myofilament change (hazard ratio [HR], 13.53; 95% CI, 5.34 to 34.26) and negative autophagic vacuole (HR, 0.21; 95% CI, 0.11 to 0.40) were independent predictors of readmission or death due to HF recurrence.
Figure 2.
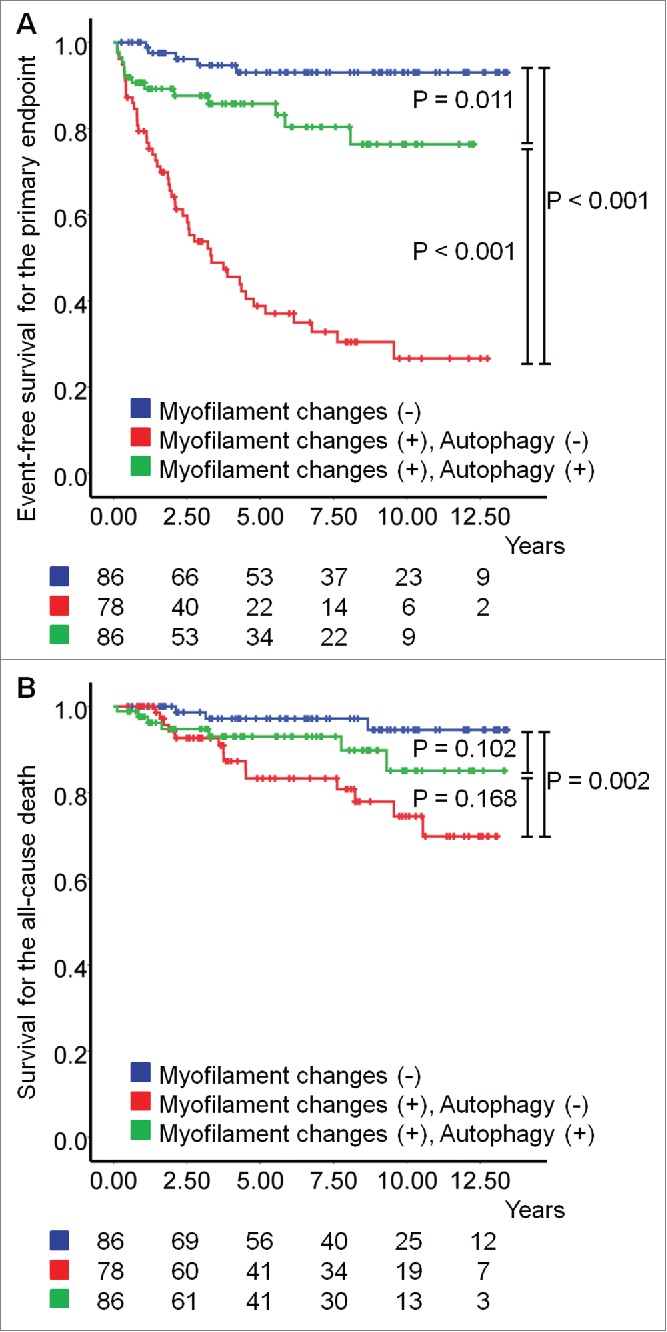
Kaplan-Meier survival curves during the follow-up period. (A) The event-free survival for the primary endpoint defined as a composite of death or readmission due to heart failure (HF). (B) The event-free survival for all-cause death (overall survival).
Table 3.
Univariate and multivariate analyses for candidate predictors of combined death or readmission due to heart failure recurrence.
| Candidate univariate analysis | Multivariate analysis | Adjusted | ||||
|---|---|---|---|---|---|---|
| Variables | Hazard ratio | 95% CI | P Value | hazard ratio | 95% CI | P Value |
| Age | 1.009 | 0.991–1.027 | 0.342 | |||
| Male sex | 1.572 | 0.803–3.078 | 0.187 | |||
| Body mass index | 0.991 | 0.948–1.036 | 0.693 | |||
| Systolic blood pressure | 0.993 | 0.984–1.002 | 0.131 | |||
| Diastolic blood pressure | 0.992 | 0.979–1.005 | 0.250 | |||
| Heart rate | 0.998 | 0.975–1.023 | 0.894 | |||
| NYHA scale III or IV | 1.031 | 0.638–1.667 | 0.900 | |||
| Family history of DCM | 2.407 | 1.400–4.138 | 0.001 | 2.117 | 1.199–3.738 | 0.010 |
| Atrial fibrillation | 1.772 | 1.098–2.862 | 0.019 | 1.484 | 0.891–2.470 | 0.129 |
| Hypertension | 1.103 | 0.683–1.783 | 0.688 | |||
| Diabetes | 1.368 | 0.841–2.224 | 0.206 | |||
| Renal dysfunction (GFR<60) | 1.330 | 0.793–2.229 | 0.280 | |||
| B-type natriuretic peptide | 1.000 | 0.999–1.309 | 0.371 | |||
| C-reactive protein | 0.920 | 0.824–1.027 | 0.136 | |||
| Hemoglobin | 0.891 | 0.802–0.991 | 0.033 | 0.845 | 0.749–0.953 | 0.006 |
| Total bilirubin | 0.839 | 0.530–1.330 | 0.455 | |||
| Left ventricular ejection fraction | 0.999 | 0.981–1.017 | 0.903 | |||
| ACE-I or ARB | 0.945 | 0.482–1.851 | 0.868 | |||
| Beta-blockers | 0.723 | 0.440–1.189 | 0.202 | |||
| Diuretics | 1.282 | 0.721–2.278 | 0.397 | |||
| Aldosterone receptor antagonists | 0.881 | 0.546–1.424 | 0.606 | |||
| Cellular diameter | 1.144 | 0.999–1.309 | 0.051 | 1.005 | 0.878–1.152 | 0.938 |
| Perinuclear halo | 1.448 | 0.894–2.346 | 0.133 | |||
| Nuclear diameter | 0.892 | 0.643–1.237 | 0.493 | |||
| Proportion of fibrosis | 1.008 | 0.984–1.032 | 0.534 | |||
| Myofilament changes | 8.840 | 3.548–22.024 | <0.001 | 13.525 | 5.340–34.255 | <0.001 |
| Autophagic vacuoles | 0.551 | 0.306–0.993 | 0.047 | 0.214 | 0.114–0.400 | <0.001 |
| Density of autophagic vacuoles | 0.919 | 0.507–1.665 | 0.779 | |||
| Mitochondrial abnormality | 0.920 | 0.530–1.595 | 0.766 | |||
| Aggregation of smooth endoplasmic reticulum | 1.326 | 0.762–2.306 | 0.318 | |||
| Lamellar body | 1.064 | 0.570–1.989 | 0.845 |
GFR, glomerular filtration rate; other abbreviations as in Table 1.
Discussion
Autophagy is a current therapeutic target in several diseases. Reports of autophagy having a cardioprotective role have been accumulating from experimental9 and human genetic examinations.10 Although autophagic vacuoles have been ultrastructurally identified in the cardiomyocytes of patients with DCM,6 there is still no evidence clarifying the clinical role of human myocardial autophagy. Previous studies have suggested autophagy induces degeneration of cardiomyocytes and “autophagic cell death;” however these studies have only seen autophagic vacuoles in highly-denatured cardiomyocytes. An endomyocardial biopsy (EMB) specimen is fixed at a certain moment in a series of biological reactions; therefore, it is difficult to determine whether autophagy is a cause or result of disease, using only the pathologic findings from an EMB section.
An effective method of evaluating the significance of myocardial autophagy is survival analysis by longitudinal observation. However, this method depends on the accuracy of detecting and interpreting autophagy. Observations without ultrastructural confirmation may be misleading and other structures may be described as autophagy. For instance, while ubiquitin appears in areas of degenerated cardiomyocytes,11 and may be stained occasionally in autophagosomes, it does not represent autophagy.12 BECN1, an autophagy-related protein, is also sometimes expressed in nonautophagy reactions.13 A study by Vigliano et al. 14 examines the association between cardiac autophagy and prognosis in patients with DCM, without using electron microscopy evaluation. As a result, the study describes a large cytoplasmic area containing ubiquitin-positive material as an “autophagic vacuole” when, in fact, it is an area of myofilament lysis. An autophagic vacuole is a double-membrane structure containing LC3; LC3-II is the lipid-conjugated form of this protein, and it serves as the primary marker of autophagosome membranes.15 Thus, we can accurately identify autophagic vacuoles observed by transmission electron microscopy as those structures enclosed by a limiting membrane and filled with glycogen granules, ribosomes, and several degenerated organelles; and then confirm the identification by combining antigen retrieval with the ABC method and detecting LC3 by electron microscopy.7 Formalin, which has weaker fixation power than glutaraldehyde, often destroys the ultrastructure of heart tissue samples including limiting membranes, and most or all of the native LC3 immunopositivity. Additionally, the membranes enclosing these vacuoles, which consist of lipoproteins, are dissolved together during deparaffinization. However, the antigen retrieval method enables the labeling of a tiny amount of LC3,7,16 so that peroxidase deposits can be seen inside or circumscribing the vacuoles, even if the limited membrane structure is unclear by electron microscopy. Lamellar bodies, also known as myelin figures or myelinosomes, (Fig. 1E) are concentric-layered structures identified in highly degenerated cardiomyocytes.17 Several studies regard these structures as autophagosomes,12,18 and these studies mention the “autophagic cell death.” However, in the present study, the lamellar bodies were negative for LC3-labeled peroxidase. These structures had no relationship with HF prognosis, so the lamellar body should be distinguished from the autophagic vacuole. Our study, therefore, is the first to evaluate the prognosis of DCM patients with or without autophagic vacuoles, and patients with autophagic vacuoles in cardiomyocytes had a much better prognosis, compared to patients without vacuoles.
Another way of evaluating the significance of the presence of autophagic vacuoles in cardiomyocytes is to consider light microscopy results and other ultrastructural variables. In the present study, autophagic vacuoles were responsible for a low proportion of intercellular fibrosis in the left ventricular (LV) myocardium as well as a high frequency of mitochondrial abnormality in the LV cardiomyocytes. Fibrosis, detected by late gadolinium enhancement cardiac magnetic resonance, is linked to a poor prognosis in patients with DCM.19 The myocardial fibrosis seen in DCM is a reactive accumulation of connective tissue in the interstitial space of the myocardium or replacement scarring to fill the space following myocyte loss.20 In the failing heart, expression of cytoskeletal proteins increases, which leads to cytoskeletal augmentation and disorganization of the myofilament.21 We previously have reported that myofilament changes, indicating cellular degeneration, are strongly associated with mortality and HF recurrence in patients with DCM.8 Autophagic vacuoles have been identified in or near the area of altered myofilament; in addition, autophagy is triggered by hypoxia and nutrient depletion,22 which are caused by HF.9 Such situations lead to mitochondrial hyperplasia and morphological abnormalities. Indeed, mitochondrial abnormalities were associated with autophagic vacuoles in the present study. Therefore, this suggests that autophagy repairs degenerated cardiomyocytes by removing damaged proteins and organelles, and prevents the loss of cardiomyocytes and the extension of intercellular fibrosis.
Limitations
The major limitation of this study is that it relies on structural evidence for autophagic vacuoles rather than biochemical evidence. Even using a combination of antigen retrieval and the avidin-biotin peroxidase complex method to detect LC3 by electron microscopy, autophagic flux could not be assessed in real-time. In addition, the limitations of EMB are well known,23 in particular the small size of the electron microscopy field;8 only a limited number of cardiomyocytes (100 to 200 cells) can be observed, so that myocytes with autophagic vacuoles may not appear in the microscopy field. However, autophagic vacuoles can be found easily by electron microscopy.6,7 Autophagosome formation has a half-time of approximately 10 min,24 and multiple autophagosomes form simultaneously within the damaged cell whereas they are hardly observed in cells that are not affected.15 Patients with DCM in end-stage HF have a number of autophagic vacuoles.6 Additionally, in the present study, EMB specimens with myofilament changes and without autophagic vacuoles were identified in 78 patients, which accounts for one-third of the study population. These patients had an obviously poor prognosis. Therefore, we propose that electron microscopy observation is an appropriate method to detect autophagy using clinical samples, even though it has limitations, as is the case with other histopathological methods.
In this study there was an absence of normal controls. However, a previous study5 has reported that myocardial ultrastructural features, including autophagic changes, can be identified in patients with DCM in end-stage HF, but are not evident in cardiac tissue samples from normal controls. In our study, patients with autophagy were older, with higher serum B-type natriuretic peptide (BNP) levels, and larger cardiomyocytes; however, multivariate analysis revealed that age, serum BNP level, and cellular diameter were not independent predictors of prognosis.
Conversely, lower hemoglobin levels and a higher frequency of family history of DCM were risk factors for combined HF death or recurrence; therefore, it is difficult to exclude the confounding effects of these complications, along with HF medications. Anemia is a well-known risk factor for HF.25 In addition, a family history of DCM is strongly associated with a genetic component of hereditary cardiomyopathy.4,26 Therefore, when such patients are identified by electron microscopy observations, careful follow-up is needed because they have a high potential for a poor prognosis. The screening of autophagic vacuoles using electron microscopy is a useful method for detecting these patients in the daily clinical situation; perhaps identifying these patients and analyzing the cause of their disorders may lead to the discovery of new treatments for DCM.
Conclusions
Autophagic vacuoles were identified in or near the area of altered myofilament in the LV cardiomyocytes of patients with DCM initially presenting with HF. In these patients, a lower frequency of autophagic vacuoles in cardiomyocytes was strongly associated with more death or readmission due to HF. This strongly suggests that myocardial autophagy may improve HF prognosis by preventing the progression of myocardial degeneration.
Patients and methods
Study population
This prospective, longitudinal study enrolled 250 patients with idiopathic DCM. All patients underwent EMB from the LV during January 1999 to December 2011 at the Nippon Medical School Hospital (Fig. 3). All patients were Japanese and presented initially with decompensated HF. Prospective patients with congenital, ischemic, or severe valvular heart disease, histological myocarditis, or secondary metabolic, drug-induced, or inflammatory cardiomyopathies, or neuromuscular disorders were excluded from the study. All patients enrolled in the study had systolic dysfunction (LV ejection fraction<50%) without significant coronary artery stenosis, as assessed by coronary angiography. Written informed consent was obtained from all remaining patients prior to their inclusion in the study. The study itself was approved by the committee on human investigation at our institution, and was performed in accordance with the Declaration of Helsinki.
Figure 3.
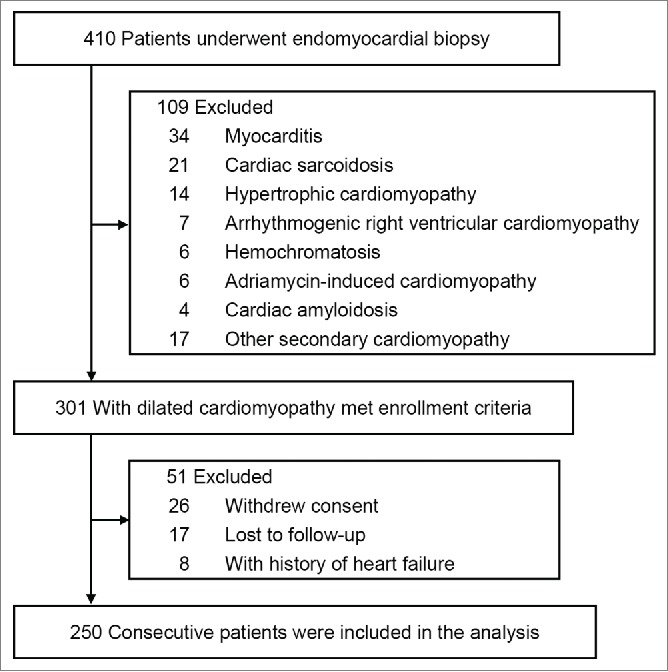
Derivation of the study cohort.
Clinical data collection and endomyocardial biopsy
Routine laboratory analyses were performed for all patients on admission. Serum BNP levels were determined using a commercially available immunoassay kit (Shionogi Inc.., MI02). At the time of admission, transthoracic echocardiography was performed to obtain morphologic and functional information. Two-dimensional, M-mode and color Doppler imaging was performed according to the standardized methods of the American Society of Echocardiography.27 Right and left catheterization was done with EMB. The EMB was performed under radiographic guidance with continuous electrocardiographic monitoring. Tissue samples were collected from the LV infero-posterior wall using a 7-Fr bioptome (Cordis; Johnson & Johnson Co, New Brunswick, NJ) and the retrograde approach. The type of medication prescribed on discharge for the treatment of HF was recorded.
Follow-up and endpoints
Patients were observed from the time of cardiac catheterization (including EMB) until death, readmission because of HF, or until June 2012. Follow-up information was obtained during routine visits and by telephone contact with the patients or their physicians. The primary endpoint was defined as a composite of death or readmission due to HF recurrence. The secondary endpoints were defined as all-cause death.
Tissue preparation and detection of autophagy
Biopsy specimens for light microscopy analysis were fixed in 20% neutral buffered formalin, embedded in paraffin, and cut into 3-μm-thick sections. Serial sections were stained using the hematoxylin and eosin, and Elastica-Masson Goldner methods.28
Photomicrographs were taken at 200× magnification in each case, including the whole tissue section. These photographs were taken using the NIS-Elements Documentation System (version 3.22; Nikon Instruments, Tokyo, Japan) and a digital microscope camera system, DS-Ri1 (Nikon Instruments, Tokyo, Japan). Parameters were calculated using ImageJ analysis software (version 1.43). The following 4 parameters were evaluated by morphometry: (1) size and (2) perinuclear halo of cardiomyocytes, (3) size of nuclei, and (4) proportion of fibrosis (%F).29 The short diameter of cardiomyocytes at the nuclear level and of nuclei was measured in 75 cells. Specimens were defined as positive for perinuclear halo if more than 50% of all cardiomyocytes in the EMB sample had this feature.30 Adobe Photoshop software version CS4 (Adobe Systems Inc., San Jose, CA) was used to identify red and green in Elastica-Masson Goldner-stained sections, and the ratio of the fibrotic area in the myocardium was calculated using the following formula29: %F = (area of fibrosis/area of fibrosis + myocardium) ×100.
For electron microscopy analysis, pieces of EMB material were fixed in 2.5% glutaraldehyde and post-fixed in 1% osmium tetroxide. Samples were dehydrated in a graded series of ethanol and embedded in Epok 812 (Ernest F. Fullam, 02–1001). Ultrathin sections were cut on an ultramicrotome with a diamond knife, stained with uranyl acetate and lead citrate, and examined under an electron microscope (JEOL-1010; JEOL, Tokyo, Japan) at 80 kV. A minimum of 50 cardiomyocytes was examined in each sample for evidence of changes to ultrastructural features.
Ultrastructural variables such as myofilament changes,8 autophagic vacuoles,7 mitochondrial abnormalities (shape, rarefaction, size, and the appearance of giant mitochondria), aggregation of smooth endoplasmic reticulum,8 and lamellar bodies17 were classified as positive (when identified in the cytoplasm of cardiomyocytes) or negative. The electron microscopy results for EMB samples were evaluated by 4 authors (T.S., S.S., A.A., and Y.S.), with each sample examined 3 times, in random order; with the examiners blinded to the clinical background of the patient. If there was a discrepancy in the evaluation of the ultrastructural findings, a final decision was reached by consensus.
Detection of autophagic vacuoles was performed only with transmission electron microscopy. Autophagic vacuoles are defined as structures enclosed by a double membrane and filled with degenerated organelles.1,5,7 To confirm that these vacuoles were autophagic vacuoles, a combination method, based on antigen retrieval and ABC techniques was performed. They contain electron-dense peroxidase reaction deposits of LC3, binding to membranous structures of the autophagic vacuole.1,7,15 The membranous structures consist of lipoprotein which may be destroyed in formalin fixation, due to inadequate fixation for electron microscopy, and may melt during deparaffinization. However, a tiny amount of LC3 can be labeled by the antigen retrieval method.7,16
The tissues were fixed in formalin, dehydrated through a graded series of ethanol, and then embedded in paraffin. Serial 3-μm paraffin sections were cut and deparaffinized by xylene. The sections on glass microscope slides were then placed in a stainless-steel staining rack, for incubating in a kitchen-style electric pot filled with 0.05% citraconic anhydride solution, pH 7.4 (Immunosaver, Nissin EM Co., Ltd, 333) at a constant temperature of 98 °C for 60 min. The sections of all patients thus prepared as A (treated antigen retrieval method) and B groups (no-treated), these sections were stained for 45 min with horseradish peroxidase-labeled rabbit anti-human LC3/MAP1LC3A (1:100 dilution; Novus Biologicals Co., Ltd, NBP1-19167). For light microscopy observation, sections were examined unstained. For electron microscopy observation, the sections were washed in phosphate buffer, pH 7.4, and then post-fixed in osmium tetroxide for 20 min, dehydrated through a graded series of ethanol, and then embedded in Epok 812. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and examined under a Hitachi H-7650 electron microscope at 80 kV. For ordinary electron microscopy observation, the samples are fixed in 2.5% glutaraldehyde.
Then, peroxidase deposits can be identified inside or in the area circumscribing the vacuoles even though the limited membrane structure is unclear by electron microscopy. Since these vacuoles are described as autophagosomes before fusing with lysosomes, and autolysosomes after fusing with lysosomes,5 we have called these structures “autophagic vacuoles.” Additionally, the density of autophagic vacuoles was calculated using the formula: number of autophagic vacuoles/number of cardiomyocytes.
Statistical analysis
Categorical variables were compared using the Chi-squared test. Continuous variables were evaluated by 2-way repeated-measures analysis of variance in the case of normally distributed data and by the Kruskal-Wallis test if data were not normally distributed. To assess the relationship between histological and ultrastructural findings, multivariable logistic regression analysis was performed; the results of 4 light microscopy morphometrical parameters (cellular diameter, perinuclear halo, nuclear diameter, and %F) and 3 ultrastructural variables (mitochondrial abnormality, aggregation of smooth endoplasmic reticulum, and lamellar body) as dependent variables and autophagy as an independent variable were analyzed. Kaplan-Meier survival curves were calculated for the presence and absence of myofilament changes and autophagy, and the log-rank test was used to compare mortality and incidence rate of readmission due to HF. Univariate Cox regression analysis was used to identify predictors of a composite of death and readmission due to recurrent HF. Variables with P < 0.1 on univariate analysis were included in the multivariate model. Statistical analyses were performed using the SPSS software package (SPSS Inc., Chicago, IL) and P < 0.05 was considered significant. All data are expressed as the mean ± SD.
Abbreviations
- ABC
techniques avidin-biotin peroxidase complex techniques
- CI
confidence interval
- DCM
dilated cardiomyopathy
- EMB
endomyocardial biopsy
- HF
heart failure
- HR
hazard ratio
- MAP1LC3
microtubule-associated protein light chain 3
- LV
left ventricular
- OR
odds ratio
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000; 290:1717-21; PMID:11099404; http://dx.doi.org/ 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med 2013; 368:651-62; PMID:23406030; http://dx.doi.org/ 10.1056/NEJMra1205406 [DOI] [PubMed] [Google Scholar]
- [3].The SOLVD Investigators . Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327:685-91; PMID:1463530; http://dx.doi.org/ 10.1056/NEJM199209033271003 [DOI] [PubMed] [Google Scholar]
- [4].Rapezzi C, Arbustini E, Caforio AL, Charron P, Gimeno-Blanes J, Heliö T, Linhart A, Mogensen J, Pinto Y, Ristic A, et al.. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34:1448-58; PMID:23211230; http://dx.doi.org/ 10.1093/eurheartj/ehs397 [DOI] [PubMed] [Google Scholar]
- [5].Eskelinen EL. Maturation of autophagic vacuoles in Mammalian cells. Autophagy 2005; 1:1-10; PMID:16874026; http://dx.doi.org/ 10.4161/auto.1.1.1270 [DOI] [PubMed] [Google Scholar]
- [6].Shimomura H, Terasaki F, Hayashi T, Kitaura Y, Isomura T, Suma H. Autophagic degeneration as a possible mechanism of myocardial cell death in dilated cardiomyopathy. Jpn Circ J 2001; 65:965-8; PMID:11716248; http://dx.doi.org/ 10.1253/jcj.65.965 [DOI] [PubMed] [Google Scholar]
- [7].Saito T, Asai K, Sato S, Takano H, Adach A, Sasaki Y, Namimatsu S, Mizuno K. Proof of myocardial autophagy by combining antigen retrieval and the avidin-biotin peroxidase complex method. Int J Cardiol 2013; 168:4843-4; PMID:23871334; http://dx.doi.org/ 10.1016/j.ijcard.2013.07.032 [DOI] [PubMed] [Google Scholar]
- [8].Saito T, Asai K, Sato S, Takano H, Mizuno K, Shimizu W. Ultrastructural features of cardiomyocytes in dilated cardiomyopathy with initially decompensated heart failure as a predictor of prognosis. Eur Heart J 2015; 36:724-33; PMID:25336212; http://dx.doi.org/ 10.1093/eurheartj/ehu404 [DOI] [PubMed] [Google Scholar]
- [9].Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, et al.. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13:619-24; PMID:17450150; http://dx.doi.org/ 10.1038/nm1574 [DOI] [PubMed] [Google Scholar]
- [10].Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, Frazier OH, Taegtmeyer H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 2009; 120:S191-7; PMID:19752367; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.108.842252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, et al.. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res 2008; 79:472-80; PMID:18375498; http://dx.doi.org/ 10.1093/cvr/cvn083 [DOI] [PubMed] [Google Scholar]
- [12].Kostin S, Pool L, Elsässer A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klövekorn WP, et al.. Myocytes die by multiple mechanisms in failing human hearts. Circ Res 2003; 92:715-24; PMID:12649263; http://dx.doi.org/ 10.1161/01.RES.0000067471.95890.5C [DOI] [PubMed] [Google Scholar]
- [13].Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res 2007; 17:839-49; PMID:17893711; http://dx.doi.org/ 10.1038/cr.2007.78 [DOI] [PubMed] [Google Scholar]
- [14].Vigliano CA, Cabeza Meckert PM, Diez M, Favaloro LE, Cortés C, Fazzi L, Favaloro RR, Laguens RP. Cardiomyocyte hypertrophy, oncosis, and autophagic vacuolization predict mortality in idiopathic dilated cardiomyopathy with advanced heart failure. Am J Coll Cardiol 2011; 57:1523-31; http://dx.doi.org/ 10.1016/j.jacc.2010.09.080 [DOI] [PubMed] [Google Scholar]
- [15].Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720-8; PMID:11060023; http://dx.doi.org/ 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Namimatsu S, Ghazizadeh M, Sugisaki Y. Reversing the effects of formalin fixation with citraconic anhydride and heat: a universal antigen retrieval method. J Histochem Cytochem 2005; 53:3-11; PMID:15637333; http://dx.doi.org/ 10.1369/jhc.4C6466.2005 [DOI] [PubMed] [Google Scholar]
- [17].Maron BJ, Ferrans VJ, Roberts WC. Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am J Pathol 1975; 79:387-434; PMID:124533 [PMC free article] [PubMed] [Google Scholar]
- [18].Fidziańska A, Bilińska ZT, Walczak E, Witkowski A, Chojnowska L. Autophagy in transition from hypertrophic cardiomyopathy to heart failure. J Electron Microsc 2010; 59:181-3; http://dx.doi.org/ 10.1093/jmicro/dfp048 [DOI] [PubMed] [Google Scholar]
- [19].Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, et al.. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013; 309:896-908; PMID:23462786; http://dx.doi.org/ 10.1001/jama.2013.1363 [DOI] [PubMed] [Google Scholar]
- [20].de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int 2001; 14:299-306; PMID:11692213; http://dx.doi.org/ 10.1007/s001470100339 [DOI] [PubMed] [Google Scholar]
- [21].Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klövekorn WP, Schlepper M, Schaper W, et al.. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res 2000; 86:846-53. [Addendum in Circ Res 2000;87:167.]; PMID:10785506; http://dx.doi.org/ 10.1161/01.RES.86.8.846 [DOI] [PubMed] [Google Scholar]
- [22].Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 2005; 120:237-48; PMID:15680329; http://dx.doi.org/ 10.1016/j.cell.2004.11.046 [DOI] [PubMed] [Google Scholar]
- [23].Mason JW, O'Connell JB. Clinical merit of endomyocardial biopsy. Circulation 1989; 79:971-9; PMID:2653662; http://dx.doi.org/ 10.1161/01.CIR.79.5.971 [DOI] [PubMed] [Google Scholar]
- [24].Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, et al.. Autophagosomes form at ER-mitochondria contact sites. Nature 2013; 495:389-93; PMID:23455425; http://dx.doi.org/ 10.1038/nature11910 [DOI] [PubMed] [Google Scholar]
- [25].Anand I, McMurray JJ, Whitmore J, Warren M, Pham A, McCamish MA, Burton PB. Anemia and its relationship to clinical outcome in heart failure. Circulation 2004; 110:149-54; PMID:15210591; http://dx.doi.org/ 10.1161/01.CIR.0000134279.79571.73 [DOI] [PubMed] [Google Scholar]
- [26].Grünig E, Tasman JA, Kücherer H, Franz W, Kübler W, Katus HA. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol 1998; 31:186-94; PMID:9426039; http://dx.doi.org/ 10.1016/S0735-1097(97)00434-8 [DOI] [PubMed] [Google Scholar]
- [27].Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2:358-67; PMID:2698218; http://dx.doi.org/ 10.1016/S0894-7317(89)80013-6 [DOI] [PubMed] [Google Scholar]
- [28].Saito T, Tamura K, Uchida D, Saito T, Togashi M, Nitta T, Sugisaki Y. Histopathological features of the resected left atrial appendage as predictors of recurrence after surgery for atrial fibrillation in valvular heart disease. Circ J 2007; 71:70-8; PMID:17186981; http://dx.doi.org/ 10.1253/circj.71.70 [DOI] [PubMed] [Google Scholar]
- [29].Kawashima T, Yokota Y, Yokoyama M, Itoh H. Pathological analysis of hypertrophic cardiomyopathy simulating dilated cardiomyopathy. Acta Pathol Jpn 1993; 43:304-12; PMID:8346707 [DOI] [PubMed] [Google Scholar]
- [30].Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997; 96:1180-4; PMID:9286947; http://dx.doi.org/ 10.1161/01.CIR.96.4.1180 [DOI] [PubMed] [Google Scholar]