Abstract
Despite intense investigation, acute respiratory distress syndrome (ARDS) remains an enormous clinical problem for which no specific therapies currently exist. In this study, we used intratracheal lipopolysaccharide or Pseudomonas bacteria administration to model experimental acute lung injury (ALI) and to further understand mediators of the resolution phase of ARDS. Recent work demonstrates macrophages transition from a predominant proinflammatory M1 phenotype during acute inflammation to an anti-inflammatory M2 phenotype with ALI resolution. We tested the hypothesis that IL-4, a potent inducer of M2-specific protein expression, would accelerate ALI resolution and lung repair through reprogramming of endogenous inflammatory macrophages. In fact, IL-4 treatment was found to offer dramatic benefits following delayed administration to mice subjected to experimental ALI, including increased survival, accelerated resolution of lung injury, and improved lung function. Expression of the M2 proteins Arg1, FIZZ1, and Ym1 was increased in lung tissues following IL-4 treatment, and among macrophages, FIZZ1 was most prominently upregulated in the interstitial subpopulation. A similar trend was observed for the expression of macrophage mannose receptor (MMR) and Dectin-1 on the surface of alveolar macrophages following IL-4 administration. Macrophage depletion or STAT6 deficiency abrogated the therapeutic effect of IL-4. Collectively, these data demonstrate that IL-4-mediated therapeutic macrophage reprogramming can accelerate resolution and lung repair despite delayed use following experimental ALI. IL-4 or other therapies that target late-phase, proresolution pathways may hold promise for the treatment of human ARDS.
Keywords: ARDS, resolution, acute lung injury, interleukin-4, macrophage
acute respiratory distress syndrome (ARDS) is a severe form of diffuse lung disease that imposes a substantial acute and chronic health burden in the United States (49, 57). Both the severity of ARDS and a failure to resolve ongoing inflammation increase mortality and prolong morbidity in survivors (49). Despite intense investigation into understanding ARDS pathogenesis, there is no effective pharmacotherapy, and management is primarily supportive (1, 23, 33, 42).
Investigation in experimental acute lung injury (ALI) models has primarily focused on interrupting the early pathophysiologic events (24–48 h) of ALI/ARDS, but this strategy has resulted in limited clinical utility of those interventions in humans with ARDS. In contrast, a focus on identifying mediators of ALI resolution may provide a clinically relevant time frame during which intervention is feasible for humans with ARDS. We demonstrated the critical importance of regulatory T cells (Tregs) to resolve experimental ALI (9). More recently, we showed that macrophages are also necessary for resolution of severe lung inflammation (4).
Lung inflammation is intimately tied to a phenotypically and functionally diverse set of monocytes and macrophages (21, 34, 50). Early macrophage classification of phenotypes included M1 (classically activated or proinflammatory) or M2 (alternatively activated or anti-inflammatory). In response to signaling via pattern recognition receptors (58, 59), macrophages become M1, possess strong microbiocidal activity, and secrete high levels of proinflammatory cytokines. However, persistence of M1 macrophages can be detrimental to wound healing (51). Experimental ALI models demonstrate macrophage transformation from a predominant M1 phenotype during acute inflammation to a proresolution, M2 phenotype with initiation of lung repair and restoration of tissue homeostasis (3, 26). M2 macrophages are elicited by IL-4 and/or IL-13 in a STAT6-dependent manner and are commonly identified in the mouse by surface expression of mannose receptor, and intracellular expression of arginase-1 (Arg1), chitinase-like 3 (Ym1), and FIZZ1 (Relmα) (20). M2 macrophages are thought to be important in wound healing and can promote tissue repair by limiting Th2-associated cellular inflammation, cytokine production, and fibroproliferation (39, 44, 45). Yet a causal role for proresolution, M2 macrophages during ALI resolution and lung repair has not been found.
Given their highly plastic nature, and the recognition of a M1 to M2 phenotypic transition during the resolution phase of acute lung inflammation, we sought to determine whether exogenously administered IL-4 could induce M2 macrophages that promote resolution of experimental ALI and enhance lung repair. Indeed, we found that IL-4 therapy decisively accelerated resolution of sterile and infection-induced lung inflammation and required macrophages and STAT6 expression to orchestrate this response.
METHODS
Animals.
Male C57BL/6J and BALB/cJ wild-type (WT) mice (8–10 wk old) were purchased from Jackson Laboratories (Bar Harbor, ME). Stat6−/− mice on a BALB/c background (gift of Dr. Alan Scott), and Foxp3DTR mice on a C57BL/6 background (gift of Dr. Alexander Y. Rudensky, Memorial Sloan-Kettering) were bred in our facilities. All mice were housed at the Johns Hopkins University Asthma and Allergy Building, and experiments were conducted under a protocol approved by the Johns Hopkins Animal Care and Use Committee.
Animal injections and harvest.
We performed intratracheal injections as before (9). Briefly, mice were anesthetized with intraperitoneal ketamine/acetylpromazine (100/2.5 μg/g) before exposure of the trachea. Lipopolysaccharide (LPS; 3–5 mg/kg mouse weight diluted in sterile water), Pseudomonas aeruginosa [PAO1; 1.5–2 × 106 colony-forming units (CFUs), ATCC, in 50 μl PBS], or respective vehicle controls were instilled intratracheally through a 20-gauge endotracheal catheter on experiment day 0. After 4, 5, or 6 days, groups of mice were anesthetized with intraperitoneal ketamine/acetylpromazine and euthanized by exsanguination from the inferior vena cava. The lungs were perfused with 1 ml of phosphate-buffered saline (PBS), followed by bronchoalveolar lavage (BAL) of the right lung following each of two aliquots of 0.7 ml PBS without calcium that was instilled via a 20-gauge endotracheal catheter. The left lung was processed for histology, collagen, immunoblot, or mRNA. BAL samples were routinely cultured to assess for bacterial infection. For quantitative measures of bacteria, whole lungs were homogenized without prior lavage, and the lysates were diluted in PBS and streaked on agar plates. After 24 h at 37°C, colonies were counted.
IL-4 complex preparation and antibody injections.
IL-4 was complexed to an anti-IL-4 antibody to prolong bioavailability, extending the half-life in mice from 0.5 to 24 h (16). Each dose of IL-4 complex contained 2.5 μg of recombinant IL-4 cytokine (PeptroTech) and 15 μg of an anti-IL-4 antibody (clone 11B11; BioXcell) suspended in 150 μl of sterile PBS and was administered by intraperitoneal injection on days 2–4 after intratracheal LPS or on days 2–3 after intratracheal PAO1 vs. PBS (150 μl) unless stated otherwise. For blocking experiments, anti-IL-4 antibody (200 μg; clone 11B11; BioXcell) suspended in 150 μl of sterile PBS or PBS alone (150 μl) was delivered intraperitoneal on days 1–5 after intratracheal LPS. Where specified, isotype antibody (Rat IgG1; clone BE0088; BioXcell) replaced anti-IL-4 Ab at the complex dose (15 μg) or in blocking experiments (200 μg).
Diphtheria toxin and clodronate liposome injections.
Diphtheria toxin (lot no. 15043A1; List Biological Laboratories) was diluted in PBS and administered via intraperitoneal injection on days −2 (50 μg/kg mouse) and −1 (10 μg/kg) before intratracheal LPS, and on days +1, +3, and +5 (10 μg/kg) following intratracheal LPS as previously described (52). Clodronate liposomes (Cl2MDP) or PBS liposomes (control) were prepared as previously described (55), followed by intravenous instillation after being diluted in 150 μl of PBS on days 0 and +3 relative to intratracheal LPS exposure.
BAL analysis.
BAL was centrifuged at 700 g for 10 min at 4°C. The cell-free supernatants were stored at −80°C until further analysis. The cell pellet was diluted in PBS, and total cell number was counted with a hemacytometer using trypan blue exclusion. Cell differentials (300 cells per sample) were counted on cytocentrifuge preparation with Diff-Quik stain (Baxter Diagnostics, McGaw Park, IL). Total protein was measured in the cell-free supernatant by the Lowry method (2). Albumin (Bethyl Laboratories, Montgomery, TX), TNF-α, IL-4, or KC (R&D Systems) was quantified in the cell-free supernatant by ELISA.
Lung histology: hematoxylin and eosin.
Lungs were inflated to a pressure of 25 cmH2O using 1% low melting agarose (Invitrogen, Carlsbad, CA) before sectioning and staining with hematoxylin and eosin (24).
Assessment of lung collagen content.
On day 6 after intratracheal LPS, left lungs were homogenized in 1 ml of Complete Lysis Buffer (Roche, Indianapolis, IN) using an UltraTurrax tissue homogenizer (Janke and Kunkel, Wilmington, NC). Collagen was measured by Sircol Assay (BioColor, Carrickferguss, UK) according to the manufacturer's instructions as before (18).
Lung function measurements.
On day 6 after intratracheal LPS, diffusing capacity was assessed by calculating the diffusion factor for carbon monoxide (DFCO) using a published method (13). In short, the lungs were inflated with 0.8 ml of a gas mixture [∼0.5% carbon monoxide (CO), 0.5% neon (Ne), and 99% room air]. After a 9-s exposure, the gas mixture was withdrawn from the lungs and diluted to 2 ml with room air. After 15 s the recovered gas was injected into a Micro GC gas chromatograph (Micro GC Model 3000A; INFICON, East Syracuse, NY) and the concentrations of Ne and CO were determined. The DFCO was defined as 1-(CO9/COC)/(Ne9/NeC), where COC and NeC represent the concentration of CO and Ne in the calibration gas and CO9 and Ne9 reflect the concentrations of CO and Ne after the 9-s breath hold. After DFCO measurements, mice were paralyzed by administration of 75 mg/kg succinylcholine and connected to a flexiVent system (Scireq, Montreal, QC, Canada). Mice were ventilated with 100% oxygen at a tidal volume of 10 ml/kg, a rate of 150 breaths per min and a positive end-expiratory pressure of 3 cmH2O. After 3 min of ventilation, the lungs were inflated to 30 cmH2O for 5 s and returned to normal ventilation for 1 min, and single-compartment mechanics were measured with a 2.5-Hz sinusoidal oscillation (Snapshot-150) to obtain respiratory system dynamic compliance (Crs).
Whole lung homogenate RNA and protein isolation.
On days 4 and 6 following LPS exposure, lung tissues were homogenized in Trizol Reagent (Life Technologies) and RNA and protein were extracted following phase-separation with chloroform. The aqueous phase was removed and total RNA was precipitated with 100% isopropyl alcohol, washed with 75% ethanol, and redissolved in DEPC-treated water. DNA was removed from the interphase/organic phase with 100% ethanol before protein was precipitated from the remaining phenol-ethanol supernatant by 100% isopropyl alcohol. Protein pellets were washed three times with 0.3 M guanidine hydrochloride in 95% ethanol, once with 100% ethanol, air-dried, and resuspended in 1% sodium dodecyl sulfate (SDS).
Real-time PCR.
Purified RNA sample concentrations were determined on a NanoDrop 1000 (Thermo Scientific). One microgram of total RNA from whole lung homogenates was reverse-transcribed into cDNA with oligo-dT and random primers using an iScript cDNA synthesis kit (Bio-Rad). Gene expression was assessed utilizing TaqMan Gene Expression Assays-On-Demand primer/probe sets and TaqMan Universal Master Mix (Life Technologies) on the Applied Biosystems 7500 real-time PCR system complete with SDS software. Fifteen-microliter PCR reactions were performed using 2 μl of cDNA, 0.5 μl of primer/probe set, 7.5 μl of master mix, and 5 μl of DEPC-treated water by initially heating the samples to 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of heating to 95°C for 15 s and 60°C for 1 min. Target gene expression levels were normalized to the housekeeping gene Actinb and the fold change was calculated using the 2−▵▵Ct method.
Western blotting.
Purified protein concentrations were determined by standard BCA assay (Pierce). SDS-PAGE using 50 μg of total protein was carried out with the Mini-Protean II System (Bio-Rad) before Western blotting. Samples were heated at 95°C for 20 min in Laemmli sample buffer (Bio-Rad) containing 5% 2-mercaptoethanol before being loaded onto AnykD Mini-Protean TGX polyacrylamide gels (Bio-Rad). Samples were run at 60 V through the stacking gel and then 90 V through the resolving gel in the presence of 1× Tris/glycine/SDS buffer (Bio-Rad). Gels were subsequently transferred in 1× Tris/glycine buffer (Bio-Rad) onto 0.2-μm PVDF membranes (Bio-Rad) for 1 h using 250 mA of constant current on ice. Membranes were blocked in SuperBlock T20 blocking buffer (Pierce) for 30 min at room temperature and then incubated overnight at 4°C with polyclonal rabbit anti-mouse Ym1 (STEMCELL Technologies), rabbit anti-mouse FIZZ1 (Peprotech), mouse anti-mouse Arg1 (BD Transduction Laboratories), or rabbit anti-mouse β-actin (Abcam) antibodies diluted 1:1,500 in blocking solution. After three washes in 1× PBS with 0.05% Tween-20 (Promega), blots were incubated with either goat anti-rabbit-HRP or goat anti-mouse-HRP (1:5,000; Thermo Scientific) for 1 h at room temperature, washed again, and developed using ECL reagents (GE Healthcare) according to manufacturer's instructions. Blots were imaged using the FluorChem Q system and software (Protein Simple). Digital images were enhanced for clarity by uniformly adjusting the tonal range across samples for each band of interest using the black point slider of the Levels Tool in Adobe Photoshop.
Flow cytometry.
On days 4, 5, or 6, cells were isolated from the BAL fluid or lung tissue. To isolate cells, lung tissue was minced with scissors into fine pieces and digested for 30 min in a 1 ml solution containing 1 mg/ml collagenase Type I (Invitrogen), 1 mg/ml DNase I (Roche Applied Science), and RPMI 1640 medium (Invitrogen). Lung tissue was ground through a 70-μm cell strainer (BD Biosciences) to form a single-cell suspension, washed, and suspended in ACK buffer (Invitrogen) to lyse red blood cells. The remaining leukocytes were passed through a 70-μm cell strainer, washed, and suspended in 1 ml of PBS. For surface staining, 1 × 106 cells were stained with the LIVE/DEAD Fixable Blue viability kit according to manufacturer's instructions (Life Technologies), washed, and then incubated in FACS buffer with Fc Block-2.4G2 (BD Pharmingen) antibody to block Fcγ III/II receptors for 10 min before the addition of fluorochrome-conjugated anti-mouse antibodies.
To identify macrophages and monocytes, cells were stained with anti-CD11b PE-Texas Red (Invitrogen), anti-F4/80-PE-Cy7 (Biolegend), anti-mouse mannose receptor (MMR; CD206)-Alexa Fluor 647 (Biolegend), anti-Dectin-1-Alexa Fluor 700 (R&D Systems), anti-Ly6c-BV605 (Biolegend), anti-CD64-PE (Biolegend), and anti-Siglec-F-BV421 (BD Biosciences). For intracellular staining of monocytes/macrophages, isolated cells were first cultured in 1 ml of complete RPMI media containing 5% mouse serum (Jackson Immuno Research) for 4 h at 37°C with Brefeldin A (eBioscience) and washed twice before surface staining. Then, 1 × 106 surface-stained cells were washed, fixed, and permeabilized with a Cytofix/Cytoperm kit (BD Biosciences) before intracellular staining for 30 min with rabbit anti-mouse primary antibodies to FIZZ1 (1:100; Peprotech). Intracellular-stained cells were washed once, stained for an additional 30 min with a donkey anti-rabbit-BV510 secondary antibody (1:100; Biolegend) for anti-FIZZ1, and washed again before analysis. Treg number and activation were assessed by surface staining with anti-CD4-Ax700 (Biolegend) before fixation/permeabilization with a FoxP3 Staining Buffer Set (eBioscience) and intracellular staining with anti-Foxp3-APC (eBioscience) antibody. A FACSAria instrument equipped with FACSDiva software (BD Biosciences) was used for data acquisition and FlowJo software was utilized for analysis (Tree Star, San Carlos, CA). Instrument compensation was performed before data acquisition using UltraComp eBeads (eBioscience). Positive staining of live cells was determined against fluorescence-minus-one (FMO) controls after first gating away from debris and dead cells.
Statistical analyses.
All values are reported as mean ± SE. Parametric or nonparametric testing was performed as indicated. Changes in body weight between groups were compared using a repeated-measures one-way ANOVA with Fisher's protected least significant difference test. Kaplan-Meier survival curves were assessed by a log-rank Mantel-Cox test. All other analyses use a Student's t-test when comparing two groups at one time point or a one-way ANOVA with Tukey's post-hoc test when comparing multiple groups at one time point. P < 0.05 was used as the cut-off point for significance, with *P < 0.05, **P < 0.01, and ***P < 0.001.
RESULTS
IL-4 treatment improves mortality and accelerates resolution of experimental lung injury.
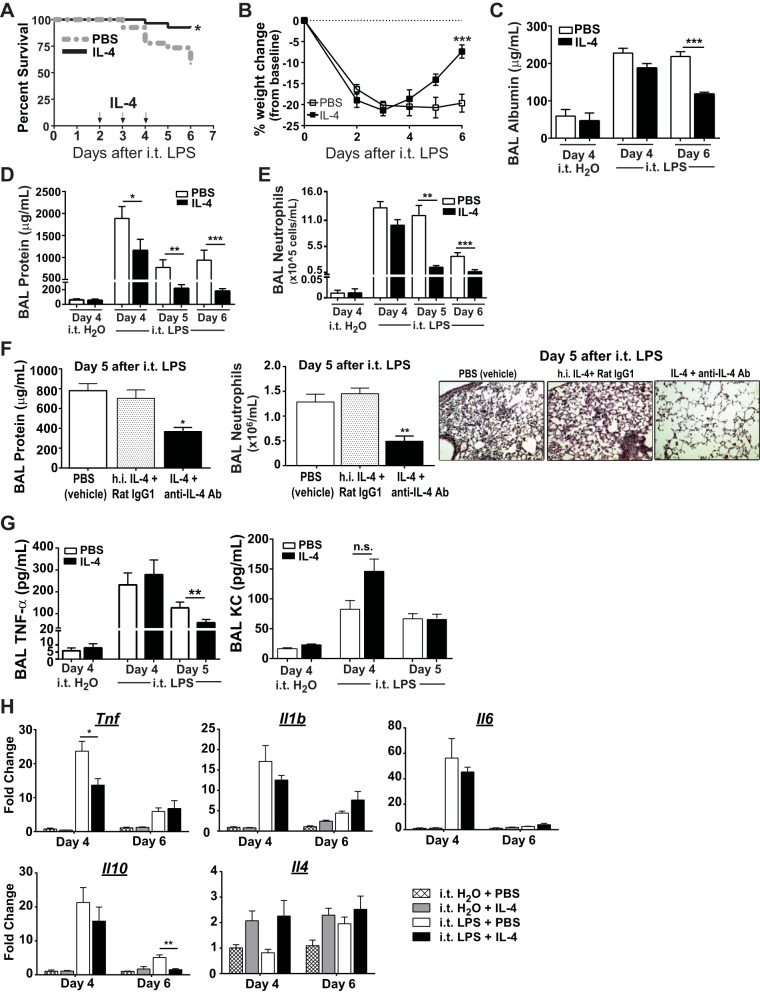
We treated WT C57BL/6 mice with high-dose intratracheal LPS (4 mg/kg) on day 0, followed by delayed IL-4 treatment on days 2–4. Compared with PBS-treated (vehicle) mice, IL-4-treated mice had significantly improved survival (92 vs. 57%; Fig. 1A) and improved weight gain (Fig. 1B) by day 6 after intratracheal LPS. Measurement of ALI parameters revealed as much as a twofold reduction in BAL albumin (Fig. 1C), a threefold reduction in BAL protein (Fig. 1D), and a fivefold reduction in BAL neutrophils (Fig. 1F) at latter time points (days 5–6) in mice treated with IL-4. IL-4 treatment following intratracheal H2O did not alter day 4 BAL protein, albumin, or neutrophil numbers compared with intratracheal H2O plus PBS treatment, and both groups had much less lung inflammation compared with LPS-exposed mice. To verify the specificity of the IL-4 effect, we compared IL-4 complex-treated mice (rIL-4 + anti-IL-4 Ab) to those treated with the same dose(s) of heat-inactivated IL-4 plus isotype Ab (heat-inactivated IL-4 + Rat IgG1). Compared with mice treated with IL-4 complex (IL-4 + anti-IL-4 Ab), mice treated with heat-inactivated IL-4 + Rat IgG1 had significantly increased BAL neutrophils and BAL protein, as well as increased histopathologic evidence of lung injury at day 5 after intratracheal LPS (Fig. 1F). Mice treated with PBS (vehicle) or heat-inactivated IL-4 + Rat IgG1 were similar for all parameters measured.
Fig. 1.
Wild-type (WT; C57BL/6) mice treated with IL-4 demonstrate improved survival and accelerated acute lung injury (ALI) resolution. A: Kaplan-Meier survival curves for mice exposed to intratracheal (i.t.) LPS (4 mg/kg) and treated with IL-4 or PBS (n = 27–30, P = 0.006 by Mantel-Cox). B: daily percent weight change from baseline for each group following intratracheal LPS (n = 6–10, ***P < 0.001 by repeated-measures ANOVA). Bronchoalveolar lavage (BAL) albumin (C) and BAL protein (D) values, as well as BAL neutrophil (E) numbers at day 4 in intratracheal H2O-exposed mice, or at days 4–6 in intratracheal LPS-exposed mice (n = 4 for intratracheal H2O groups, 6–10 for intratracheal LPS groups, *P < 0.05, **P < 0.01 by one-way ANOVA). F: following intratracheal LPS, mice received either PBS (vehicle), heat-inactivated (h.i.) IL-4 + Rat IgG1 (protein control), or IL-4 + anti-IL-4 Ab (active IL-4) on days 2–4. BAL protein and BAL neutrophils were quantified at day 5 after intratracheal LPS (n = 7 per group, *P < 0.05, **P < 0.01 by one-way ANOVA). Representative hematoxylin and eosin stain of the lung at day 5 after intratracheal LPS in all 3 groups at ×20 magnification. G: BAL TNF-α and KC after intratracheal H2O or LPS (**P < 0.01 by t-test, n = 4 for intratracheal H2O groups, 5–6 for LPS groups). H: gene expression changes for Tnf, Il1b, Il6, Il10, and Il4 in lung tissues following intratracheal LPS or H2O in IL-4- or PBS-treated mice (n = 3–6, *P < 0.05, **P < 0.01 by one-way ANOVA).
To assess the potential effects of IL-4 therapy on the production of inflammatory mediators in the lung, we monitored secretion and gene expression of select cytokines and chemokines in the BAL and lung. IL-4 treatment reduced alveolar levels of the proinflammatory cytokine TNF-α at day 5 but did not significantly alter levels of the chemokine KC despite significantly fewer alveolar neutrophils at these later time points (Fig. 1G). Intratracheal LPS-treated mice had dramatically increased expression of the proinflammatory genes Tnf, Il1b, and Il6, as well as the anti-inflammatory gene Il10 (Fig. 1H). The addition of IL-4 was found to significantly reduce day 4 Tnf and day 6 Il10 expression but had little impact on the levels of Il1b and Il6 mRNA. Irrespective of IL-4 treatment, LPS exposure induced minimal, nonsignificant changes in Il4 gene expression at days 4 and 6, suggesting there is little endogenous IL-4 production during later phases of ALI.
IL-4 treatment reduces fibroproliferation and improves lung mechanics after lung injury.
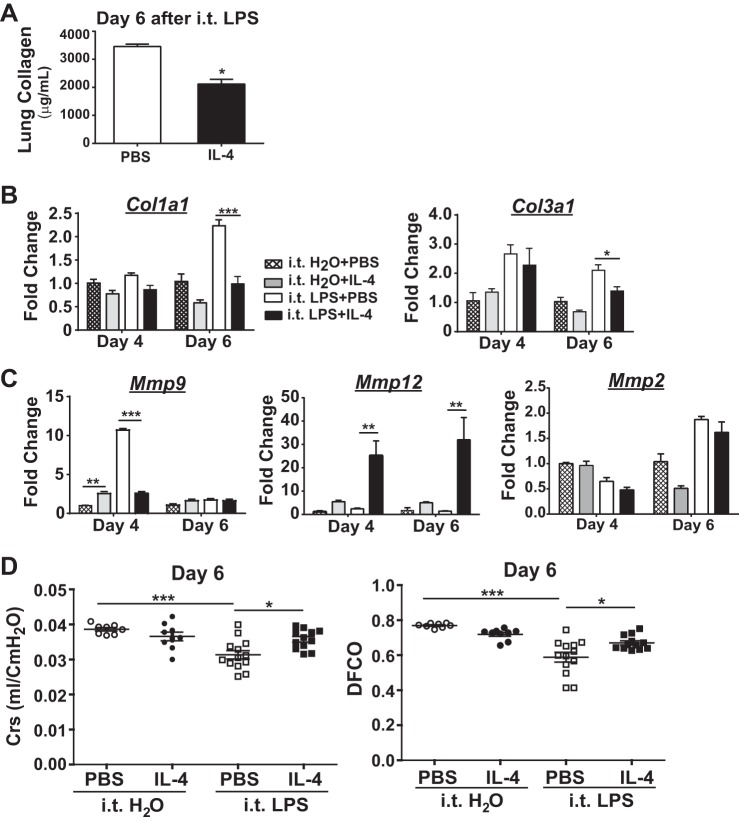
To determine other proresolution effects of IL-4 therapy, we further investigated its effects on lung repair and remodeling by assessing its impact on changes associated with fibroproliferation. In the intratracheal LPS ALI model, peak lung collagen levels coincide with maximal lung injury and are significantly reduced with resolution of lung inflammation and lung repair (9). At day 6 after intratracheal LPS, IL-4-treated mice had significantly reduced lung collagen compared with PBS-treated mice (Fig. 2A). To help determine if IL-4 treatment also reduced production of collagen, we measured whole lung Col1a1 and Col3a1 mRNA (Fig. 2B). At day 4, we observed similar expression of Col1a1 mRNA and Col3a1 expression among the intratracheal LPS-exposed and IL-4- and PBS-treated groups. By day 6, however, both Col1a1 expression and Col3a1 mRNA expression were significantly lower in LPS-exposed mice treated with IL-4 compared with LPS-exposed mice treated with PBS. This reduction in collagen gene expression correlated with the reduction in total collagen levels and thus identifies a potential mechanism for IL-4 to enhance lung repair.
Fig. 2.
Fibroproliferative changes are reduced in WT (C57BL/6) mice treated with IL-4 after ALI. A: lung collagen at day 6 following intratracheal (i.t.) LPS (n = 5–6, *P < 0.05). Gene expression for the collagen genes Col1a1 and Col3a1 (B) and the matrix metalloproteinase (MMP) genes Mmp9, Mmp12, and Mmp2 (C) quantified in lung tissue and normalized to Actinb at days 4 and 6 after intratracheal LPS or intratracheal H2O (n = 3–5, *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA). D: dynamic lung compliance (Crs; ml/cmH2O) and diffusing capacity (DFCO) after intratracheal LPS or intratracheal H2O (n = 8–12, *P < 0.05, ***P < 0.001 by one-way ANOVA).
Matrix metalloproteinases (MMPs) have been shown to influence lung injury and repair in a variety of inflammatory models by regulating extracellular matrix components such as collagen and elastin (22). MMP 2, 9, and 12 are among those that appear to dynamically regulate fibrotic lung processes (19). To determine whether IL-4 treatment altered lung MMPs as another potential mechanism to limit fibroproliferation following intratracheal LPS, we measured whole lung gene expression for Mmp2, Mmp9, and Mmp12 (Fig. 2C). Mice exposed to intratracheal LPS plus PBS expressed significantly elevated levels of Mmp9 mRNA at day 4, yet with IL-4 treatment, Mmp9 expression in LPS-exposed mice was reduced back to baseline levels. In marked contrast, Mmp12 expression was significantly upregulated in intratracheal LPS-exposed mice treated with IL-4 at both days 4 and 6 compared with other groups. Unlike dynamic changes with Mmp9 and Mmp12 in response to IL-4, Mmp2 mRNA levels were similar between all groups.
To assess whether differences in ALI resolution and fibroproliferative processes correlated with physiologic lung recovery and repair, we measured select lung mechanics at day 6 following either intratracheal H2O or intratracheal LPS (Fig. 2D). There were no marked differences in Crs or DFCO between intratracheal H2O-exposed groups. However, mice exposed to intratracheal LPS demonstrated a significant, ∼25% reduction in Crs and DFCO compared with intratracheal H2O-exposed mice and approached a DFCO of 0.45 which is associated with extremely poor gas exchange (similar values are obtained from deceased and exsanguinated mice) (13). With IL-4 treatment in intratracheal LPS-exposed mice, Crs recovered to the level observed in intratracheal H2O groups, and the DFCO was also significantly improved. Therefore, the beneficial effects of IL-4 therapy on lung function following experimental ALI strongly support its positive impact on resolution of lung inflammation and enhanced repair.
IL-4 blockade impairs endogenous ALI resolution.
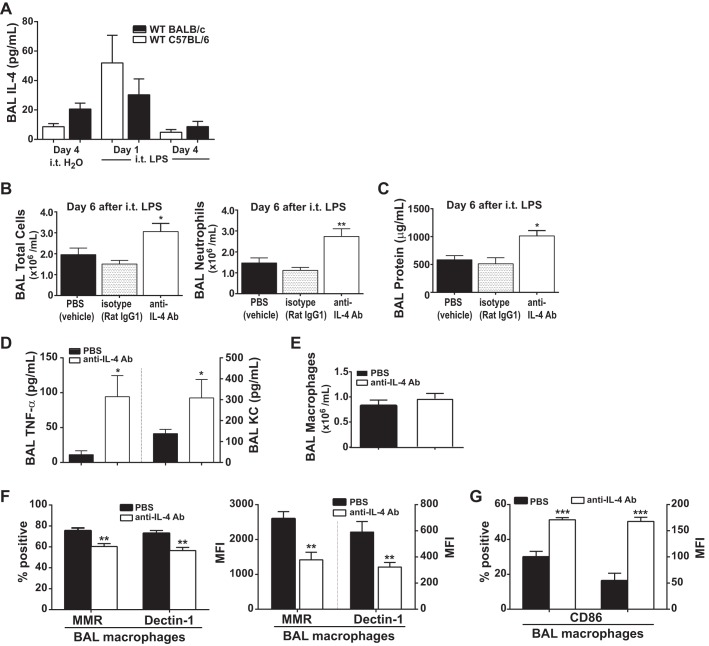
We were interested in knowing whether IL-4 contributed to endogenous ALI resolution. Following intratracheal LPS (4 mg/kg), IL-4 levels in the alveolar space were highest at day 1 and were reduced by day 4 (Fig. 3A). To ascertain whether endogenous IL-4 is necessary for ALI resolution in the intratracheal LPS ALI model, we utilized once daily antibody-mediated blockade of IL-4 starting on day 1 after intratracheal LPS (5 mg/kg) exposure to BALB/c mice, which are more Th2-prone and have been reported to produce more IL-4 at baseline (30). We utilized a higher LPS dose to ensure that a difference in ALI resolution could be seen with IL-4 blockade. Mice treated with anti-IL-4 antibody (200 μg) had significantly impaired resolution of ALI with a significant increase in the total number of BAL cells, a twofold increase in BAL neutrophils (Fig. 3B), and a twofold increase in BAL protein (Fig. 3C) compared with PBS-treated and isotype antibody-treated (Rat IgG1) mice at day 6 after intratracheal LPS. Mice treated with Rat IgG1 isotype or PBS had similar levels of ALI.
Fig. 3.
Following intratracheal LPS exposure, blocking IL-4 delays endogenous ALI resolution. A: BAL IL-4 levels were quantified following intratracheal LPS exposure to WT C57BL/6 and BALB/c mice (n = 4). Following intratracheal LPS (5 mg/kg) exposure to BALB/c mice, mice received either PBS (vehicle), Rat IgG1 (isotype antibody), or anti-IL-4 Ab (blocking antibody) on days 1–5. BAL total cell count and neutrophils (B) as well as BAL protein (C) were quantified at day 6 after intratracheal LPS (n = 5–6, *P < 0.05, **P < 0.01 by one-way ANOVA). BAL TNF-α (D) and BAL macrophages (E) were also quantified at day 6 after intratracheal LPS in PBS- and anti-IL-4 Ab-exposed groups (n = 4, *P < 0.05 by t-test). Percent positive and MFI expression of the M2 macrophage markers MMR and Dectin-1 (F) and the M1 marker CD86 (G) among F4/80+ macrophages (n = 4–5, **P < 0.01 and ***P < 0.001 by t-test). MFI, mean fluorescence intensity.
Furthermore, use of anti-IL-4 antibody resulted in a fourfold increase in BAL TNF-α, and a twofold increase in BAL KC compared with PBS-treated mice (Fig. 3D), suggestive of higher levels of proinflammatory signaling. Interestingly, although the number of alveolar macrophages recovered at day 6 was similar (Fig. 3E), blocking IL-4 reduced the number of macrophages expressing MMR and Dectin-1, markers of M2 activation (Fig. 3F), yet increased the number of macrophages expressing CD86, a marker of M1 activation (Fig. 3G). These data suggest that blocking endogenous IL-4 blunts ALI resolution, an effect that is associated with impaired M1 to M2 macrophage transition.
Macrophages robustly express M2 markers in response to IL-4 and are required to accelerate ALI resolution.
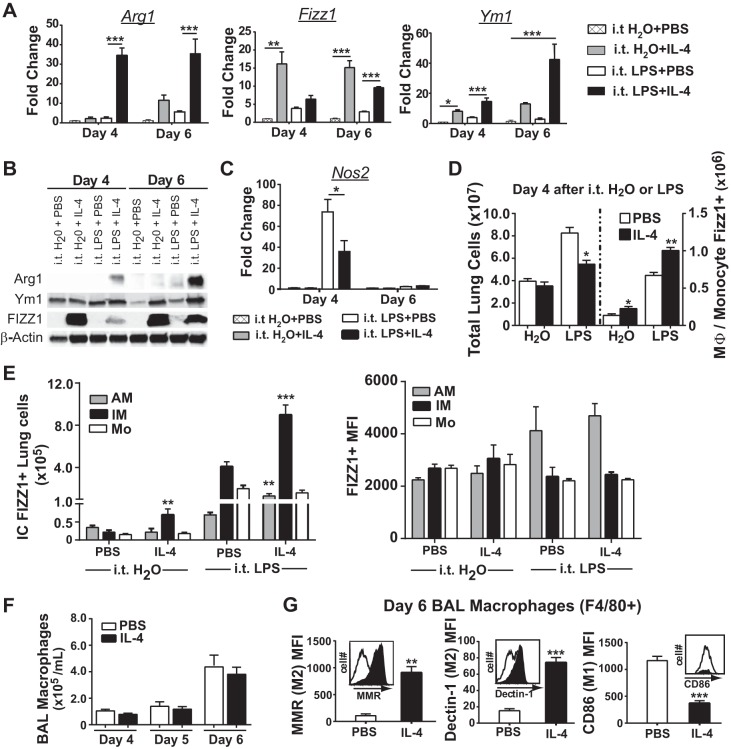
We further sought to determine whether IL-4 directly mediated M2 reprogramming of lung macrophages and whether this could be a mechanism to accelerate ALI resolution and lung repair. Mice treated with IL-4 after intratracheal LPS had significantly increased levels of M2 markers Arginase 1 (Arg1), Fizz1 (Retnla), and Ym1 (Chil3) (Fig. 4A), which were validated at the protein level utilizing lung immunoblots (Fig. 4B). In contrast, IL-4 therapy reduced whole lung mRNA levels of inducible nitric oxide synthase (Nos2), a classical M1 marker, at day 4 (Fig. 4C). Furthermore, mice exposed to intratracheal LPS plus IL-4 had significantly more FIZZ1-expressing monocytes or macrophages within the lung (Fig. 4D). To determine how IL-4 induced M2 programming in resident and recruited macrophage subpopulations, we quantified intracellular FIZZ1 expression among alveolar macrophages, interstitial macrophages, and monocytes (Fig. 4E). In intratracheal LPS-exposed mice, IL-4 treatment resulted in a significant twofold increase in the number of FIZZ1-expressing interstitial macrophages and a smaller, yet significant, increase in FIZZ1-expressing alveolar macrophages compared with PBS. The MFI of FIZZ1 expression among FIZZ1-positive cells was similar. Although a similar number of BAL macrophages were present in PBS- and IL-4-treated mice (Fig. 4F), we also quantified M2 and M1 surface marker expression among alveolar macrophages (F4/80+) at day 6 in intratracheal LPS-treated mice to determine whether IL-4 could sustain macrophage reprogramming 2 days after the last exposure (Fig. 4G). In fact, alveolar macrophages from IL-4-treated mice expressed fourfold higher levels of MMR and Dectin-1 and threefold lower levels of CD86 compared with PBS-treated mice.
Fig. 4.
Lung macrophages prominently express M2 proteins following IL-4-treatment in intratracheal LPS-exposed WT mice. A: mRNA expression for the M2 markers Arg1, Fizz1, and Ym1 quantified in the whole lung at days 4 and 6 among intratracheal H2O- or LPS-exposed mice with IL-4 or PBS treatment (n = 3–6, *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA). B: whole lung tissue immunoblots for Arg1, Ym1, FIZZ1, and β-actin after intratracheal LPS or H2O. C: mRNA expression of the M1 marker Nos2 quantified in the whole lung of mice at days 4 and 6 among the 4 groups (n = 3–6, *P < 0.05 by one-way ANOVA). D: total lung cells (left) and the number of lung monocytes and macrophages that expressed intracellular FIZZ1 using flow cytometry (right) at day 4 after intratracheal H2O or intratracheal LPS (n = 4–5, *P < 0.05, and **P < 0.01 by one-way ANOVA). E: intracellular (IC) FIZZ1 expression quantified by the number of positive cells and MFI for monocyte (Mo; Ly6c+CD11b+CD64low/−), interstitial macrophage (IM; CD64+CD11b+F4/80+), and alveolar macrophage (AM; CD64+SiglecF+CD11b−) populations in the whole lung of each group (n = 4–5, **P < 0.01 and ***P < 0.001 by t-test comparing H2O or LPS groups for AM, IM, or Mo populations). F: BAL macrophage numbers in intratracheal LPS-exposed mice followed by IL-4 or PBS (n = 6–10). G: M2 markers MMR and Dectin-1, and the M1 marker CD86, quantified on the surface of F4/80+ macrophages at day 6 after intratracheal LPS. Representative flow histogram plots of the number of cells (y-axis) and fluorescence intensity (x-axis, log scale) for each marker are shown (n = 4–5, **P < 0.01 and ***P < 0.001 by t-test).
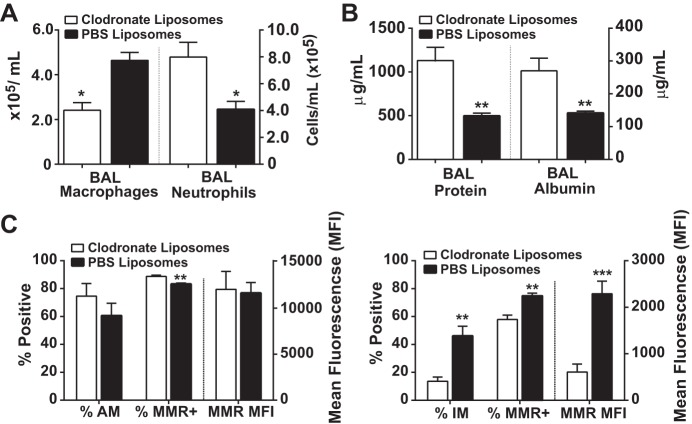
To address the importance of IL-4-stimulated macrophages to accelerate ALI resolution, we depleted macrophages using intravenous liposomal clodronate on days 0 and 3, timed as to not disrupt the onset of ALI in mice exposed to intratracheal LPS. Along with a 40–50% reduction in lung macrophages, significantly fewer macrophages were recovered by BAL in the macrophage-depleted group, leading to a twofold increase in BAL neutrophils (Fig. 5A), BAL protein (Fig. 5B), and BAL albumin despite IL-4 treatment. In IL-4-treated mice, liposomal clodronate exposure did not markedly change the percentage of alveolar macrophages (F4/80+CD11c+CD11b−) or their MMR expression (Fig. 5C). In contrast, liposomal clodronate exposure significantly reduced the percentage of interstitial macrophages (F4/80+CD11b+CD11c+/−) and their MMR expression compared with control mice exposed to PBS liposomes. Collectively, these data support the importance of the interstitial macrophage subpopulation for the beneficial effects of IL-4 therapy.
Fig. 5.
Macrophage depletion mitigates IL-4 benefits on ALI resolution. A: BAL macrophage and BAL neutrophil number at day 5 after intratracheal LPS and IL-4 in mice receiving either liposomal clodronate (macrophage depleted) or PBS liposomes (control) (n = 4–5, *P < 0.05 by t-test). B: BAL protein and BAL albumin at day 5 quantified in both groups following IL-4 therapy (n = 4–5, **P < 0.01 by t-test). C: percent depletion of alveolar (AM; F4/80+CD11c+) and interstitial (IM; F4/80+CD11b+) macrophage subpopulations, as well as the percentage and MFI of MMR expression among these cells (n = 4–5, *P < 0.05, **P < 0.01, and ***P < 0.001 by t-test).
IL-4 therapeutic effects are negated in mice with impaired M2 macrophage activation.
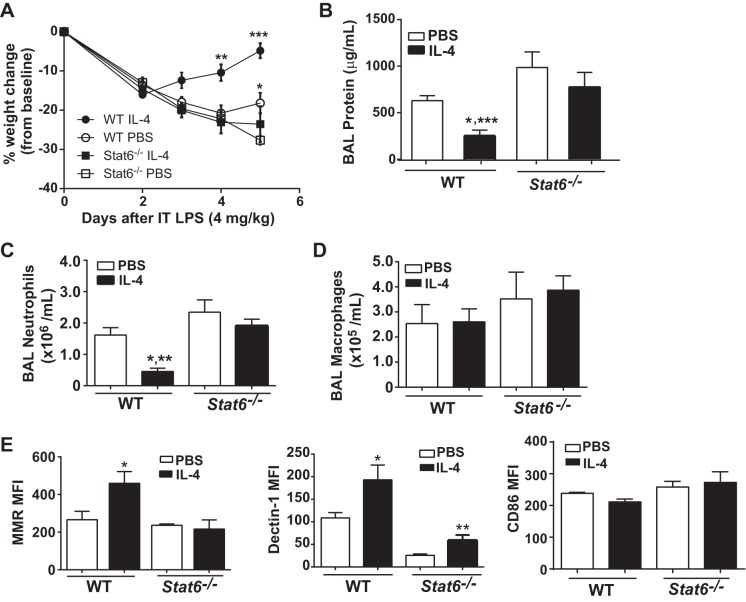
We further sought to determine whether the success of IL-4 therapy requires M2-specific macrophage activation. To test this hypothesis, we compared the response to intratracheal LPS between WT mice and Stat6−/− mice, which are deficient in a master transcriptional regulator of M2 differentiation and activation (28). Following intratracheal LPS, IL-4-treated WT mice had significant weight gain compared with all other groups at days 4 and 5 (Fig. 6A), as well as a greater than twofold reduction in BAL protein (Fig. 6B) and BAL neutrophils (Fig. 6C) at day 5. Compared with IL-4-treated WT mice, Stat6−/− mice demonstrated impaired resolution with persistently elevated BAL protein and neutrophils and a blunted response to IL-4. Furthermore, despite a similar number of macrophages recovered by BAL compared with WT mice (Fig. 6D), alveolar macrophages from Stat6−/− mice expressed significantly lower levels of MMR and Dectin-1 (Fig. 6E). In contrast to reduced M2 marker expression in Stat6−/− mice, we observed similar CD86 expression between groups (Fig. 6E). Overall, these data support the importance of macrophage STAT6-dependent M2 protein upregulation as necessary for IL-4 to accelerate resolution following experimental ALI.
Fig. 6.
IL-4 does not accelerate ALI resolution in Stat6−/− mice. Weight change (A), BAL protein (B), neutrophils (C), and macrophages (D) quantified in WT and Stat6−/− mice with IL-4 or PBS treatment on day 5 after intratracheal LPS (n = 7–10. For BAL protein,*P < 0.05 compared with WT sham and Stat6−/− IL-4 and ***P < 0.001 compared with Stat6−/− PBS by t-test. For BAL neutrophils, *P < 0.05 to WT PBS, and **P < 0.01 compared with Stat6−/− by t-test). E: among BAL macrophages (F4/80+), MFI for mannose receptor (MMR), Dectin-1, and CD86 quantified by flow cytometry (n = 4–5, *P < 0.05 and **P < 0.01 by t-test).
Tregs are not necessary for IL-4 to accelerate ALI resolution.
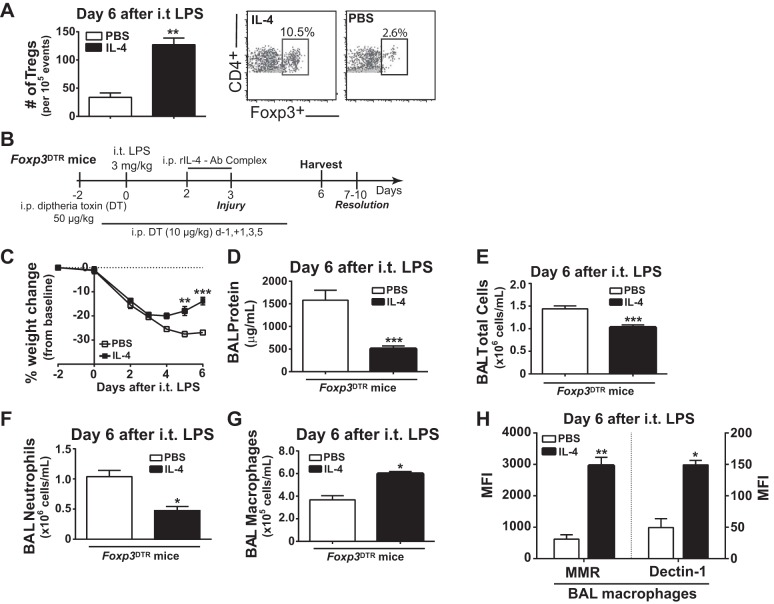
We have previously defined the critical importance of CD4+CD25+Foxp3+ Tregs for experimental ALI resolution and lung repair (9). Compared with PBS-treated mice, IL-4-treated mice demonstrated a significant threefold increase in Tregs recovered by BAL at day 6 after intratracheal LPS (Fig. 7A). Because IL-4 can induce proliferation and maintain full suppressive function of Tregs (41), we sought to understand whether Tregs contributed to the beneficial effects of IL-4 therapy on ALI resolution. We used Foxp3DTR mice and treated them with intraperitoneal diphtheria toxin to selectively deplete Tregs (Fig. 7B). We used a lower LPS dose because diphtheria toxin-treated Foxp3DTR mice have increased susceptibility to intratracheal LPS and achieved similar depletion in PBS- and IL-4-treated groups (<0.2% of all CD4+ cells in spleen, lung, BAL) as before (52). Despite Treg absence, IL-4 therapy in Foxp3DTR mice significantly increased weights (Fig. 7C) and significantly reduced parameters of ALI including BAL protein (Fig. 7D), BAL total cell count (Fig. 7E), and BAL neutrophils (Fig. 7F) compared with PBS controls in Foxp3DTR mice when assessed at day 6 after intratracheal LPS. We also observed a significant increase in BAL macrophages in IL-4-treated Foxp3DTR mice compared with PBS-treated mice (Fig. 7G), as well as higher M2 macrophage surface marker expression (Fig. 7H). Collectively, these data demonstrate that Tregs are not required for IL-4 to accelerate ALI resolution and to reprogram lung macrophages.
Fig. 7.
Regulatory T cells (Tregs) are not necessary for IL-4 to accelerate ALI resolution. A: alveolar Tregs were enumerated at day 6 following intratracheal LPS in PBS or IL-4 treated mice (n = 4–5, **P < 0.01 by t-test), following identification by dual positive expression of CD4 and Foxp3 using flow cytometry. B: experimental design for Treg depletion in Foxp3DTR mice using intraperitoneal injection of diphtheria toxin (DT). All Foxp3DTR mice received DT to deplete Tregs and either IL-4 or PBS treatment following intratracheal LPS. C: Daily weight difference compared with baseline (day 2) expressed as percent change (n = 7–8, **P < 0.01, ***P < 0.001 by repeated-measures ANOVA). At day 6 after intratracheal LPS, ALI markers including total protein (D), cell count (E), neutrophils (F), and macrophages (G) were quantified following BAL in IL-4 and PBS-treated Foxp3DTR mice (n = 8, *P < 0.05, ***P < 0.001 by t-test). H: among BAL macrophages (F4/80+), the MFI for MMR and Dectin-1 (n = 4, *P < 0.05, **P < 0.01 by t-test).
IL-4 therapy can also accelerate resolution following infection-induced ALI.
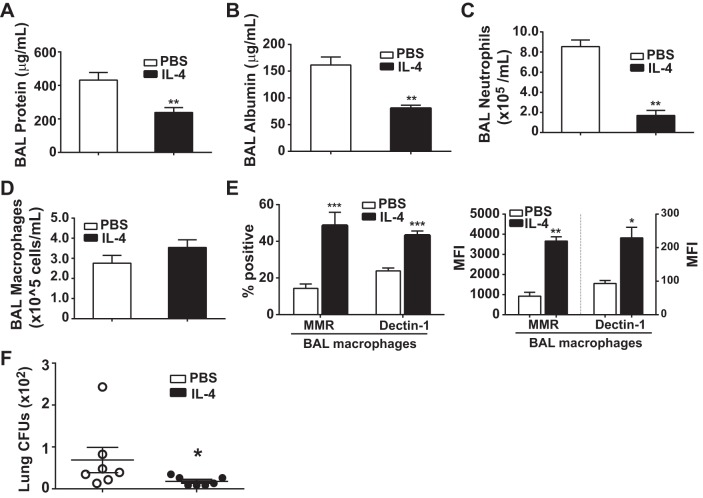
As is the case with any experimental ALI model, intratracheal LPS cannot recapitulate all of the pathologic features of human ARDS (36). Furthermore, there are reports that IL-4 programming of M2 macrophages may be a detriment towards macrophage-derived bacterial clearance (29). Therefore, we tested whether IL-4 would be beneficial in experimental ALI in a live bacteria model induced by intratracheal administration of the Pseudomonas aeruginosa O1 strain (intratracheal PAO1). We have previously shown reproducible lung injury and resolution patterns by days 4–5 following intratracheal PAO1, with reduced lung CFUs by day 2 (4). Mice were exposed to intratracheal PAO1 on day 0, followed 2 and 3 days later with IL-4 or PBS treatment. At day 4, IL-4-treated mice had significant, twofold reductions in BAL protein (Fig. 8A) and BAL albumin (Fig. 8B). In addition, we observed a fourfold reduction in BAL neutrophils (Fig. 8C). Despite similar numbers of BAL macrophages (Fig. 8D), IL-4 treatment more than doubled the percentage of alveolar macrophages (F4/80+) that expressed the M2 surface markers MMR and Dectin-1 and also significantly increased the MFI for each marker compared with macrophages from PBS-treated mice (Fig. 8E). Interestingly, IL-4-treated mice had significantly reduced PAO1 CFUs in the lung at day 4 compared with PBS-treated mice (Fig. 8F), although the majority of PAO1 had been cleared from the lungs of both groups.
Fig. 8.
IL-4 accelerates ALI resolution following lung Pseudomonas (PAO1) challenge. BAL protein (A), albumin (B), neutrophils (C), and macrophages (D) were quantified (n = 6, **P < 0.01 by t-test.) at day 4 in WT mice exposed to intratracheal PAO1 [∼2 × 106 colony-forming units (CFUs)] followed by IL-4 or PBS on days 2 and 3. E: among BAL macrophages (F4/80+), the percent positive and MFI for the M2 markers MMR and Dectin-1 (n = 6, *P < 0.05, **P < 0.01, ***P < 0.001 by t-test). F: lung CFUs were quantified at day 4 after WT mice were exposed to intratracheal PAO1 (∼1.5 × 106 CFUs) followed by IL-4 or PBS on days 2 and 3 (n = 7, *P < 0.05 by t-test).
DISCUSSION
Delayed IL-4 therapy offered dramatic benefits following both sterile and infection-induced experimental ALI. Specifically, IL-4 improved mortality, accelerated resolution of lung injury, and restored lung function. Lung macrophages and STAT6 transcription factor expression were necessary for IL-4 to exert its therapeutic effects on lung injury resolution; Tregs, on the other hand, did not appear to be required. Before ALI resolution and lung repair, the M2 proteins Arg1, FIZZ1, and Ym1 were increased in the lung with IL-4 treatment, and among macrophages FIZZ1 upregulation was most pronounced in the interstitial subpopulation. In addition, the importance of the interstitial macrophage sub-population towards IL-4-derived ALI resolution is further supported by their preferential depletion in intravenous liposomal clodronate experiments.
Study of experimental ALI models is requisite to gain understanding of relevant biological pathways in human ARDS. Since the majority of ARDS cases are diagnosed after patients are hospitalized with acute lung inflammation (31), using IL-4 as a therapy to target late-phase, proresolution pathways may be more effective in improving survival and restoring lung function (14). Researchers in ARDS have begun to focus on understanding the resolution phase to generate pharmacologic targets and even potential therapeutics, with an emphasis on epithelial and endothelial barrier restoration as well as neutrophil clearance (35). Despite a prominent role for macrophages in neutrophil clearance, however, little effort has been invested to determine the significance of macrophages to accelerate resolution of lung inflammation and enhance lung repair. For some time, we have known that increased BAL macrophage number and phenotypic maturity are associated with improved outcomes in human ARDS (48, 53) but have yet to define specific macrophage contributions to the active process of ARDS resolution.
Although measuring secreted IL-4 may have limited utility due to its high binding affinity to myeloid cells that are abundantly present in circulation and in inflamed tissue (25), we observed that endogenous alveolar IL-4 levels are highest at day 1 in the intratracheal LPS model, findings supported by previous work (5). Therefore, by delaying exogenous IL-4 delivery until 2 days after intratracheal LPS when endogenous IL-4 levels appear to be lower, our findings support the therapeutic benefits of exogenous IL-4 when precomplexed to its antibody at ∼2:1 molar ratio, which is reported to prolong in vivo IL-4 activity from ∼30 min to >24 h (16). Furthermore, blocking endogenous IL-4 impaired ALI resolution and reduced expression of M2 macrophage proteins and therefore offers strong support as to the importance of IL-4 in endogenous ALI resolution. As such, our enthusiasm for the therapeutic potential of IL-4 is not diminished by prior reports that demonstrate a lack of consistent association between serum or BAL IL-4 levels with better outcome in human ARDS (6, 37). Because our focus was to understand whether exogenous IL-4 could accelerate resolution and lung repair in a macrophage-dependent manner, we did not attempt to identify cellular sources of IL-4, although this will be important to investigate in future work.
Mitigating influx and enhancing removal of accumulated alveolar neutrophils is a hallmark of resolution of lung inflammation (32). M2 macrophages are effective at phagocytosis (12, 26), and IL-4 can restore impaired macrophage phagocytosis (15). Following infectious and sterile insults to the lung, IL-4 therapy resulted in a severalfold reduction in alveolar neutrophils at later time points despite similar production of neutrophil chemokines in the alveolar space. Although we cannot exclude direct effects of IL-4 on neutrophil apoptosis, signaling through the IL-4 receptor has been shown to actually delay apoptosis of human neutrophils (47). Therefore, we suggest a potential IL-4-mediated benefit would be to enhance macrophage efferocytosis of apoptotic neutrophils and accelerate experimental ALI resolution.
Aberrant or excessive fibroproliferation results in impaired lung repair following acute lung inflammation (7, 10). IL-4-treated mice had reduced lung collagen and expression of collagen genes, demonstrating a potential mechanism for IL-4 to enhance lung repair by limiting fibroproliferation and remodeling the extracellular matrix. Furthermore, with reduced Mmp9 and increased Mmp12 lung mRNA levels, IL-4 treatment altered expression of macrophage-derived matrix metalloproteinases (19), indicating a possible mechanism for IL-4-reprogrammed macrophages to enhance lung repair. In support of this hypothesis, prior work demonstrated that increased macrophage-derived MMP9 enhanced bleomycin-induced pulmonary fibrosis (40), and MMP12 produced by macrophages promoted matrix remodeling (8). Previous work has also highlighted the potential for M2 macrophages expressing Arg1 and FIZZ1 genes to limit fibrosis (39, 44), further evidence that IL-4-polarized M2 macrophages can promote lung repair. Work by Huaux et al. (27) also surmised an antifibrotic and immunoprotective effect of endogenous IL-4 early after bleomycin-induced lung injury but possibly a profibrotic role during the reparative phase. The variable effects of IL-4 on fibrosis during different phases of the bleomycin model highlight that timing is of critical importance in lung inflammatory models, and emphasizes that testing therapies in more than one experimental ALI model is an important step towards translation to human ARDS (36).
Although M1 macrophages are typically associated with enhanced microbial phagocytosis and killing of intracellular bacteria via inducible nitric oxide synthase, TNF-α, and IL-12 production (26), IL-4 reprogramming of M2 macrophages was able to accelerate ALI resolution in an infection ALI model with a possible benefit towards bacterial clearance. M2 macrophages are reported to have increased phagocytic activity via upregulation of Fc or scavenger receptors (12). Although on-going bacterial replication occurring simultaneously with IL-4 therapy may be a cause for concern in the clinical realm or in other infectious experimental models, macrophages are a highly plastic cell and therefore would likely reprogram to a predominant M1 phenotype in response to infection-induced signaling of pattern recognition receptors. Furthermore, in mice, and in at least some reports in humans, a significant percentage of quiescent alveolar macrophages display an M2 phenotype with prominent mannose receptor expression (38, 43, 60) yet have undiminished capacity to initiate a robust proinflammatory response to infectious and noninfectious stimuli. Therefore, we surmise that not only does IL-4 therapy accelerate ALI resolution and lung repair, it may also promote homeostasis by reprogramming lung macrophages back to their quiescent, predominantly M2 phenotypic state.
To our surprise, the salutary effects of IL-4 on lung repair did not require Tregs. Although this finding seemed at odds with our prior work, in fact we have shown that Tregs can resolve lung inflammation by abrogating proinflammatory macrophage responses and, like IL-4, by reprogramming macrophages towards an M2 phenotype (9). Indeed, the work by Taams and colleagues (54, 56) has shown that human Tregs dampened LPS-induced M1 monocyte proinflammatory responses while promoting an M2 phenotype. Moreover, we cannot exclude the possibility that Tregs are an important IL-4 source and, as such, may regulate macrophage phenotype and function during lung inflammation as a mechanism to support endogenous resolution. Therefore, IL-4 treatment may obviate the need for endogenous Treg-derived IL-4 and potentially explain the lack of Treg requirement. With the ability of IL-4 to induce endogenous Treg proliferation and maintain their suppressive function (41), we further postulate that IL-4 treatment may have a synergistic prorepair effect by programming macrophages and Tregs.
We elected to use IL-4 treatment instead of IL-13, which can also signal through a macrophage IL-4 receptor to promote M2 protein expression, because IL-4 can induce more robust expression of M2 genes (25) and may be less likely to induce fibrosis (26). In addition to IL-4- or IL-13-derived M2 macrophages, there are several other subtypes of anti-inflammatory macrophages that are not addressed here yet may be important for endogenous repair pathways (3). Recent work has identified macrophages derived from stimulation with high-density immune complexes express elevated levels of IL-10 and can protect from lethal endotoxemia in a STAT6-independent fashion (17). Signaling pathways that do not require STAT6 to induce M2 marker expression include IL-6/IL-10/STAT3- and TLR/MyD88/C/EBP, and both may also be of significance in experimental ALI (11, 46). From this, we suggest the possibility that the therapeutic benefits of IL-4 and M2 marker expression may be amplified by similar STAT6-independent autocrine or paracrine signals. However, in the intratracheal LPS model, IL-4 signaling through macrophage STAT6 appears necessary to accelerate ALI resolution, suggesting IL-4-specific cytokine activity is critical. Given that our data merely associate select M2 markers with accelerated ALI resolution, however, a detailed molecular characterization of endogenous and IL-4-elicited macrophages supported by additional mechanistic studies is necessary to further delineate a critical role for specific M2-associated genes.
Despite intensive investigation in ARDS pathogenesis, translation of therapies has been largely disappointing in human ARDS. We propose a novel therapeutic agent, IL-4, that potently resolves sterile and nonsterile lung inflammation. By reprogramming macrophages into a critical prorepair phenotype, we also identify a novel cellular player to promote ALI resolution and lung repair. Importantly, IL-4 treatment was administered as a rescue therapy well after the onset of experimental ALI and thus offers translational potential.
GRANTS
The study was supported by National Heart, Lung, and Blood Institute Grants R00-HL-103973 (to F. R. D'Alessio), F32-HL-124823 (to J. M. Craig), P01-HL-10342 (to W. Mitzner), F32-HL-120400 (to B. D. Singer), and K08-HL-128867 (to B. D. Singer), R00HL096897 (to N. M. Heller), American Heart Association Grant 11FTF7280014 (to N. R. Aggarwal), and the Flight Attendant Medical Research Institute Young Clinical Science Awards Program (to N. R. Aggarwal).
DISCLOSURES
PCT application “Compositions and Methods to Accelerate Resolution of Acute Lung Inflammation,” PCT/US2016/012621 has been filed and is currently pending.
AUTHOR CONTRIBUTIONS
F.R.D., J.M.C., B.D.S., D.C.F., J.R.M., B.T.G., J.F., V.K.S., N.M.H., L.S.K., and N.R.A. conception and design of research; F.R.D., J.M.C., B.D.S., D.C.F., A.T., P.M., and N.R.A. analyzed data; F.R.D., J.M.C., V.K.S., N.M.H., W.M., L.S.K., and N.R.A. interpreted results of experiments; F.R.D., J.M.C., and N.R.A. drafted manuscript; F.R.D., J.M.C., B.D.S., D.C.F., J.R.M., B.T.G., J.F., A.T., P.M., J.H.G., N.L., V.K.S., N.M.H., W.M., L.S.K., and N.R.A. edited and revised manuscript; F.R.D., J.M.C., B.D.S., D.C.F., J.R.M., B.T.G., J.F., A.T., P.M., J.H.G., N.L., V.K.S., N.M.H., W.M., L.S.K., and N.R.A. approved final version of manuscript; J.M.C., B.D.S., J.R.M., B.T.G., J.F., A.T., P.M., J.H.G., N.L., and N.R.A. performed experiments; J.M.C., A.T., P.M., J.H.G., and N.R.A. prepared figures.
ACKNOWLEDGMENTS
We thank Raffaello Cimbro for assistance in the Johns Hopkins Bayview Flow Core, Dr. Alan Scott for donation of Stat6−/− mice, and Dr. Alexander Rudensky for donation of Foxp3DTR mice.
Present address of B. D. Singer: Division of Pulmonary and Critical Care, Northwestern University Feinberg School of Medicine, Chicago, IL.
Present address of J. R. Mock: Division of Pulmonary and Critical Care, University of North Carolina, Chapel Hill, NC.
REFERENCES
- 1.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes compared with traditional tidal volumes for acute lung injury, and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal NR, D'Alessio FR, Tsushima K, Files DC, Damarla M, Sidhaye VK, Fraig MM, Polotsky VY, King LS. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 298: L371–L381, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal NR, King LS, D'Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol 306: L709–L725, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggarwal NR, Tsushima K, Eto Y, Tripathi A, Mandke P, Mock JR, Garibaldi BT, Singer BD, Sidhaye VK, Horton MR, King LS, D'Alessio FR. Immunological priming requires regulatory T cells and IL-10-producing macrophages to accelerate resolution from severe lung inflammation. J Immunol 192: 4453–4464, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosmann M, Russkamp NF, Ward PA. Fingerprinting of the TLR4-induced acute inflammatory response. Exp Mol Pathol 93: 319–323, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouros D, Alexandrakis MG, Antoniou KM, Agouridakis P, Pneumatikos I, Anevlavis S, Pataka A, Patlakas G, Karkavitsas N, Kyriakou D. The clinical significance of serum and bronchoalveolar lavage inflammatory cytokines in patients at risk for acute respiratory distress syndrome. BMC Pulm Med 4: 6, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnham EL, Janssen WJ, Riches DW, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J 43: 276–285, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: matrix metalloproteinases in COPD. Eur Respir J 39: 197–209, 2012. [DOI] [PubMed] [Google Scholar]
- 9.D'Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, King LS. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J Clin Invest 119: 2898–2913, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dos Santos CC. Advances in mechanisms of repair and remodelling in acute lung injury. Intensive Care Med 34: 619–630, 2008. [DOI] [PubMed] [Google Scholar]
- 11.El Kasmi KC, Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, Murray PJ. Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 9: 1399–1406, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 101: 890–898, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallica J, Das S, Horton M, Mitzner W. Application of carbon monoxide diffusing capacity in the mouse lung. J Appl Physiol (1985) 110: 1455–1459, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, Pronovost PJ, Needham DM. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med 42: 849–859, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, Henson PM, Bratton DL. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood 113: 2047–2055, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol 151: 1235–1244, 1993. [PubMed] [Google Scholar]
- 17.Fleming BD, Chandrasekaran P, Dillon LA, Dalby E, Suresh R, Sarkar A, El-Sayed NM, Mosser DM. The generation of macrophages with anti-inflammatory activity in the absence of STAT6 signaling. J Leukoc Biol 98: 395–407, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garibaldi BT, D'Alessio FR, Mock JR, Files DC, Chau E, Eto Y, Drummond MB, Aggarwal NR, Sidhaye V, King LS. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am J Respir Cell Mol Biol 48: 35–43, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 7: 193–203, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 32: 593–604, 2010. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev 87: 69–98, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, Group PS. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368: 2159–2168, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Halbower AC, Mason RJ, Abman SH, Tuder RM. Agarose infiltration improves morphology of cryostat sections of lung. Lab Invest 71: 149–153, 1994. [PubMed] [Google Scholar]
- 25.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, Keegan AD. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal 1: ra17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2: 65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol 170: 2083–2092, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Lazarski CA, Ford J, Katzman SD, Rosenberg AF, Fowell DJ. IL-4 attenuates Th1-associated chemokine expression and Th1 trafficking to inflamed tissues and limits pathogen clearance. PLoS One 8: e71949, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol 14: 1146–1154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest 135: 936–943, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol 76: 467–492, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, Hubmayr RD, Gajic O. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. Am J Respir Crit Care Med 183: 59–66, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229: 176–185, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest 122: 2731–2740, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107: 1062–1073, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Melgert BN, ten Hacken NH, Rutgers B, Timens W, Postma DS, Hylkema MN. More alternative activation of macrophages in lungs of asthmatic patients. J Allergy Clin Immunol 127: 831–833, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ, Goldschmidt M, Swain GP, Yancopoulos GD, Valenzuela DM, Murphy A, Karow M, Stevens S, Pearce EJ, Artis D. Alternatively activated macrophage-derived RELM-α is a negative regulator of type 2 inflammation in the lung. J Exp Med 206: 937–952, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 204: 594–604, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Pace L, Rizzo S, Palombi C, Brombacher F, Doria G. Cutting edge: IL-4-induced protection of CD4+CD25- Th cells from CD4+CD25+ regulatory T cell-mediated suppression. J Immunol 176: 3900–3904, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guerin C, Prat G, Morange S, Roch A; ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363: 1107–1116, 2010. [DOI] [PubMed] [Google Scholar]
- 43.Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, Friedrich K, Muller-Quernheim J, Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol 137: 89–101, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5: e1000371, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pesce JT, Ramalingam TR, Wilson MS, Mentink-Kane MM, Thompson RW, Cheever AW, Urban JF Jr, Wynn TA. Retnla (relmalpha/fizz1) suppresses helminth-induced Th2-type immunity. PLoS Pathog 5: e1000393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, Murray PJ. Arginine usage in mycobacteria-infected macrophages depends on autocrine-paracrine cytokine signaling. Sci Signal 3: ra62, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratthe C, Girard D. Investigation of the interleukin (IL)−4/IL-4 receptor system in promyelocytic leukaemia PLB-985 cells during differentiation toward neutrophil-like phenotype: mechanism involved in IL-4-induced SOCS3 protein expression. Br J Haematol 140: 59–70, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schutte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 279: L25–L35, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121: 985–997, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singer BD, Mock JR, Aggarwal NR, Garibaldi BT, Sidhaye VK, Florez MA, Chau E, Gibbs KW, Mandke P, Tripathi A, Yegnasubramanian S, King LS, D'Alessio FR. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol 52: 641–652, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinberg KP, Milberg JA, Martin TR, Maunder RJ, Cockrill BA, Hudson LD. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med 150: 113–122, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol 66: 222–230, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 170: 499–509, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci USA 104: 19446–19451, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Wynn TA. IL13 effector functions. Annu Rev Immunol 21: 425–456, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Wynn TA, Cheever AW. Cytokine regulation of granuloma formation in schistosomiasis. Curr Opin Immunol 7: 505–511, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 49: 180–189, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]