ABSTRACT
Autophagy plays an important role in the removal of membrane bound organelles during the last stage of erythropoiesis as the enucleate reticulocyte matures into the erythrocyte. Autophagic vesicles are expelled from the reticulocyte as intact, inside-out, phosphatidylserine (PS) decorated vesicles and are subsequently removed during splenic passage. Failure to remove these vesicles causes the elevation in PS exposed red cells in Sickle Cell Disease.
The maturation of reticulocytes involves a reduction in cellular size and volume and the purging of residual membrane-bound organelles. Utilizing an in vitro erythroid culture system we have produced a homogenous population of human reticulocytes to study the process of reticulocyte maturation. We previously reported that maturing reticulocytes internalize plasma membrane in GYPA (glycophorin A [MNS blood group])-containing vesicles, which fuse with autophagosomes prior to exocytosis. Subsequent investigations have shown that during the final phase of reticulocyte maturation autophagic and exocytic pathways converge resulting in the extrusion of large, intact, inside-out vesicles that are decorated with phosphatidylserine (PS). Approximately 0.5% of red cells in circulating blood are reticulocytes and we observed these PS-positive vesicles on red cells in the peripheral blood of a normal donor. In patients after splenectomy or in patients with sickle cell disease (SCD) the number of red cells positive for PS is elevated. We showed that in these patients the increased PS decoration is not uniform over the red cell surface but found in small discrete areas corresponding to the expulsion of vesicles that are identical to the autophagic vesicles observed extruding from cultured reticulocytes. Our data suggest that in vivo the extrusion of inside-out autophagic vesicles from maturing reticulocytes is facilitated by passage through the spleen. It is likely that the known increased risk of thrombosis observed in splenectomized individuals and patients with haemoglobinopathies results from elevated levels of circulating reticulocytes expressing inside-out PS-exposed autophagic vesicles on their surface.
Reticulocytes are formed after the enucleation of orthochromatic erythroid cells during erythropoiesis and they are broadly grouped into 2 types, R1 and R2. R1 are immature reticulocytes, which are normally confined to the bone marrow for 24 h post enucleation where they become nonmotile and more mechanically stable. Once they have matured into R2 reticulocytes they are released into circulation where they take a further 24 h to mature into erythrocytes. During the whole maturation process they lose around 20% of their surface area, reduce their volume and eliminate any remaining membrane-bound cytosolic organelles. We have shown that in R2 reticulocytes the autophagic, endocytic, and exocytic processes are linked. The reduction in surface area is caused by endocytosis of plasma membrane to form a large internal GYPA-decorated vesicle, which then fuses with autophagosomes containing organelles for removal. The autophagic vesicles are extruded from the reticulocyte intact and the surface membrane of the extruded vesicle exposes the cytoplasmic domains of major red cell membrane proteins (GYPA and SLC4A1/AE1) and PS and therefore has an “inside-out” orientation. This novel process is depicted in Figure 1. It remains to be determined if this process is unique to the reticulocyte, is found in other cells where enucleation occurs (lens epithelia and keratinocytes) or is a common aspect of autophagy not previously recognized.
Figure 1.
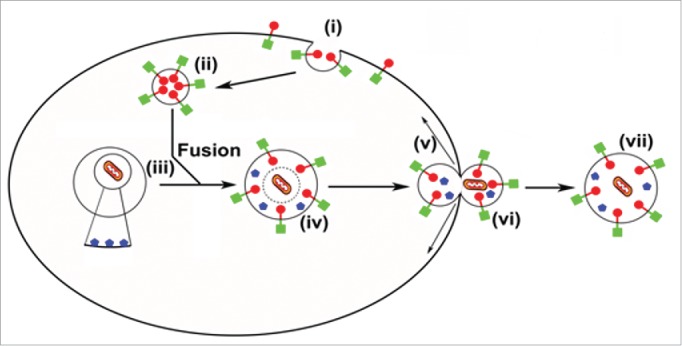
Model for the formation and release of autophagic vesicles. GYPA (extracellular domain [red circle] and intracellular domain [green square]) is internalized by endocytosis of the plasma membrane (i) to produce large GYPA-decorated vesicles (ii) that fuse with an LC3 (blue pentagon)-positive autophagosome (iii) forming a large LC3-GYPA-positive hybrid inside-out vesicle (iv). Temporary weakening of the cytoskeleton (v) allows extrusion (vi) releasing a PS-positive inside-out vesicle (vii) for phagocytosis in the spleen.
An abnormally high number of circulating PS-positive red cells is found in patients after splenectomy and those with haemoglobinopathies. We studied the red cells from 2 surgically splenectomized patients and a cohort of 20 patients with SCD in comparison with 7 normal controls. The number of red cells with external PS-positive autophagic vesicles was elevated in both patient groups when compared to controls. PS was only observed in the discrete patches represented by an autophagic vesicle rather than uniform across the plasma membrane. As well as initiating phagocytosis, PS is also a focal point for the activation of proF2/prothrombin and thereby activation of the coagulation cascade. The presence of elevated numbers of reticulocytes with PS-exposed autophagic vesicles and vesicles released from the reticulocyte into plasma likely accounts for much of the severe vascular pathology seen in SCD.
Our data describe a novel aspect of autophagy occurring in enucleated human reticulocytes. Extrusion of the autophagic vesicle occurs in the absence of lysosomes and this process allows the reticulocyte to reduce its surface area and volume while simultaneously expelling residual organelles. Through this process the reticulocyte becomes an erythrocyte. The spleen appears to play an essential role in removing autophagic vesicles from the circulating reticulocyte and consequently, in asplenic patients, autophagic vesicles remain in the peripheral circulation where they contribute to the pathology of their disease.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.


