Abstract
Aim and Objectives:
To investigate the efficacy of 0.1% tacrolimus with Colgate Oraguard-B paste for the treatment of patients with oral lichen planus (OLP).
Materials and Methods:
One hundred and fifty patients with symptomatic OLP participated in the study, who had clinically and histopathologically proven OLP. In this study, patients were provided with 0.1% tacrolimus ointment with Colgate Oraguard-B paste as the study medication. Patients were asked to use the medication over the areas three times a day until resolution of the lesion. Patients were recalled to assess the drug response every 15 days. The duration of treatment ranged from 3 months to 4 months, with follow-up of 2 years and 6 months.
Statistical Analysis:
The Wilcoxon signed-rank test was performed, which is a nonparametric statistical hypothesis test for comparing two related samples, matched samples or repeated measurements on a single sample to assess whether their population mean ranks differ (i.e., it is a paired difference test). In our study, the pre- and post-Visual Analogue Scale (VAS) values were compared and the mean, standard deviation and P values were calculated.
Results:
Of 150 patients, 71 (47.33%) patients had complete resolution of the lesion to the topical tacrolimus therapy. Sixty-five (43.33%) patients had marked resolution of their lesions, i.e., the size of the lesion was decreased. Fourteen (9.33%) patients had remission of lesion (reduction in burning sensation and size of lesion) in symptoms as recorded by the VAS.
Conclusion:
Topical tacrolimus ointment 0.1% in Oraguard-B paste is an effective treatment for different types of OLP.
Keywords: Orabase, tacrolimus, oral lichen planus
INTRODUCTION
Oral lichen planus (OLP) is a T-cell-mediated chronic inflammatory oral mucosal disease of unknown etiology, but it is believed to result from an abnormal T-cell-mediated immune response in which basal epithelial cells are recognized as foreign body because of changes in the antigenicity of their cell surface. The lymphocytic infiltrate in OLP is composed almost exclusively of T-cells, and the majority of T-cells within the epithelium and adjacent to damaged basal keratinocytes are activated CD8+ lymphocytes.[1] There are no consistent serological changes associated with OLP.[2,3,4]
OLP is diagnosed on the basis of the presence of Wickham's striae that produce either a lacelike pattern or radiating lines that can be faint or prominent, which presents a symmetrical and bilateral distribution on the buccal mucosa. They appear as white papules, plaques, erythema, erosions or blisters, affecting predominantly the buccal mucosa, tongue and gingivae.[5,6] Lesions are usually bilateral, and atrophic and erosive lesions are often sensitive or painful.[7,8]
The most widely accepted treatment is topical and systemic corticosteroids. Alternative treatments include retinoids, ultraviolet phototherapy, steroid-sparing agents (hydroxychloroquine, azathioprine, mycophenolate mofetil) and pimecrolimus.[8] Although the above-mentioned drugs have shown positive results in the treatment of OLP, resistance to treatment and a high risk of toxicities limit their use. Tacrolimus (FK506/Prograph; Fujisawa Inc., Deerfield, IL, USA) is an immunosuppressive agent currently available worldwide for the prevention of organ transplant rejection. In recent years, tacrolimus ointment 0.1% and cream 0.1% (Protopic; Fujisawa Inc.) have received FDA approval for the treatment of atopic dermatitis in children, as has pyoderma gangrenosum because of its clinical benefits and safety profile. Tacrolimus is 10–100-times as potent as cyclosporine in its ability to inhibit IL-2 mRNA synthesis, and it inhibits mediator release from basophils and mast cells. It inhibits enzyme calcineurin phosphatase activity, resulting in decreased IL-2 synthesis and secretion hence inhibiting T cell multiplication [Figure 1].[9] With such an insight, this investigation was performed to assess the efficacy of 0.1% tacrolimus ointment with Oraguard-B paste for the treatment of patients with symptomatic OLP. Colgate Oragard-B paste is a powerful reliever and mucoadhesive with a protective barrier to give your patients fast pain relief.[10]
Figure 1.
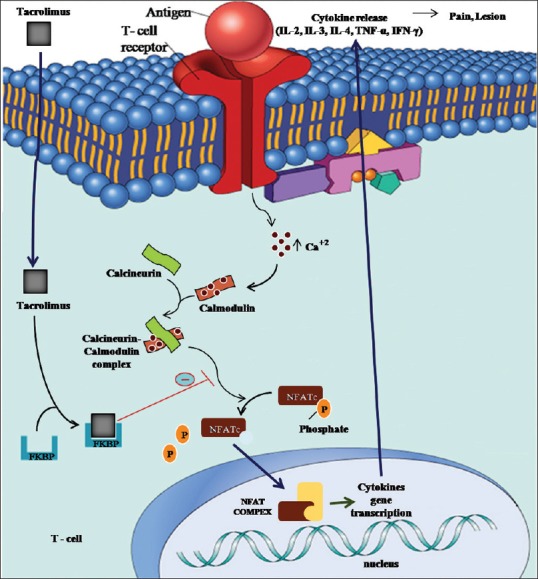
Mechanism of action of tacrolimus
MATERIALS AND METHODS
This study was conducted at the Department of Oral Medicine and Radiology after receiving ethical clearance from the institute, with 150 patients participating in the study. The study was explained to the patients and informed consent was obtained. A minimum data set (patient age, gender, medical history and habits) [Table 1] was documented by means of case history performa and clinical photographs of the affected site were taken. The patients for the study were selected among the patients attending the outpatient department.
Table 1.
Demographics of patients with oral lichen planus

Patients with clinical evidence of the lesion followed by histopathological confirmation were included in the study. Patients on medication for other systemic diseases or with any history of renal, hepatobiliary or malignant disease, uncontrolled hypertension or recurrent acute infection, etc., were excluded from the study group.
The patients’ subjective assessment for symptoms of pain and burning was performed by means of a Visual Analogue Scale (VAS) [Table 2]. Pain intensity can be measured by the VAS rating. The pain VAS is a unidimensional measure of pain intensity, which has been widely used in diverse adult populations. The pain VAS is a continuous scale comprised of a horizontal (HVAS) or vertical (VVAS) line, usually 10 cm (100 mm) in length, anchored by two verbal descriptors, one for each symptom extreme. Instructions were given to the patient that VAS consists of a 10 cm line on which 0 cm is “no pain” and 10 cm is “pain as bad it could be.” The patient is asked to mark the point along the line to know his best experience of pain score and then it is measured from “no pain” to the “worst pain” till the end of the scale.
Table 2.
Visual Analogue Scale (VAS) (0-10)
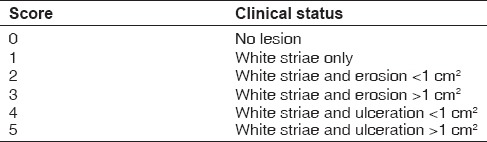
The extent and types of lichen planus were recorded by using a scoring system [Table 3]. In this study, patients were provided with 0.1% tacrolimus ointment with Colgate Oraguard-B paste for the study medication. Patients were asked to use the medication over the symptomatic areas three times a day until resolution of the lesion. Patients were recalled to assess the drug response every 15 days.
Table 3.
Clinical scoring (adopted from J. Am. Acad. Dermatology. 2002; 46:35-41)[11]
RESULTS
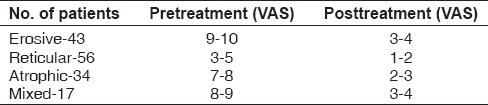
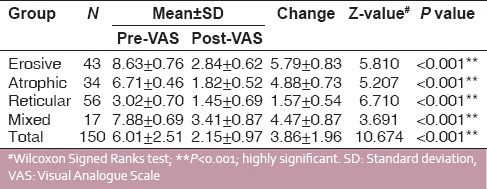
The Wilcoxon signed-rank test was performed, which is a nonparametric statistical hypothesis test used when comparing two related samples, matched samples or repeated measurements on a single sample to assess whether their population mean ranks differ (i.e. it is a paired difference test). A total of 150 patients were enrolled in the study, of whom 43 patients had erosive OLP, 56 patients had reticular OLP, 34 patients had atrophic OLP and 17 patients had mixed lesions. All the patients had clinical evidence lesion followed by histopathological confirmation. The VAS was used to assess the improvement in pain intensity among different forms of lichen planus by recording the pre- and posttreatment values [Table 2 and Graph 1]. The demographic data were recorded along with type and site of lesion given in Table 1 and clinical scoring in Table 3.
Graph 1.
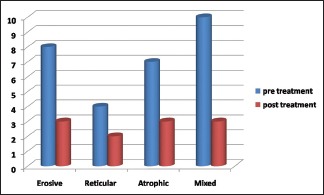
Pre- and post-treatment response of tacrolimus therapy in different types of oral lichen planus
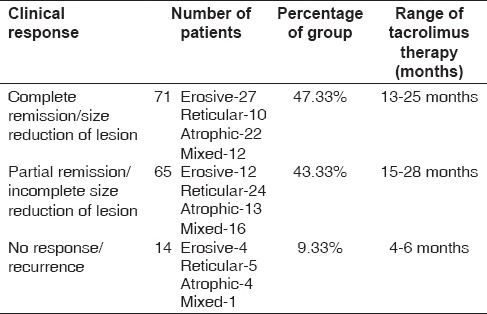
The clinical response of 150 patients with different types of OLP was recorded following topical application of tacrolimus therapy for 2 years and 6 months. In 150 patients, 71 (47.33%) patients had complete remission or complete reduction in size of lesion after tacrolimus therapy for the duration of 13–25 months. Sixty-five (43.33%) patients had partial remission of lesion after tacrolimus therapy for the duration of 15–28 months [Figure 2]. Fourteen (9.33%) patients had no response or recurrence after tacrolimus therapy for a duration of 4–6 months.
Figure 2.
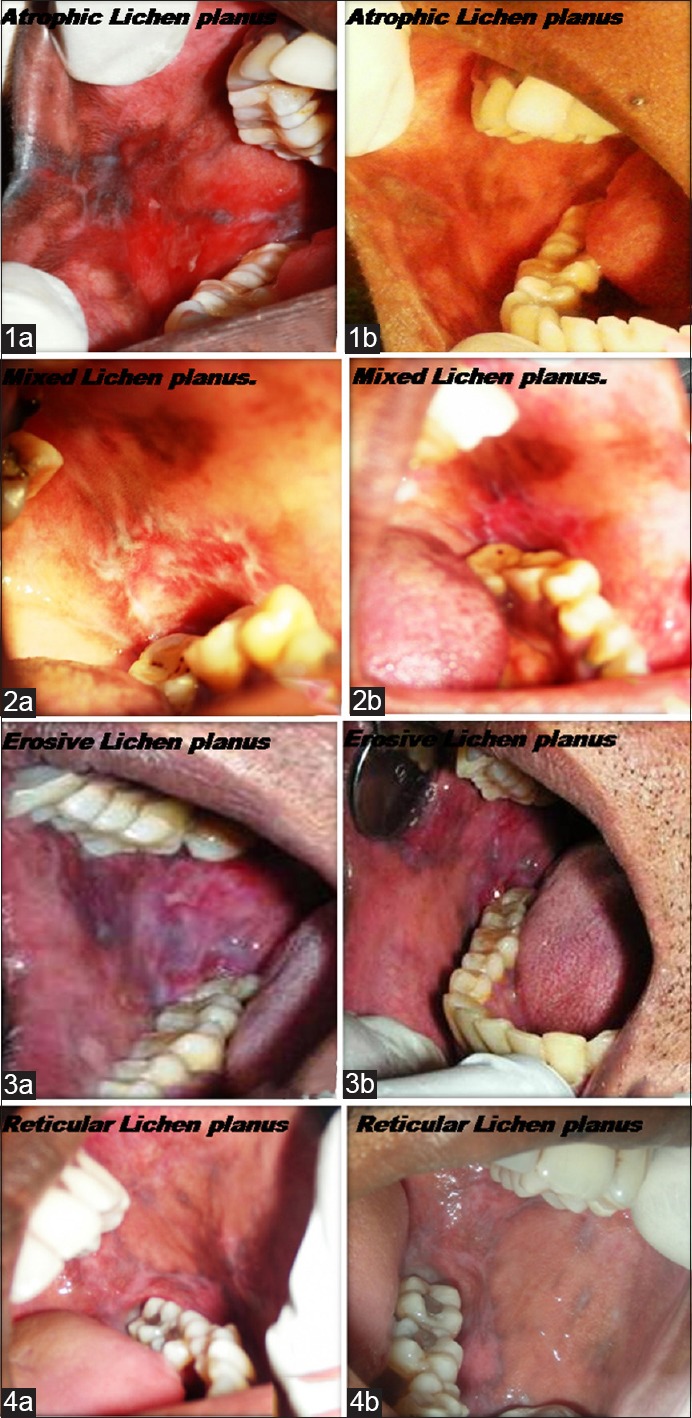
Different types of lichen planus (pre- and posttreatment). (1a) Atrophic lichen planus (pre-operative), (1b) atrophic lichen planus (post-operative), (2a) mixed lichen planus (pre-operative), (2b) mixed lichen planus (post-operative), (3a) erosive lichen planus (pre-operative), (3b) erosive lichen planus (post-operative) (4a) reticular lichen planus (pre-operative), (4b) reticular lichen planus (post-operative)
The values of the VAS, both pre- and posttreatment, for male and female patients in terms of mean ± standard deviation and Wilcoxon signed-rank test to test the significant differences in pre- and postobservational scores are listed in Table 4. The pre- and post-VAS values were compared and statistically highly significant difference (P < 0.001%) in mean VAS and clinical scores was observed.
Table 4.
VAS scoring among different forms of oral lichen planus
DISCUSSION
Topical tacrolimus is one of a new class of medications, the noncorticosteroid topical immunomodulators. The present study significantly extends previous reported findings regarding the role of 0.1% topical tacrolimus ointment in the management of symptomatic erosive/ulcerative OLP by both increasing the number of treated patients and duration of therapy.[11]
Tacrolimus ointment in 0.1% Orabase paste appears to be an effective and promising alternative in the treatment of patients with OLP. Colgate Oragard-B paste is a powerful reliever and mucoadhesive with a protective barrier to give fast pain relief to the patient. Tacrolimus, also a calcineurin inhibitor, is a steroid-free topical immunosuppressive agent approved for the treatment of atopic dermatitis. It is 10–100-times as potent as cyclosporine and has greater percutaneous absorption than cyclosporine. This substance is produced by Streptomyces tsukubaensis and belongs to the macrolide family.[12,13] Adverse effects associated with the therapy were mild and transient; they were limited only to a burning sensation. The reason for fewer adverse effects may be attributed to the fact that compounds having a mass unit greater than approximately 500 Da and scarcely penetrate the epidermis or epithelium of normal skin mucosa. Inflamed mucosa, due to increased permeability, allows penetration of molecules of higher molecular weight such as tacrolimus 823 Da. Once the inflammation (and permeability) decreases and the lesion improves due to the anti-inflammatory activity of topical tacrolimus, the penetration of the compound into the epithelium decreases, thereby limiting the potential side-effects of this particular regime.[14]
Tacrolimus treatment is revealed as an effective and secure alternative due to its low systemic absorption and the low incidence and importance of its secondary effects.[15]
This study was designed to determine the safety of tacrolimus with Colgate Orabase in 150 patients for a period of 2 years and 6 months. In our study, topical tacrolimus was found to be safe and effective in all the 150 patients who participated in the study.
In the present study, the demographic characteristics of the patients were taken on the basis of gender, age, type and site of OLP [Table 1], which were similar to a previously reported study.[11]
In the present study, patient's assessment for the symptoms of pain and burning sensations was recorded by means of a VAS. The intensity of pain and burning sensation was worst, scoring 9–10 by VAS assessment during the first visit and, after 2 months of therapy, the pain and burning sensation was drasticallyreduced up to 1–2 [Table 2]. Highly significant results were obtained between pre- and posttreatment values on the VAS scale. This is because tacrolimus blocks the activation of T-lymphocytes by targeting calcineurin, an important activator of T-lymphocyte, and Colgate Oragard-B Paste, which is a powerful reliever and mucoadhesive, retains the contact of drug for a longer duration.[16]
In the present study, 47.33% (71 of 150 patients) had complete resolution, including erosive, ulcerated and even the reticular form of OLP. Partial remission in the symptoms of burning and pain were observed in 43.33% (65 of 150 patients) patients. In 9.33% (14 of 150 patients) patients, there was recurrence of the lesion in erosive and atrophic type of OLP, but the intensity of recurrence observed was mild and the patients were symptomless [Table 5]. Few studies have shown the role of tacrolimus therapy in the treatment of symptomatic reticular, erosive and atrophic OLP.[15,16]
Table 5.
Clinical response of 150 patients with oral lichen planus following topical tacrolimus therapy for 2 years and 6 months
In the present study, Oraguard-B has been a promising base, showing good mucoadhesive properties through patients’ responses and is a powerful reliever with a protective barrier to give your patients fast pain relief. Because the study was nonblinded and noncomparative, whether topical tacrolimus can be used as the first line of treatment or used in other concentrations needs to be assessed.
In the present study, Oraguard-B has showed promising base through patients’ responses due to its good mucoadhesive properties, protective barrier and powerful reliever to give patients fast relief from pain.
CONCLUSION
The encouraging results of this study lead us to conclude that topical tacrolimus ointment 0.1% with Oraguard-B paste is an effective treatment for symptomatic OLP. Most patients experienced symptomatic improvement in less than 1 month. However, the effect is temporary and, when topical tacrolimus is discontinued in a few patients, the lesion may flare again.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jungell P, Konttinen YT, Nortamo P, Malmstrom M. Immunoelectron microscopic study of distribution of T cell subsets in oral lichen planus. Scand J Dent Res. 1989;97:361–7. doi: 10.1111/j.1600-0722.1989.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 2.Scully C, Carrozzo M. Oral mucosal disease: Lichen planus. Br J Oral Maxillofac Surg. 2008;46:15–21. doi: 10.1016/j.bjoms.2007.07.199. [DOI] [PubMed] [Google Scholar]
- 3.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 4.Sugerman PB, Savage NW. Oral lichen planus: Causes, diagnosis and management. Aust Dent J. 2002;47:290–7. doi: 10.1111/j.1834-7819.2002.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 5.Silverman S, Jr, Gorsky M, Lozada-Nur F. A prospective follow-up study of 570 patients with oral lichen planus: Persistence, remission and malignant association. Oral Surg Oral Med Oral Pathol. 1985;60:30–4. doi: 10.1016/0030-4220(85)90210-5. [DOI] [PubMed] [Google Scholar]
- 6.Vincent SD, Fotos PG, Baker KA, Williams TP. Oral lichen planus: The clinical, historical, and therapeutic features of 100 cases. Oral Surg Oral Med Oral Pathol. 1990;70:165–71. doi: 10.1016/0030-4220(90)90112-6. [DOI] [PubMed] [Google Scholar]
- 7.Scully C, el-Kom M. Lichen planus: Review and update on pathogenesis. J Oral Pathol. 1985;14:431–58. doi: 10.1111/j.1600-0714.1985.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 8.Eisen D. The therapy of oral lichen planus. Crit Rev Oral Biol Med. 1993;4:141–58. doi: 10.1177/10454411930040020101. [DOI] [PubMed] [Google Scholar]
- 9.Patil US, Jaydeokar AV, Bandawane DD. Immunomodulators: A pharmacological review. Int J Pharm Pharm Sci. 2012;4:30–6. [Google Scholar]
- 10.Lopez-Jornet P, Camacho-Alonso F, Salazar-Sanchez N. Topical tacrolimus and pimecrolimus in the treatment of oral lichen planus: An update. J Oral Pathol Med. 2010;39:201–5. doi: 10.1111/j.1600-0714.2009.00830.x. [DOI] [PubMed] [Google Scholar]
- 11.Malik U, Gupta S, Malik SD, Vashishth S, Zaheeruddin, Raju MS. Treatment of symptomatic oral lichen planus (OLP) with 0.1% tacrolimus powder in Oraguard-B-A pilot prospective study. Saudi Dent J. 2012;24:143–8. doi: 10.1016/j.sdentj.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: An update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127–32. doi: 10.4103/0973-029X.84474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisen D, Carrozzo M, Bagan Sebastian JV, Thongprasom K. Oral lichen planus: Clinical features and management. Oral Dis. 2005;11:338–49. doi: 10.1111/j.1601-0825.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaliakatsou F, Hodgson TA, Lewsey JD, Hegarty AM, Murphy AG, Porter SR. Management of recalcitrant ulcerative oral lichen planus with topical tacrolimus. Jam Acad Dermatol. 2002;46:35–41. doi: 10.1067/mjd.2002.120535. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson TA, Sahni N, Kaliakatsou F, Buchanan JA, Porter SR. Long-term efficacy and safety of topical tacrolimus in the management of ulcerative/erosive oral lichen planus. Eur J Dermatol. 2003;13:466–70. [PubMed] [Google Scholar]
- 16.Lozada-Nur FI, Sroussi HY. Tacrolimus powder in Orabase 0.1% for the treatment of oral lichen planus and oral lichenoid lesions: An open clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:744–9. doi: 10.1016/j.tripleo.2006.02.033. [DOI] [PubMed] [Google Scholar]


