Abstract
The generation of patient-specific induced pluripotent stem (iPS) cells permits the development of next-generation patient-specific systems biology models reflecting personalized genomics profiles to better understand pathophysiology. In this chapter, we describe how to create a patient-specific iPS cell line. There are three major steps: (1) performing a skin biopsy procedure on the patient; (2) extracting human fibroblast cells from the skin biopsy tissue; and (3) reprogramming patient-specific fibroblast cells into the pluripotent stem cell stage.
Keywords: Human iPS, Skin biopsy, Fibroblast, Reprogramming, Sendai virus
1 Introduction
Induced pluripotent stem (iPS) cells reprogrammed from somatic cells have allowed for the generation of patient-specific disease cells, which carry disease phenotype in vitro. Interest in generating human-induced pluripotent stem (hiPS) cells for stem cell based disease modeling has overtaken that of patient-specific human embryonic stem (ES) cells due to the ethical, technical, and political concerns associated with the latter (1). Patient-specific iPS cells have potential in human disease modeling and regenerative medicine. The in vitro phenotype of disease-specific iPS-derived cells can be used to bridge the knowledge gap between the clinical phenotype and molecular or cellular pathophysiology, along with further applications, such as creating new strategies for drug screening or developing novel therapeutic agents. By using patient-specific hiPS cells, we can prove that a disease is caused by a novel gene mutation and hypothesize potential treatment options before moving to more expensive animal studies (1). The hiPS cell-based disease models may also assist in the development of novel treatments for clinical trials (2, 3).
The generation of human iPS cell lines has already been widely described since Takahashi's and Yamanaka's report on obtaining pluripotent cells from somatic cells (4). At the time, the scientific community's primary concern regarding the limitation of Takahashi and Yamanaka's technique was that the integration of retroviral-mediated transduction into the host genome includes the risk of insertional mutagenesis (5). In our protocol, we use the Sendai virus (6, 7), a footprint-free RNA virus that carries no risk of altering the host genome (8), to generate patient-specific iPS cell lines.
Successfully establishing a patient-specific iPS cell line requires three steps: (1) obtaining human subject committee approval and performing a skin biopsy on patient; (2) deriving human fibroblast cells from the skin biopsy tissue; and (3) reprogramming patient-specific fibroblast cells into the pluripotent stem cell stage. Here, we summarize the materials and methods necessary for the entire procedure.
2 Materials
All the reagents, medium, components, and tools are sterile.
2.1 Basic Supplies
Laminar flow hood.
Water bath, 37 °C.
Pipet-Aid.
Plastic serological pipets: 1, 2, 5, and 10 ml.
Incubator, 5 % CO2.
Tabletop centrifuge.
Spray bottle, 70 % ethanol.
2.2 Materials for Skin Biopsy Procedure
Biopsy collect medium: RPMI1640, 1× Anti-Anti.
Disposable biopsy punches, diameter 4 mm.
Xylocaine (lidocaine HCL and epinephrine injection, USP).
Sterile fenestrated towel.
Povidone–iodine, USP swab stick.
Sterile, powder-free surgical gloves.
5-0 VICRYL coated suture.
3/10 ml insulin syringe.
Sterile gauze pad.
Autoclaved forceps, needle holder, scissors.
15 ml conical tube.
Band-Aid.
70 % ethanol wipes.
2.3 Materials for Preparation of Human Fibroblast Cultures from Skin Biopsy Tissue
6-well cell culture plate.
Plastic serological pipets.
Biopsy plating medium: DMEM, 10 % fetal bovine serum (FBS), 1× nucleosides, 1× l-glutamine, 1× nonessential amino acids, 1× Anti-Anti, 0.1 mM β-mercaptoethanol.
Microscope coverslips.
Sterile micro-forceps and scissors.
Sterile scalpel.
Silicone grease.
Phosphate buffered saline (PBS).
0.05 % trypsin–EDTA.
Fibroblast culture medium: DMEM, 10 % FBS, 1× l-glutamine, 1× sodium pyruvate, 1× penicillin–streptomycin.
10 cm petri dish.
0.5 % gelatin coating solution (in water).
2.4 Materials of Human iPS Cells Generation
KO-DMEM medium: KnockOut DMEM, 15 % KnockOut serum replacement, 1× l-glutamine, 1× nonessential amino acids, 1× penicillin–streptomycin, 0.1 mM β-Mercaptoethanol, basic fibroblast growth factor (bFGF) 10 ng/ml.
Fibroblast medium: DMEM (high glucose), 10 % FBS, 1× l-glutamine, 1× penicillin–streptomycin, 1× sodium pyruvate.
12-well cell culture plate.
Y-27632 (ROCK inhibitor).
0.1 % gelatin solution.
Freezing solution for ES cells.
TrypLE Express (1×).
Phosphate buffered saline (PBS).
Mitomycin-C treated mouse embryonic fibroblast (MEFs, feeders).
CytoTune-iPS reprogramming Kit (Life technologies, A13780).
4-well cell culture plate.
2.5 Materials for Characterization Assay
2.5.1 Materials for Teratoma Assay
Matrigel matrix.
1 ml insulin syringe.
Hypodermic disposable needle, 18G.
Ice and ice bucket.
SCID mice.
2.5.2 Materials and Markers for Immunofluorescent Assay
Materials for immunofluorescent assay have been widely available and primary antibodies of pluripotent markers are: OCT4 Santa Cruz sc-9081 Rabbit poly, SOX2 R&D Systems 245610 Mouse IgG, TRA-1-60 Millipore (Chemicon) MAB4381, Mouse, IgM, SSEA4 Millipore (Chemicon) MAB4304 Mouse IgG, Nanog R&D Systems AF1997 Goat poly.
3 Methods
All procedures excluding the skin biopsy surgery are preformed in the laminar flow hood.
3.1 Skin Biopsy Procedure
Here (see Note 1), we briefly describe this procedure. It should be done by a dermatologist or an appropriately trained physician.
The biopsy must be performed under sterile conditions. Place a sterile fenestrated towel over the biopsy area. The biopsy site will be at the center of the fenestrated location.
Anesthetize the skin locally by subcutaneous injection of 1 % lidocaine.
The site of biopsy should be disinfected with 5 % povidone–iodine twice. For disinfection, use a povidone–iodine swab to wipe the site of biopsy beginning from the center in circular movements and proceeding to the outer edges. Air-dry. Take 15 s for this procedure and repeat it using a new swab.
Skin biopsy is performed with a sterile 4 mm skin punch and cut off by a sterile scissors. Transfer the skin biopsy with sterile forceps into a sterile 15 ml plastic tube containing 10 ml biopsy collect medium. Place the tube under room temperature.
Suture the edges of the wound with 6.0 VICRYL coated dissolvable suture and bury the knots.
Clean the sutured area with sterile 70 % ethanol wipes and then place a Band-Aid to cover the suturing site.
3.2 Fibroblast Culture and Collection from Skin Biopsy Tissue
3.2.1 Preparation of Human Fibroblast Cultures from Skin Biopsies
The entire procedure usually takes about 4 weeks: 2 or 3 weeks for fibroblasts to expand to cover most of the area underneath the coverslips, then another week to collect the fibroblasts from the plating dish and passage them to cover a 10 cm culture dish.
Rinse the biopsy once in PBS or collection medium.
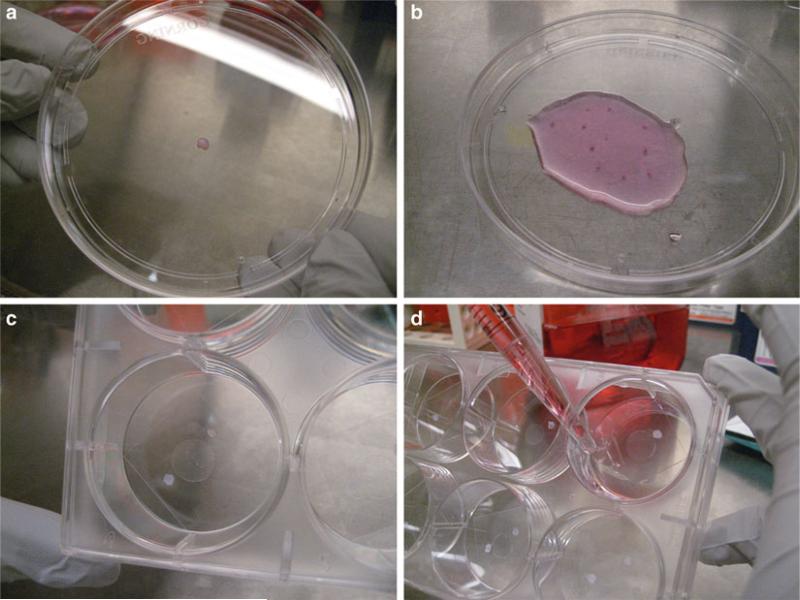
Place biopsy tissue in a 10 cm petri dish with a little bit of medium (Fig. 1a).
Carefully hold the biopsy punch with sterile forceps and remove the scarfskin (see Notes 2 and 3), then carefully mince the biopsy core into 10–12 pieces (Fig. 1b).
Using a sterile 1,000 μl pipet tip, transfer autoclaved silicone grease and place one drop in the center of each well in a 6-well culture plate.
Using sterile forceps, place two to three pieces of minced biopsy around the silicon grease drop. Plate all tissue.
Take a coverslip and place over the grease and minced biopsy pieces. Press on coverslip (Fig. 1c) (see Note 4).
Tilt the dish slightly. Slowly add 5 ml of biopsy plating medium. Be careful to not lift off the cover slip (Fig. 1d). The air below the cover slip should be displaced by medium. Gently apply pressure on each cover slip to ensure that it is well attached. Place into incubator. Do not disturb the cultures for at least 4 days.
Change medium every 5–7 days.
Fig. 1.
Key steps of plating human skin biopsy sample on culture dish. (a) Place biopsy tissue in a 10 cm petri dish. (b) Mince the biopsy core into 10–12 pieces. (c) Take a coverslip and place over the grease and minced biopsy pieces. (d) Tilt the dish slightly. Slowly add biopsy plating media into the dish
3.2.2 Collection of Fibroblast Cells from Biopsy Plating Dish
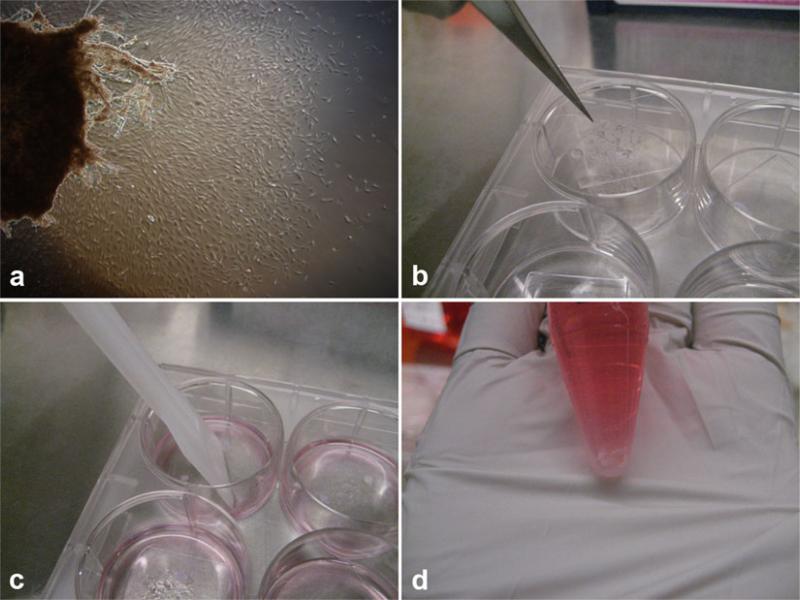
Cultures are ready to be split when the entire coverslip is covered with fibroblasts (in 2–3 weeks). Fibroblasts grow on the glass as well as on the plastic (Fig. 2a).
Pre-warm trypsin, prior to use. Gelatinize 6-well culture plate for every 6-well biopsy plating plate.
Remove topic medium and wash the plate surface with PBS. With a sterile forceps, carefully peel off the coverslip from the bottom of each well and overturn inside the well. The cell growth surface should be facing up (Fig. 2b). Add 1 ml of pre-warmed trypsin, ensuring the overturn coverslip is covered with trypsin, and replace into incubator for 5 min.
Check trypsin digestion after 5 min. When the cells are no longer attached to the coverslip, harvest the cells and biopsy tissue together by a sterile scalpel, and then transfer them into a 15 ml conical tube (Fig. 2c). Inactivate trypsin with 1:1 volume of fibroblast culture medium.
Spin down at 500 × g for 4 min. Aspirate supernatant and resuspend the cell pellet and tissue with fibroblast culture medium. Wait 30 s to allow the tissue to settle to the bottom of the conical tube (Fig. 2d).
Remove gelatin from pre-coated 6-well plate and transfer cell suspension to each well (discard the tissue).
Change medium every 1 or 2 days for regular culture until the cell grow to confluency for the next passage.
Fig. 2.
Key steps of collecting fibroblast cells from biopsy plating dish. (a) Fibroblasts cells growing out from skin tissue. Fourteen days post plating. (b) Using a sterile forceps, carefully peel off the coverslip from the bottom of each well and overturn inside the well. (c) After digestion, harvest the cells by a sterile scalpel. (d) After resuspending cell pellet and tissue, wait the tissue to settle to the bottom of the conical tube
3.3 iPS Generation Protocol with Sendai Virus
Plate 5 × 104 fibroblast cells (see Note 5) in each well of a 12-well plate one day ahead the transfection day.
Culture fibroblast cells in an incubator (37 °C, 5 % CO2) overnight to make sure that the cells extend and adhere to the dish.
Take out the Sendai viruses (see Note 6) expressing the four Yamanaka factors (OCT3/4, SOX2, KLF4, and c-MγC) from stock (CytoTune-iPS reprogramming, Life Technologies, USA) at −80 °C and thaw them following manufacturer instruction.
Calculate volumes of each virus used for one well of cells (5 × 104 cells per well) at a multiplicity of infection (MOI) of 3.
Aliquot the appropriate volume of each virus for every 5 × 104 cells, as decided in step 4, to 500 μl fibroblast culture medium (every 500 μl virus–medium mixture contains the four Yamanaka factors for one well of cells).
Remove the culture medium completely from the cells prepared in step 1. For every 5 × 104 cells (each well) apply 500 μl virus–medium mixture gently to each well. Swirl the plate slightly to make the mixture covers the entire cell layer.
Place the plate into an incubator (37 °C, 5 % CO2) overnight.
The next day, add another 500 μl of fibroblast culture medium to each well. Place the plate into incubator (37 °C, 5 % CO2) overnight.
On the following day, remove the virus-containing medium and replace with KO-DMEM medium. Continue incubation (37 °C, 5 % CO2) for an additional 6–7 days, changing the medium every day with KO-DMEM medium.
One day before the day of cell passage in step 8, prepare a feeder cell-coated plate by inoculating Mitomycin-C treated MEF cells on gelatin-coated cells. To coat cells with gelatin, add 2 ml of 0.1 % gelatin solution per well of a 6-well, swirl to cover the entire surface with the solution, and let stand at 37 °C for 30 min. Remove the gelatin solution immediately before plating. MEF cells should be plated in 6-well plates at 2 × 105 cells per well. On the following day, change the medium×with fibroblast culture medium.
7–8 days after Sendai transduction, remove the medium, wash the cells once with PBS, add 500 μl per well of TrypLE express and let it incubate at 37 °C for 4 min. After 4 min, take the plate out of the incubator, remove the TrypLE express carefully and leaves the half-detached cells in the wells. Apply 2 ml KO-DMEM medium containing 10 μM ROCK inhibitor in each well and resuspend the cells by gently pipette up and down. Chunks of cells may remain in this step. Transfer cells onto the feeder plate. Cells from one well of a 12-well plate should be transferred to one well of 6-well feeder plate.
Return the culture plates to the incubator (37 °C, 5 % CO2). After 24 h, change the medium with KO-DMEM medium (without ROCK inhibitor). Change medium every day with freshly prepared KO-DMEM medium.
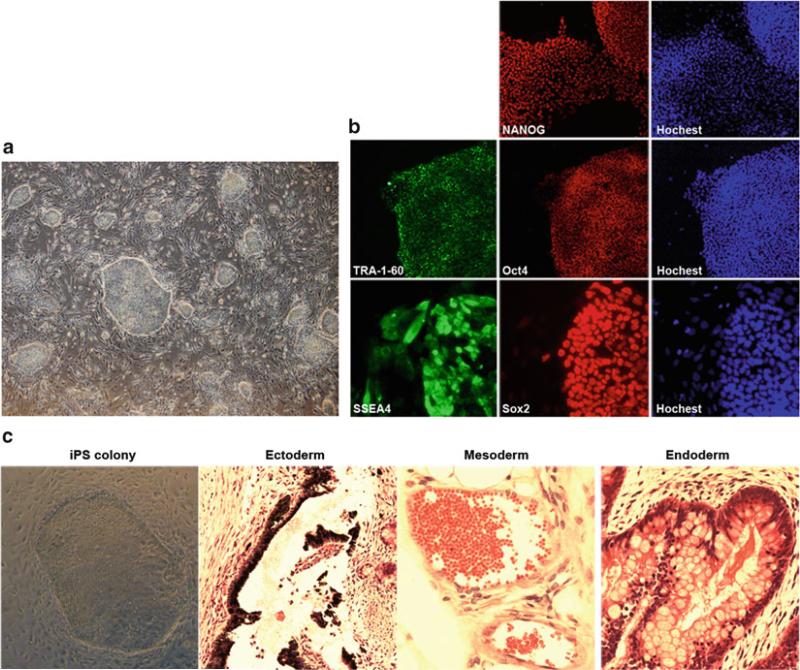
Colonies should be observed 6–7 days after passage (Fig. 3a). One day before passaging colonies, prepare feeder cells by inoculating MEF cells at 4 × 104 cells per well (4-well plate). The wells should be pre-coated with gelatin.
Apply 750 μl pre-warmed 10 μM ROCK inhibitor contained KO-DMEM medium to each well of 4-well plate right before use.
Microdissect each iPS colony into chunks of about 100–150 cells using sterile glass hooks under microscope. The hook is used to gently split apart pieces of the colony. Cut a grid into the colony with the back of the hook to pull the pieces away from the colony. The size of each division should be sufficiently large to survive the cutting and adhering to the feeder layer (see Note 7).
Transfer four to five colony chunks into one well of a 4-well plate, prepared in step 12, using 200 μl micropipets.
Replace the 4-well plate to the incubator (37 °C, 5 % CO2).
On the next day, change the medium with KO-DMEM medium. Change medium daily with the KO-DMEM medium.
Passage cells 1 week after the colony transfer in step 15 using conventional methods for iPS cell cultures (9) (see Note 8).
Fig. 3.
Generation and characterization of human iPS cells. (a) iPS cell colonies start to appear on infection plate, 20 days post infection. (b) Anticipated results of iPS Characterization assay: immunofluorescent assay, human iPS cells express surface markers of human ES cells. (c) Anticipated results of iPS Characterization assay: teratoma assay, teratoma generated from human iPS cells containing tissues derived from three primary germ layers
3.4 iPS Characterization Assay
There are two commonly used assays for iPS cells. Immunocytochemistry assays are established means for scoring stem cell pluripotency (Fig. 3b). The ability for iPS cells to generate teratomas in immune-deficient mice is used to assess their capabilities to differentiate into the three primary germ layers (Fig. 3c).
3.4.1 Immunofluorescent Assay
Protocols for immunofluorescence are widely available. Here we describe briefly this application for cells in culture on coverslip plates, clear-bottom dishes for brief imaging (Fig. 3b).
Wash plates once in 1× PBS.
Fix in about 2 ml of 4 % PFA for 20 min at room temperature (RT).
Wash two times in 1× PBS. The dishes can be stored at 4 °C if sealed with Parafilm.
Block with 3 % normal serum (species dictated by host of secondary antibody, usually Donkey or Goat for Alexa conjugated secondaries) with 0.1 % Triton X in 1× PBS (minus) for 30 min at RT.
Remove block and add primary antibodies at 1:100 mentioned in Section 2.5.2.
Incubate at 4 °C overnight.
Wash three times, with at least 3 min per wash, in PBST (PBS with 0.1 % Tween 20) at RT.
Add secondary diluted in Block.
Incubate at RT for 30 min or overnight at 4 °C.
Wash twice in PBST for each 3 min wash.
If counterstaining with SytoxOrange (or other nuclear counterstain), incubate in PBST. SytoxOrange is diluted at 1:25,000.
Wash twice in PBST and leave in PBST. They can be stored at 4 °C sealed with Parafilm or imaged. If photobleaching is a problem, the PBST can be removed and a drop of VECTA-SHIELD mounting medium added. A coverslip can be placed on top to prevent evaporation.
3.4.2 Teratoma Assay
iPS cells are harvested as described in previous procedures for passaging and suspension in medium (250 μl per injection), mixed with an equal volume of thawed Matrigel and transferred to cold cryotubes. The mixture is held on ice until loaded into the syringe just before injecting.
The iPS cells are loaded into syringes fitted with an 18G needle. Load the cells into the syringe by drawing in a small amount of medium followed by the iPS cell suspension before attaching the needle.
The suspension is injected subcutaneously, targeting the needle beneath the skin on the rear flank of the SCID mouse.
Monitor the mice and their sites of injection weekly for 6–22 weeks. The mice should be weighed weekly and watched for signs of infection during the incubation period.
Teratomas can be recovered by dissection with surrounding tissue and usually arise between 6 and 8 weeks after grafting. They are fixed in formalin and sent for histological examination by a pathologist.
Acknowledgments
The Barbara & Donald Jonas Laboratory is supported by NIH R01EY018213, the Research to Prevent Blindness Physician-Scientist Award, the Schneeweiss Stem Cell Fund, New York State (N09G-302 and N13G-275), and the Foundation Fighting Blindness New York Regional Research Center Grant (C-NY05-0705-0312), the Joel Hoffman Fund, Gale and Richard Siegel Stem Cell Fund, Charles Culpeper Scholarship, Laszlo Bito and Olivia Carino Foundation, Irma T. Hirschl Charitable Trust, Bernard and Anne Spitzer Stem Cell Fund, Professor Gertrude Rothschild Stem Cell Foundation, and Gebroe Family Foundation. H.V.N. is supported by the RPB Medical Student Fellowship.
Footnotes
We described briefly the procedure for obtaining a skin biopsy from a patient. A dermatologist or other appropriately trained physician should perform skin biopsy under human subjects ethical guidelines.
Rinse with medium when cutting the skin tissue into pieces, in case the small skin piece dries out.
Remove the epidermis to avoid growth of keratinocytes.
After putting coverslips on the top of the silicone gel and skin tissue, use pipets to press the coverslip to attach it well. There should be barely any space between the coverslip and the culture dish.
Use cells with as early passage number if possible since the passage number may affect the efficiency of reprogramming.
The iPS cells generated by the use of Sendai do not retain any vector DNA sequences.
Concerning the size of iPS chunks during iPS colony picking, if a piece is too large, it will tend to form an embryoid body-like structure on the feeder layer and it will take too long for the entirety of a large colony to come into contact with the feeders. The resulting colony will have an area of differentiation in the center arising from the embryoid body-like structure.
Complete growth medium is exchanged on the growing colonies every day as the feeder layer can use up nutrients quickly. The cell cycle for this line is about 24–36 h. The lines should culture for no more than 6 days to a week.
References
- 1.Li Y, Wu WH, Hsu CW, Nguyen HV, Tsai YT, Chan L, Nagasaki T, Maumenee IH, Yannuzzi LA, Hoang QV, Hua H, Egli D, Tsang SH. Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Mol Ther. 2014 doi: 10.1038/mt.2014.100. doi:10.1038/mt.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh R, Shen W, Kuai D, Martin JM, Guo X, Smith MA, Perez ET, Phillips MJ, Simonett JM, Wallace KA, Verhoeven AD, Capowski EE, Zhang X, Yin Y, Halbach PJ, Fishman GA, Wright LS, Pattnaik BR, Gamm DM. iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013;22(3):593–607. doi: 10.1093/hmg/dds469. doi:10.1093/hmg/dds469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Li Y, Chan L, Tsai YT, Wu WH, Nguyen HV, Hsu CW, Li X, Brown LM, Egli D, Sparrow JR, Tsang SH. Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Hum Mol Genet. 2014;23(13):3445–3455. doi: 10.1093/hmg/ddu053. doi:10.1093/hmg/ddu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripo-tent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. doi:10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 6.Kuroya M, Yoshinari Y, Ishida N, Noda K, Koseki E. Isolation of a virus from a sporadic case of Izumi fever. Tohoku J Exp Med. 1953;58(1):22. doi: 10.1620/tjem.58.22. [DOI] [PubMed] [Google Scholar]
- 7.Li HO, Zhu YF, Asakawa M, Kuma H, Hirata T, Ueda Y, Lee YS, Fukumura M, Iida A, Kato A, Nagai Y, Hasegawa M. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J Virol. 2000;74(14):6564–6569. doi: 10.1128/jvi.74.14.6564-6569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripo-tent stem cells. Nat Protoc. 2008;3(7):1180–1186. doi: 10.1038/nprot.2008.92. doi:10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]