Abstract
Triple-negative breast cancer is a type of breast cancer that does not express the genes for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2). It is an important and clinically relevant condition as it has a poor prognosis and is difficult to treat. Basal-like triple-negative cancer is highly prevalent in both African-Americans and adolescents. We therefore examined whether such a cancer likewise occurs in specific breeds and age groups in dogs, focusing on basal-like triple-negative cancer in particular. In this study, 181 samples from dogs with malignant mammary carcinoma from the 5 most common breeds and 2 age groups in Korea were analyzed. Histological classification and molecular subtyping, including assessment of immunohistochemical findings, were carried out. Twenty-five of 28 (89.3%) triple-negative carcinomas were identified as basal-like triple-negative carcinomas. Analysis of associations of classified factors revealed that the shih tzu breed (9/25, 36.0%) and advanced-age (19/25, 76.0%) groups were characterized by higher prevalence of basal-like triple-negative tumors with diverse histological types and of a higher grade. These results suggest that breed- and age-related differences can be identified in canine mammary carcinoma and, notably, in the shih tzu breed and at older ages. Further investigation of these distinguishing characteristics of the shih tzu breed is warranted.
Résumé
Le cancer mammaire triple-négatif est un type de cancer mammaire qui n’exprime pas les gènes pour les récepteurs de l’oestrogène (RO), les récepteurs de la progestérone (RP), et les récepteurs pour le facteur de croissance épidermique humain-2 (REH-2). Il s’agit d’une condition importante et cliniquement significative étant donné son mauvais pronostic et la difficulté à le traiter. Le cancer triple-négatif de type basal est très prévalent chez les afro-américains et les adolescents. Nous avons donc voulu savoir si chez les chiens ce type de cancer est rencontré également dans des races spécifiques et dans des groupes d’âge précis, en se concentrant sur des cancers triple-négatifs de type basal en particulier. Dans la présente étude réalisée en Corée, 181 échantillons provenant de chiens avec des carcinomes mammaires malins des cinq races les plus communes et de deux groupes d’âge ont été analysés. Une classification histologique et un sous-typage moléculaire, incluant une évaluation des trouvailles immunohistochimiques, ont été réalisés. Vingt-cinq des 28 (89,3 %) des carcinomes triple-négatifs ont été identifiés comme étant des carcinomes triple-négatifs de type basal. Une analyse des associations des facteurs classés a révélé que les groupes de chiens de la race Shih tsu (9/25, 36,0 %) et d’un âge avancé (19/25, 76,0 %) étaient caractérisés par une prévalence plus élevée de tumeurs triple-négatives de type basal avec des types histologiques variés et de grade plus élevé. Ces résultats suggèrent que des différences reliées à la race et à l’âge peuvent être identifiés dans les carcinomes mammaires canins et, notamment, chez la race Shih tsu et à un âge plus avancé. Des études supplémentaires de ces caractéristiques distinctives de la race Shih tsu sont justifiées.
(Traduit par Docteur Serge Messier)
Introduction
Breast cancer is one of the most common cancers in women and a frequent cause of death from cancer (1–2). Classification of breast cancer helps in understanding and treating this condition. Molecular phenotyping to determine the expression pattern of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) is widespread and routinely evaluated in women with breast cancer (3–4). Receptor expression is closely associated with the development and progression pathway of breast cancer (5) and contributes significantly to the treatment outcome and prognosis (6–8). For example, ER-positive and HER-2-positive breast cancers can be treated with tamoxifen and trastuzumab, respectively, by means of blocking the specific receptor pathway (9).
Triple-negative cancer, however, in which expression for all 3 of the receptors ER, PR, and HER-2 is absent, cannot be treated with such tumor-specific treatments because the lack of receptor expression precludes inhibition of receptor-dependent pathways (10). Studies on breast cancer have therefore attempted to determine characteristics of and ways to overcome this type of tumor. More recent studies differentiated triple-negative breast cancer from basal-like breast cancer (11). Not all triple-negative cancers are basal-like (12). Triple-negative cancer can be differentiated into basal-like and normal-like phenotypes according to the expression of basal markers such as CK5/6, CK14, CK17, and EGFR (11). Because of a particularly poor prognosis, basal-like triple-negative tumors warrant thorough investigation (10,11,13).
In humans, the occurrence of breast cancer shows race- and age-related differences (14,15). The discovery that African-American female patients with breast cancer tended to have a lower survival rate than Caucasian female patients triggered studies of racial differences in breast cancer (14,15). Different aspects of breast cancer related to race and age have since been reported (16). The total number of breast cancer patients was not remarkable in African-American women or younger subjects, but breast cancers with worse prognosis, particularly the triple-negative cancers, tended to occur more often in African-American female patients and younger patients (17–21). These findings are presumed to be related to genetic differences among races.
Recently, basal-like triple-negative tumors have also been identified in dogs (22,23). The dog may be a reliable animal model for human breast cancer (23,24). Therefore, establishing breed-related and age-related differences in mammary tumors in dogs would be meaningful for both human and canine studies. This study aimed to determine the differences in expressions of histological types, molecular types, and other applicable factors in canine mammary cancer and to determine the characteristics of canine mammary carcinoma, with particular reference to the race- and age-related differences in expressions of basal-like triple-negative cancer.
Materials and methods
Sample selection
Entire samples used to diagnose canine mammary tumors were obtained from the Department of Veterinary Pathology, Konkuk University Animal Teaching Hospital, Seoul, Korea. Primary selection was limited to canine malignant mammary carcinoma diagnosed from 2003 to 2013 and consisted of 672 samples. All 672 samples were analyzed according to breed and the 5 most frequent breeds (Maltese, 148; Yorkshire terrier, 99; shih tzu, 72; poodle, 63; and Cocker spaniel, 53) were divided by age into subjects below and above 10 y of age, with 20 samples extracted randomly for each subgroup (< 10 and ≥ 10 y). The final study population consisted of 20 dogs in each age subgroup for each of the 5 breed groups, i.e., 40 dogs in each breed group for a total of 200 samples. As samples excluded after initial screening were not artificially replaced, 181 samples were analyzed.
Immunohistochemistry
Four-micrometer sections of formalin-fixed paraffin-embedded tissues were fixed on slides and deparaffinated by xylene, followed by serial rehydration by graded ethanol. Slides were washed 3 times with phosphate-buffered saline (PBS). A 3% hydrogen peroxide solution diluted with PBS was used to block endogenous peroxidase activity. Antigen retrieval was then carried out by the microwave retrieval method or enzyme retrieval method, selected according to the primary antibody.
Microwave retrieval (750W, 60Hz, 15 min) in pH 9.0 Trisethylenediamine tetra-acetic acid (EDTA) buffer was applied to the sample for anti-ER (clone: ER88; BioGenex, Fremont, California, USA), PR (PR10A9; Immunotech SAS, Marseille, France), HER-2/neu (CB1L; BioGenex), CK5/6 immunostaining (D5/16/B4; Dako, Glostrup, Denmark), and CK14 (LL002; Abcam, Cambridge, UK); 0.05% proteinase K (37°C, 30 min) enzyme digestion was applied for staining with anti-EGFR antibody (31G7; Abcam).
After PBS washing, slides were covered with 5% normal goat serum for 30 min for anti-ER staining to block nonspecific binding. Primary antibody staining was conducted. Incubation for 3 h at room temperature was applied for anti-ER (1:60), HER-2/neu (1:100), and CK14 (1:300) and overnight at 4°C for anti-PR (1:500), CK5/6 (1:100), and EGFR (1:50) antibody staining. PBS washing was carried out, followed by 2-step immunolabeling with secondary antibody-HRP conjugation for 40 min. Visualization was achieved using DAB+ chromogen (REAL EnVision Kit; Dako). Finally, slides were washed with distilled water and counterstained with Gill’s hematoxylin and coverslips were applied.
Tumor classification
Histological subtyping was carried out using HE-stained slides according to previously proposed criteria (25). Histological subtypes included carcinoma arising in a mixed tumor, carcinoma-complex type, simple carcinoma, intraductal papillary carcinoma, solid carcinoma, ductal carcinoma, carcinoma-anaplastic, carcinoma-micropapillary invasive, comedocarcinoma, and squamous cell carcinoma.
In the molecular classification of tumors, types of tumors that are positive for hormone receptor expression are called luminal types. Tumors with positive expression for ER or PR and negative expression for HER-2 were classified as luminal A type and tumors with positive expression for ER or PR and negative expression for HER-2 were classified as luminal B type. Types of tumors that were hormone-receptor negative, but were positive for HER-2 receptor expression, were classified as HER-2-overexpressing type and tumors that were negative for the expression of all 3 receptors were classified as triple-negative type. After identifying triple-negative cancer samples, basal-marker expression was sequentially evaluated for subtyping basal-like triple negative cancers. Triple-negative cancers expressing at least 1 basal marker were considered basal-like triple-negative cancers.
Grading was done by evaluating the sum of the scores of tubule formation, mitoses, and nuclear pleomorphism (26). Grade 1 indicated well differentiated tumors, Grade 2 indicated moderately differentiated tumors, and Grade 3 indicated poorly differentiated tumors.
Immunohistochemical analysis
Anti-ER or anti-PR staining was considered positive if there was more than 10% expression of clear nuclear staining. Evaluation of anti-HER-2 staining was based on the Hercep test and more than 10% of complete plasma membrane expression was considered positive. CK5/6, CK14, and EGFR were used as basal markers and areas of interest with more than 5% of membrane expression were considered basal-positive.
Statistical analysis
The Statistical Package for Social Science 17.0 software (SPSS, Chicago, Illinois, USA) was used for statistical analysis. Analysis of frequency, Pearson’s Chi-square test, and Fisher’s exact test were applied and P-values less than 0.05 (P < 0.05) were considered significant.
Results
Clinical data
The 181 dogs with malignant mammary carcinoma ranged in age from 3 to 17 y (mean: 9.4 ± 2.72 y). Ninety-two dogs (50.8%) were < 10 y and 89 dogs (49.2%) were ≥ 10 y. The 181 dogs were divided into 5 groups based on breeds with the highest prevalence for canine malignant mammary carcinoma as follows: 38 Cocker spaniels (20 < 10 y, 18 ≥ 10 y), 36 Maltese (18 < 10 y, 17 ≥ 10 y), 36 poodles (18 < 10 y, 18 ≥ 10 y), 34 shih tzus (18 < 10 y, 16 ≥ 10 y), and 35 Yorkshire terriers (18 < 10 y, 17 ≥ 10 y).
Histological classification and molecular phenotype
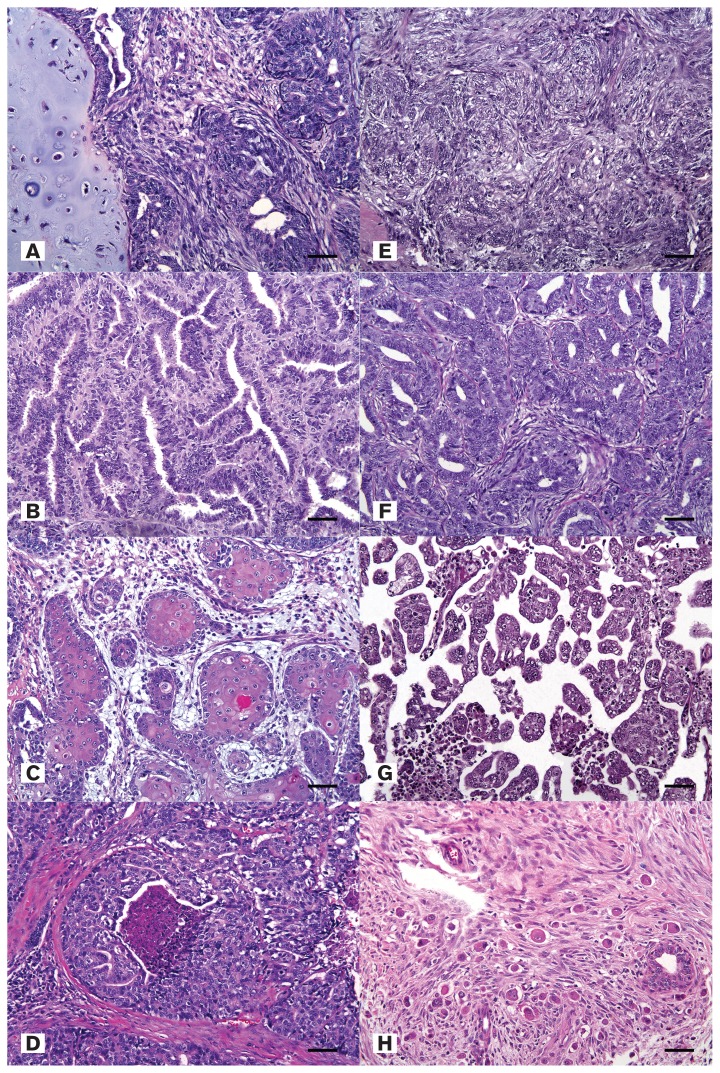
The malignant canine mammary carcinomas were categorized into histological types according to the classification proposed by Goldschmidt et al (25) based on hematoxylin and eosin (HE) staining (Figure 1). The 181 samples were divided into the following histological types: 71 (39.2%) carcinoma arising in a mixed tumor; 47 (26.0%) carcinoma-complex types; 36 (19.9%) simple carcinomas; 8 (4.4%) intraductal papillary carcinomas; 7 (3.09%) solid carcinomas; 5 (2.8%) ductal carcinomas; 4 (2.2%) squamous cell carcinomas; 1 (0.6%) carcinoma-micropapillary invasive; 1 (0.6%) comedocarcinoma; and 1 (0.6%) carcinoma-anaplastic.
Figure 1.
A — Carcinoma arising in a mixed tumor, mammary gland, canine. Hematoxylin and eosin (H&E). Bar = 35 μm. B — Simple carcinoma, mammary gland, canine. H&E. Bar = 35 μm. C — Squamous cell carcinoma, mammary gland, canine. H&E. Bar = 35 μm. D — Comedocarcinoma, mammary gland, canine. H&E. Bar = 35 μm. E — Carcinoma-complex type, canine. H&E. Bar = 35 μm. F — Ductal carcinoma, mammary gland, canine. H&E. Bar = 35 μm. G — Carcinoma-micropapillary invasive, mammary gland, canine. H&E. Bar = 35 μm. H — Carcinoma-anaplastic, mammary gland, canine. H&E. Bar = 35 μm.
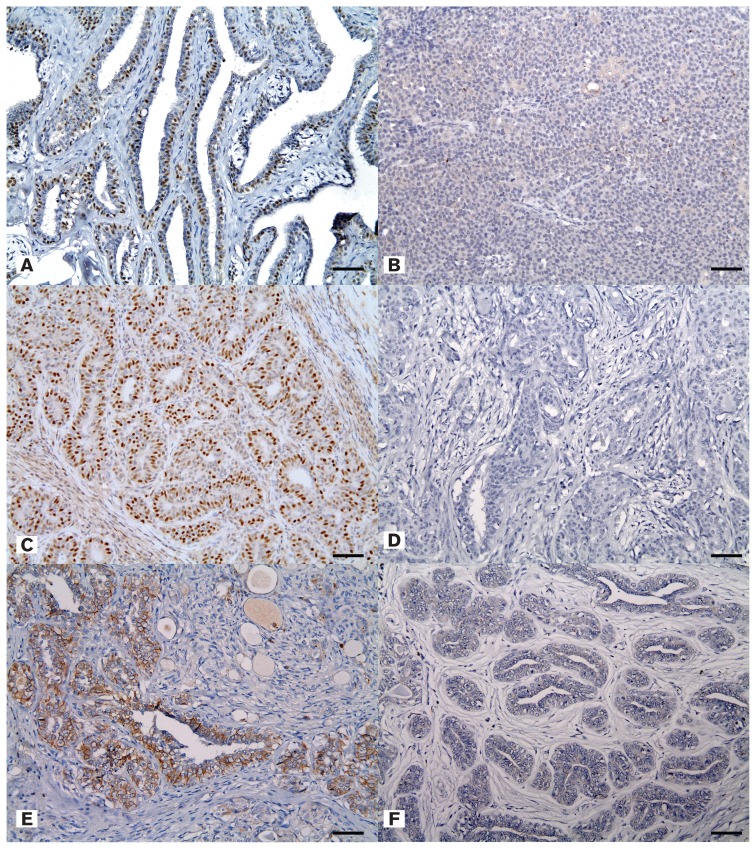
Two major types of tumor, carcinoma arising in a mixed tumor and complex type carcinoma, accounted for more than half (65%) of the samples. According to molecular phenotype, the luminal A type was the most prevalent at 42.5%, followed by the luminal B and triple-negative types (15.5%), with the HER-2-overexpressing type being the least common (6.1%). Overall, hormonal receptor positive type (ER positive or PR positive) mammary tumors accounted for 78% of the samples and the triple-negative type, indicating negative expression for anti-ER, anti-PR, and anti-HER-2 antibodies, accounted for 15.5% (Figure 2).
Figure 2.
A — Positive expression of estrogen receptor in simple carcinoma. Immunohistochemistry (IHC), mammary gland, canine. Bar = 35 μm. B — Negative expression of estrogen receptor in solid carcinoma. Immunohistochemistry (IHC), mammary gland, canine. Bar = 35 μm. C — Positive expression of progesterone receptor in ductal carcinoma. IHC, mammary gland, canine. Bar = 35 μm. D — Negative expression of progesterone receptor in complex carcinoma. IHC, mammary gland, canine. Bar = 35 μm. E — Positive expression of human epithelial growth factor receptor-2 in ductal carcinoma. IHC, mammary gland, canine. Bar = 35 μm. F — Negative expression of human epithelial growth factor receptor-2 in ductal carcinoma. IHC, mammary gland, canine. Bar = 35 μm.
Characteristics of tumor according to breed
Correlations between breeds and characteristics of malignant canine mammary carcinoma are listed in Table I. Statistically significant associations were not found between the breed and the histological classification and molecular phenotype. Histological grade revealed decreasing prevalence from Grade 1 to Grade 3 for all the breeds (P < 0.05). Grade 1 was most common (65.2%), followed by Grade 2 (28.7%), and Grade 3 (6.0%). The prevalence of Grade 3 was higher in the shih tzu breed, however, than in the other breeds (7/34, 63.6%).
Table I.
Breed-based statistical analysis of malignant mammary carcinoma of canines
| Breed | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Cocker spaniel (n = 38) | Maltese (n = 36) | Poodle (n = 36) | Shih tzu (n = 34) | Yorkshire terrier (n = 35) | P | |
| Histological type | ||||||
| Carcinoma — anaplastic (n = 1) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.115 |
| Carcinoma — complex type (n = 47) | 12 (25.5) | 13 (27.7) | 8 (17.0) | 5 (10.6) | 9 (19.1) | |
| Carcinoma — micropapillary invasive (n = 1) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Simple carcinoma (n = 36) | 11 (30.6) | 7 (19.4) | 7 (19.4) | 5 (13.9) | 6 (16.7) | |
| Solid carcinoma (n = 7) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 3 (42.9) | 1 (14.3) | |
| Carcinoma arising in a mixed tumor (n = 71) | 9 (12.7) | 15 (21.1) | 18 (25.4) | 13 (18.3) | 16 (22.5) | |
| Comedocarcinoma (n = 1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| Ductal carcinoma (n = 5) | 1 (20.0) | 1 (20.0) | 0 (0.0) | 1 (20.0) | 2 (40.0) | |
| Intraductal papillary carcinoma (n = 8) | 3 (37.5) | 0 (0.0) | 2 (25.0) | 2 (25.0) | 1 (12.5) | |
| Squamous cell carcinoma (n = 4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (100) | 0 (0) | |
| Molecular type | ||||||
| HER-2 overexpressinga (n = 11) | 5 (45.5) | 1 (9.1) | 0 (0.0) | 1 (9.1) | 4 (36.4) | 0.096 |
| Luminal Ab (n = 77) | 18 (23.4) | 14 (18.2) | 16 (20.8) | 13 (16.9) | 16 (20.8) | |
| Luminal Bc (n = 65) | 12 (18.5) | 19 (29.2) | 14 (21.5) | 10 (15.4) | 10 (15.4) | |
| Triple negatived (n = 28) | 3 (10.7) | 4 (14.3) | 6 (21.4) | 10 (35.7) | 5 (17.9) | |
| Triple negative | ||||||
| Non-triple negative (n = 153) | 35 (22.9) | 34 (22.2) | 30 (19.6) | 24 (15.7) | 30 (19.6) | 0.111 |
| Triple negative (n = 28) | 3 (10.7) | 4 (14.3) | 6 (21.4) | 10 (35.7) | 5 (17.9) | |
| Grade | ||||||
| 1 (n = 118) | 29 (24.6) | 25 (21.2) | 23 (19.5) | 19 (16.1) | 22 (18.6) | 0.017 |
| 2 (n = 52) | 8 (15.4) | 13 (25.0) | 11 (21.2) | 8 (15.4) | 12 (23.1) | |
| 3 (n = 11) | 1 (9.1) | 0 (0.0) | 2 (18.2) | 7 (63.6) | 1 (9.1) | |
ER−/PR−/HER-2+.
ER+ or PR+/HER-2−.
ER+ or PR+/HER-2+.
ER−/PR−/HER-2−.
P < 0.05.
Characteristics of tumor according to age
Data from the 2 age groups are summarized in Table II. Luminal A (44/77, 57.1%) and luminal B type (38/65, 58.5%) tumors were associated with the group of younger dogs (under 10 y), whereas HER-2-overexpressing (8/11, 72.7%) and triple-negative (21/28, 75.0%) types were related to the older group (10 y and over) (P < 0.05). Grade 1 accounted for 65.2% of all subjects, followed by Grade 2 at 28.7%, and Grade 3 at 6.1%. Of note, Grade 3 was seen more in the older group (≥ 10 y) (10/11, 90.9%) than in the younger group (< 10 y) (1/11, 9.1%).
Table II.
Age-based statistical analysis of malignant mammary carcinoma of canines
| Age groups | |||
|---|---|---|---|
|
|
|||
| < 10 y (n = 92) | ≥ 10 y (n = 89) | P | |
| Histological type | |||
| Carcinoma — anaplastic (n = 1) | 0 (0.0) | 1 (100.0) | 0.061* |
| Carcinoma — complex type (n = 47) | 32 (68.1) | 15 (31.9) | |
| Carcinoma — micropapillary invasive (n = 1) | 1 (100.0) | 0 (0.0) | |
| Simple carcinoma (n = 36) | 18 (50.0) | 18 (50.0) | |
| Solid carcinoma (n = 7) | 3 (42.9) | 4 (57.1) | |
| Carcinoma arising in a mixed tumor (n = 71) | 32 (45.1) | 39 (54.9) | |
| Comedocarcinoma (n = 1) | 0 (0.0) | 1 (100.0) | |
| Ductal carcinoma (n = 5) | 3 (60.0) | 2 (40.0) | |
| Intraductal papillary carcinoma (n = 8) | 3 (37.5) | 5 (62.5) | |
| Squamous cell carcinoma (n = 4) | 0 (0.0) | 4 (100.0) | |
| Molecular type | |||
| HER-2 overexpressinga (n = 11) | 3 (27.3) | 8 (72.7) | 0.005 |
| Luminal Ab (n = 77) | 44 (57.1) | 33 (42.9) | |
| Luminal Bc (n = 75) | 38 (58.5) | 27 (41.5) | |
| Triple negatived (n = 28) | 7 (25.0) | 21 (75.0) | |
| Triple negative | |||
| Non-triple negative (n = 153) | 85 (55.6) | 68 (44.4) | 0.003 |
| Triple negative (n = 28) | 7 (25.0) | 21 (75.0) | |
| Grade | |||
| 1 (n = 118) | 61 (51.7) | 57 (48.3) | 0.013 |
| 2 (n = 52) | 30 (57.7) | 22 (42.3) | |
| 3 (n = 11) | 1 (9.1) | 10 (90.9) | |
ER−/PR−/HER-2+.
ER+ or PR+/HER-2−.
ER+ or PR+/HER-2+.
ER−/PR−/HER-2−.
P < 0.05.
Fisher’s exact test.
Analysis of tumor characteristics according to grade
There were significant correlations (P < 0.05) among grade and dog breed, age group, histological type, and molecular phenotype. The results of the Chi-square test based on grade are given in Table III. Complex-type carcinoma (27/47, 57.4%), simple carcinoma (28/36, 77.8%), carcinoma arising in a mixed tumor (54/71, 76.1%), ductal carcinoma (4/5, 80.0%), and intraductal papillary carcinoma (5/8, 62.5%) were more predominant in the lower grades. There was a tendency for higher grade expression to be in the solid carcinoma (4/7, 57.1%) and squamous cell carcinoma (3/4, 75.0%). Although there was only 1 case of each, the anaplastic carcinoma (1/1, 100%) and comedocarcinoma (1/1, 100%) were both Grade 3. Grade 1 tended to dominate in all molecular phenotypes, but the triple-negative subtype accounted for 45.4% (5/11) of Grade 3.
Table III.
Statistical analysis of malignant canine mammary carcinoma based on grade
| Grade | ||||
|---|---|---|---|---|
|
|
||||
| 1 (n = 118) | 2 (n = 42) | 3 (n = 11) | P | |
| Histological type | ||||
| Carcinoma — anaplastic (n = 1) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0.000 |
| Carcinoma — complex type (n = 47) | 27 (57.4) | 20 (42.6) | 0 (0.0) | |
| Carcinoma — micropapillary invasive (n = 1) | 0 (0.0) | 1 (100.0) | 0 (0.0) | |
| Simple carcinoma (n = 36) | 28 (77.8) | 8 (22.2) | 0 (0.0) | |
| Solid carcinoma (n = 7) | 0 (0.0) | 3 (42.9) | 4 (57.1) | |
| Carcinoma arising in a mixed tumor (n = 71) | 54 (76.1) | 15 (21.1) | 2 (2.8) | |
| Comedocarcinoma (n = 1) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Ductal carcinoma (n = 5) | 4 (80.0) | 1 (20.0) | 0 (0.0) | |
| Intraductal papillary carcinoma (n = 8) | 5 (62.5) | 3 (37.5) | 0 (0.0) | |
| Squamous cell carcinoma (n = 4) | 0 (0.0) | 1 (25.0) | 3 (75.0) | |
| Molecular type | ||||
| HER-2 overexpressinga (n = 11) | 10 (90.9) | 0 (0.0) | 1 (9.1) | 0.010* |
| Luminal Ab (n = 77) | 45 (58.4) | 28 (36.4) | 4 (5.2) | |
| Luminal Bc (n = 65) | 46 (70.8) | 18 (27.7) | 1 (1.5) | |
| Triple negatived (n = 28) | 17 (60.7) | 6 (21.4) | 5 (17.9) | |
| Triple negative | ||||
| Non-triple negative (n = 153) | 101 (66.0) | 46 (30.1) | 6 (3.9) | 0.016 |
| Triple negative (n = 28) | 17 (60.7) | 6 (21.4) | 5 (17.9) | |
ER−/PR−/HER-2+.
ER+ or PR+/HER-2−.
ER+ or PR+/HER-2+.
ER−/PR−/HER-2−.
P < 0.05.
Fisher’s exact test.
Characteristics of basal-like triple-negative mammary cancer
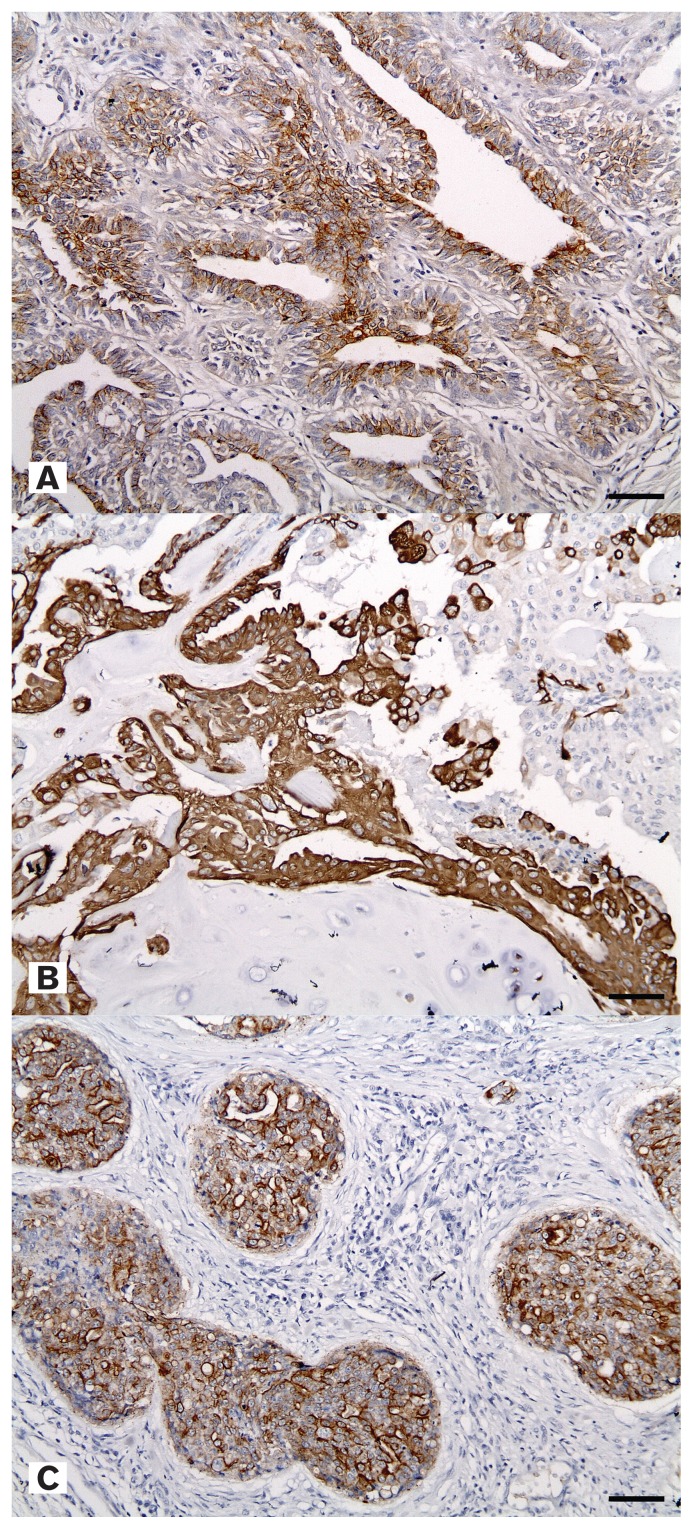
Twenty-five of the tumors were basal-like triple-negative tumors (Figure 3), which represented 89.3% of the triple-negative type cancers. The most frequent histological type was carcinoma arising in a mixed tumor (12/25, 48%) and the most common breed was the shih tzu (9/25, 36%) (Table IV). Grade 1 (16/25, 64%) was the predominant grade within the basal-like triple-negative tumors and Grade 3 was solely expressed by the shih tzu breed, except for 1 Poodle. The shih tzu breed had the broadest spectrum of histological types. By age, 76% (19/25) of the total cases were ≥ 10 y of age and 36.8% (7/19) of these dogs were shih tzus.
Figure 3.
A — Positive expression of epidermal growth factor receptor in simple carcinoma. IHC, mammary gland, canine. Bar = 35 μm. B — Positive expression of cytokeratin 14 in carcinoma arising in a mixed tumor. IHC, mammary gland, canine. Bar = 35 μm. C — Positive expression of cytokeratin 5/6 in squamous cell carcinoma, metastasized. IHC, mammary gland, canine. Bar = 35 μm.
Table IV.
Characteristics of distributions of basal-like triple-negative mammary cancer
| Breed | |||||
|---|---|---|---|---|---|
|
|
|||||
| Cocker spaniel | Maltese | Poodle | Shih tzu | Yorkshire terrier | |
| Frequency | 2 (8.0) | 3 (12.0) | 6 (24.0) | 9 (36.0) | 5 (20.0) |
| Histological type | |||||
| Carcinoma — complex type (n = 4) | 1 (25.0) | 1 (25.0) | 0 (0.0) | 1 (25.0) | 1 (25.0) |
| Simple carcinoma (n = 1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 100 (100.0) |
| Solid carcinoma (n = 4) | 0 (0.0) | 1 (25.0) | 1 (25.0) | 2 (50.0) | 0 (0.0) |
| Carcinoma arising in a mixed tumor (n = 12) | 1 (8.3) | 1 (8.3) | 4 (33.3) | 2 (16.7) | 4 (33.3) |
| Intraductal papillary carcinoma (n = 2) | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) |
| Squamous cell carcinoma (n = 2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.0) | 0 (0) |
| Grade | |||||
| 1 (n = 16) | 2 (12.5) | 2 (12.5) | 4 (25.0) | 4 (25.0) | 4 (25.0) |
| 2 (n = 4) | 0 (0.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) | 1 (25.0) |
| 3 (n = 5) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 4 (80.0) | 0 (0.0) |
Discussion
Determining and categorizing the characteristics of breast cancer may help to treat this disease and to establish the causative factors of the cancer (27). A molecular-based classification scheme has been proposed (28), in which triple-negative breast cancer and basal-like breast cancer are problematic in terms of having comparatively poor prognoses and therapeutic challenges (19).
Many factors have been implicated in the pathogenesis of breast cancer, including hormonal influences, environmental and hereditary factors, and age (18,29). Investigations into the characteristics of tumor development yielded higher incidences of triple-negative breast cancer in young patients and African-American women (12,14). These predilections of race and age appear to be related to the hereditary factors causing breast cancer.
The dog is one of the most common species that develops mammary tumors and can therefore serve as an acceptable model for human breast cancer. Basal-like triple-negative tumors have also been identified in dogs (22,23). In this study, we investigated associations between types and characteristics of tumors. We especially focused on breed and age group by using previously reported criteria. Among the tumor types, particular emphasis was placed on the basal-like triple-negative tumor. Characteristics of basal-like triple-negative cancers were analyzed by frequency and relations between certain clinicopathological parameters were evaluated.
While the types of tumor expression did not clearly differ according to breed, the shih tzu breed exhibited some distinguishing characteristics. In analyzing histological type, types of tumors known to have better prognoses were not commonly expressed in shih tzus, but higher expression rates of types of tumors known to have worse prognoses were the most outstanding characteristics of the shih tzu breed compared with the other breeds. Tumors with better prognoses are carcinoma arising in a mixed tumor, carcinoma-complex type, and simple carcinoma, while tumors with worse prognoses include all of the other types of tumors. In molecular phenotype analysis, triple-negative type tumors were more common in the shih tzu than in other breeds. Overall, the number of subjects decreased from Grade 1 to Grade 3, but the shih tzu group had the highest percentage of Grade 3 subjects. Of the 25 basal-like triple-negative tumors in 28 triple-negative mammary tumors, 36% (9/25) were from shih tzu dogs. The shih tzu group had a broader distribution of histological types, whereas histological types of tumors in other breeds tended towards better prognostic types, such as carcinoma-complex type and carcinoma arising in a mixed tumor (Figure 3). Although expressions of grade in other groups were primarily Grade 1 or Grade 2, a higher proportion of Grade 3 was a distinguishing characteristic of the shih tzu breed.
When analyzing the characteristics of tumors according to age groups, we ascertained that histological types of mammary tumors with the worse prognosis tended to develop in older dogs (≥ 10 y), particularly in the case of basal-like triple-negative tumors. Regarding molecular phenotype, both luminal A and luminal B types of tumors were more likely to be expressed in the younger age group, while HER-2-overexpressing types and triple-negative types of tumors tended to be expressed in the older age group. Analysis by Fisher’s exact test revealed that the histological types with better prognoses, such as complex carcinoma, carcinoma arising in a mixed tumor, and simple-type carcinoma, were significantly associated with younger age.
In contrast, triple-negative tumors, which are known to have a worse prognosis in human studies and poor response to treatment, were more common in the older group of dogs. Basal-like triple-negative tumors were expressed more frequently in the older group [19 of 25 cases (76.0%)], with the shih tzu being the most common breed among these 19 subjects [36.8% (7/19)].
Additional analysis was carried out according to tumor grade. An association was identified between tumor types with worse prognosis and higher grades and relations between triple-negative tumors and Grade 3. Some histological types of tumors known to have worse prognoses showed higher grades (P < 0.05). Molecular phenotype analysis that showed grade 3 tumors had the highest percentage of triple-negative type tumors.
To summarize the results, breeds did not show statistical significance by tumor molecular phenotypes, but the shih tzu breed most frequently developed basal-like triple-negative tumors and was significantly associated with Grade 3. In age, the older group of ≥ 10 y was associated with basal-like triple-negative and Grade 3 tumors.
Breed-related differences in the development of basal-like triple-negative tumors in dogs are similar to the race-related characteristics of African-Americans in the development of human breast cancer. The shih tzu breed was not the most common breed to develop mammary carcinoma, but it showed a higher prevalence of basal-like triple-negative mammary tumors than all the other breeds, just as African-Americans have the highest prevalence of human triple-negative breast cancer.
Basal-like triple-negative carcinomas were associated with both older non-shih tzu subjects and older shih tzu breed subjects. Differences were revealed between humans and dogs in developing basal-like triple-negative tumors because there was a higher prevalence of these tumors in the older dogs (> 10 y). In humans, younger patients are usually more likely to develop these tumors.
Menopause is one of the important standards in classifying when breast cancer develops in humans. When breast cancer develops during the postmenopausal period, it does not develop under conditions of typical cyclic hormonal influence. As there is no physiological menopausal period in dogs, they do not experience cessation of ovarian hormone release and the accompanying lobular involution of the mammary tissue. To correctly determine the age-related differences between dogs and humans, further studies will be needed to clarify and exclude the effects of a change in hormone status, accompanied by the effects of aging on tumor development or to provide correspondent factors in the dog, such as an ovariohysterectomy.
Although the results were not identical to those in human cases, there were considerable breed- and age-related differences in canine tumor characteristics. There were enough disparities in the expression of tumor types by breed to consider that certain hereditary factors may influence cancer development. We can therefore infer that this prevalence is based on genetic inequality.
Associations between triple-negative tumors and the BRCA1 mutation have recently been investigated in humans (30–33). The BRCA1 mutation is a hereditary factor and representative mutation of genes that can cause breast cancers. In the present study, associations of canine breed and age with basal-like triple-negative tumors have been investigated. In addition, associations of the BRCA mutation with canine mammary tumors have been investigated in many other studies (34–35). One study found noticeable characteristics of the shih tzu breed in overexpression of BRCA1 (36). Because the antibody used in this study was limited to exon 11 mutations of the human BRCA1 gene, these results cannot fully reflect the various BRCA1 mutations in dogs. Considering that 84% of the human and canine BRCA1 genes are homologous (37), however, these common characteristics of the shih tzu breed are noteworthy.
Previous studies suggest that there are racial differences in triple-negative breast cancer (29), that BRCA mutation locus varies among races (38–40), and that there is an association between the occurrence of triple-negative tumors and BRCA mutation (6,33). Further studies must be conducted on canine breeds, however, to better understand breed-related hereditary factors that can influence the type of tumor that develops, basal-like triple-negative cancers of specific breeds, and the specific locus of BRCA1 gene mutation, as well as to establish associations between certain breeds and basal-like triple-negative cancers with BRCA1 mutations. When considering those common characteristics in the shih tzu breed, triple-negative tumors and BRCA1 mutation seem to be affected by certain hereditary influences in tumor development. There may be an association between basal-like triple-negative cancers and BRCA1 mutation as well as other genetic factors in tumor development. Further study to investigate and understand canine mammary cancers may be useful for canine mammary cancer to serve as an effective model in human studies.
Acknowledgments
The authors thank Rae-Hwa Jang for her excellent technical assistance. This article is part of the PhD thesis of Hyun-Woo Kim. This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) and funded by the Ministry of Science, Information/Communication Technology (ICT) and Future Planning (2014R1A2A2A01003470).
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy L, Watson P. Steroid receptors in human breast tumorigenesis and breast cancer progression. Biomed Pharmacother. 2002;56:65–77. doi: 10.1016/s0753-3322(01)00157-3. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Sorenmo K, Rasotto R, Zappulli V, Goldschmidt MH. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet Path. 2011;48:85–97. doi: 10.1177/0300985810389480. [DOI] [PubMed] [Google Scholar]
- 8.van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 9.Choo JR, Nielsen TO. Biomarkers for basal-like breast cancer. Cancers. 2010;2:1040–1065. doi: 10.3390/cancers2021040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 11.Rakha EA, Elsheikh SE, Aleskandarany MA, et al. Triple-negative breast cancer: Distinguishing between basal and nonbasal subtypes. Clin Cancer Res. 2009;15:2302–2310. doi: 10.1158/1078-0432.CCR-08-2132. [DOI] [PubMed] [Google Scholar]
- 12.Reis-Filho JS, Tutt A. Triple negative tumours: A critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 13.Tischkowitz M, Brunet JS, Bégin LR, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007;7:134. doi: 10.1186/1471-2407-7-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2005. Bethesda: National Cancer Institute; 2008. [Google Scholar]
- 15.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: Update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 16.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 17.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women. Cancer. 2011;117:2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Chen Z, Anderson GL, et al. Ethnicity and breast cancer: Factors influencing differences in incidence and outcome. J Natl Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 19.Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: A population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan N, Nemoto T, Mettlin C, Murphy GP. Race-related differences in breast cancer patients. Results of the 1982 national survey of breast cancer by the American College of Surgeons. Cancer. 1985;56:1704–1709. doi: 10.1002/1097-0142(19851001)56:7<1704::aid-cncr2820560740>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10:1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim NH, Lim HY, Im KS, Kim JH, Sur JH. Identification of triple-negative and basal-like canine mammary carcinomas using four basal markers. J Comp Path. 2013;148:298–306. doi: 10.1016/j.jcpa.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Gama A, Alves A, Schmitt F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: Application of the human classification. Virchows Arch. 2008;453:123–132. doi: 10.1007/s00428-008-0644-3. [DOI] [PubMed] [Google Scholar]
- 24.Queiroga FL, Raposo T, Carvalho MI, Prada J, Pires I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. In Vivo. 2011;25:455–465. [PubMed] [Google Scholar]
- 25.Goldschmidt M, Pena L, Rasotto R, Zappulli V. Classification and grading of canine mammary tumors. Vet Path. 2011;48:117–131. doi: 10.1177/0300985810393258. [DOI] [PubMed] [Google Scholar]
- 26.Peña L, De Andrés PJ, Clemente M, Cuesta P, Pérez-Alenza MD. Prognostic value of histological grading in noninflammatory canine mammary carcinomas in a prospective study with two-year follow-up relationship with clinical and histological characteristics. Vet Path. 2013;50:94–105. doi: 10.1177/0300985812447830. [DOI] [PubMed] [Google Scholar]
- 27.Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Path. 2008;216:141–150. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 29.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 30.Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control. 2010;17:173–176. doi: 10.1177/107327481001700305. [DOI] [PubMed] [Google Scholar]
- 31.Turner NC, Reis-Filho JS. Basal-like breast cancer and the BRCA1 phenotype. Oncogene. 2006;25:5846–5853. doi: 10.1038/sj.onc.1209876. [DOI] [PubMed] [Google Scholar]
- 32.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814–819. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 33.Young SR, Pilarski RT, Donenberg T, et al. The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Cancer. 2009;9:86. doi: 10.1186/1471-2407-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieto A, Pérez-Alenza MD, Del Castillo N, Tabanera E, Castaño M, Peña L. BRCA1 expression in canine mammary dysplasias and tumours: Relationship with prognostic variables. J Comp Pathol. 2003;128:260–268. doi: 10.1053/jcpa.2002.0631. [DOI] [PubMed] [Google Scholar]
- 35.Rivera P, Melin M, Biagi T, et al. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009;69:8770–8774. doi: 10.1158/0008-5472.CAN-09-1725. [DOI] [PubMed] [Google Scholar]
- 36.Im KS, Kim IH, Kim NH, Lim HY, Kim JH, Sur JH. Breed-related differences in altered BRCA1 expression, phenotype and subtype in malignant canine mammary tumors. Vet J. 2013;195:366–372. doi: 10.1016/j.tvjl.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Szabo CI, Wagner LA, Francisco LV, et al. Human, canine and murine BRCA1 genes: Sequence comparison among species. Hum Mol Genet. 1996;5:1289–1298. doi: 10.1093/hmg/5.9.1289. [DOI] [PubMed] [Google Scholar]
- 38.Gao Q, Neuhausen S, Cummings S, Luce M, Olopade OI. Recurrent germ-line BRCA1 mutations in extended African American families with early-onset breast cancer. Am J Hum Genet. 1997;60:1233–1236. [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Q, Tomlinson G, Das S, et al. Prevalence of BRCA1 and BRCA2 mutations among clinic-based African American families with breast cancer. Hum Genet. 2000;107:186–191. doi: 10.1007/s004390000290. [DOI] [PubMed] [Google Scholar]
- 40.Panguluri RC, Brody LC, Modali R, et al. BRCA1 mutations in African Americans. Hum Genet. 1999;105:28–31. doi: 10.1007/s004399900085. [DOI] [PubMed] [Google Scholar]