Abstract
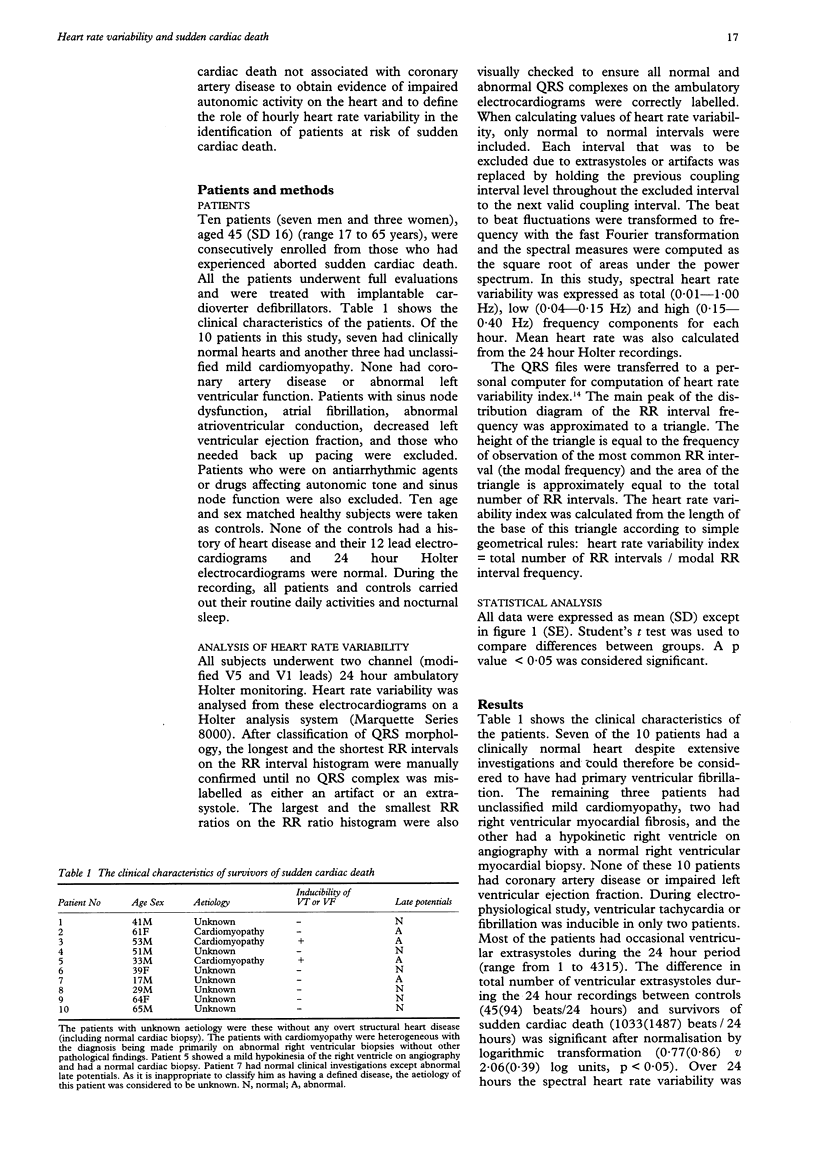
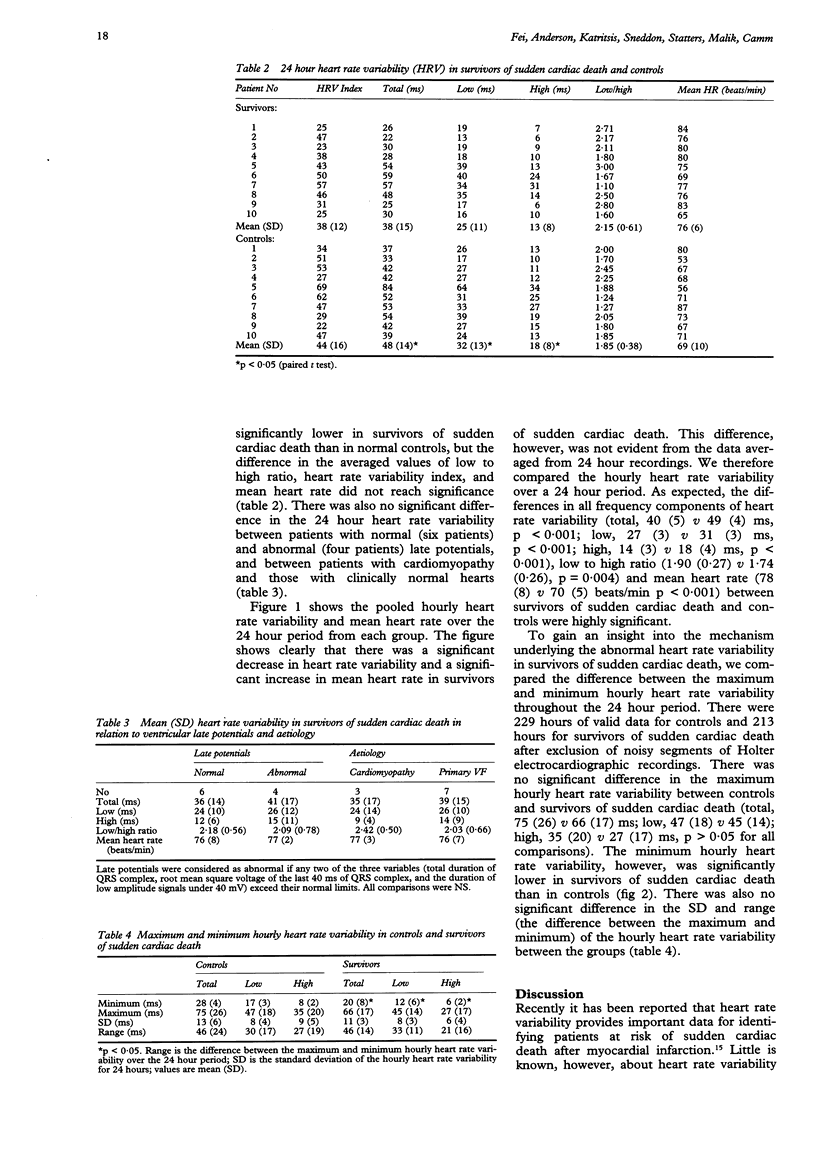
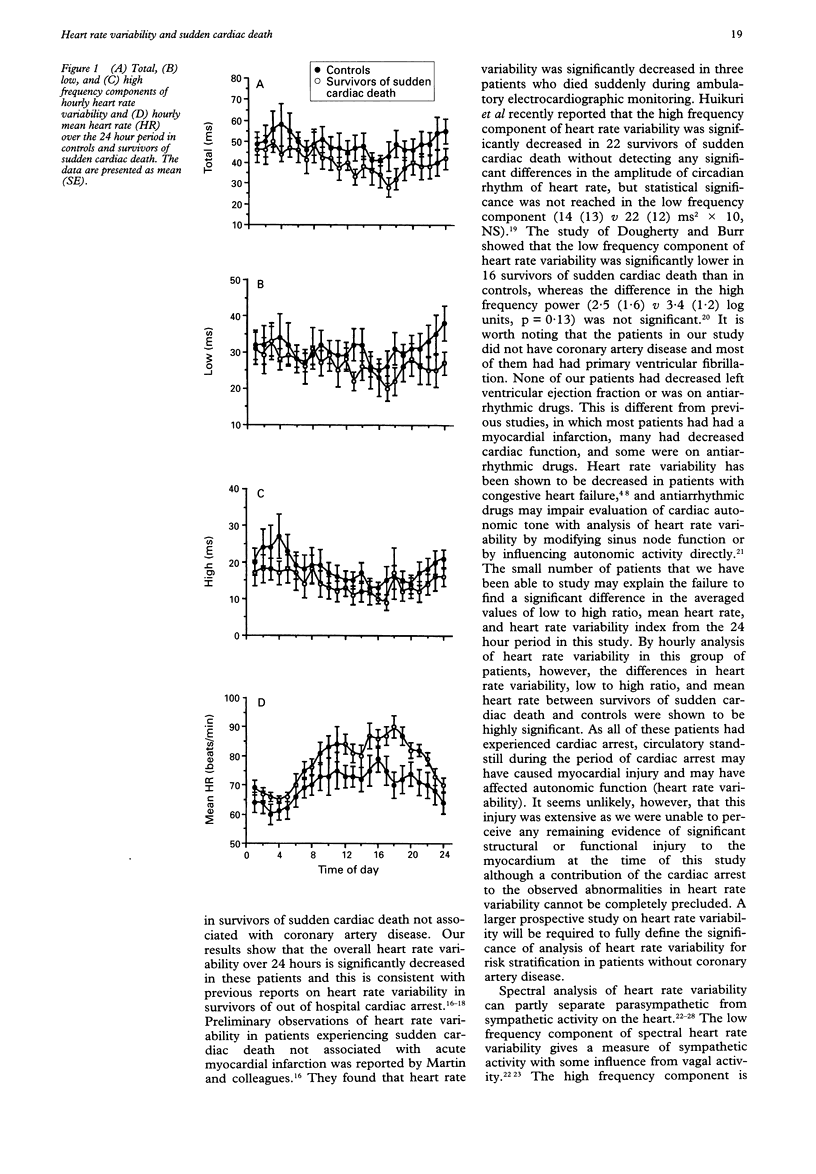
BACKGROUND--Although heart rate variability has already been studied in survivors of sudden cardiac death secondary to coronary artery disease, an assessment of heart rate variability in survivors of sudden cardiac death not associated with coronary artery disease has not been made. METHODS--10 patients with aborted sudden cardiac death not associated with coronary artery disease (seven patients with primary ventricular fibrillation and three with unclassified mild cardiomyopathy) underwent two channel 24 hour Holter monitoring in a drug free state. All subjects were in sinus rhythm and had normal atrioventricular conduction and normal cardiac function. Spectral heart rate variability was analysed on a Holter analysis system and was expressed as total (0.01-1.00 Hz), low (0.04-0.15 Hz) and high (0.15-0.40 Hz) frequency components for each hour. Heart rate variability index was calculated for the 24 hour periods. 10 age and sex matched healthy subjects were taken as a control group. RESULTS--The spectral heart rate variability over 24 hours was significantly lower in survivors of sudden cardiac death than in controls (total 38(15) v 48(14) ms; low, 25(11) v 32(13) ms; and high, 13(8) v 18(8) ms; p < 0.05 for all comparisons). The differences in the ratio of low/high (2.19(0.76) v 1.98(0.50), p = 0.132), mean heart rate (77(12) v 69(12) beats/min, p = 0.070), and heart rate variability index (38(12) v 44(16), p = 0.287) over 24 hours between survivors of sudden cardiac death and controls did not reach significance. Comparisons of the hourly heart rate variability over the 24 hour period between the two groups showed that the differences in all components of heart rate variability, low/high ratio and mean heart rate were highly significant. Furthermore, there was no significant difference in the maximum hourly heart rate variability over the 24 hour period. The minimum hourly heart rate variability was, however, significantly lower in survivors of sudden cardiac death than in controls (total, 20(8) v 28(4) ms; low, 12(6) v 17(3) ms; high, 6(2) v 8(2) ms; p < 0.05 for all comparisons). CONCLUSIONS--These findings suggest that there is abnormal autonomic influence on the heart in patients without coronary artery disease at risk of sudden cardiac death. Hourly analysis of heart rate variability throughout the 24 hour period may provide additional information important in the identification of high risk patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airaksinen K. E., Ikäheimo M. J., Linnaluoto M. K., Niemelä M., Takkunen J. T. Impaired vagal heart rate control in coronary artery disease. Br Heart J. 1987 Dec;58(6):592–597. doi: 10.1136/hrt.58.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akselrod S., Gordon D., Ubel F. A., Shannon D. C., Berger A. C., Cohen R. J. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981 Jul 10;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Bigger J. T., Jr, Fleiss J. L., Steinman R. C., Rolnitzky L. M., Kleiger R. E., Rottman J. N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992 Jan;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Böcker D., Shenasa M., Borggrefe M., Fetsch T., Breithardt G. Late potentials, heart rate variability, and electrocardiography. Curr Opin Cardiol. 1993 Jan;8(1):39–53. doi: 10.1097/00001573-199301000-00006. [DOI] [PubMed] [Google Scholar]
- Casolo G., Balli E., Taddei T., Amuhasi J., Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989 Nov 15;64(18):1162–1167. doi: 10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]
- Cripps T. R., Camm A. J. Prediction of arrhythmic events in patients following myocardial infarction. Clin Cardiol. 1989 Nov;12(11):661–665. doi: 10.1002/clc.4960121109. [DOI] [PubMed] [Google Scholar]
- Cripps T. R., Malik M., Farrell T. G., Camm A. J. Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br Heart J. 1991 Jan;65(1):14–19. doi: 10.1136/hrt.65.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty C. M., Burr R. L. Comparison of heart rate variability in survivors and nonsurvivors of sudden cardiac arrest. Am J Cardiol. 1992 Aug 15;70(4):441–448. doi: 10.1016/0002-9149(92)91187-9. [DOI] [PubMed] [Google Scholar]
- Farrell T. G., Bashir Y., Cripps T., Malik M., Poloniecki J., Bennett E. D., Ward D. E., Camm A. J. Risk stratification for arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables and the signal-averaged electrocardiogram. J Am Coll Cardiol. 1991 Sep;18(3):687–697. doi: 10.1016/0735-1097(91)90791-7. [DOI] [PubMed] [Google Scholar]
- Fleiss J. L., Bigger J. T., Jr, Rolnitzky L. M. The correlation between heart period variability and mean period length. Stat Med. 1992 Jan 15;11(1):125–129. doi: 10.1002/sim.4780110111. [DOI] [PubMed] [Google Scholar]
- Huikuri H. V., Kessler K. M., Terracall E., Castellanos A., Linnaluoto M. K., Myerburg R. J. Reproducibility and circadian rhythm of heart rate variability in healthy subjects. Am J Cardiol. 1990 Feb 1;65(5):391–393. doi: 10.1016/0002-9149(90)90308-n. [DOI] [PubMed] [Google Scholar]
- Huikuri H. V., Linnaluoto M. K., Seppänen T., Airaksinen K. E., Kessler K. M., Takkunen J. T., Myerburg R. J. Circadian rhythm of heart rate variability in survivors of cardiac arrest. Am J Cardiol. 1992 Sep 1;70(6):610–615. doi: 10.1016/0002-9149(92)90200-i. [DOI] [PubMed] [Google Scholar]
- Kiowski W., Linder L., Kleinbloesem C., van Brummelen P., Bühler F. R. Blood pressure control by the renin-angiotensin system in normotensive subjects. Assessment by angiotensin converting enzyme and renin inhibition. Circulation. 1992 Jan;85(1):1–8. doi: 10.1161/01.cir.85.1.1. [DOI] [PubMed] [Google Scholar]
- Kleiger R. E., Miller J. P., Bigger J. T., Jr, Moss A. J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987 Feb 1;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- Lipsitz L. A., Mietus J., Moody G. B., Goldberger A. L. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990 Jun;81(6):1803–1810. doi: 10.1161/01.cir.81.6.1803. [DOI] [PubMed] [Google Scholar]
- Lombardi F., Sandrone G., Pernpruner S., Sala R., Garimoldi M., Cerutti S., Baselli G., Pagani M., Malliani A. Heart rate variability as an index of sympathovagal interaction after acute myocardial infarction. Am J Cardiol. 1987 Dec 1;60(16):1239–1245. doi: 10.1016/0002-9149(87)90601-1. [DOI] [PubMed] [Google Scholar]
- Malliani A., Schwartz P. J., Zanchetti A. Neural mechanisms in life-threatening arrhythmias. Am Heart J. 1980 Nov;100(5):705–715. doi: 10.1016/0002-8703(80)90238-0. [DOI] [PubMed] [Google Scholar]
- Northcote R. J., MacFarlane P., Kesson C. M., Ballantyne D. Continuous 24-hour electrocardiography in thyrotoxicosis before and after treatment. Am Heart J. 1986 Aug;112(2):339–344. doi: 10.1016/0002-8703(86)90272-3. [DOI] [PubMed] [Google Scholar]
- Pagani M., Lombardi F., Guzzetti S., Rimoldi O., Furlan R., Pizzinelli P., Sandrone G., Malfatto G., Dell'Orto S., Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986 Aug;59(2):178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Sands K. E., Appel M. L., Lilly L. S., Schoen F. J., Mudge G. H., Jr, Cohen R. J. Power spectrum analysis of heart rate variability in human cardiac transplant recipients. Circulation. 1989 Jan;79(1):76–82. doi: 10.1161/01.cir.79.1.76. [DOI] [PubMed] [Google Scholar]
- Saul J. P., Arai Y., Berger R. D., Lilly L. S., Colucci W. S., Cohen R. J. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol. 1988 Jun 1;61(15):1292–1299. doi: 10.1016/0002-9149(88)91172-1. [DOI] [PubMed] [Google Scholar]
- Shin S. J., Tapp W. N., Reisman S. S., Natelson B. H. Assessment of autonomic regulation of heart rate variability by the method of complex demodulation. IEEE Trans Biomed Eng. 1989 Feb;36(2):274–283. doi: 10.1109/tbme.1989.1201373. [DOI] [PubMed] [Google Scholar]
- Van Hoogenhuyze D., Martin G. J., Weiss J. S., Schaad J., Fintel D., Singer D. H. Heart rate variability 1989. An update. J Electrocardiol. 1989;22 (Suppl):204–208. doi: 10.1016/s0022-0736(07)80125-7. [DOI] [PubMed] [Google Scholar]
- Vybiral T., Bryg R. J., Maddens M. E., Boden W. E. Effect of passive tilt on sympathetic and parasympathetic components of heart rate variability in normal subjects. Am J Cardiol. 1989 May 1;63(15):1117–1120. doi: 10.1016/0002-9149(89)90089-1. [DOI] [PubMed] [Google Scholar]


