Abstract
Cystic fibrosis (CF) is caused by homozygous mutations of the CF transmembrane conductance regulator (CFTR) Cl− channel, which result in chronic pulmonary infection and inflammation, the major cause of morbidity and mortality. Although these processes are clearly related to each other, each is likely to contribute to the pathology differently. Understanding the contribution of each of these processes to the overall pathology has been difficult, because they are usually so intimately connected. Various CF mouse models have demonstrated abnormal immune responses compared with wild-type (WT) littermates when challenged with live bacteria or bacterial products acutely. However, these studies have not investigated the consequences of persistent inflammation on lung tissue in CF mice, which may better model the lung pathology in patients. We characterized the lung pathology and immune response of Cftr−/− (CF) and Cftr+/+ (WT) mice to chronic administration of Pseudomonas aeruginosa lipopolysaccharide (LPS). We show that, after long-term repeated LPS exposure, CF mice develop an abnormal and persistent immune response, which is associated with more robust structural changes in the lung than those observed in WT mice. Although CF mice and their WT littermates develop lung pathology after chronic exposure to LPS, the inflammation and damage resolve in WT mice. However, CF mice do not recover efficiently, and, as a consequence of their chronic inflammation, CF mice are more susceptible to morphological changes and lung remodeling. This study shows that chronic inflammation alone contributes significantly to aspects of CF lung pathology.
Keywords: cystic fibrosis, inflammation, IFN-γ-induced protein 10
in cystic fibrosis (CF), the most common lethal genetic disorder in the Caucasian population, major morbidity and mortality are due to chronic obstructive lung disease. Mutations in the CF transmembrane conductance regulator (CFTR) affect its ability to function as a Cl− channel, resulting in decreased Cl− and bicarbonate permeability. In the respiratory tract, CFTR dysfunction leads to alterations in the airway surface liquid and the airway microenvironment, which lead to impaired mucociliary clearance. This impaired clearance is observed very early in life, as the lungs of infants with CF appear to be colonized with bacteria. Although these organisms include Staphylococcus aureus and Haemophilus influenza, colonization with Pseudomonas aeruginosa (PA) is most associated with significant clinical decline (18). Over time, the inability to clear bacteria results in chronic infection and chronic inflammation. It is believed that, together, these processes lead to further airway destruction and, eventually, respiratory failure and premature death (11).
The robust inflammatory response in CF is characterized by increased neutrophil migration into the lung, higher levels of cytokines such IL-8 and TNF-α in the bronchoalveolar lavage (BAL) fluid (BALF), and a robust inflammatory response. Most CF patients with pulmonary manifestations have considerable neutrophilic inflammation, even in the absence of bacterial infection, which has led many to believe that one of the consequences of defective CFTR is a hyperresponsive, proinflammatory immune response. This hyperresponsiveness may be associated with changes in the lung environment that, in the long term, could contribute to the establishment of the chronic infection (21). The agarose bead method of chronic Pseudomonas endobronchial infection (32, 33) has been extensively used. These studies highlighted the effects of chronic infection on lung remodeling in CF mice. Although it is considered a model of chronic infection, the agarose bead method assessed the effects of infection for a relatively short period of time (2 wk), and the consequences of lung remodeling in CF for further acute inflammatory insults were not described.
To better understand the effects of chronic inflammation in the complex cascade of events involved in lung damage and remodeling leading to lung pathology, we compared the effects of repeated exposure to LPS in CF and wild-type (WT) lungs. This protocol is tolerated by the fragile CF mouse model and allows assessment of the effects of more prolonged (up to 6 wk) chronic inflammatory insults in CF.
Since CF mice do not develop the chronic bacterial infection, they represent a unique model for dissecting aspects of the CF lung pathology associated with the defective handling of chronic inflammatory events. Indeed, chronic hyperinflammation is observed in the lungs (2, 8, 22, 23, 31, 32), pancreas (12), and gastrointestinal tract (1) of these mice.
We use chronic LPS treatment, because this approach is well tolerated in CF mice (8) and has been associated with several structural and functional lung modifications (3, 4, 34). The goal of this study was to determine if lack of functional CFTR influences the in vivo response to chronic LPS challenge, leading to more severe structural lung changes.
MATERIALS AND METHODS
Mouse breeding and in vivo procedures.
Transgenic CFTR knockout (KO; Cftr−/−) mice (B6.129P2-KOCftrtm1Unc) (29) were purchased from the Jackson Laboratory and bred in the Yale University Animal Facility in pathogen-free ventilated cages. Mice were backcrossed on a C57BL/6 background for up to 10 generations. WT mice were littermates. Upon weaning, experimental (WT and CF) mice were fed a liquid diet (Peptamen, Nestle, Deerfield, IL), as previously described (15).
Two-month-old WT and CF mice were exposed to aerosolized lipopolysaccharide (LPS; catalog no. L8643, Sigma) three times a week for 6 wk (Fig. 1A). LPS (12.5 mg) was dissolved in 5.0 ml of warm (70–80°C) PBS. After vigorous mixing, the solution was cooled to 37°C and administered using a nebulizer (Pulmo-Aide compressor) connected to a container with vent holes. Five milliliters of solution were administered over 15 min. WT and CF mice were treated simultaneously in each experiment. WT and CF mice were randomized into three different groups that were euthanized at different times: group 1 at the end of the initial 6 wk of LPS exposure (T1, n = 6 for each genotype), group 2 after 10 wk of “recovery” from LPS nebulizations (T2, n = 4 for each genotype), and group 3, which was allowed to recover for 10 wk and then received three acute doses of LPS over 3 days, as described previously (8), at 6 h after the last LPS dose (T3, n = 4 for each genotype). Age-matched WT and CF mice were used as control (T0). A schematic of LPS administration time points is shown in Fig. 1A.
Fig. 1.
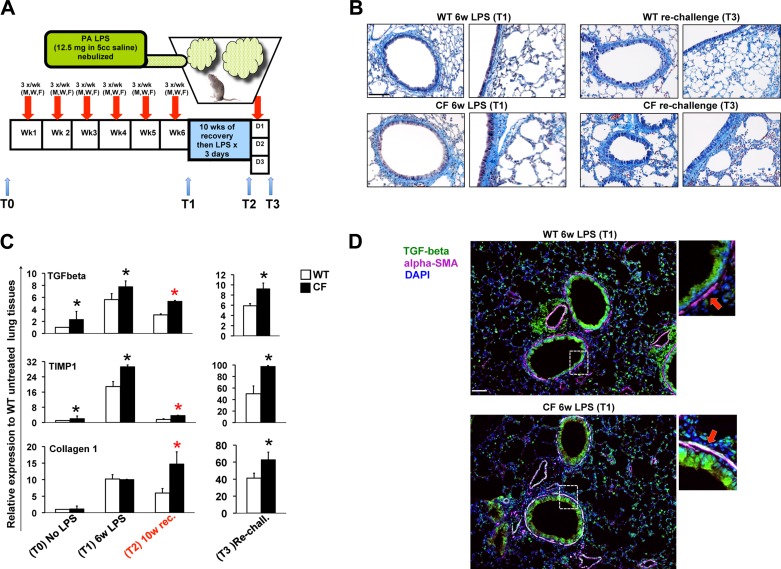
Chronic LPS exposure induces profound lung remodeling in cystic fibrosis transmembrane conductance regulator knockout [Cftr−/− (CF)] mice. A: cartoon representation of the in vivo treatment. B: representative Masson's trichrome staining of paraffin-embedded lung tissues from wild-type (WT, top) and CF (bottom) mice after 6 wk of exposure to Pseudomonas aeruginosa (PA) LPS (T1) and 6 wk of LPS exposure followed by 10 wk of recovery and rechallenge with LPS exposure for 3 days (T3). C: quantitative PCR for transforming growth factor (TGF)-β, tissue inhibitor of metalloprotease (TIMP)-1, and collagen 1 in lung lysates from WT and CF mice untreated or treated with LPS before LPS challenge (T0), at T1, after 6 wk of LPS exposure followed by 10 wk of recovery (T2), and at T3; relative expression levels were normalized to S18. Values are means ± SD. *P < 0.05 vs. WT. D: immunofluorescence staining for TGF-β (green), α-smooth muscle actin (α-SMA, magenta), and 4′,6-diamidino-2-phenylindole (DAPI, blue) in paraffin-embedded lung tissues from WT and CF mice at T1. Colocalization of TGF-β and α-SMA is shown in white. Enlargements of image details (dotted square) are shown at right. Scale bars = 20 μm.
Bone marrow (BM) transplantation was performed as previously described (7, 27). Briefly, BM cells were isolated from the femurs of WT or CF mice. Cells were suspended in serum-containing medium at 5 × 106 cells/100 μl. Recipient mice were subjected to whole body irradiation (900 cGy) via a cesium irradiator. About 5 × 106 BM cells were injected intravenously at 4 h postirradiation. At 2–3 mo after transplantation, mice were treated with chronic LPS for 6 wk, as described above (Fig. 1A).
All procedures were performed in compliance with relevant laws and institutional guidelines and were approved by the Yale University Institutional Animal Care and Use Committee.
BALF collection analysis.
Mice were anesthetized, and 200 μl of blood were recovered by retro-orbital puncture. BALF was collected using standard methods. The dilution of epithelial lining fluid recovered with BAL was calculated on the basis of the blood urea nitrogen concentration in the plasma, as previously described (7, 20). BALF was stored in single-use aliquots at −80°C until analysis. The total number of cells recovered in the BAL was determined using an automated cell counter (Hemavet multispecies hematology system, Drew Scientific). For differential cell counting, cells were cytospun onto slides (20,000 cells/slide, 2 slides per BAL) and stained with Wright Giemsa. At least 200 cells per slide were classified. Values are expressed as percentage of each different cell type, and the total number of neutrophils, lymphocytes, and macrophages for each sample was calculated.
Lung histology.
Lungs were collected for histopathology via a midline incision from sternum to diaphragm, and blood was removed from the pulmonary circulation by perfusion of PBS via the right ventricle using a 20-gauge needle. After cardiac perfusion with PBS supplemented with heparin, the left lung lobes were inflated with 0.5% low-melting agarose in PBS at constant pressure. The left lung was then harvested, fixed overnight in 10% neutral buffered formalin, and embedded in paraffin. The right lobes were snap-frozen in liquid nitrogen and used for RNA isolation. Paraffin-embedded tissues were stained with hematoxylin-eosin, Alcian-periodic acid-Schiff, and Masson's trichrome for morphological analysis.
For septa length measurements, sagittal sections of the whole left lobe were stained with hematoxylin-eosin. For each lung tissue, 10 pictures were taken at ×10 magnification. Morphometric quantitation of the air space enlargement was performed with the ImageJ software.
Immunofluorescence on lung tissues.
Briefly, formalin-fixed, paraffin-embedded lung tissue sections were deparaffinized with xylene, rehydrated gradually with graded alcohol solutions, and then washed with deionized water. After antigen retrieval and blocking, sections were incubated with a 1:100 dilution of the mouse monoclonal antibodies anti-transforming growth factor (TGF)-β (rabbit polyclonal) and anti-α-smooth muscle actin (SMA; both obtained from Abcam) at 4°C overnight. Sections were washed in PBS and then incubated with a 1:500 dilution of fluorescent-labeled anti-mouse (Alexa 568) and anti-rabbit (Alexa 488) antibodies at room temperature for 2 h. Slides were then washed with PBS and mounted with mounting medium containing 4′,6-diaminido-2-phenylindole (Vector Laboratories). Images were acquired with an Olympus BX51 fluorescence microscope and IPLab 4.0.8 software.
Cytokine quantification.
Cytokine concentration in the BAL was assessed by LINCOplex following the manufacturer's instructions (MCYTO-70K-PMX22, Linco Research). The following cytokines were simultaneously assessed from 50 μl of BALF: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13, TNF-α, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1α, monocyte chemoattractant protein (MCP)-1, regulated on activation, normal T cell expressed and secreted, IL-1α, IL-7, IL-15, IL-17, IFN-γ-induced protein 10 (IP-10), and granulocyte colony-stimulating factor (G-CSF).
Expression analysis.
Total RNA was isolated using RNA Mini kits (Qiagen, Valencia, CA). After RNase-free DNaseI treatment (Roche Molecular Biochemical, Mannheim, Germany), 2 μg of total RNA were reverse-transcribed using SuperScript II RNaseH− reverse transcriptase (GIBCO BRL, Grand Island, NY) with 100 μg of random hexamers. TaqMan or SYBR Green-based real-time PCR analysis was performed with a Bio-Rad iCycler or on a BioMark 48 “lab-on-a-chip” system (ServiceXS, Leiden, The Netherlands). Relative expression levels in the samples were calculated using the cycle threshold (ΔΔCt) method, with 18S, hypoxanthine-guanine phosphoribosyltransferase 1, β-glucuronidase, or GAPDH as reference genes as internal normalization control. The expression level is shown as fold increase in experimental samples over untreated age-matched WT mice (y-axis). Primers were purchased from Applied Biosystems (Life Technology). Some of the primer sequences used for quantitative PCR were provided by Dr. Brass and have been used in similar studies (3).
Lung function tests.
Lung volumes and airway resistance were assessed using a flexiVent apparatus (SCIREQ). After pentobarbital sodium anesthesia, an endotracheal tube was connected to a volume-cycled ventilator. Mice were closely monitored, and body temperature was maintained. Mice were ventilated at a rate of 160–200 breaths/min and a tidal volume of 0.2 ml with positive end-expiratory pressure of 2–3 cmH2O. Baseline airway resistance was established and measured for 6 min by the forced oscillation technique, as previously described (26, 28).
Statistical analysis.
Statistical analyses were conducted using one-sided two-sample t-tests or two-sample unequal-variance t-tests (in vivo studies). All experiments were performed in triplicate, unless indicated. Data are expressed as either means ± SD or means ± SE as stated in the figure legend. P < 0.05 was considered statistically significant.
RESULTS
To examine the consequences of chronic inflammation on lung tissue integrity and innate immune regulation in a mouse model for CF, we exposed Cftr+/+ and Cftr−/− (hereafter WT and CF, respectively) mice to 18 doses of LPS over 6 wk (3 times per week, every other day). Mice were allowed to recover for 10 wk prior to an acute rechallenge with LPS. Chronic exposure to LPS has been reported to induce lung tissue remodeling (3, 4). WT and CF mice were euthanized at each time point to assess inflammation and structural changes: T1 (time 0, immediately after the last of 18 LPS doses, which are given over 6 wk), T2 [10 wk after last LPS dose (recovery time)], and T3 [after recovery, when mice were acutely rechallenged with LPS (acute rechallenge)]. The experimental groups were compared and contrasted with the outcomes from age-matched WT and CF mice that had not been exposed to LPS [control (T0); Fig. 1A].
Cftr−/− mice are more susceptible to lung remodeling, fibrosis, and lung function decline in response to chronic LPS exposure.
The 6-wk chronic LPS treatment course was well tolerated, and neither CF nor WT mice demonstrated significant weight loss, alterations in respiratory pattern, distress behaviors, or increased mortality during the treatment. However, lungs of CF mice developed a more pronounced fibrotic signature than lungs of WT mice. Masson's trichrome staining revealed persistent extracellular matrix (ECM) deposition in the submucosa, which was more robust in lungs from CF than WT mice. Matrix deposition was highest in the small/medium airways but was also apparent in the larger airways. These structural lung changes worsened after acute rechallenge with LPS (Fig. 1B). Consistent with the immunocytochemistry data, after the chronic LPS exposure, expression of the profibrotic growth factor TGF-β and the ECM gene tissue inhibitor of metalloproteases 1 (TIMP1), which impairs matrix metalloproteinase (MMP) function and collagen breakdown, was increased in lungs of CF mice compared with controls. Collagen 1 expression was induced in both genotypes (Fig. 1C).
After 10 wk of recovery, expression of these remodeling-associated genes decreased in WT and CF mice. However, the levels of TGF-β and TIMP1 remained significantly elevated in CF mice compared with controls. Also, while collagen 1 expression decreased in WT lungs, in the CF lungs it increased threefold, suggesting ongoing matrix deposition during the recovery phase of our study. Moreover, differences in expression of these ECM genes between genotypes were further exacerbated by the acute LPS rechallenge (Fig. 1C, right). Interestingly, prior to LPS exposure, basal levels of TGF-β, TIMP1, and collagen 1 expression were higher in age-matched untreated CF than WT mice (Fig. 1C), in agreement with findings by Durie et al. (14) showing that CF mice develop spontaneous lung remodeling with aging.
To further evaluate the profibrotic signature, we examined expression of TGF-β by immunofluorescence in lung tissues after chronic LPS challenge (T1). TGF-β expression was abundant in the airway epithelium of WT and CF mice. However, in CF lungs we observed robust expression of TGF-β in myofibroblasts (TGF-β colocalization with α-SMA-positive cells) surrounding the airways. In contrast, in WT mice, TGF-β colocalization with α-SMA-positive cells in myofibroblasts was minimal (Fig. 1D). These findings are consistent with a recent study performed on human lung biopsies that revealed an increase in TGF-β signaling and myofibroblast differentiation in CF lungs (19). Thus the chronic exposure to LPS recapitulates the TGF-β-driven lung fibrosis in severe CF pulmonary disease.
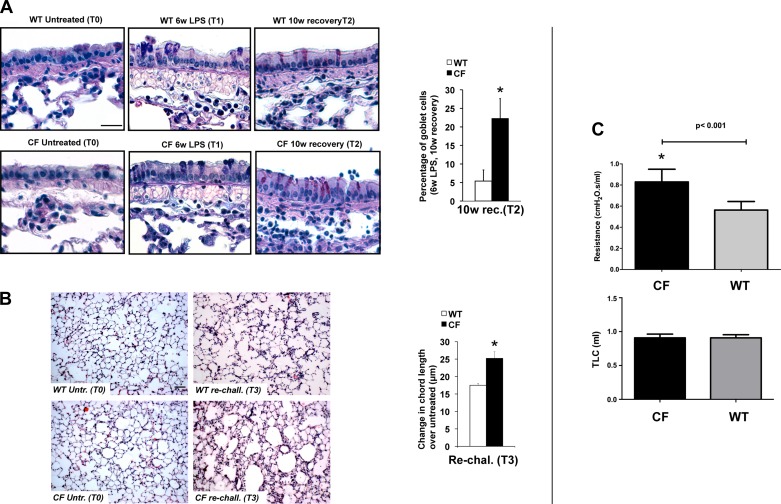
We observed several additional structural changes in the lungs of LPS-treated mice, all of which, including an increase in mucus-producing goblet cells, were more pronounced in a CF mouse background. CF mice showed a significant persistence in the number of goblet cells along the larger airways compared with controls. The difference in goblet cell number between WT and CF animals was most dramatic at the end of the recovery period (T2), when the percentage of goblet cells in WT and CF mice was 5.4 ± 3.0% and 22.3 ± 13.0%, respectively (P = 0.002; Fig. 2A).
Fig. 2.
CF mice have more severe lung function decline in response to chronic LPS exposure. A: representative Alcian/periodic acid-Schiff staining of paraffin-embedded lung tissues from WT and CF mice at T0, T1, and T2 (left) and percentage of goblet cells in CF and WT lungs at T2 (right). B: representative hematoxylin-eosin staining of lung parenchyma of WT and CF mice at T0 and T3 (left), at which time alveoli of CF mice showed the highest degree of enlargement and disruption, and changes in chord length from T0 to T3 in WT and CF mice. Scale bars = 20 μm. C: pulmonary function tests. Top: airway resistance. Bottom: total lung capacity (TLC). Values are means ± SD. *P < 0.05.
The chronic LPS treatment in both genotypes also induced enlargement of the air spaces distal to the terminal bronchioles accompanied by distortion of the normal alveolar architecture. Morphometric quantitation of the air space enlargement revealed a statistically significant increase in chord lengths in CF mice compared with their WT littermates, supporting a more pronounced emphysematous status in CF lungs or more pronounced air trapping (Fig. 2B). These structural changes correlate with functional changes, as the pulmonary function data from these animals show increased resistance in the CF animals compared with WT littermates. The total lung capacity was not different between groups, suggesting that structural changes are likely the result of air trapping due to airway resistance, and not emphysematous changes and increased compliance (Fig. 2C).
Taken together, these data confirm that chronic LPS exposure is associated with lung remodeling, including air trapping, increased mucus production, and increased expression of ECM proteins. Moreover, these data demonstrate that these changes are far more severe in a CF background. Importantly, although the differences between genotypes early in the inflammatory course may appear relatively subtle, profound differences are found with time, suggesting an inefficient repair process in CF lungs in response to a period of chronic inflammation. In addition, these data show that CF mice are more susceptible to more profound damage with subsequent acute inflammatory insults.
Lung remodeling in CF mice is associated with unresolved lung inflammation.
The morphological changes in the CF lungs after chronic LPS exposure were associated with more robust lung inflammation, characterized by increased immune cells in the lungs, than in WT mice (Fig. 3A). These findings are consistent with the observations of Vernooy et al. (34). After chronic LPS exposure, both genotypes demonstrated dense lymphocytic aggregates, localized to peribronchial and perivascular areas of the lung, which persisted even after weeks of recovery time (Fig. 3A, right).
Fig. 3.
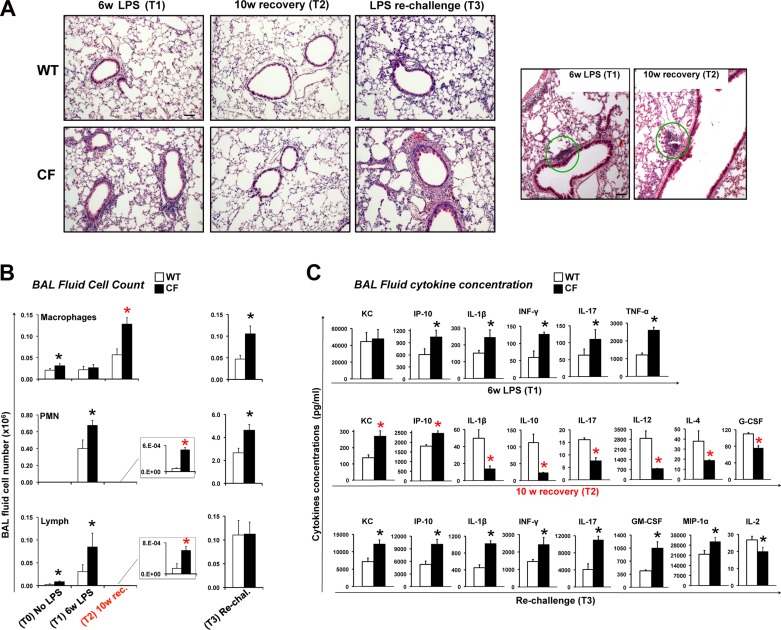
Chronic LPS exposure leads to unresolved lung inflammation in CF mice. A: representative hematoxylin-eosin staining of paraffin-embedded lung tissues from WT and CF mice at T1, T2, and T3. Representative images showing lung lymphocyte aggregates (T1 and T2), which were similarly distributed in both genotypes, are shown at left. Dense lymphocytic aggregation is shown by green circle. Scale bars = 20 μm. B and C: differential bronchoalveolar lavage (BAL) fluid cell number and BAL fluid cytokine concentration in WT and CF mice at T1, T2, and T3. Note difference in scale bars in B, right and left. KC, keratinocyte chemoattractant; IP-10, IFN-γ-induced protein 10; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MIP-1α, macrophage inflammatory protein-1α. Values are means ± SE. *P < 0.05.
To assess the degree of lung inflammation in WT and CF mice in response to LPS, we evaluated total and differential cell number and cytokine concentration in BALF. BALF of untreated mice contained very few cells, which were, as expected, predominantly macrophages. Even though the absolute number of cells is modest in untreated animals, at baseline, the number of macrophages and lymphocytes was increased in CF lungs compared with age-matched WT controls (Fig. 3C), consistent with our previous findings (8). Importantly, an increase in the number of alveolar macrophages has been also observed in the lungs of children with CF (6). At the end of chronic LPS treatment (T1), the total number of BAL cells increased in both groups of mice (WT and CF), and most of the BAL cells were neutrophils (PMNs). The numbers of lymphocytes were also increased compared with baseline. A robust increase in the number of PMNs and lymphocytes was observed in lungs from CF compared with WT mice (Fig. 3B).
After 10 wk of recovery (T2), PMN and lymphocyte numbers decreased to baseline values in CF and WT mice. Baseline values were significantly increased in CF compared with WT mice (Fig. 3B). Importantly, the number of macrophages in the CF animals was significantly elevated from baseline. The increased number of macrophages (despite the recovery time) in CF lungs may contribute to the persistent lung inflammation and remodeling.
With the acute LPS rechallenge, there was a pronounced lung neutrophilia (Fig. 3B), and the PMN and macrophage numbers were elevated in lungs from CF compared with WT mice.
Next, the levels of 22 cytokines [GM-CSF, INF-γ, IL-12p70, IL-13, IL-15, IL-17, IL-1β, IL-1α, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IP-10, MCP-1, G-CSF, MIP-1α, keratinocyte chemoattractant (KC), regulated on activation normal T cell expressed and secreted, and TNF-α] in the BALF of the experimental groups were assessed by Luminex assay. After chronic LPS treatment, most of the cytokines, except IL-15 and IL-7, which were not detected with this assay, were elevated in CF and WT mice. IP-10, IL-1β, INF-γ, IL-17, and TNF-α were significantly elevated in BALF from CF compared with WT mice (Fig. 3C, top). Despite the elevated number of PMNs recovered in the BALF of CF mice, KC concentrations (IL-8 homologous and strong PMN chemoattractant) were not different between WT and CF mice after chronic LPS treatment (Fig. 3C). It should be noted that KC was markedly elevated in both groups. In contrast, IL-17, a cytokine secreted mainly by T helper (Th) 17 (Th17) T cells, which orchestrate neutrophil recruitment to inflamed tissues, was elevated in BALF from CF compared with control mice. Thus the increased number of PMNs in CF lungs exposed chronically to LPS may be sustained by the higher levels of IL-17 (Fig. 3C). This is consistent with the human disease, as Th17 cells have been found to be elevated in the lung of CF patients (10). In turn, the increased number of Th17-producing lymphocytes in CF lungs may be driven by elevated levels of IP-10, which is a potent chemoattractant for T cells, monocytes/MΦs, and natural killer (NK) cells, and has been associated with the CF lung exacerbations (30).
After recovery (T2), many cytokines were undetectable in the BALF of CF and WT mice. However, a number of key cytokines, including KC, IP-10, IL-1β, IL-10, IL-17, IL-12, IL-4, and G-CSF, remained altered in CF mice compared with age-matched wild-type (controls). For instance, levels of the PMN and lymphocyte chemattractants KC and IP-10 were elevated in CF mice compared with WT animals, which is consistent with the higher levels of MΦs and PMNs that were still detectable in the lungs of CF mice (Fig. 3B). However, the concentrations of several other cytokines (IL-1β, IL-10, IL-17, IL-12, IL-4, and G-CSF) were reduced in BALF from CF mice compared with WT controls. Most of these downregulated cytokines are involved in driving T cell differentiation, suggesting the potential for impaired T cell signaling in CF lungs. Taken together, these findings show that, after a prolonged recovery from repetitive inflammatory stimuli, CF lungs are characterized by unresolved inflammation associated with persistently high levels of immune cells, mostly macrophages. In addition, the cytokine milieu of CF lungs suggests impaired T cell activation (Fig. 3C, middle).
In response to the acute rechallenge with LPS (T3), cytokine concentrations were elevated in BALF from CF and WT mice, and, at various levels, all cytokines analyzed were detected. Statistically significant differences between WT and CF mice were found for KC, IP-10, IL-1β, INF-γ, IL-17, GM-CSF, MIP-1α, and IL-2. IL-2 was the only cytokine reduced in CF compared with WT mice; all the others were statistically significantly increased (Fig. 3C, bottom). Thus the acute rechallenge with LPS in CF mice reestablishes a robust inflammatory hyperresponsiveness, which, ultimately, will further damage the lung tissue, as shown in Figs. 1B and 2.
Taken together, these data suggest that CF mice do not recover efficiently from a period of chronic inflammation. The residual inflammation/lung structural changes that persist in CF lungs may drive the increased susceptibility to lung fibrosis. Moreover, this situation primes CF mice to respond more robustly to subsequent acute inflammatory stimuli; perhaps this can be compared with the robust inflammatory response in an acute exacerbation in CF patients. Also, these data suggest that the lung CF environment may alter T cell differentiation, which has been observed in lungs of CF patients (10, 13). Importantly, in the recovery period, we observed an increased number of macrophages in CF lungs, suggesting that these cells may contribute to the persistent inflammation/lung remodeling observed in CF lung pathology.
Elevated secretion of IP-10 is driven by the CF epithelium.
Among the different time points (6 wk of LPS exposure, recovery, and LPS rechallenge), IP-10 was the only cytokine to be elevated persistently in BALF from CF mice compared with controls. IP-10 is secreted by several cell types (monocytes, epithelial cells, endothelial cells, and fibroblasts) in response to IFN-γ and works as chemoattractant for monocytes/MΦs, T cells, NK cells, and dendritic cells into sites of tissue inflammation. Expression of IP-10 is seen in many Th1-type inflammatory diseases, and, interestingly, IP-10 has been recently identified as a potential biomarker for CF acute pulmonary exacerbation (30). Upregulation of IP-10 expression is also a signature in fibrotic injury and repair (5).
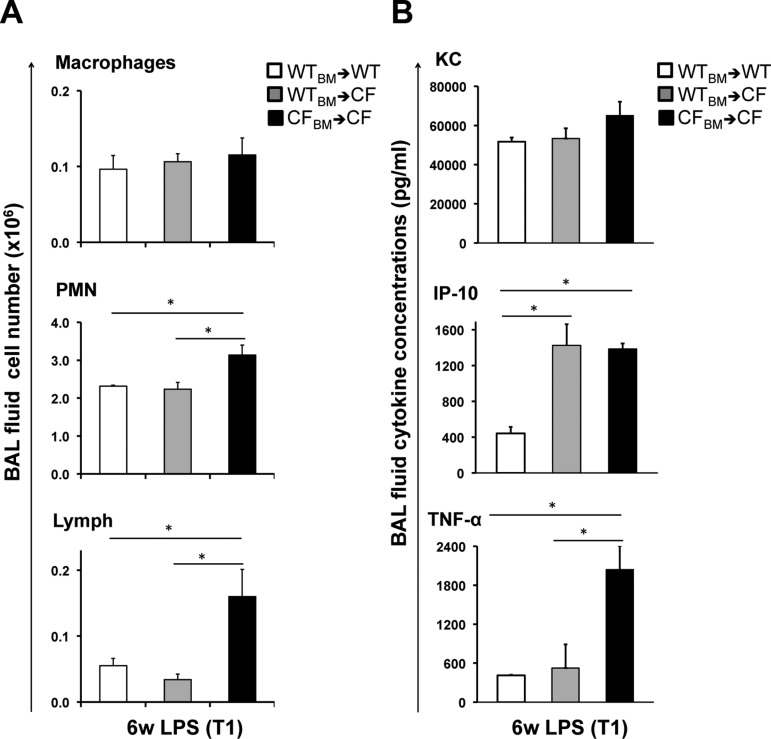
In our earlier work examining the acute inflammatory response in the lung of CF mice, we identified IP-10 as one of the few cytokines for which the dysregulation was driven by the lack of Cftr in the parenchyma, rather than in immune cells (8). To assess whether this would be consistent in a model of chronic LPS treatment, CF mice were transplanted with BM from WT or CF mice (WT→CF or CF→CF); as a control, WT mice were transplanted with BM from WT mice (WT→WT). At 3 mo after transplantation, all groups of mice were chronically exposed to LPS for 6 wk and euthanized 3 h after the last LPS treatment (T1). We then looked at cytokine concentration in the BALF of experimental animals. KC concentration was not different among groups, whereas IP-10 and TNF-α were elevated in the BALF from CF→CF compared with WT→WT mice. These data are consistent with data from nontransplanted mice (Fig. 3C, top). The level of TNF-α in WT→CF mice was comparable to that in WT→WT mice, which suggests that the exaggerated production of TNF-α is driven by the lack of Cftr in BM-derived cells. In contrast, WT Cftr in the BM population was not sufficient to decrease IP-10 concentrations to levels observed in WT→WT mice, thus suggesting that the elevated production of IP-10 is driven by the lack of Cftr in the parenchyma. Numbers of PMNs and lymphocytes were increased in CF→CF compared with WT→WT and WT→CF mice, suggesting that the lack of Cftr in the immune cells contributes to the increased migration of immune cells into the lung in this model (Fig. 4A).
Fig. 4.
Elevated secretion of IP-10 is driven by CF epithelium. A and B: differential BAL fluid cell number and BAL fluid cytokine concentration in WT mice transplanted with WT bone marrow (WTBM→WT), CF mice transplanted with WT BM (WTBM→CF), and CF mice transplanted with CF BM (CFBM→CF) at T1. Values are means ± SE. *P < 0.05.
DISCUSSION
The CF mouse model has not adequately recapitulated the human lung phenotype. CF mice do not develop the spontaneous life-threatening lung disease that occupies the lives of patients; rather, they die of intestinal obstruction, which occurs in a minority of infants with CF. Differences in anatomy and a significant alternative Cl− secretory pathway lessen the lower respiratory tract ion transport abnormalities in mice, leading many to conclude that the murine model is not susceptible to chronic obstructive lung disease. Given that the chronic obstructive lung disease observed in CF patients is a slow and progressive process, we hypothesized that the CF mouse model might develop structural changes observed in the lungs of CF patients after significant cumulative exposure to a known inflammatory stimulus such as PA LPS. Brass and colleagues (3, 4) demonstrated that repeated exposure to aerosolized LPS daily for several weeks results in persistent pathology, including increased goblet cells and mucus production, enlargement of air spaces, and airway wall thickening, in normal mouse lungs. These findings of Brass and colleagues were associated with increased and persistent expression of procollagen I, III, and IV and an increase in apoptosis within the lung parenchyma, which, together, suggest that cumulative exposure results in significant pathology in normal mice. We hypothesized that this response would be more pronounced in our CF animals, such that they would develop pathology that is reminiscent of CF.
In our present study we aimed to examine the long-term consequences of the hyperinflammatory response in the CF mouse after repeated doses of LPS and whether the lungs of CF mice are able to fully recover from chronic inflammatory injuries. We found that chronic LPS exposure resulted in a persistent proinflammatory state, which was more robust in CF animals. Although initial differences were modest between WT and CF mice after 6 wk of chronic LPS challenge, resolution of the inflammatory response was slower and incomplete in CF animals compared with WT littermates. The inability to recover from the chronic LPS challenge after 10 wk of recovery was associated with increased neutrophils, lymphocytes, and macrophages in the BAL, increased air trapping, increased goblet cells with associated mucus production, elevated levels of IP-10 and KC, and persistence of lymphocytic infiltrates in the airways. After 10 wk of recovery, while inflammation was resolved and expression of fibrogenic genes (TGF-β1, TIMP1, and collagen) was decreased to basal levels in WT mice, inflammation in CF mice remained unresolved, characterized by increased numbers of MΦs, PMNs, and lymphocytes and an ongoing fibrotic signature (high expression of TGF-β1 and collagen; Fig. 2, B–E). Intriguingly, at this time, the cytokine profile in CF mice suggests a skewed Th17 cytokine environment, with high TGF-β1/IL-6, low IL-12 (a Th1 cytokine), and low IL-4 (a Th2 cytokine) expression. High levels of IP-10 (CXCL10) were observed in CF BALF. IP-10 is secreted by several cell types (monocytes, endothelial cells, and fibroblasts) in response to IFN-γ and works as a chemoattractant for monocytes/MΦs, T cells, NK cells, and dendritic cells. Interestingly, IP-10 has been recently identified as a potential biomarker for CF acute pulmonary exacerbation (30), and upregulation of this gene is also a signature in fibrotic injury and repair (5). Lastly, after LPS rechallenge, expression of KC, IL-1β, INF-γ, and IL-17, as well as profibrotic genes (Fig. 2, D and E), remained elevated in BALF from CF mice, and trichrome staining revealed profound fibrosis, with abundant subepithelial collagen deposition and alveolar space destruction in CF mice (Fig. 2B, right), suggesting that CF mice do not recover efficiently from the chronic inflammation. As a consequence, CF mice are more susceptible to lung fibrosis. In our recent studies we found that signaling pathways involved in the regulation of TGF-β signaling are altered in CF macrophages (35). We also observed an increase over baseline in expression of Muc5a, as well as higher levels of a number of ECM proteins, in WT and CF mice. CF mice expressed higher levels of mRNA for collagen 1 and TGF-β than WT mice, which could prime CF mice for more profound pathological changes in response to future challenges. Importantly, this CF mouse model reveals that persistent lung remodeling in CF emerges independently from the excessive mucus production, which the CF mouse model does not recapitulate. The lymphocytic infiltrates, present in WT and CF mice, were similar to those described by Vernooy and colleagues (34) after repeated LPS administration, and we postulate that they may comprise predominantly CD4+ T cells. Further studies are necessary for understanding whether the composition of lymphocytic infiltrates is different between WT and CF mice. We should mention that PA can modify its LPS as it encounters different environments. Ernst et al. showed that modifications in the lipid A structure of PA LPS in CF isolates can increase the inflammatory response (16, 17, 24); therefore, the response to repeated LPS from CF isolates may actually be more robust than that reported in the present study.
Thus these results suggest that the lack of CFTR expression drives the inadequate resolution of inflammatory challenge and predisposes mice to lung fibrosis. Interestingly, using a F508del CF mouse model, Scholte and colleagues (9) showed increased damage in CF airway epithelia after naphthalene injury in CF mice that was slow to repair compared with WT controls, suggesting that the murine CF lung may be more prone to damage and remodeling after exposure to a wide variety of triggers, perhaps including PA. Van Heeckeren and colleagues (32) used an agarose PA bead model to recapitulate a chronic PA colonization in Cftr−/− mice. In this model the agarose beads allow for retention of the PA in the airway, which allows for an increased inflammatory response that fails to resolve quickly, because the beads are retained (32). Although this is a shorter time frame than our model, there are a number of histological changes similar to those described in the current report at intermediate time points (2, 25). In addition, acute LPS administration in Cftr−/− and CftrF508del/F508del mice resulted in a similar lung hyperinflammatory response (8, 36). Together, these data suggest that the hyperinflammatory response to acute inflammatory challenges and extensive lung remodeling with chronic inflammation are driven by lack of functional CFTR independent of the class of mutation: null mutations, which are mimicked by the Cftr−/− mouse, or class 2 mutations (F508del/F508del). These data also suggest that the lung hyperinflammation and remodeling in CF mice are driven by persistent activation of inflammatory signaling from a pathogen-associated molecular pattern, independent of the presence of the live bacteria. The persistent presence of a high number of alveolar macrophages in the lung of CF mice during recovery also highlights the possibility that these cells may contribute to the unresolved lung remodeling and predispose the mice to a more robust response to acute inflammatory stimuli. In future studies we will characterize the phenotype of the macrophage that remained elevated in the CF mice to determine if they underlie this response.
There are several correlations between the number of alveolar macrophages and chemokine secretion in this murine model of CF and in children with CF. Brennan et al. (6) found an increase in MIP-1α, as well as an abundance of neutrophils and macrophages in the airway. Hilliard and colleagues (20) demonstrated that, in children with CF, evidence of airway remodeling begins early, with marked increases in elastase, MMP9, and TGF-β, which were elevated in our animal model. Similar to the increases in IL-17 that we observe in our model, Dubin and Kolls (13) showed that the concentration of IL-17 in the BAL is elevated in pediatric CF patients compared with control subjects. Furthermore, they demonstrated that the sputum of adults with CF, who are chronically infected with PA, contains higher concentrations of IL-17A and IL-17F, which decrease once antibiotic therapy is instituted. Interestingly, these sputum samples were also remarkable for increased levels of IL-8 (KC in the murine model) and GM-CSF, which were notably elevated in our model during rechallenge. Taken together, these data suggest that Cftr−/− mice develop lung disease after chronic exposure to LPS, which demonstrates many similarities to the observed phenotype in children with CF. Thus this model may provide insight into the early-stage CF lung disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL-093004 (to E. M. Bruscia and M. E. Egan), Cystic Fibrosis Foundation Grant EGAN1010 (to M. E. Egan), and the Dutch Cystic Fibrosis Foundation (NCFS and EUROCARE CF Grant LSHM-018932).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.B., D.K., and M.E.E. developed the concept and designed the research; E.B., P.X.Z., C.B., and B.S. performed the experiments; E.B., R.J.H., and M.E.E. analyzed the data; E.B., B.S., D.K., and M.E.E. interpreted the results of the experiments; E.B. and M.E.E. prepared the figures; E.B. and M.E.E. drafted the manuscript; E.B., B.S., D.K., and M.E.E. edited and revised the manuscript; E.B. and M.E.E. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Lauren Cohn and Daniel Kiridly for help and assistance with murine pulmonary function; Dr. David Brass for the sequence of the primers used in the study; and Jeffrey Wisner, Elisa Rodriguez, Justin B. Cohen, Ruvalic M. Buijs-Offerman, and Stefanie Donaldson for animal husbandry.
REFERENCES
- 1.Becker KA, Tummler B, Gulbins E, Grassme H. Accumulation of ceramide in the trachea and intestine of cystic fibrosis mice causes inflammation and cell death. Biochem Biophys Res Commun 403: 368–374, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol 92: 1111–1122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brass DM, Hollingsworth JW, Cinque M, Li Z, Potts E, Toloza E, Foster WM, Schwartz DA. Chronic LPS inhalation causes emphysema-like changes in mouse lung that is associated with apoptosis. Am J Respir Cell Mol Biol 39: 584–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brass DM, Savov JD, Gavett SH, Haykal-Coates N, Schwartz DA. Subchronic endotoxin inhalation causes persistent airway disease. Am J Physiol Lung Cell Mol Physiol 285: L755–L761, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Brass DM, Yang IV, Kennedy MP, Whitehead GS, Rutledge H, Burch LH, Schwartz DA. Fibroproliferation in LPS-induced airway remodeling and bleomycin-induced fibrosis share common patterns of gene expression. Immunogenetics 60: 353–369, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Brennan S, Sly PD, Gangell CL, Sturges N, Winfield K, Wikstrom M, Gard S, Upham JW. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur Respir J 34: 655–661, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC, Egan ME, Krause DS. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci USA 103: 2965–2970, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am J Respir Cell Mol Biol 40: 295–304, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho-Oliveira IM, Charro N, Aarbiou J, Buijs-Offerman RM, Wilke M, Schettgen T, Kraus T, Titulaer MK, Burgers P, Luider TM, Penque D, Scholte BJ. Proteomic analysis of naphthalene-induced airway epithelial injury and repair in a cystic fibrosis mouse model. J Proteome Res 8: 3606–3616, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Chan YR, Chen K, Duncan SR, Lathrop KL, Latoche JD, Logar AJ, Pociask DA, Wahlberg BJ, Ray P, Ray A, Pilewski JM, Kolls JK. Patients with cystic fibrosis have inducible IL-17+IL-22+ memory cells in lung draining lymph nodes. J Allergy Clin Immunol 131: 1117–1129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chmiel JF, Davis PB. Why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res 4: 8, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimagno MJ, Lee SH, Hao Y, Zhou SY, McKenna BJ, Owyang C. A proinflammatory, antiapoptotic phenotype underlies the susceptibility to acute pancreatitis in cystic fibrosis transmembrane regulator−/− mice. Gastroenterology 129: 665–681, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Dubin PJ, Kolls JK. IL-17 in cystic fibrosis: more than just Th17 cells. Am J Respir Crit Care Med 184: 155–157, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Durie PR, Kent G, Phillips MJ, Ackerley CA. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol 164: 1481–1493, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckman EA, Cotton CU, Kube DM, Davis PB. Dietary changes improve survival of CFTR S489X homozygous mutant mouse. Am J Physiol Lung Cell Mol Physiol 269: L625–L630, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Ernst RK, Adams KN, Moskowitz SM, Kraig GM, Kawasaki K, Stead CM, Trent MS, Miller SI. The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J Bacteriol 188: 191–201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst RK, Moskowitz SM, Emerson JC, Kraig GM, Adams KN, Harvey MD, Ramsey B, Speert DP, Burns JL, Miller SI. Unique lipid A modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J Infect Dis 196: 1088–1092, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168: 918–951, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Harris WT, Kelly DR, Zhou Y, Wang D, Macewen M, Hagood JS, Clancy JP, Ambalavanan N, Sorscher EJ. Myofibroblast differentiation and enhanced TGF-β signaling in cystic fibrosis lung disease. PLos One 8: e70196, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilliard JB, Konstan MW, Davis PB. Inflammatory mediators in CF patients. Methods Mol Med 70: 409–431, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151: 1075–1082, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Legssyer R, Huaux F, Lebacq J, Delos M, Marbaix E, Lebecque P, Lison D, Scholte BJ, Wallemacq P, Leal T. Azithromycin reduces spontaneous and induced inflammation in ΔF508 cystic fibrosis mice. Respir Res 7: 134, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol 12: 863–875, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz SM, Ernst RK. The role of Pseudomonas lipopolysaccharide in cystic fibrosis airway infection. Subcell Biochem 53: 241–253, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paroni M, Moalli F, Nebuloni M, Pasqualini F, Bonfield T, Nonis A, Mantovani A, Garlanda C, Bragonzi A. Response of CFTR-deficient mice to long-term chronic Pseudomonas aeruginosa infection and PTX3 therapy. J Infect Dis 208: 130–138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, Cochran BH, Simon AR. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol 286: L1282–L1292, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Sharkis SJ, Cahill R, Ahmed A, Jedrzejczak WW, Sell KW. Genetic requirements for bone marrow transplantation for stem-cell-defective W/Wv mice. Transplant Proc 11: 511–516, 1979. [PubMed] [Google Scholar]
- 28.Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol 178: 6191–6199, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science 257: 1083–1088, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Solomon GM, Frederick C, Zhang S, Gaggar A, Harris T, Woodworth BA, Steele C, Rowe SM. IP-10 is a potential biomarker of cystic fibrosis acute pulmonary exacerbations. PLos One 8: e72398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soltys J, Bonfield T, Chmiel J, Berger M. Functional IL-10 deficiency in the lung of cystic fibrosis (cftr−/−) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J Immunol 168: 1903–1910, 2002. [DOI] [PubMed] [Google Scholar]
- 32.van Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest 100: 2810–2815, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, Haxhiu MA, Ferkol TW. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med 161: 271–279, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol 26: 152–159, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Zhang PX, Cheng J, Zou S, D'Souza AD, Koff JL, Lu J, Lee PJ, Krause DS, Egan ME, Bruscia EM. Pharmacological modulation of the AKT/microRNA-199a-5p/CAV1 pathway ameliorates cystic fibrosis lung hyper-inflammation. Nat Commun 6: 6221, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang PX, Murray TS, Villella VR, Ferrari E, Esposito S, D'Souza A, Raia V, Maiuri L, Krause DS, Egan ME, Bruscia EM. Reduced caveolin-1 promotes hyperinflammation due to abnormal heme oxygenase-1 localization in lipopolysaccharide-challenged macrophages with dysfunctional cystic fibrosis transmembrane conductance regulator. J Immunol 190: 5196–5206, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]