Abstract
Bovine milk and its products (e.g. cheese, yoghurt) are an important part of human diet with beneficial effects for all ages. Although analyses of different milk components (e.g. proteins, lipids) pose huge challenges, the use of mass spectrometric (MS)-based techniques is steadily improving our understanding of the complexity of the biological traits that effect milk yield and its components to meet the global demand arising from population growth. In addition, different milk constituents have various applications in veterinary research and medicine, including early disease diagnosis. The aim of the review is to present an overview of the progress made in MS-based analysis of milk, and suggest a multi-pronged MS strategy to better explore different milk components for translational and clinical utilities.
Keywords: Bovine, Milk, Proteomics, Lipidomics, Mass spectrometry, Livestock
Introduction
Agriculture, a pivotal sector for ensuring food and nutritional security, is undergoing a radical change in India and at the global level. Conventional crop and animal production methods are facing enormous pressure related to increased grain and animal production to meet the growing demand of population increase [1]. Although Indian agriculture performed better than expected during the global food crisis in 2008, the agriculture sector needs to envision future challenges as potential opportunities to make it more sustainable to provide food security and alleviate poverty [2].
In India, livestock as a sub-sector of agriculture contributes significantly to the economy by ranking first in world milk production, as well as producing vast amounts of milk products, meat, eggs, wool, hide and skin [3]. Livestock in spite of sustained pressure from climate change and increased demand of animal protein has consistently contributed significantly to the agricultural gross domestic product. For example, animal husbandry involves approximately 5.5 % of the total work force in the country, as well as providing gender equity and women empowerment [4].
There is no program in place anywhere in the world including India that considers appropriate husbandry practices to develop milk as functional food by altering its individual components which has been previously reported to have significant association with genotype [5] and environmental factors [6]. Therefore, to keep up with the demand and supply chain of animal products, it is critical to understand the challenges for improving animal health, production, and their welfare by adopting better husbandry and management practices [7]. In particular, early and quick disease diagnosis, especially at farms is a huge challenge for veterinary physicians.
The advancement of proteomics technology has enabled researchers to analyze different body fluid such as milk [8] saliva [9] and urine to better understand etiology and pathogenesis of disease. Although the use of mass spectrometry (MS)-based proteomics in translational veterinary research is steadily increasing, information about the frequency, onset and progression of different markers (e.g. proteins, lipids) due to exogenous (e.g. season) and endogenous (age, lactation) factors which influence the dynamic nature of different milk components have not been sufficiently explored. Thus, it is critical to consider these normal differences in expression when searching for clinically relevant, disease specific markers. In this review, we provide an update on the progress made in the application of MS-based proteomics over the last 5 years in bovine milk analysis, as well as point out the possible challenges and considerations for improving livestock production and management.
Alternate diagnostic body fluid
Historically, blood has been used as the first choice of body fluid for analyzing changes in its constituents associated with pathophysiological conditions. However, due to the limitations of analyzing low abundance proteins in blood due to its complex nature, it is imperative to explore alternate diagnostic fluids such as milk, urine, and saliva to reflect local or systemic illness. In addition, due to the variability of the sources and composition of body fluids, different approaches are required to compile a comprehensive catalogue of potential markers. To this end, MS-based proteomic methods have great potential because they are unbiased and require no prior knowledge of fluid composition. In the context of this review, milk as an alternate diagnostic fluid including its different components has been discussed with respect to its diverse applicability in livestock proteomics.
Milk
Bovine milk is a complex biological fluid secreted by a dynamic and complex organ composed of various cell types working together for synthesis and secretion of milk as shown in Fig. 1. Milk is responsible with multifaceted functionality for the nourishment of young and provides a vital source of nutrition for humans of all ages. Bovine milk composition is dynamic in nature containing proteins and peptides, lipids, and complex carbohydrates with health benefits beyond the expected nutritional content. Its composition varies continuously due to different factors such as breed, feed, age, season and stage of lactation [11–13]. Although milk has evolved as a natural food under selective pressure to meet nutritional needs of different species, limited knowledge is available about changes in its components (e.g. proteins, lipids) in health and disease due to different environmental and physiological factors. Changes in the expression of these components alter normal functional properties of milk and would be expected to be indicative of systemic or local illness. However, much of the studies to date, have focused on the alteration of different milk components of exotic breeds (e.g. Jersey and Holstein–Friesian) [14, 15] with limited reports on Indian pure breed cows (e.g. Sahiwal, Tharparker) and buffaloes (e.g. Murrah, Jafarabadi), which are large contributors to Indian dairy industry.
Fig. 1.
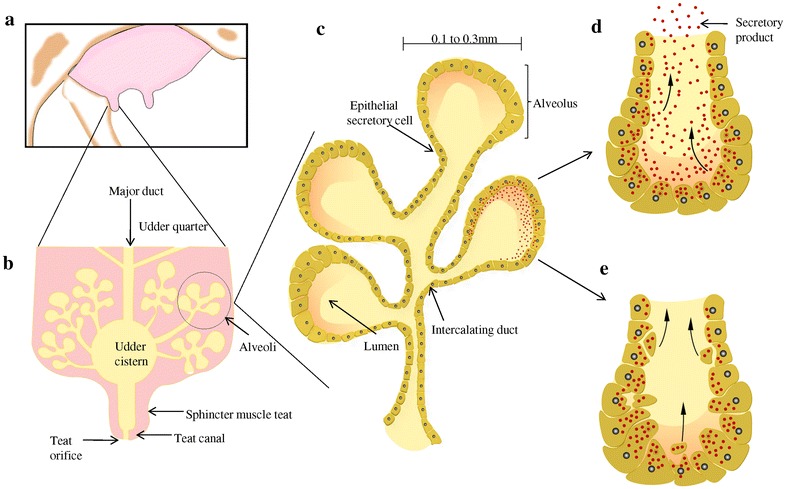
Schematic representation of the structure of mammary gland. a A general model of udder, b image of udder with complex tissue comprised of many ducts and alveoli, c an alveolus comprising of many cell types such as secretory and intercalating ducts, d an alveolus showing merocrine mode of secretion for protein component of the milk, e an alveolus showing apocrine mode of secretion for lipid component of the milk
Protein markers
Over the past decade, a number of groups using proteomics methodologies have made significant progress in characterizing abundant milk whey proteins [16–18], while detection of medium to low abundant proteins has been a bottle-neck due to its dynamic nature [19–21]. Similarly, early detection of mastitis, inflammation of the mammary gland by biomarkers or patterns of biomarkers has had limited success [7]. Mastitis, both clinical and subclinical, is the most devastating bovine disease causing staggering economic losses worldwide to the dairy industry. Unhygienic milking practices, diverse production systems, inadequate treatments and other factors are contributing to higher incidence of mastitis [22], while the lack of early diagnostic test has led to a lag where symptoms precede diagnosis by weeks and months resulting in spread of infection to other uninfected udder and cows [18, 23]. Currently, diagnosis of mastitis relies on visual signs such as redness, swelling of the infected quarter or altered consistency of milk (thickened or watery), increased somatic cell count (SCC) or clots [24]. In contrast, the subclinical form of mastitis is more difficult to diagnose due to lack of visual signs either in the udder or in milk and is generally based on the detection of bacteria or SCC in milk [25] measuring electrical conductivity [26], lactate dehydrogenase activity [27, 28], and decreased milk production [25].
Although the majority of previous studies have reported analysis of milk and its different components, in-depth analysis requires newer technologies such as proteomics. To date, the majority of bovine milk protein analysis including post-translational modifications of different proteins (PTMs) from healthy and diseased animals have been performed using 2-dimensional gel electrophoresis (2DE), differential gel electrophoresis (2DE-DIGE) followed by MALDI-time-of-flight (TOF)-MS, and/or liquid chromatography coupled tandem mass spectrometry (LC–MS/MS) [16–18, 29–32]. In addition, to maximize protein identifications and expand the analysis of the milk proteome, multiple analytical approaches including fractionation techniques have been adopted. For example, casein which makes up 80 % of overall milk protein content was extracted using hydrophobic and hydrophilic procedures followed by size exclusion fractionation to identify low molecular weight molecules [33]. Similarly, enhanced identification of whey proteins was reported after precipitation of casein [34, 35]. Nissen et al. [19] performed different fractionation techniques such as acidification, filtration, and centrifugation followed by LC–MS/MS and identified 635 bovine whey proteins. Similarly, Molle et al. [36] applied electrospray (ESI) and matrix-assisted laser desorption (MALDI) ionization in parallel for complementary proteome coverage in bovines. Using LC–MS/MS Boehmer et al. [37] were successful in identifying proteins from complex mixtures, while Zhang et al. [17] reported change in abundance of acute phase protein abundance in colostrum and mature milk. Similar to shotgun proteomics, MALDI-TOF has gained success in bovine milk proteomics, for example, molecular weight of proteins was determined without any fractionation [38] MALDI-TOF has also been used to determine N-linked glycosylation patterns for milk proteins of milk-fat-globules [39], immunoglobulins [40], α-lactalbumin [41, 42], κ-casein [43] and lactoferrin [44, 45]. Furthermore, MALDI-TOF has achieved success in determining changes in N-glycans in early lactation [40] as well as top-down sequencing of complex O-glycans at the protein level [46].
For mastitis milk proteome analysis, the majority of studies have used 2DE followed by MS [7, 8, 47–50]. For example, Hogarth et al. [34] reported down-regulation of caseins, α-lactalbumin and β-lactalbumin while up-regulation of serum albumin and serotransferrin. Similarly, differential expression of proteins including acute phase proteins (APP), lactotransferrin and immunoglobulins was reported during infection [47, 51]. Quantitative analysis of infected milk using isobaric tag for relative and absolute quantification (iTRAQ) has significantly increased protein identifications. For instance, Reinhardt et al. [49] were successful in identifying 2971 proteins significantly expanding the milk proteome. Of these proteins, more than 300 were associated with host defense via neutrophil extracellular traps (NETs) thereby increasing our understanding of mammary gland immune function [52]. Similarly, a number of differentially expressed proteins (e.g. IL-8, IFN-γ) were identified using 2DE in milk collected from post-intramammary infection with Staphylococcus aureus [53]. Huang et al. [50] characterized S. aureus infected mammary gland using proteomics resulting in the identification of 768 proteins, indicative of the epithelial changes occurring due to infection. Apart from proteins, peptides (n = 154) were identified in mastitis milk caused by S. aureus and Escherichia coli as potential markers for early and differentially diagnosed mastitis caused by two bacterial sources [8]. Analysis of milk from sub-clinical mastitis revealed changes in abundance of proteins including β-1,4 galactosyltransferase, β-2 microglobulin, complement 3, α-1-acid glycoprotein, and serotransferrin precursor [22]. Inspite of these reported markers, validation of a single biomarker specific to bovine mastitis has not been feasible and presents a unique challenge and opportunity.
The economic consequences of mastitis influences the dairy industry immensely [54]. The cost associated with delayed diagnosis of mastitis includes factors like loss of milk production, discarded milk, veterinary services, labour, product quality, materials and investments, culling and therapeutics [55]. Rollin et al. [56] reported spending of $444 during the first 30 days of lactation, mainly associated with productive losses in milk and culling. Similarly, Cha et al. [57] reported average cost per case associated with different types of mastitis caused by gram-positive, gram-negative bacteria and other clinical mastitic organism to be $133.73, $211.03, $95.31, respectively. Thus, it is critical to adopt a combinatorial approach involving better husbandry and diagnostic methods to monitor animal’s health status including udder before it transitions to clinical mastitis.
Bovine milk has been used for clinical diagnosis, monitoring, control and eradication of infectitious disease such as bovine viral diarrhoea (BVDV) [58]. The causative agent belongs to pestivirus genus and spreads through milk, urine, saliva, nasal discharge, fetal fluids and semen causing acute infection [59]. Infection with BVDV during pregnancy causes huge financial losses as well as increase in incidence of secondary bacterial infections [60]. Furthermore, poor compliance of farmers in implementing control measures has lead to persistence of infection in the herd and spread of virus to uninfected animals within and across herds [58]. Currently, a number of diagnostic tests are available for the detection of virus including enzyme linked immunosorbent assay (ELISA), immunohistochemistry (IHC), reverse transcriptase polymerase chain reaction (RT-PCR), agarose-gel immunodiffusion and viral neutralization test [61]. For example, Gates et al. [58] collected milk samples from female breeding cattle and performed ELISA to detect infected animals. However, there are pitfalls with conventional diagnostic techniques such as higher false positive cases were observed when virus was isolated by culture methods [62]. In addition, due to differences in epidemiology it is pertinent to adopt sensitive detection strategies to identify key molecules that are involved in the pathophysiology of BVDV infection. To this end, MS can play a significant role in qualitative and quantitative characterization of target molecules which could enable clinicians in early disease diagnosis, treatment and control of infection.
During the transition of a pregnant dairy cow from late gestation to early lactation, it experiences a negative energy balance due to rise in demand of milk which cannot be met by feed alone and at risk of developing metabolic disorder known as ketosis [63–65]. This condition is characterized by the increased concentration of ketone bodies such as acetone, acetoacetic acid and β-hydroxybutric acid (βHBA) in blood, milk, urine [66]. Ketosis causes huge financial losses due to treatment cost and decreases in milk production as well as makes the animal susceptible to periparturient diseases such as metritis, mastitis, displaced abomasums [63, 67, 68]. Although a number of diagnostic kits are commercially available, they provide semi-quantitative results [69, 70]. Similarly, the diagnostic test by dipstick using urine have limitations due to difficulty in urine collection compared to milk [71], animal failing to urinate within a reasonable time increasing labour cost [72]. In contrast, accurate measurements from milk by nitroprusside reaction are not sensitive [73]. Currently, the gold standard for diagnosis of ketosis is based on detection of βHBA in serum or plasma using a commercially available instrumentation used in humans for detection of diabetes [74]. However, commercially available kits have not been successful in veterinary practice due to the differences in blood types and antigen expression between humans and animals [75]. Thus, to overcome these limitations, more recently, Weng et al. [75] developed a handheld microfluidic device, which relies on photometric detection of βHBA to confirm ketosis. Similarly, Weng et al. [76] developed quantum dots (QD) to monitor βHBA in cow’s blood and milk. Since newer technologies are being developed for the diagnosis of ketosis with distinct advantages of low cost and detection limits, it is worth trying out the effectiveness of MS-based proteomics to identify as well as validate markers from different biological fluids for routine diagnostic assay in large animal cohort.
Conventionally bovine pregnancy has been detected by palpation per rectum at 60 days after artificial insemination (AI) or ultrasonography at 35 days after AI [77, 78]. However, more recently, 2DE DIGE has been used to separate pregnancy specific proteins from serum [79]. In this study, Lee et al. [79] reported up-regulation of seven protein spots (e.g. modified bovine fibrinogen), while down-regulation of six protein spots (e.g. complement). Similarly, significant change in protein abundance (n = 32) were detected in corpus luteum (CL), an organ formed in the ovary, responsible for the maintainance of pregnancy by 2DE and MALDI [80, 81]. Furthermore, Forde et al. [82] by MS-based proteomics reported 30 unique proteins specific to uterine luminal fluid which could be involved in the interaction between conceptus and the endometrium and potentially play a role in pregnancy detection. Similarly, GC–MS has been extensively used in the detection of volatile compounds from urine of cows and buffaloes. For example, Barman et al. [83] reported identification of six pregnancy-specific compounds such as 2-butenedioic acid-dimethyl, 2-piperidinone, eicosane, nonacosane, octadecanoic acid, butyl ester, and thiazole, 2,4-dimethyl. Thus, unlike traditional approaches, MS can provide an accurate, rapid and non-invasive method of determining pregnancy and can be a valuable tool in improved management of bovine pregnancy.
Quality of milk is a major issue to the dairy industry including consumers due to deliberate addition of adulterants like vegetable fats and oils, melamine and nitrogen-containing compounds like urea and anhydrous milk products such as milk protein concentrate, caseins and whey proteins to milk [84–87]. Furthermore, adulteration of high-value goat and buffalo milk with low priced bovine milk due to easy accessibility has also been found to be an area of foremost concern [88, 89]. These unethical practices have led to serious health concerns to consumers due to addition of unknown allergens. Thus, to check milk adulterants, and safeguard the interests of consumers, different strategies such as polymerase chain reaction (PCR) [89, 90], high performance liquid chromatography (HPLC) [91], infrared spectroscopy [92], immunoassays, nuclear magnetic resonance (NMR) [93] and electrophoretic methods like capillary electrophoresis (CE), urea-polyacrylamide gel electrophoresis [94, 95] have been used. Recently, bovine milk adulteration with goat cheese was successful by amplification of species-specific ribosomal RNA by PCR [89]. Infrared spectroscopy has provided a non-destructive fingerprinting approach to examine and quantify adulterants like whey, urea, caustic soda and hydrogen peroxide in milk [87]. Although these techniques are effective, but have few limitations like co-elution of major proteins in HPLC from bovine, caprine, ovine and buffalo milk leading to inadequate protein identifications [91]. Furthermore, electrophoretic techniques alone cannot differentiate overlapping species specific low abundant proteins [95].
However, recently a number of studies reported the application of MS-based proteomics in detection of adulterations [96, 97]. For example, MALDI-TOF mass spectrometric determination of adulterated milk was found to be a rapid, more competent and cost effective technique. Calvano et al. [88] reported the use of phospholipids as markers of bovine milk adulteration using MALDI-TOF. Similarly 2DE gels coupled with MALDI-TOF has enabled to detect cow milk adulteration in mixtures of buffalo, yak, camel milk mixtures by observing the distribution patterns of α-lactalbumin and β-lactoglobulin, and αs1-casein [95]. In addition, adulteration of milk with vegetable fats and oils was identified by MALDI-TOF MS by studying the intact triacylglycerols (TAG) profile [85]. Similarly, LC–MS has been used to estimate the profitable adulteration caused by nitrogen containing compounds like melamine, biuret and urea-based fertilizers in milk allowing detection of contaminants up to 0.5 ppm [86]. More recently, MALDI-TOF was used to analyze antibiotic like benzyl penicillin in dairy milk, using titanium oxide (TiO2) nanowires as solid matrix [97]. Thus, the applicability of MS in examining milk quality is increasing due to its rapid and robust screening and characterization of adulterants with minimum sample preparation and does not require any prior modification and derivatization.
MALDI biotyping
Traditionally, phenotypic properties of microorganisms have been identified by antigen–antibody reaction, Gram staining, and colony morphology, while genotypic traits were characterized using PCR, pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST), restriction fragment length polymorphism (RFLP), and microarrays [98, 99]. Nevertheless, most of these methodologies are expensive, time consuming, laborious, require special skills and are unsuitable for use in routine clinical laboratories. Due to these limitations, MALDI-TOF has recently gained momentum and revolutionalised clinical microbiology laboratories across the world in identifying bacteria, yeast and fungi directly from colonies which were previously misidentified thereby reducing the time for secondary phenotypic identifications [100]. The principle of MALDI-TOF based biotyping relies on unique ribosomal protein profiles matched to a database [101–104]. Genus level identification of unknown microbes is performed by matching peptide mass fingerprint (PMF) with PMFs of known isolated in the database [99]. For species level identification of microorganisms, a spectra of mass range 2–20 kDa represented by abundant ribosomal proteins is matched with PMFs of ribosomal proteins in the database [105]. For example, classical procedures used for detection of Listeria monocytogenes take at least 1 week, while MALDI-TOF confirmed by rapid and sensitive analysis within 4–5 h, expanding the applicability of MALDI-TOF for the identification of pathogens [106, 107]. Similarly, detection of antimicrobial resistance using MALDI-TOF has been reported for S. aureus, Acinetobacter baumannii, and E. coli [108, 109]. In addition, MALDI-based identification has been used in biodefense and environmental microbiology and epidemiological studies [110]. In spite of the progress, it must be noted that identification of organisms is database dependent, which are commercially available limiting researchers accessing the ever increasing in size and regularly updated database with discovery of new microbial species. For example, Carbonnelle et al. [111] reported inability of identification of few microorganism due to absence of the organism in the database and not due to methodological error. However, to overcome these limitations, a number of open-source softwares and databases such as mMASS [112], pkDACLASS [113], MALDIquant [114], SpectraBank [115] and BIOSPEAN [116] are freely available.
For human studies, MALDI-TOF based identification of clinical isolates has been extensively used but limited explorations have been performed in veterinary microbial diagnostics. Consequently, the benefit of MALDI-TOF can be used to routinely monitor milk microbiota, create a repository of existing and emerging microorganism, and perform surveillance for dissemination of pathogens in preventing an outbreak including regular screening of milk microbiota for its quality to improve milk as a functional food.
Lipid markers
To date, the majority of the studies have focused their efforts on analyzing different components of milk such as whey proteins [16–18, 117], milk fat globule membrane (MFGM) [118, 119], and milk exosomes [49, 52], while minimal focus has been on milk lipids. For example, the role of different exogenous and endogenous factors influencing the composition of a particular lipid, a potential source of functional food is limited in both cows and buffaloes. Consequently, researchers and dairy industry are keen to study lipids and its numerous fatty acids (FAs) due to its potential in early disease diagnosis and altering different components to enhance milk quality [120].
Gas chromatography coupled mass spectrometry (GC–MS) has been used to identify milk lipids and FAs as fatty acids methyl esters (FAME) [121]. Stefanov et al. [121] using dichloromethane-ethanol as a solvent identified 49 FAs from bovine milk. Furthermore, Feng et al. [122] identified 108 FAs from milk using a CP-SIL column, while Delmonte et al. [123] were successful in enhancing separation of short-chain FAs and poly-unsaturated fatty acids (PUFAs). In addition, GC has been successfully applied to differentiate FAs based on their positional and geometrical isomers (e.g. isomers of conjugated linoleic acid) [124]. Nevertheless, there are inherent limitations of GC–MS, such as underivatization of FAs into FAME, formation of artifacts and conversion of cis to trans form FAs which in turn alters the composition of fat, during the process of esterification and leads to low lipid recovery and erroneously identified peaks [125].
Recently, MALDI-TOF has been progressively used to study milk lipids as it does not require an additional step of derivatization and results in rapid, accurate detection of lipids. In addition, application of new matrices such as 2,5-dihydroxybenzoic acid, 9-aminoacridine results in well resolved spectra allowing easy characterization of different lipid classes [126]. Similarly, Calvano et al. [127], have characterized phospholipids as species-specific markers in bovine milk using a new matrix, α-cyno-4-chlorocinnamic acid (CCICA). In addition, MALDI-TOF has proven to be a reliable method for high throughput forensic screening of adulterated bovine milk sample with vegetable fats [128]. However, in order to achieve comprehensive lipidome coverage, research groups have used LC–MS/MS [16]. For example, Sommer et al. [129] validated identification and quantification of previously unidentified FAs using LC–MS/MS. Similarly, previously unidentified short chain FAs from cow milk and milk products were identified by LC–MS/MS [130]. Furthermore, significant insights about structural aspects of FAs were reported using LC–MS/MS [131]. In addition, Liu et al. [131] reported a new LC–MS method using a HILIC column for characterization of phospholipids. More recently, MS-based techniques have been used to characterize FAs present in trace amounts in cow milk to maximize the compositional differences between milk samples analyzed across different seasons, lactation periods for identification of potential markers indicative of healthy and pathological condition of secretory cells [132].
Milk fat globule membrane
Bovine milk fat is dispersed in the form of spherical droplets or globules in the aqueous phase of milk and are found abundantly in milk secreting cells of mammary gland varying in size between 0.2 and 15 µm [133, 134]. The cytoplasmic lipid droplets are made of TAG and encapsulated by membrane of epithelial cell of lactating mammary gland are called as MFGM [135]. The size and distribution of MFGM is influenced by factors such as lactation, age, season, bacteriological quality of milk and breeds [134]. This three-layered complex has been reported to be functionally and nutritionally active as it contains membrane specific proteins including glycoproteins, phospholipids and bioactive sphingolipids [136–138].
The MFGM contains a unique composition of polar lipids and membrane proteins which not only is intriguing as a model to study membrane lipids and proteins but function as markers of biological processes of the cow’s udder cells [139]. For example, Reinhardt et al. [49] reported accumulation of host defense proteins and presence of NETs in MFGM preparations indicative of biology and immune function of the infected mammary gland [49, 140–143]. Similarly, comparative profiling of milk lipids and proteins in healthy versus disease conditions showed contrasting expression of serpin A3-1, vitronectin-like protein and complement factor H [8, 144]. Furthermore, differential expression of lipids and proteins have been used as potential markers (e.g. vitronectin, prostaglandin-D synthase) and presence of oxidative stress response serum amyloid A (SAA) for early detection of mastitis [48, 132, 144, 145]. The presence of phospholipids (e.g. sphingolipids, phosphatidyl ethanolamine) in the MFG membrane imparts a zeta potential to the globules [146, 147], which changes upon contact with reactive oxygen species released by bacteria in infected milk [144]. Thus, MFGM in practice can be used as a valuable tool to test sub-clinical mastitis [49].
Exosomes
Exosomes are small heterogeneous, extracellular organelles approximately 40–100 nm in diameter [148, 149] found in a variety of body fluids such as blood [150], saliva [151, 152], urine [153], milk [52, 154, 155], and bronchoalveolar lavage fluid [156]. Exosomes contain ubiquitous and cell specific molecules such as proteins, lipids, miRNA and mRNA mediating diverse biological functions, including antigen presentation, signaling, immune function and a source of biomarkers for disease [157–160].
Bovine milk exosomes were partially characterized by Plantz et al. [161], however technical advances in isolation and purification methodology has led to their successful characterization. For example, Reinhardt et al. [49] identified 2350 proteins from exosomes by MS-based proteomics significantly expanding the milk proteome. Of these proteins, a number of proteins were identified as part of neutrophil extracellular trap (NETs) suggesting their role in defense and mammary immune function in mastitis [49]. Exosomes are also reported to be involved in the transmission of pathogens including Leishmani spp. and human immunodeficiency virus [162, 163]. Furthermore, different proteins from exosomes such as cytokines, growth factors, hormones, and IgA have been reported to play a significant role in the development of neonatal intestine [164], stimulate secretion of intrinsic growth factors [165] and protection against infection [166]. Taken together, it is also conceivable that exosomal proteins could play an important role to better understand lactation physiology, defense, milk composition and abundance indicative of health and disease.
Mass spectrometry-based proteomic approach for sample analysis
Sample preparation and identification
Sample preparation is the most critical and challenging step in proteomics. The sample must be cleaned-up and/or fractionated at the protein or peptide level to unmask medium and low-copy proteins to identify potential markers. Along these lines, different depletion strategies have been used to separate abundant proteins in cow’s milk [21, 118] and urine [10] samples. Subsequently, 2DE has been mainly used to document changing patterns of protein followed by their identification by MS, however, it is limited due to its dynamic range and poor reproducibility [167, 168].
Qualitative protein identification
Protein identifications can be carried out by tandem mass spectrometry (MS/MS) using TOF/TOF analyzer, with peptide fragmented by post source decay [169] or collision induced dissociation (CID) [170]. However the generation of singly charged peptides by MALDI-TOF leads to preferential cleavage of the peptide backbone with loss of sequence information [171]. This kind of fragmentation may not be a problem for protein identification using adequate software analysis, but can lead to ambiguous protein identification by de novo sequencing. Alternatively, tandem mass spectrometry can be carried out for protein identification using hybrid mass analyzers, such as a combination with quadrupole-time of flight (Q-TOF). In this method, C18 is interfaced as on-line reversed-phase (RP) microcapillary liquid chromatography (LC) electrospray ionization (ESI) [172] or nano-ESI [173] greatly increasing sensitivity, efficiency, and analysis of small sample volume [174]. In this instrument, fragmentation occurs in a predictable manner between the amino acids bonds, enabling identification using software, such as MASCOT [175] or SEQUEST [168]. LC–MS/MS generates multiply charged peptide ions which readily fragment generating high quality and informative tandem mass spectra for confident protein identification [176].
For large scale proteomic analysis, multidimensional protein identification technology (MudPIT) holds great potential. In this technique, a strong cation exchange resin is in line with the RP column. Digested peptides are eluted onto the column at low pH facilitating binding to the cation exchange column and subsequently salt steps are used in an incremental manner to elute peptides onto the C18 RP column for further analysis by MS.
If the above techniques fail to provide any positive protein identifications, de novo sequencing followed by BLAST searching provides an alternative identification strategy [177, 178]. By this analysis the amino acid sequence is obtained by evaluating the mass difference between two adjacent y- and b-ion series in the fragmentation spectra of the precursor ion [173]. Alternatively, specialized software can be used to create amino acid sequence to interpret tandem mass spectra of peptides [178]. Figure 2 shows possible ways of sample analysis to maximize confident protein identification.
Fig. 2.
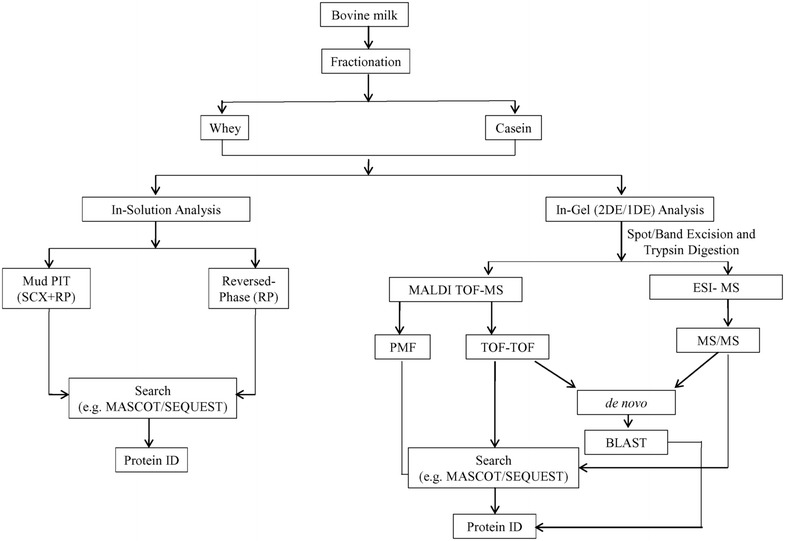
Possible pathways for protein identification. A combination of strategies for characterization of bovine milk whey and casein for maximizing successful protein identification
Quantitation
In addition to protein identifications, MS can be used to quantify differential expressed proteins between two different conditions (healthy vs. disease) either by a label-free or a labeled approach (incorporation of stable isotopes). To date, a number of studies have been performed to quantify milk proteins. For example, mastitis milk proteins were quantified either using a label-free approach [179, 180], or by isobaric tags for relative and absolute quantification (iTRAQ) [49, 50, 118, 142]. In contrast, limited studies have been performed using other labeling techniques such as peptide labeling via metabolic incorporation into cell or tissue culture (15N/14N), stable isotope labeling by amino acids in cell culture (SILAC), amino group labeling using isotope-coded affinity tags (ICAT), tandem mass tags (TMT) and enzymatically catalyzed incorporation (18O labeling).
For targeted quantification of potential markers, although techniques such as ELISA and Western blots are most commonly used, there are limitations including availability, sensitivity and specificity of antibodies for proteins, and multiplexing immunoassays in large animal populations [181]. However, to overcome these limitations, targeted quantification of markers, either by label-free or isotope labeling, can be performed using triple-quadrupole mass spectrometers by single reaction monitoring (SRM) or multiple reaction monitoring (MRM).
Conclusion
The dairy industry in India has progressed steadily and is the world’s largest milk producer. More recently, there has been an increase in awareness of consumers about milk quality from a health perspective, while little attention has been paid to changing individual constituents due to environmental and physiological factors for enhanced beneficial effect. These compositional variations add to the complexity and diversity of different milk components providing a compelling reason to investigate their changes in abundance for their beneficial effect and markers for early disease diagnosis for timely therapeutic intervention and subsequently diverting attention to better management practices.
From a MS-based proteomic analysis perspective, it is critical and imperative for researchers to combine strategies to increase the likelihood of maximizing positive protein identification. For example, a high-throughput approach for discovery will enable analyses of samples collected from much larger populations followed by a targeted quantification to validate potential markers. Taken together, results of a number of early studies on milk proteomics have reported promising results and also present a challenge to further develop effective proteomic tools for improving livestock productivity and fertility.
Authors’ contributions
AV and KA wrote the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
This work is supported by Department of Biotechnology (DBT), Government of India; Grant No. BT/PR12721/AAQ/1/618/2015 (KA). AV is supported by DBT, Government of India fellowship (DBT/2014/IITR-R/110). We gratefully acknowledge Dr. Peter Burbelo, Dental and Craniofacial Institute, National Institutes of Health, USA, for critical proof-reading of the manuscript. We also thank Narender Kumawat for assistance in making figure one.
Competing interests
Both authors declare that they have no competing interests.
Contributor Information
Aparna Verma, Email: aparndbt@iitr.ac.in.
Kiran Ambatipudi, Phone: +91-1332-284786, Email: kiranfbt@iitr.ac.in, Email: srinivaskiran78@gmail.com.
References
- 1.Boggess MV, Lippolis JD, Hurkman WJ, Fagerquist CK, Briggs SP, Gomes AV, Righetti PG, Bala K. The need for agriculture phenotyping: “moving from genotype to phenotype”. J Proteomics. 2013;93:20–39. doi: 10.1016/j.jprot.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 2.National agriculture research system: Vision 2030. http://www.icar.org.in/NationalAgriculturalResearchSystem/Vision2030. (Accessed 30 Jan 2011).
- 3.Borah M, Halim R. Economic analysis of milk production in Rewari district of Haryana. Indian J Dairy Sci. 2015;68:496–501. [Google Scholar]
- 4.Vision 2050. http://www.nrce.gov.in/Vision2050. (Accessed 2015).
- 5.Bhattacharya T, Misra S, Sheikh F, Sukla S, Kumar P, Sharma A. Effect of butyrophilin gene polymorphism on milk quality traits in crossbred cattle. Asian Aust J Anim Sci. 2006;19:922–926. doi: 10.5713/ajas.2006.922. [DOI] [Google Scholar]
- 6.Radhika G, Ajith Kumar S, Rani A, Sathian C, Anilkumar K, Usha A, Dinesh C. Milk yield and composition of crossbred cows in the hilly Wayanad district of Kerala, India. Indian J Anim Sci. 2012;82:1251–1254. [Google Scholar]
- 7.Roncada P, Piras C, Soggiu A, Turk R, Urbani A, Bonizzi L. Farm animal milk proteomics. J Proteomics. 2012;75:4259–4274. doi: 10.1016/j.jprot.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Mansor R, Mullen W, Albalat A, Zerefos P, Mischak H, Barrett DC, Biggs A, Eckersall PD. A peptidomic approach to biomarker discovery for bovine mastitis. J Proteomics. 2013;85:89–98. doi: 10.1016/j.jprot.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Horsington J, Zhang Z, Bittner H, Hole K, Singanallur NB, Alexandersen S, Vosloo W. Early protection in sheep against intratypic heterologous challenge with serotype O foot-and-mouth disease virus using high-potency, emergency vaccine. Vaccine. 2015;33:422–429. doi: 10.1016/j.vaccine.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Bathla S, Rawat P, Baithalu R, Yadav ML, Naru J, Tiwari A, Kumar S, Balhara AK, Singh S, Chaudhary S, Kumar R, Lotfan M, Behare P, Phulia SK, Mohanty TK, Kaushik JK, Nallapeta S, Singh I, Ambatipudi SK, Mohanty AK. Profiling of urinary proteins in Karan Fries cows reveals more than 1550 proteins. J Proteomics. 2015;127:193–201. doi: 10.1016/j.jprot.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Haug A, Hostmark AT, Harstad OM. Bovine milk in human nutrition—a review. Lipids Health Dis. 2007;6:25. doi: 10.1186/1476-511X-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Månsson HL. Fatty acids in bovine milk fat. Food Nutr Res. 2008 doi: 10.3402/fnr.v52i0.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samková E, Spicka J, Pesek M, Pelikánová T, Hanus O. Animal factors affecting fatty acid composition of cow milk fat: a review. S Afr J Anim Sci. 2012;42:83–100. [Google Scholar]
- 14.Palladino RA, Buckley F, Prendiville R, Murphy JJ, Callan J, Kenny DA. A comparison between Holstein–Friesian and Jersey dairy cows and their F1 hybrid on milk fatty acid composition under grazing conditions. J Dairy Sci. 2010;93:2176–2184. doi: 10.3168/jds.2009-2453. [DOI] [PubMed] [Google Scholar]
- 15.Mapekula M, Chimonyo M, Mapiye C, Dzama K. Fatty acid, amino acid and mineral composition of milk from Nguni and local crossbred cows in South Africa. J Food Compos Anal. 2011;24:529–536. doi: 10.1016/j.jfca.2011.01.014. [DOI] [Google Scholar]
- 16.Li X, Ding XZ, Wan YL, Liu YM, Du GZ. Comparative proteomic changes of differentially expressed whey proteins in clinical mastitis and healthy yak cows. Genet Mol Res. 2014;13:6593–6601. doi: 10.4238/2014.August.28.4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Boeren S, Hageman JA, van Hooijdonk T, Vervoort J, Hettinga K. Bovine milk proteome in the first 9 days: protein interactions in maturation of the immune and digestive system of the newborn. PLoS One. 2015;10:e0116710. doi: 10.1371/journal.pone.0116710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas FC, Waterston M, Hastie P, Parkin T, Haining H, Eckersall PD. The major acute phase proteins of bovine milk in a commercial dairy herd. BMC Vet Res. 2015;11:207. doi: 10.1186/s12917-015-0533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nissen A, Bendixen E, Ingvartsen KL, Rontved CM. In-depth analysis of low abundant proteins in bovine colostrum using different fractionation techniques. Proteomics. 2012;12:2866–2878. doi: 10.1002/pmic.201200231. [DOI] [PubMed] [Google Scholar]
- 20.Nissen A, Bendixen E, Ingvartsen KL, Rontved CM. Expanding the bovine milk proteome through extensive fractionation. J Dairy Sci. 2013;96:7854–7866. doi: 10.3168/jds.2013-7106. [DOI] [PubMed] [Google Scholar]
- 21.Tacoma R, Fields J, Ebenstein DB, Lam YW, Greenwood SL. Characterization of the bovine milk proteome in early-lactation Holstein and Jersey breeds of dairy cows. J Proteomics. 2016;130:200–210. doi: 10.1016/j.jprot.2015.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bian YLY, Li Q. Identification of diagnostic protein markers of subclinical mastitis in bovine whey using comparative proteomics. Bull Vet Inst Pulawy. 2014;58:385–392. doi: 10.2478/bvip-2014-0060. [DOI] [Google Scholar]
- 23.Hogeveen H, Huijps K, Lam TJ. Economic aspects of mastitis: new developments. N Z Vet J. 2011;59:16–23. doi: 10.1080/00480169.2011.547165. [DOI] [PubMed] [Google Scholar]
- 24.Akerstedt M, Persson Waller K, Sternesjo A. Haptoglobin and serum amyloid A in relation to the somatic cell count in quarter, cow composite and bulk tank milk samples. J Dairy Res. 2007;74:198–203. doi: 10.1017/S0022029906002305. [DOI] [PubMed] [Google Scholar]
- 25.Hamadani H, Khan AA, Manday BT, Ashraf I, Handoo N, Shah AB, Hamadani A. Bovine mastitis—a disease of serious concern for dairy farmers. Int J Livest Res. 2013;3:42–55. doi: 10.5455/ijlr.20130213091143. [DOI] [Google Scholar]
- 26.de Mol RM, Ouweltjes W. Detection model for mastitis in cows milked in an automatic milking system. Prev Vet Med. 2001;49:71–82. doi: 10.1016/S0167-5877(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 27.Chagunda MG, Larsen T, Bjerring M, Ingvartsen KL. l-lactate dehydrogenase and N-acetyl-beta-d-glucosaminidase activities in bovine milk as indicators of non-specific mastitis. J Dairy Res. 2006;73:431–440. doi: 10.1017/S0022029906001956. [DOI] [PubMed] [Google Scholar]
- 28.Friggens NC, Chagunda MG, Bjerring M, Ridder C, Hojsgaard S, Larsen T. Estimating degree of mastitis from time-series measurements in milk: a test of a model based on lactate dehydrogenase measurements. J Dairy Sci. 2007;90:5415–5427. doi: 10.3168/jds.2007-0148. [DOI] [PubMed] [Google Scholar]
- 29.Smolenski G, Haines S, Kwan FY, Bond J, Farr V, Davis SR, Stelwagen K, Wheeler TT. Characterisation of host defence proteins in milk using a proteomic approach. J Proteome Res. 2007;6:207–215. doi: 10.1021/pr0603405. [DOI] [PubMed] [Google Scholar]
- 30.Affolter M, Grass L, Vanrobaeys F, Casado B, Kussmann M. Qualitative and quantitative profiling of the bovine milk fat globule membrane proteome. J Proteomics. 2010;73:1079–1088. doi: 10.1016/j.jprot.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Bund T, Allelein S, Arunkumar A, Lucey JA, Etzel MR. Chromatographic purification and characterization of whey protein–dextran glycation products. J Chromatogr A. 2012;1244:98–105. doi: 10.1016/j.chroma.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 32.Janjanam J, Singh S, Jena MK, Varshney N, Kola S, Kumar S, Kaushik JK, Grover S, Dang AK, Mukesh M, Prakash BS, Mohanty AK. Comparative 2D-DIGE proteomic analysis of bovine mammary epithelial cells during lactation reveals protein signatures for lactation persistency and milk yield. PLoS One. 2014;9:e102515. doi: 10.1371/journal.pone.0102515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheema M, Mohan MS, Campagna SR, Jurat-Fuentes JL, Harte FM. The association of low-molecular-weight hydrophobic compounds with native casein micelles in bovine milk. J Dairy Sci. 2015;98:5155–5163. doi: 10.3168/jds.2015-9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogarth CJ, Fitzpatrick JL, Nolan AM, Young FJ, Pitt A, Eckersall PD. Differential protein composition of bovine whey: a comparison of whey from healthy animals and from those with clinical mastitis. Proteomics. 2004;4:2094–2100. doi: 10.1002/pmic.200300723. [DOI] [PubMed] [Google Scholar]
- 35.D’Amato A, Bachi A, Fasoli E, Boschetti E, Peltre G, Senechal H, Righetti PG. In-depth exploration of cow’s whey proteome via combinatorial peptide ligand libraries. J Proteome Res. 2009;8:3925–3936. doi: 10.1021/pr900221x. [DOI] [PubMed] [Google Scholar]
- 36.Molle D, Jardin J, Piot M, Pasco M, Leonil J, Gagnaire V. Comparison of electrospray and matrix-assisted laser desorption ionization on the same hybrid quadrupole time-of-flight tandem mass spectrometer: application to bidimensional liquid chromatography of proteins from bovine milk fraction. J Chromatogr A. 2009;1216:2424–2432. doi: 10.1016/j.chroma.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 37.Boehmer JL, DeGrasse JA, McFarland MA, Tall EA, Shefcheck KJ, Ward JL, Bannerman DD. The proteomic advantage: label-free quantification of proteins expressed in bovine milk during experimentally induced coliform mastitis. Vet Immunol Immunopathol. 2010;138:252–266. doi: 10.1016/j.vetimm.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Ham JS, Han GS, Jeong SG, Seol KH, Jang AR, Oh MH, Kim DH, Park YW. Determination of molecular weights of caprine milk proteins by matrix-assisted laser desorption/ionization mass spectrometry. J Dairy Sci. 2012;95:15–19. doi: 10.3168/jds.2011-4543. [DOI] [PubMed] [Google Scholar]
- 39.Wilson NL, Robinson LJ, Donnet A, Bovetto L, Packer NH, Karlsson NG. Glycoproteomics of milk: differences in sugar epitopes on human and bovine milk fat globule membranes. J Proteome Res. 2008;7:3687–3696. doi: 10.1021/pr700793k. [DOI] [PubMed] [Google Scholar]
- 40.Takimori S, Shimaoka H, Furukawa J, Yamashita T, Amano M, Fujitani N, Takegawa Y, Hammarstrom L, Kacskovics I, Shinohara Y, Nishimura S. Alteration of the N-glycome of bovine milk glycoproteins during early lactation. FEBS J. 2011;278:3769–3781. doi: 10.1111/j.1742-4658.2011.08299.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Lee PJ, Stapels M, Gebler JC. The use of mass spectrometry to determine location and extent of N-glycosylation on folate binding protein from bovine milk. Rapid Commun Mass Spectrom. 2006;20:313–316. doi: 10.1002/rcm.2284. [DOI] [PubMed] [Google Scholar]
- 42.Slangen CJ, Visser S. Use of mass spectrometry to rapidly characterize the heterogeneity of bovine α-lactalbumin. J Agric Food Chem. 1999;47:4549–4556. doi: 10.1021/jf990212j. [DOI] [PubMed] [Google Scholar]
- 43.Nwosu CC, Strum JS, An HJ, Lebrilla CB. Enhanced detection and identification of glycopeptides in negative ion mode mass spectrometry. Anal Chem. 2010;82:9654–9662. doi: 10.1021/ac101856r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Leeuwen SS, Schoemaker RJ, Timmer CJ, Kamerling JP, Dijkhuizen L. N- and O-glycosylation of a commercial bovine whey protein product. J Agric Food Chem. 2012;60:12553–12564. doi: 10.1021/jf304000b. [DOI] [PubMed] [Google Scholar]
- 45.O’Riordan N, Kane M, Joshi L, Hickey RM. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology. 2014;24:220–236. doi: 10.1093/glycob/cwt162. [DOI] [PubMed] [Google Scholar]
- 46.Hanisch FG. Chemical de-O-glycosylation of glycoproteins for applications in LC-based proteomics. Methods Mol Biol. 2011;753:323–333. doi: 10.1007/978-1-61779-148-2_22. [DOI] [PubMed] [Google Scholar]
- 47.Boehmer JL. Proteomic analyses of host and pathogen responses during bovine mastitis. J Mammary Gland Biol Neoplasia. 2011;16:323–338. doi: 10.1007/s10911-011-9229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alonso-Fauste I, Andres M, Iturralde M, Lampreave F, Alava MA. Acute phase characterization by 2-DE/MALDI-TOF MS of bovine serum and whey from healthy and mastitis affected animals. FEBS J. 2012;279:232. doi: 10.1016/j.jprot.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 49.Reinhardt TA, Sacco RE, Nonnecke BJ, Lippolis JD. Bovine milk proteome: quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J Proteomics. 2013;82:141–154. doi: 10.1016/j.jprot.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Huang J, Luo G, Zhang Z, Wang X, Ju Z, Qi C, Zhang Y, Wang C, Li R, Li J, Yin W, Xu Y, Moisa SJ, Loor JJ, Zhong J. iTRAQ-proteomics and bioinformatics analyses of mammary tissue from cows with clinical mastitis due to natural infection with Staphylococci aureus. BMC Genomics. 2014;15:839. doi: 10.1186/1471-2164-15-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alonso-Fauste I, Andres M, Iturralde M, Lampreave F, Gallart J, Alava MA. Proteomic characterization by 2-DE in bovine serum and whey from healthy and mastitis affected farm animals. J Proteomics. 2012;75:3015–3030. doi: 10.1016/j.jprot.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 52.Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics. 2012;75:1486–1492. doi: 10.1016/j.jprot.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Kim Y, Atalla H, Mallard B, Robert C, Karrow N. Changes in Holstein cow milk and serum proteins during intramammary infection with three different strains of Staphylococcus aureus. BMC Vet Res. 2011;7:51. doi: 10.1186/1746-6148-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halasa T, Huijps K, Osteras O, Hogeveen H. Economic effects of bovine mastitis and mastitis management: a review. Vet Q. 2007;29:18–31. doi: 10.1080/01652176.2007.9695224. [DOI] [PubMed] [Google Scholar]
- 55.Heikkila AM, Nousiainen JI, Pyorala S. Costs of clinical mastitis with special reference to premature culling. J Dairy Sci. 2012;95:139–150. doi: 10.3168/jds.2011-4321. [DOI] [PubMed] [Google Scholar]
- 56.Rollin E, Dhuyvetter KC, Overton MW. The cost of clinical mastitis in the first 30 days of lactation: an economic modeling tool. Prev Vet Med. 2015;122:257–264. doi: 10.1016/j.prevetmed.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Cha E, Bar D, Hertl JA, Tauer LW, Bennett G, González RN, Schukken YH, Welcome FL, Gröhn YT. The cost and management of different types of clinical mastitis in dairy cows estimated by dynamic programming. J Dairy Sci. 2011;94:4476–4487. doi: 10.3168/jds.2010-4123. [DOI] [PubMed] [Google Scholar]
- 58.Gates MC, Woolhouse MEJ, Gunn GJ, Humphry RW. Relative associations of cattle movements, local spread, and biosecurity with bovine viral diarrhoea virus (BVDV) seropositivity in beef and dairy herds. Prev Vet Med. 2013;112:285–295. doi: 10.1016/j.prevetmed.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Meyling A, Houe H, Jensen A. Epidemiology of bovine virus diarrhoea virus. Rev sci tech (Int Off Epizoot) 1990;9:75–93. doi: 10.20506/rst.9.1.489. [DOI] [PubMed] [Google Scholar]
- 60.Grooms DL. Reproductive losses caused by bovine viral diarrhea virus and leptospirosis. Theriogenology. 2006;66:624–628. doi: 10.1016/j.theriogenology.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Lanyon SR, Hill FI, Reichel MP, Brownlie J. Bovine viral diarrhoea: pathogenesis and diagnosis. Vet J. 2014;199:201–209. doi: 10.1016/j.tvjl.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 62.Edwards S. The diagnosis of bovine virus diarrhoea-mucosal disease in cattle. Rev sci tech (Int Off Epizoot) 1990;9:115–130. doi: 10.20506/rst.9.1.486. [DOI] [PubMed] [Google Scholar]
- 63.Dohoo IR, Martin SW. Disease, production and culling in Holstein–Friesian cows III. Disease and production as determinants of disease. Prev Vet Med. 1984;2:671–690. doi: 10.1016/0167-5877(84)90013-8. [DOI] [Google Scholar]
- 64.Oetzel GR. Monitoring and testing dairy herds for metabolic disease. Vet Clin N Am Food Anim Pract. 2004;20:651–674. doi: 10.1016/j.cvfa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Leblanc S. Monitoring metabolic health of dairy cattle in the transition period. J Reprod Dev. 2010;56:S29–S35. doi: 10.1262/jrd.1056S29. [DOI] [PubMed] [Google Scholar]
- 66.Enjalbert F, Nicot MC, Bayourthe C, Moncoulon R. Ketone bodies in milk and blood of dairy cows: relationship between concentrations and utilization for detection of subclinical ketosis. J Dairy Sci. 2001;84:583–589. doi: 10.3168/jds.S0022-0302(01)74511-0. [DOI] [PubMed] [Google Scholar]
- 67.Duffield TF, Lissemore KD, McBride BW, Leslie KE. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci. 2009;92:571–580. doi: 10.3168/jds.2008-1507. [DOI] [PubMed] [Google Scholar]
- 68.Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci. 2007;90:2788–2796. doi: 10.3168/jds.2006-560. [DOI] [PubMed] [Google Scholar]
- 69.Samiei A, Liang JB, Ghorbani GR, Hirooka H, Yaakub H, Tabatabaei M. An evaluation of beta-hydroxybutyrate in milk and blood for prediction of subclinical ketosis in dairy cows. Pol J Vet Sci. 2010;13:349–356. [PubMed] [Google Scholar]
- 70.Larsen M, Kristensen NB. Effect of a lucerne feeding strategy in the first week postpartum on feed intake and ketone body profiles in blood plasma, urine, and milk in Holstein cows. Acta Agric Scand Sect A. 2010;60:239–249. [Google Scholar]
- 71.Osborne T, Leslie K, Duffield T, Petersson C, Ten Hag J, Okada Y. Evaluation of Keto-test in urine and milk for the detection of subclinical ketosis in periparturient Holstein dairy cattle. In: Proceedings of the 35th conference of the American Association of Bovine Practitioners, Rome, GA, USA; 2002. p. 188–9.
- 72.Carrier J, Stewart S, Godden S, Fetrow J, Rapnicki P. Evaluation and use of three cowside tests for detection of subclinical ketosis in early postpartum cows. J Dairy Sci. 2004;87:3725–3735. doi: 10.3168/jds.S0022-0302(04)73511-0. [DOI] [PubMed] [Google Scholar]
- 73.Rollin F. Tools for a prompt cowside diagnosis: what can be implemented by the bovine practitioner. In: World buiatrics congress. Citeseer; 2006.
- 74.Oetzel GR. Herd-level ketosis–diagnosis and risk factors. In: Proceedings of the 40th annual conference of bovine practitioners, Vancouver, BC, Canada; 2007.
- 75.Weng X, Zhao W, Neethirajan S, Duffield T. Microfluidic biosensor for β-Hydroxybutyrate (βHBA) determination of subclinical ketosis diagnosis. J Nanobiotechnol. 2015;13:13. doi: 10.1186/s12951-015-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weng X, Chen L, Neethirajan S, Duffield T. Development of quantum dots-based biosensor towards on-farm detection of subclinical ketosis. Biosens Bioelectron. 2015;72:140–147. doi: 10.1016/j.bios.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Oltenacu PA, Ferguson JD, Lednor AJ. Economic evaluation of pregnancy diagnosis in dairy cattle: a decision analysis approach. J Dairy Sci. 1990;73:2826–2831. doi: 10.3168/jds.S0022-0302(90)78970-9. [DOI] [PubMed] [Google Scholar]
- 78.Nation DP, Malmo J, Davis GM, Macmillan KL. Accuracy of bovine pregnancy detection using transrectal ultrasonography at 28–35 days after insemination. Aust Vet J. 2003;81:63–65. doi: 10.1111/j.1751-0813.2003.tb11435.x. [DOI] [PubMed] [Google Scholar]
- 79.Lee JE, Lee JY, Kim HR, Shin HY, Lin T, Jin DI. Proteomic analysis of bovine pregnancy-specific serum proteins by 2D fluorescence difference gel electrophoresis. Asian Australas J Anim Sci. 2015;28:788–795. doi: 10.5713/ajas.14.0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kankofer M, Wawrzykowski J, Miller I, Hoedemaker M. Usefulness of DIGE for the detection of protein profile in retained and released bovine placental tissues. Placenta. 2015;36:246–249. doi: 10.1016/j.placenta.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Chung HJ, Kim KW, Han DW, Lee HC, Yang BC, Chung HK, Shim MR, Choi MS, Jo EB, Jo YM, Oh MY, Jo SJ, Hong SK, Park JK, Chang WK. Protein profile in corpus luteum during pregnancy in Korean native cows. Asian Australas J Anim Sci. 2012;25:1540–1545. doi: 10.5713/ajas.2012.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forde N, Bazer FW, Spencer TE, Lonergan P. ’Conceptualizing’ the endometrium: identification of conceptus-derived proteins during early pregnancy in cattle. Biol Reprod. 2015;92:156. doi: 10.1095/biolreprod.115.129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barman P, Yadav MC, Kumar H, Meur SK, Ghosh SK. Gas chromatographic–mass spectrometric analysis of chemical volatiles in buffalo (Bubalus bubalis) urine. Theriogenology. 2013;80:654–658. doi: 10.1016/j.theriogenology.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 84.Gutiérrez R, Vega S, Díaz G, Sánchez J, Coronado M, Ramírez A, Pérez J, González M, Schettino B. Detection of non-milk fat in milk fat by gas chromatography and linear discriminant analysis. J Dairy Sci. 2009;92:1846–1855. doi: 10.3168/jds.2008-1624. [DOI] [PubMed] [Google Scholar]
- 85.Garcia JS, Sanvido GB, Saraiva SA, Zacca JJ, Cosso RG, Eberlin MN. Bovine milk powder adulteration with vegetable oils or fats revealed by MALDI–QTOF MS. Food Chem. 2012;131:722–726. doi: 10.1016/j.foodchem.2011.09.062. [DOI] [Google Scholar]
- 86.Abernethy G, Higgs K. Rapid detection of economic adulterants in fresh milk by liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2013;1288:10–20. doi: 10.1016/j.chroma.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 87.Santos PM, Pereira-Filho ER, Rodriguez-Saona LE. Application of hand-held and portable infrared spectrometers in bovine milk analysis. J Agric Food Chem. 2013;61:1205–1211. doi: 10.1021/jf303814g. [DOI] [PubMed] [Google Scholar]
- 88.Calvano CD, De Ceglie C, Aresta A, Facchini LA, Zambonin CG. MALDI-TOF mass spectrometric determination of intact phospholipids as markers of illegal bovine milk adulteration of high-quality milk. Anal Bioanal Chem. 2013;405:1641–1649. doi: 10.1007/s00216-012-6597-z. [DOI] [PubMed] [Google Scholar]
- 89.Golinelli LP, Carvalho AC, Casaes RS, Lopes CS, Deliza R, Paschoalin VM, Silva JT. Sensory analysis and species-specific PCR detect bovine milk adulteration of frescal (fresh) goat cheese. J Dairy Sci. 2014;97:6693–6699. doi: 10.3168/jds.2014-7990. [DOI] [PubMed] [Google Scholar]
- 90.Guerreiro JS, Fernandes P, Bardsley RG. Identification of the species of origin of milk in cheeses by multivariate statistical analysis of polymerase chain reaction electrophoretic patterns. Int Dairy J. 2012;25:42–45. doi: 10.1016/j.idairyj.2012.01.002. [DOI] [Google Scholar]
- 91.Rodriguez N, Ortiz MC, Sarabia L, Gredilla E. Analysis of protein chromatographic profiles joint to partial least squares to detect adulterations in milk mixtures and cheeses. Talanta. 2010;81:255–264. doi: 10.1016/j.talanta.2009.11.067. [DOI] [PubMed] [Google Scholar]
- 92.Nicolaou N, Xu Y, Goodacre R. Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J Dairy Sci. 2010;93:5651–5660. doi: 10.3168/jds.2010-3619. [DOI] [PubMed] [Google Scholar]
- 93.Lamanna R, Braca A, Di Paolo E, Imparato G. Identification of milk mixtures by 1H NMR profiling. Magn Reson Chem. 2011;49(Suppl. 1):S22–S26. doi: 10.1002/mrc.2807. [DOI] [PubMed] [Google Scholar]
- 94.Mayer HK. Milk species identification in cheese varieties using electrophoretic, chromatographic and PCR techniques. Int Dairy J. 2005;15:595–604. doi: 10.1016/j.idairyj.2004.10.012. [DOI] [Google Scholar]
- 95.Yang Y, Zheng N, Yang J, Bu D, Wang J, Ma L, Sun P. Animal species milk identification by comparison of two-dimensional gel map profile and mass spectrometry approach. Int Dairy J. 2014;35:15–20. doi: 10.1016/j.idairyj.2013.09.008. [DOI] [Google Scholar]
- 96.Nicolaou N, Xu Y, Goodacre R. MALDI-MS and multivariate analysis for the detection and quantification of different milk species. Anal Bioanal Chem. 2011;399:3491–3502. doi: 10.1007/s00216-011-4728-6. [DOI] [PubMed] [Google Scholar]
- 97.Kim JI, Park JM, Noh JY, Hwang SJ, Kang MJ, Pyun JC. Analysis of benzylpenicillin in milk using MALDI-TOF mass spectrometry with top-down synthesized TiO2 nanowires as the solid matrix. Chemosphere. 2016;143:64–70. doi: 10.1016/j.chemosphere.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 98.Gekenidis MT, Studer P, Wuthrich S, Brunisholz R, Drissner D. Beyond the matrix-assisted laser desorption ionization (MALDI) biotyping workflow: in search of microorganism-specific tryptic peptides enabling discrimination of subspecies. Appl Environ Microbiol. 2014;80:4234–4241. doi: 10.1128/AEM.00740-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Normand A-C, Cassagne C, Ranque S, L’Ollivier C, Fourquet P, Roesems S, Hendrickx M, Piarroux R. Assessment of various parameters to improve MALDI-TOF MS reference spectra libraries constructed for the routine identification of filamentous fungi. BMC Microbiol. 2013;13:76. doi: 10.1186/1471-2180-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Claydon MA, Davey SN, Edwards-Jones V, Gordon DB. The rapid identification of intact microorganisms using mass spectrometry. Nat Biotechnol. 1996;14:1584–1586. doi: 10.1038/nbt1196-1584. [DOI] [PubMed] [Google Scholar]
- 102.Krishnamurthy T, Ross PL, Rajamani U. Detection of pathogenic and non-pathogenic bacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1996;10:883–888. doi: 10.1002/(SICI)1097-0231(19960610)10:8<883::AID-RCM594>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 103.Fenselau C, Demirev PA. Characterization of intact microorganisms by MALDI mass spectrometry. Mass Spectrom Rev. 2001;20:157–171. doi: 10.1002/mas.10004. [DOI] [PubMed] [Google Scholar]
- 104.Dieckmann R, Strauch E, Alter T. Rapid identification and characterization of Vibrio species using whole-cell MALDI-TOF mass spectrometry. J Appl Microbiol. 2010;109:199–211. doi: 10.1111/j.1365-2672.2009.04647.x. [DOI] [PubMed] [Google Scholar]
- 105.Murray PR. What is new in clinical microbiology–microbial identification by MALDI-TOF mass spectrometry: a paper from the 2011 William Beaumont Hospital symposium on molecular pathology. J Mol Diagn. 2012;14:419–423. doi: 10.1016/j.jmoldx.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jadhav S, Sevior D, Bhave M, Palombo EA. Detection of Listeria monocytogenes from selective enrichment broth using MALDI-TOF mass spectrometry. J Proteomics. 2014;97:100–106. doi: 10.1016/j.jprot.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 107.Jadhav S, Gulati V, Fox EM, Karpe A, Beale DJ, Sevior D, Bhave M, Palombo EA. Rapid identification and source-tracking of Listeria monocytogenes using MALD-TOF mass spectrometry. Int J Food Microbiol. 2015;202:1–9. doi: 10.1016/j.ijfoodmicro.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 108.Kostrzewa M, Sparbier K, Maier T, Schubert S. MALDI-TOF MS: an upcoming tool for rapid detection of antibiotic resistance in microorganisms. Proteomics Clin Appl. 2013;7:767–778. doi: 10.1002/prca.201300042. [DOI] [PubMed] [Google Scholar]
- 109.Hrabak J, Chudackova E, Walkova R. Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry for detection of antibiotic resistance mechanisms: from research to routine diagnosis. Clin Microbiol Rev. 2013;26:103–114. doi: 10.1128/CMR.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sandrin TR, Goldstein JE, Schumaker S. MALDI TOF MS profiling of bacteria at the strain level: a review. Mass Spectrom Rev. 2013;32:188–217. doi: 10.1002/mas.21359. [DOI] [PubMed] [Google Scholar]
- 111.Carbonnelle E, Grohs P, Jacquier H, Day N, Tenza S, Dewailly A, Vissouarn O, Rottman M, Herrmann JL, Podglajen I, Raskine L. Robustness of two MALDI-TOF mass spectrometry systems for bacterial identification. J Microbiol Methods. 2012;89:133–136. doi: 10.1016/j.mimet.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 112.Strohalm M, Hassman M, Kosata B, Kodicek M. mMass data miner: an open source alternative for mass spectrometric data analysis. Rapid Commun Mass Spectrom. 2008;22:905–908. doi: 10.1002/rcm.3444. [DOI] [PubMed] [Google Scholar]
- 113.Ndukum J, Atlas M, Datta S. pkDACLASS: open source software for analyzing MALDI-TOF data. Bioinformation. 2011;6:45–47. doi: 10.6026/97320630006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gibb S, Strimmer K. MALDIquant: a versatile R package for the analysis of mass spectrometry data. Bioinformatics. 2012;28:2270–2271. doi: 10.1093/bioinformatics/bts447. [DOI] [PubMed] [Google Scholar]
- 115.Bohme K, Fernandez-No IC, Barros-Velazquez J, Gallardo JM, Canas B, Calo-Mata P. SpectraBank: an open access tool for rapid microbial identification by MALDI-TOF MS fingerprinting. Electrophoresis. 2012;33:2138–2142. doi: 10.1002/elps.201200074. [DOI] [PubMed] [Google Scholar]
- 116.Raus M, Šebela M. BIOSPEAN: a freeware tool for processing spectra from MALDI intact cell/spore mass spectrometry. J Proteomics Bioinform. 2013;6:282–287. [Google Scholar]
- 117.Hettinga K, van Valenberg H, de Vries S, Boeren S, van Hooijdonk T, van Arendonk J, Vervoort J. The host defense proteome of human and bovine milk. PLoS One. 2011;6:e19433. doi: 10.1371/journal.pone.0019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Y, Zheng N, Zhao X, Zhang Y, Han R, Ma L, Zhao S, Li S, Guo T, Wang J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J Proteomics. 2015;116:34–43. doi: 10.1016/j.jprot.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 119.Sui S, Zhao J, Wang J, Zhang R, Guo C, Yu T, Li N. Comparative proteomics of milk fat globule membrane proteins from transgenic cloned cattle. PLoS One. 2014;9:e105378. doi: 10.1371/journal.pone.0105378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benbrook CM, Butler G, Latif MA, Leifert C, Davis DR. Organic production enhances milk nutritional quality by shifting fatty acid composition: a United States-wide, 18-month study. PLoS One. 2013;8:e82429. doi: 10.1371/journal.pone.0082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stefanov I, Vlaeminck B, Fievez V. A novel procedure for routine milk fat extraction based on dichloromethane. J Food Compos Anal. 2010;23:852–855. doi: 10.1016/j.jfca.2010.03.016. [DOI] [Google Scholar]
- 122.Feng S, Lock AL, Garnsworthy PC. Technical note: a rapid lipid separation method for determining fatty acid composition of milk. J Dairy Sci. 2004;87:3785–3788. doi: 10.3168/jds.S0022-0302(04)73517-1. [DOI] [PubMed] [Google Scholar]
- 123.Delmonte P, Fardin-Kia AR, Kramer JK, Mossoba MM, Sidisky L, Tyburczy C, Rader JI. Evaluation of highly polar ionic liquid gas chromatographic column for the determination of the fatty acids in milk fat. J Chromatogr A. 2012;1233:137–146. doi: 10.1016/j.chroma.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Ecker J, Scherer M, Schmitz G, Liebisch G. A rapid GC–MS method for quantification of positional and geometric isomers of fatty acid methyl esters. J Chromatogr B Anal Technol Biomed Life Sci. 2012;897:98–104. doi: 10.1016/j.jchromb.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 125.Simionato JI, Garcia JC, dos Santos GT, Oliveira CC, Visentainer JV, de Souza NE. Validation of the determination of fatty acids in milk by gas chromatography. J Braz Chem Soc. 2010;21:520–524. doi: 10.1590/S0103-50532010000300018. [DOI] [Google Scholar]
- 126.Calvano CD, Zambonin CG. MALDI-Q-TOF-MS ionization and fragmentation of phospholipids and neutral lipids of dairy interest using variable doping salts. J Adv Dairy Res. 2013;1:101. [Google Scholar]
- 127.Calvano CD, Monopoli A, Loizzo P, Faccia M, Zambonin C. Proteomic approach based on MALDI-TOF MS to detect powdered milk in fresh cow’s milk. J Agric Food Chem. 2013;61:1609–1617. doi: 10.1021/jf302999s. [DOI] [PubMed] [Google Scholar]
- 128.Garcia JS, Sanvido GB, Saraiva SA, Zacca JJ, Cosso RG, Eberlin MN. Bovine milk powder adulteration with vegetable oils or fats revealed by MALDI–QTOF MS. Food Chem. 2012;131:722–726. doi: 10.1016/j.foodchem.2011.09.062. [DOI] [Google Scholar]
- 129.Sommer U, Herscovitz H, Welty FK, Costello CE. LC–MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res. 2006;47:804–814. doi: 10.1194/jlr.M500506-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Sokol E, Ulven T, Faergeman NJ, Ejsing CS. Comprehensive and quantitative profiling of lipid species in human milk, cow milk and a phospholipid-enriched milk formula by GC and MS/MS. Eur J Lipid Sci Technol. 2015;117:751–759. doi: 10.1002/ejlt.201400575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu Z, Moate P, Cocks B, Rochfort S. Comprehensive polar lipid identification and quantification in milk by liquid chromatography–mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2015;978–979:95–102. doi: 10.1016/j.jchromb.2014.11.036. [DOI] [PubMed] [Google Scholar]
- 132.Sun HZ, Wang DM, Wang B, Wang JK, Liu HY, le Guan L, Liu JX. Metabolomics of four biofluids from dairy cows: potential biomarkers for milk production and quality. J Proteome Res. 2015;14:1287–1298. doi: 10.1021/pr501305g. [DOI] [PubMed] [Google Scholar]
- 133.El-Loly M. Composition, properties and nutritional aspects of milk fat globule membrane—a review. Pol J Food Nutr Sci. 2011;61:7–32. [Google Scholar]
- 134.Lopez C, Briard-Bion V, Ménard O, Beaucher E, Rousseau F, Fauquant J, Leconte N, Robert B. Fat globules selected from whole milk according to their size: different compositions and structure of the biomembrane, revealing sphingomyelin-rich domains. Food Chem. 2011;125:355–368. doi: 10.1016/j.foodchem.2010.09.005. [DOI] [Google Scholar]
- 135.Heid HW, Keenan TW. Intracellular origin and secretion of milk fat globules. Eur J Cell Biol. 2005;84:245–258. doi: 10.1016/j.ejcb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 136.Contarini G, Povolo M. Phospholipids in milk fat: composition, biological and technological significance, and analytical strategies. Int J Mol Sci. 2013;14:2808–2831. doi: 10.3390/ijms14022808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bezelgues JB, Morgan F, Palomo G, Crosset-Perrotin L, Ducret P. Short communication: milk fat globule membrane as a potential delivery system for liposoluble nutrients. J Dairy Sci. 2009;92:2524–2528. doi: 10.3168/jds.2008-1725. [DOI] [PubMed] [Google Scholar]
- 138.Singh H. The milk fat globule membrane—a biophysical system for food applications. Curr Opin Colloid Interface Sci. 2006;11:154–163. doi: 10.1016/j.cocis.2005.11.002. [DOI] [Google Scholar]
- 139.Chiaradia E, Valiani A, Tartaglia M, Scoppetta F, Renzone G, Arena S, Avellini L, Benda S, Gaiti A, Scaloni A. Ovine subclinical mastitis: proteomic analysis of whey and milk fat globules unveils putative diagnostic biomarkers in milk. J Proteomics. 2013;83:144–159. doi: 10.1016/j.jprot.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 140.Lu J, van Hooijdonk T, Boeren S, Vervoort J, Hettinga K. Identification of lipid synthesis and secretion proteins in bovine milk. J Dairy Res. 2014;81:65–72. doi: 10.1017/S0022029913000642. [DOI] [PubMed] [Google Scholar]
- 141.Almeida A, Albuquerque P, Araujo R, Ribeiro N, Tavares F. Detection and discrimination of common bovine mastitis-causing streptococci. Vet Microbiol. 2013;164:370–377. doi: 10.1016/j.vetmic.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 142.Danielsen M, Codrea MC, Ingvartsen KL, Friggens NC, Bendixen E, Rontved CM. Quantitative milk proteomics–host responses to lipopolysaccharide-mediated inflammation of bovine mammary gland. Proteomics. 2010;10:2240–2249. doi: 10.1002/pmic.200900771. [DOI] [PubMed] [Google Scholar]
- 143.Addis MF, Pisanu S, Ghisaura S, Pagnozzi D, Marogna G, Tanca A, Biosa G, Cacciotto C, Alberti A, Pittau M, Roggio T, Uzzau S. Proteomics and pathway analyses of the milk fat globule in sheep naturally infected by Mycoplasma agalactiae provide indications of the in vivo response of the mammary epithelium to bacterial infection. Infect Immun. 2011;79:3833–3845. doi: 10.1128/IAI.00040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Turk R, Piras C, Kovacic M, Samardzija M, Ahmed H, De Canio M, Urbani A, Mestric ZF, Soggiu A, Bonizzi L, Roncada P. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J Proteomics. 2012;75:4412–4428. doi: 10.1016/j.jprot.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 145.Baeker R, Haebel S, Schlatterer K, Schlatterer B. Lipocalin-type prostaglandin D synthase in milk: a new biomarker for bovine mastitis. Prostaglandins Other Lipid Mediat. 2002;67:75–88. doi: 10.1016/S0090-6980(01)00175-7. [DOI] [PubMed] [Google Scholar]
- 146.Conway V, Gauthier SF, Pouliot Y. Buttermilk: much more than a source of milk phospholipids. Anim Front. 2014;4:44–51. doi: 10.2527/af.2014-0014. [DOI] [Google Scholar]
- 147.Ménard O, Ahmad S, Rousseau F, Briard-Bion V, Gaucheron F, Lopez C. Buffalo vs. cow milk fat globules: size distribution, zeta-potential, compositions in total fatty acids and in polar lipids from the milk fat globule membrane. Food Chem. 2010;120:544–551. doi: 10.1016/j.foodchem.2009.10.053. [DOI] [Google Scholar]
- 148.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 150.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 151.Michael A, Bajracharya SD, Yuen PST, Zhou H, Star RA, Illei GG, Alevizos I. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 155.Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N. Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun. 2010;396:528–533. doi: 10.1016/j.bbrc.2010.04.135. [DOI] [PubMed] [Google Scholar]
- 156.Admyre C, Grunewald J, Thyberg J, Gripenback S, Tornling G, Eklund A, Scheynius A, Gabrielsson S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur Respir J. 2003;22:578–583. doi: 10.1183/09031936.03.00041703. [DOI] [PubMed] [Google Scholar]
- 157.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 158.Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 159.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 160.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 161.Plantz PE, Patton S, Keenan TW. Further evidence of plasma membrane material in skim milk. J Dairy Sci. 1973;56:978–983. doi: 10.3168/jds.S0022-0302(73)85292-0. [DOI] [PubMed] [Google Scholar]
- 162.Silverman JM, Clos J, de’Oliveira CC, Shirvani O, Fang Y, Wang C, Foster LJ, Reiner NE. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123:842–852. doi: 10.1242/jcs.056465. [DOI] [PubMed] [Google Scholar]
- 163.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Reeds PJ. Amino acid composition of the milk of some mammalian species changes with stage of lactation. Br J Nutr. 1994;72:845–853. doi: 10.1079/BJN19940089. [DOI] [PubMed] [Google Scholar]
- 165.Izumi H, Ishizuka S, Inafune A, Hira T, Ozawa K, Shimizu T, Takase M, Hara H. Alpha-lactalbumin hydrolysate stimulates glucagon-like peptide-2 secretion and small intestinal growth in suckling rats. J Nutr. 2009;139:1322–1327. doi: 10.3945/jn.109.106401. [DOI] [PubMed] [Google Scholar]
- 166.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156:S3–S7. doi: 10.1016/j.jpeds.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 167.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., III Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 168.Eng JK, McCormack AL, Yates JR. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 169.Spengler B, Kirsch D, Kaufmann R, Cotter RJ. Metastable decay of peptides and proteins in matrix-assisted laser-desorption mass spectrometry. Rapid Commun Mass Spectrom. 1991;5:198–202. doi: 10.1002/rcm.1290050412. [DOI] [Google Scholar]
- 170.Papayannopoulos IA. The interpretation of collision-induced dissociation tandem mass spectra of peptides. Mass Spectrom Rev. 1995;14:49–73. doi: 10.1002/mas.1280140104. [DOI] [Google Scholar]
- 171.Wattenberg A, Organ AJ, Schneider K, Tyldesley R, Bordoli R, Bateman RH. Sequence dependent fragmentation of peptides generated by MALDI quadrupole time-of-flight (MALDI Q-TOF) mass spectrometry and its implications for protein identification. J Am Soc Mass Spectrom. 2002;13:772–783. doi: 10.1016/S1044-0305(02)00414-2. [DOI] [PubMed] [Google Scholar]
- 172.Hunt DF, Yates JR, Shabanowitz J, Winston S, Hauer CR. Protein sequencing by tandem mass spectrometry. Proc Natl Acad Sci USA. 1986;83:6233–6237. doi: 10.1073/pnas.83.17.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 174.Alexander JN, Schultz GA, Poli JB. Development of a nano-electrospray mass spectrometry source for nanoscale liquid chromatography and sheathless capillary electrophoresis. Rapid Commun Mass Spectrom. 1998;12:1187–1191. doi: 10.1002/(SICI)1097-0231(19980915)12:17<1187::AID-RCM300>3.0.CO;2-T. [DOI] [Google Scholar]
- 175.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 176.Aebersold R, Goodlett DR. Mass spectrometry in proteomics. Chem Rev. 2001;101:269–296. doi: 10.1021/cr990076h. [DOI] [PubMed] [Google Scholar]
- 177.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taylor JA, Johnson RS. Sequence database searches via de novo peptide sequencing by tandem mass spectrometry. Rapid Commun Mass Spectrom. 1997;11:1067–1075. doi: 10.1002/(SICI)1097-0231(19970615)11:9<1067::AID-RCM953>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 179.Boehmer JL, Bannerman DD, Shefcheck K, Ward JL. Proteomic analysis of differentially expressed proteins in bovine milk during experimentally induced Escherichia coli mastitis. J Dairy Sci. 2008;91:4206–4218. doi: 10.3168/jds.2008-1297. [DOI] [PubMed] [Google Scholar]
- 180.Boehmer JL, Ward JL, Peters RR, Shefcheck KJ, McFarland MA, Bannerman DD. Proteomic analysis of the temporal expression of bovine milk proteins during coliform mastitis and label-free relative quantification. J Dairy Sci. 2010;93:593–603. doi: 10.3168/jds.2009-2526. [DOI] [PubMed] [Google Scholar]
- 181.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, Zimmerman LJ, Ham AJ, Keshishian H, Hall SC, Allen S, Blackman RK, Borchers CH, Buck C, Cardasis HL, Cusack MP, Dodder NG, Gibson BW, Held JM, Hiltke T, Jackson A, Johansen EB, Kinsinger CR, Li J, Mesri M, Neubert TA, Niles RK, Pulsipher TC, Ransohoff D, Rodriguez H, Rudnick PA, Smith D, Tabb DL, Tegeler TJ, Variyath AM, Vega-Montoto LJ, Wahlander A, Waldemarson S, Wang M, Whiteaker JR, Zhao L, Anderson NL, Fisher SJ, Liebler DC, Paulovich AG, Regnier FE, Tempst P, Carr SA. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]


